Compound preparation of cefotaxime sodium and sulbactam sodium as well as preparation method and application thereof
A technology of cefotaxime sodium and compound preparations, which is applied in the directions of medical preparations containing active ingredients, pharmaceutical formulas, powder delivery, etc. Dispensing, short validity period and other issues, to achieve the effect of easy transportation and long-term storage, improving drug safety and economic benefits, and easy storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Prescription composition:
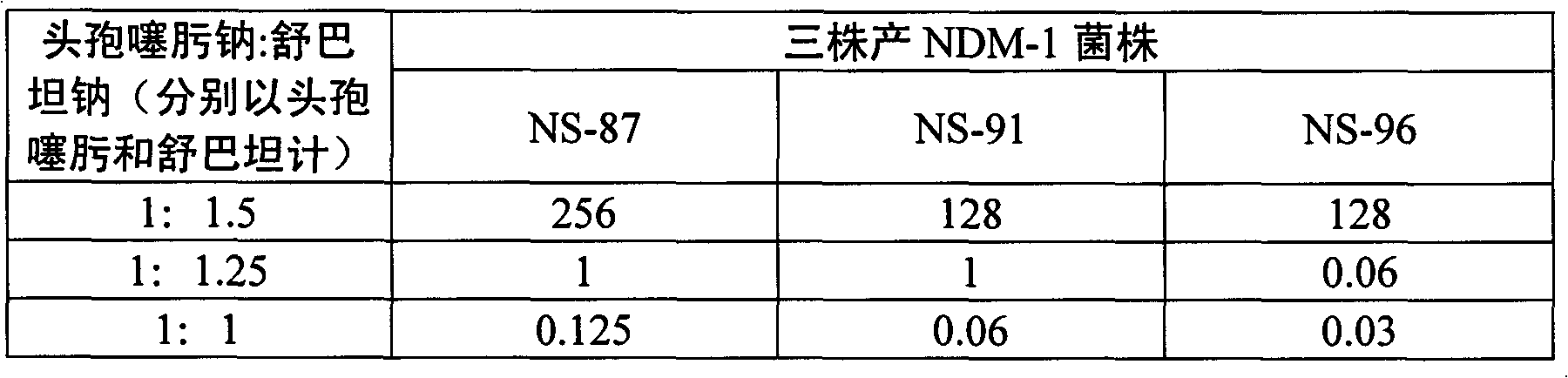
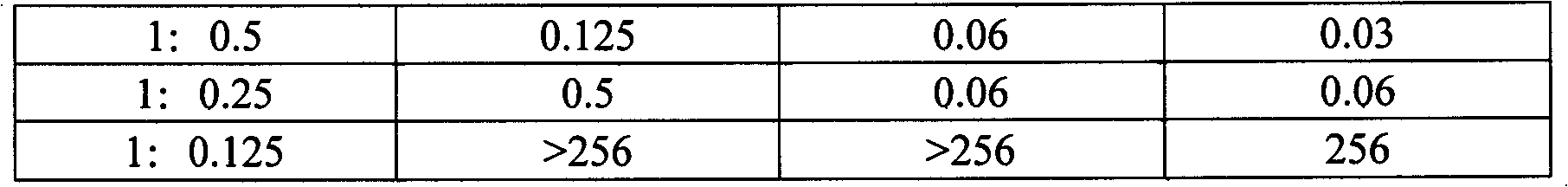
[0035] Cefotaxime sodium (calculated as cefotaxime) 1g
[0036]Sulbactam sodium (calculated as sulbactam) 1.5g
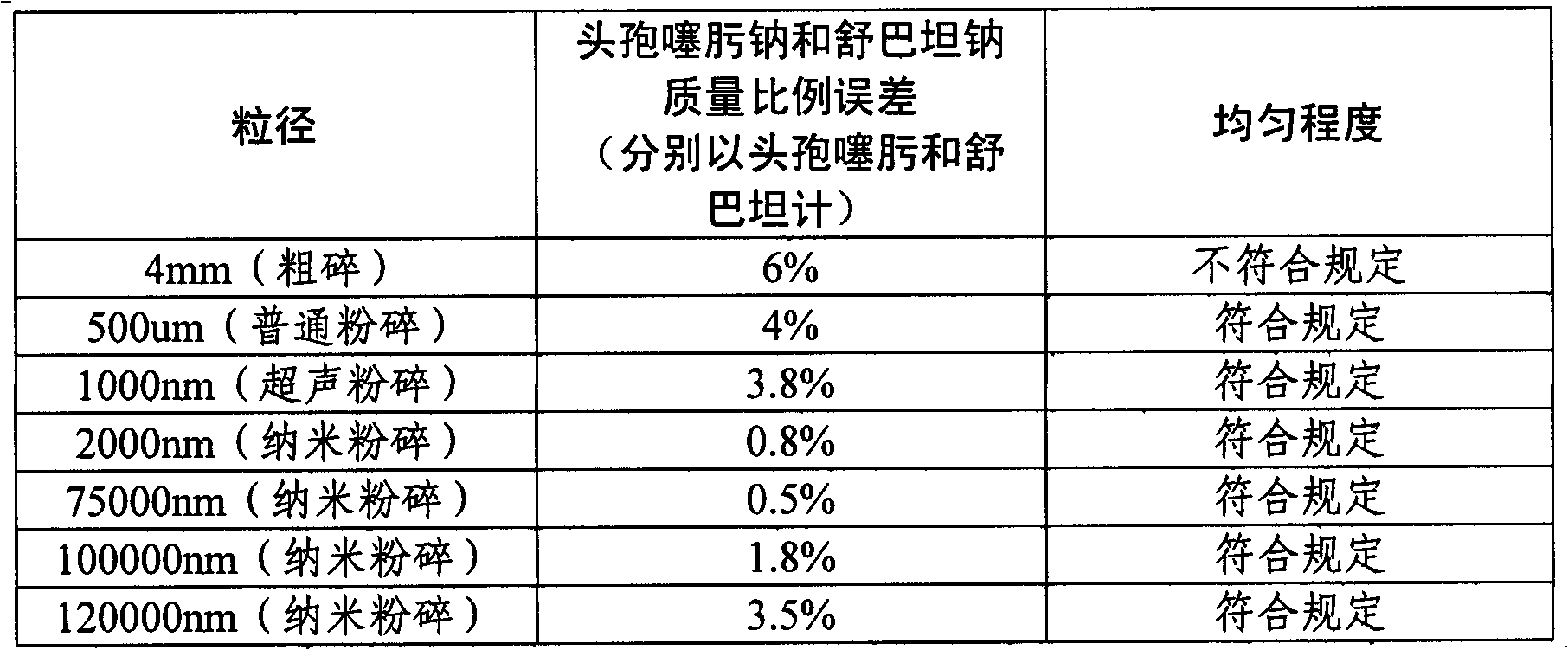
[0037] Preparation method: Under GMP aseptic conditions, cefotaxime sodium and sulbactam sodium are respectively micronized with a nano pulverizer, so that the particle size is 2000nm, and the ratio is calculated according to the composition requirements of the prescription before feeding. A high-efficiency mixer with a rotating speed of 17r / min was used for mixing, and the mixing time was 20 minutes. Filling, subpackaging and lamination of film butyl rubber stoppers were carried out under nitrogen filling to obtain the target powder injection.
Embodiment 2
[0039] Prescription composition:
[0040] Cefotaxime sodium (calculated as cefotaxime) 1g
[0041] Sulbactam sodium (calculated as sulbactam) 1.25g
[0042] Preparation method: Under GMP aseptic conditions, cefotaxime sodium and sulbactam sodium are respectively micronized with a nano pulverizer, so that the particle size is 5000nm, and the ratio is calculated according to the composition requirements of the prescription. A high-efficiency mixer with a rotating speed of 15r / min was used for mixing, and the mixing time was 30 minutes. Filling and subpackaging and lamination of film butyl rubber stoppers were carried out under nitrogen filling to obtain the target powder injection.
Embodiment 3
[0044] Prescription composition:
[0045] Cefotaxime sodium (calculated as cefotaxime) 1g
[0046] Sulbactam sodium (as sulbactam) 1g
[0047] Preparation method: Under GMP aseptic conditions, cefotaxime sodium and sulbactam sodium were micronized with a nano pulverizer, so that the particle size was 10000nm, and the ratio was calculated according to the composition requirements of the prescription before feeding. A high-efficiency mixer with a rotating speed of 25r / min was used for mixing, and the mixing time was 25 minutes. Filling and subpackaging and lamination of film butyl rubber stoppers were carried out under nitrogen filling to obtain the target powder injection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com