Method for detecting polymers in cefotaxime sodium and injection thereof
A technology of cefotaxime and thioxime sodium, which is applied in the field of rapid determination of polymer content in cefotaxime sodium drugs, can solve the problems of low column efficiency and long analysis time, and achieve high accuracy, good repeatability, and linearity wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
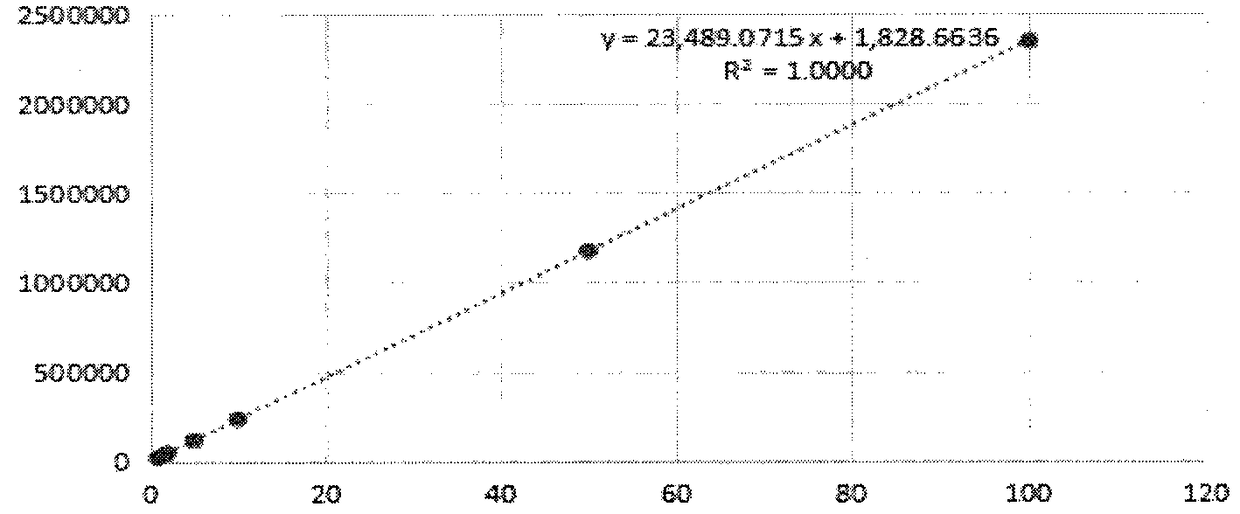
[0020] Example 1 Determination of polymer content in cefotaxime sodium and its injection by external standard method.
[0021] 1.1 Chromatographic conditions
[0022] Chromatographic column: a chromatographic column (SRTSEC-150, 7.8*300mm, 5μm) filled with a spherical silica gel matrix bonded to the surface of a nano-neutral hydrophilic film; mobile phase: 0.1mol / L phosphate buffer [0.1mol / L phosphoric acid Disodium hydrogen-0.1mol / L sodium dihydrogen phosphate solution (61:39)]; flow rate: 0.8mL / min; detection wavelength: 235nm. Injection volume: 10 μL. Column temperature: 35°C.
[0023] 1.2 Experimental steps
[0024] Preparation of reference solution
[0025] Take an appropriate amount of cefotaxime reference substance, accurately weigh it, add water to dissolve and quantitatively dilute to make a solution containing about 10 μg of cefotaxime sodium in every 1 ml, to obtain the product.
[0026] Preparation of the test solution
[0027] Take an appropriate amount of t...
Embodiment 2
[0044] The identification of each impurity peak of embodiment 2
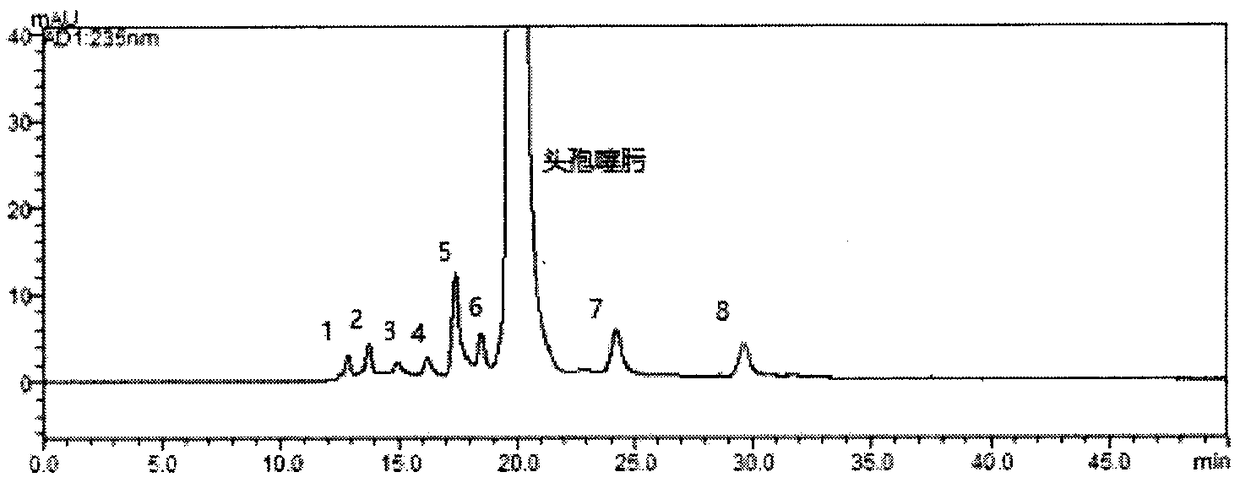
[0045] 2.1 Impurities with impurity reference substances: (A, B, E) (impurities 6, 5, 8)
[0046] Using cefotaxime existing reference substance cefotaxime impurity A (deacetyl cefotaxime), cefotaxime impurity B (deacetyl cefotaxime), cefotaxime impurity E (deacetyl cefotaxime lactone ) for sample injection analysis, impurity 6, 5, and 8 are respectively consistent with impurity A, B, E retention time in the chromatogram of need testing solution.
[0047] 2.2 Impurities of the reference substance without impurities: (impurities 1, 2, 3, 4, 7)
[0048]2.2.1 Chromatography and mass spectrometry conditions The chromatographic conditions are 0.02mol / L ammonium acetate as the mobile phase, the detection wavelength is 235nm, the column temperature is 35°C, and the flow rate is 0.3mL / min. Packed chromatographic column (SRT SEC-150, 7.8*300mm, 5μm).
[0049] Mass Spectrometry Conditions Electrospray ionization source ...
Embodiment 3
[0054] Embodiment 3 The typical chromatogram comparison of the inventive method and " Chinese Pharmacopoeia " 2015 edition cefotaxime sodium polymer method
[0055] 3.1 "Chinese Pharmacopoeia" 2015 edition cefotaxime polymer Sephadex G10 gel chromatography conditions
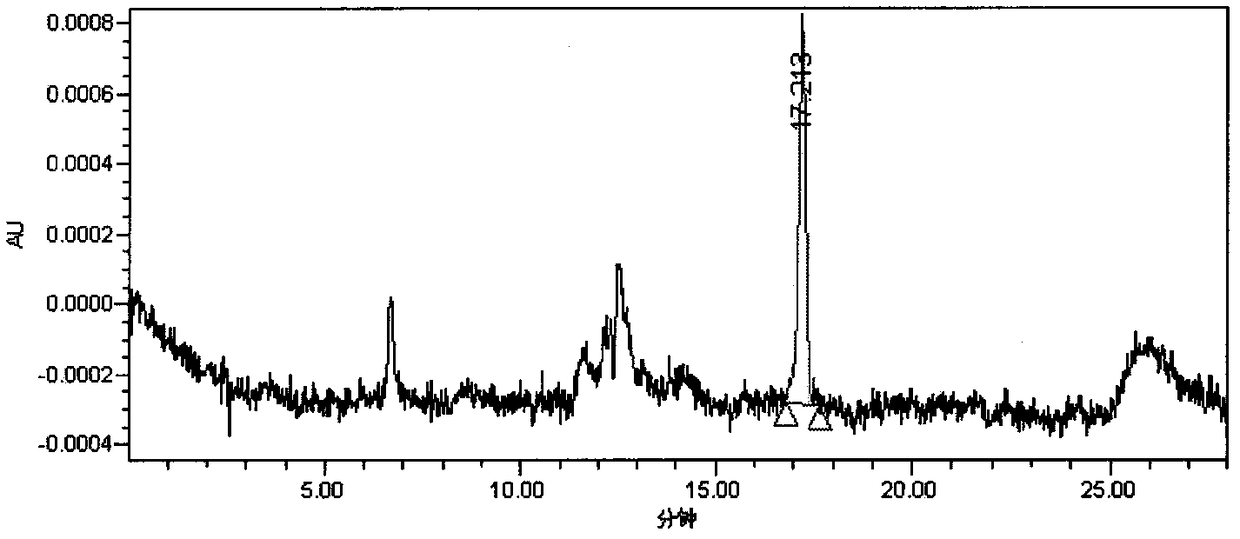
[0056] "Chinese Pharmacopoeia" 2015 edition cefotaxime polymer Sephadex G10 gel chromatography salt phase: 0.1mol / L phosphate buffer [0.1mol / L disodium hydrogen phosphate-0.1mol / L sodium dihydrogen phosphate (61:39 )], detection wavelength: 254nm; injection volume 100μl. Sample concentration: 20mg / ml, the typical chromatogram obtained is as attached Figure 5 , the polymer impurity peak is enriched, and the method of the present invention is used for reanalysis, and the results are as attached Figure 6 , show that the method of the present invention has better separation ability, obtain 4 impurity peaks before main peak cefotaxime, and No. 1, 2, 3 and No. 4 peaks obtained by separation of the aforementioned n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com