Establishing method of fingerprint of traditional Chinese medicinal composition
A technique for fingerprinting and establishing methods, which can be used in measuring devices, instruments, scientific instruments, etc., and can solve the problems of inability to fully characterize the chemical characteristics and quality of Shujinjianyao Pills
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Example 1 Establishment of the method for preparing the test solution
[0096] 1.1 Selection of extraction solvent:
[0097] Get 5g of the Shujinjianyaopill sample of batch number D23095, pulverize, accurately weigh, place in 50ml tool stopper conical flask, add 25ml methanol, 75% methanol (volume percent concentration), 50% methanol (volume percent concentration) respectively accurately Concentration), 95% ethanol (volume percentage concentration), dilute ethanol (volume percentage concentration 50%), after ultrasonic 30min, allow to cool to room temperature, weigh, make up the lost weight with extraction solvent respectively, acquired.
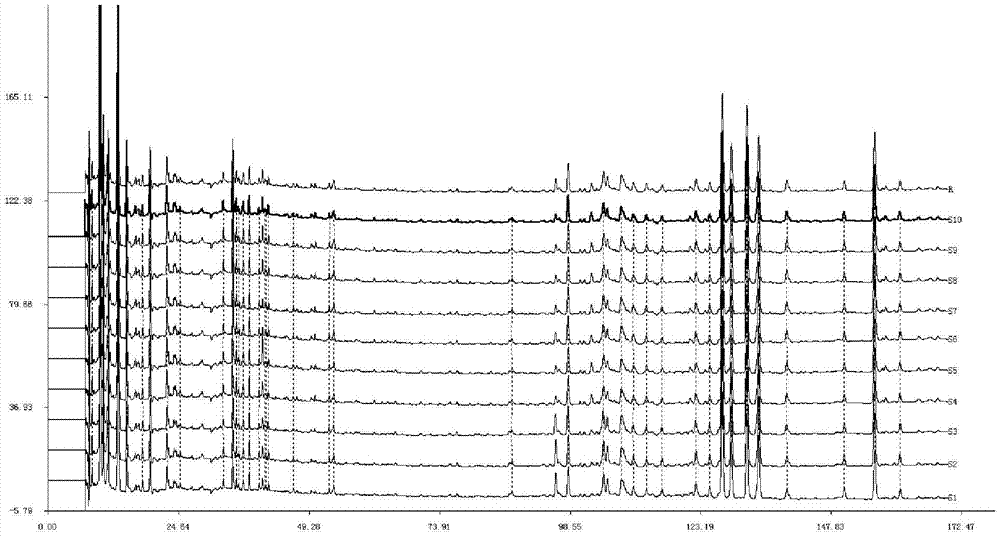
[0098] Under the following chromatographic conditions, measure the test solution prepared by different extraction solvents respectively, and record the chromatogram of 0-170min:
[0099] Chromatographic column: Waters Atlantis chromatographic column, 250×4.6mm, the average particle size of the stationary phase is 5μm;
[0100] Mob...
Embodiment 2
[0110] Example 2 Establishment of Chromatographic Conditions
[0111] 2.1 Determination of mobile phase
[0112] According to the preferred preparation method of the test solution in Example 1, about 5 g of the sample of batch number D23095 was taken to prepare the test solution. Column temperature is 30°C, injection volume is 10 μl, flow rate: 1ml / min, acetonitrile-water, acetonitrile-0.1% phosphoric acid, methanol-0.1% phosphoric acid and acetonitrile-methanol-0.1% phosphoric acid are used as mobile phases, and the elution time is 170 minutes , investigate the chromatogram of need testing solution under 254nm. Wherein, the volume percentage of the organic phase of the two-phase system changes from 5% to 90% at a constant speed, and the volume percentage of the water or phosphoric acid solution changes from 95% to 10% at a corresponding uniform speed.
[0113] The results showed that acetonitrile-0.1% phosphoric acid was used as the mobile phase, the baseline was stable a...
Embodiment 3
[0119] Example 3 Composition testing of Shujinjianyao Pills
[0120] 1. Chromatographic column: Waters Atlantis chromatographic column (5μm, 250×4.6mm);
[0121] 2. Mobile phase: use acetonitrile-0.1% phosphoric acid as the mobile phase, and the volume percentage of acetonitrile in the mobile phase increases from 5% to 90% at a constant speed in 0-170 minutes;
[0122] 3. Flow rate: 1.0mL / min;
[0123] 4. Detection wavelength: 254nm;
[0124] 5. Column temperature: 30°C;
[0125] 6. the preparation of need testing solution: prepare according to the preferred method of embodiment 1;
[0126] 7. Preparation of reference solution: Precisely weigh protocatechuic acid, protocatechualdehyde, privetin, schizandrin A, acetyl schisandrin A, (2′S)-schisandrin lignin The reference substance, made by adding methanol, has a concentration of 90 μg / ml protocatechuic acid, 30 μg / ml protocatechuic aldehyde, 50 μg / ml privetin, 20 μg / ml schizandrin A, and 20 μg / ml acetylated schisandrin A ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com