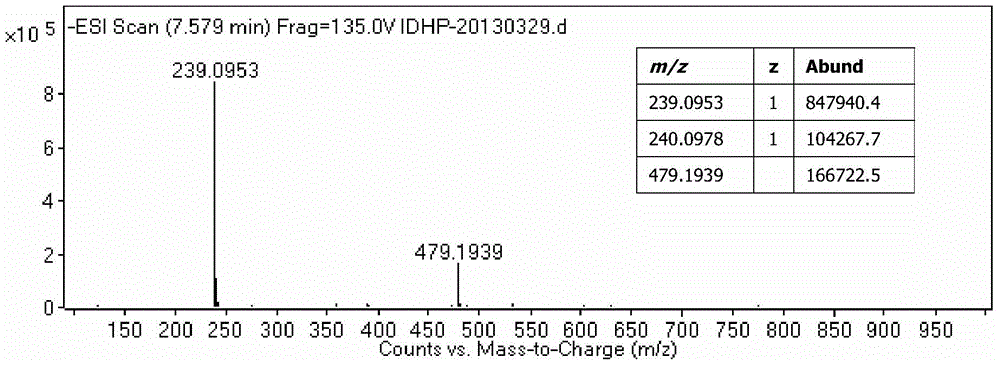
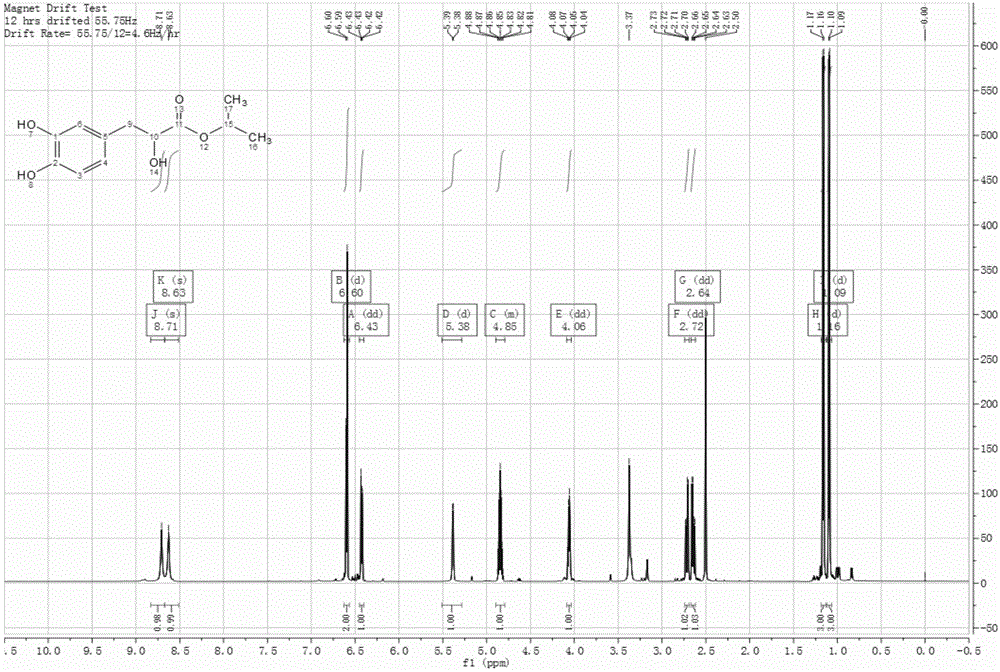
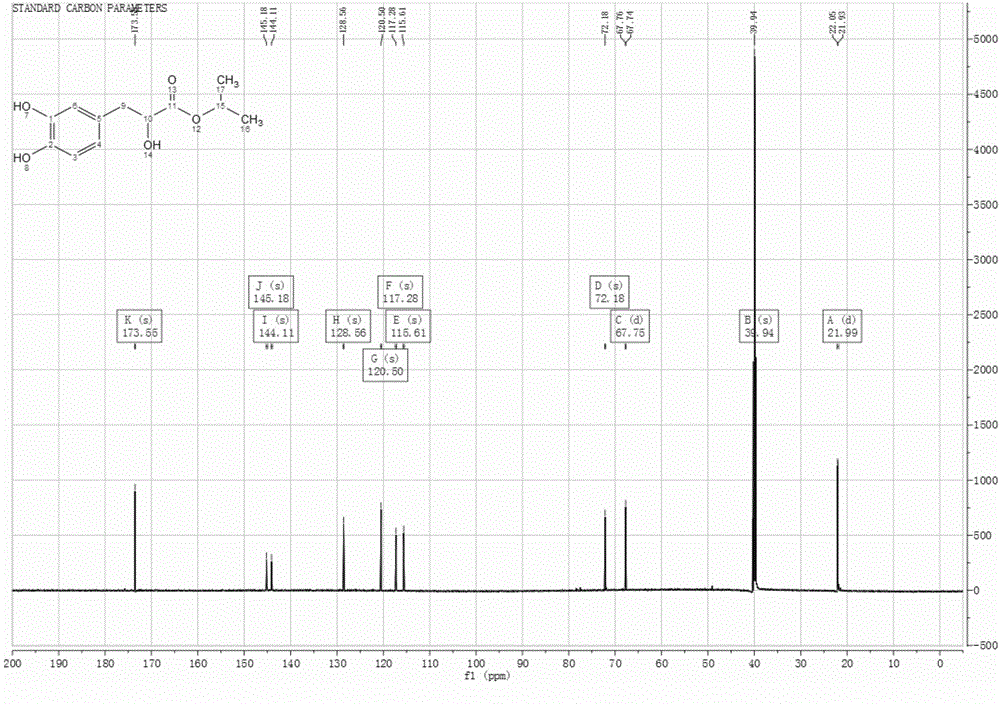
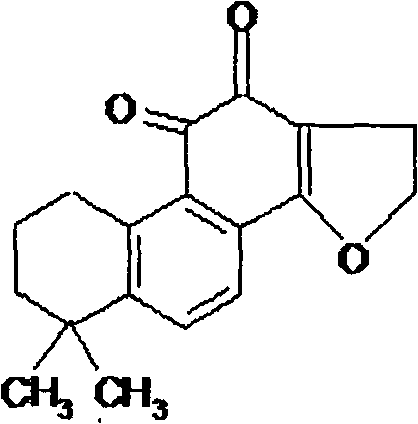
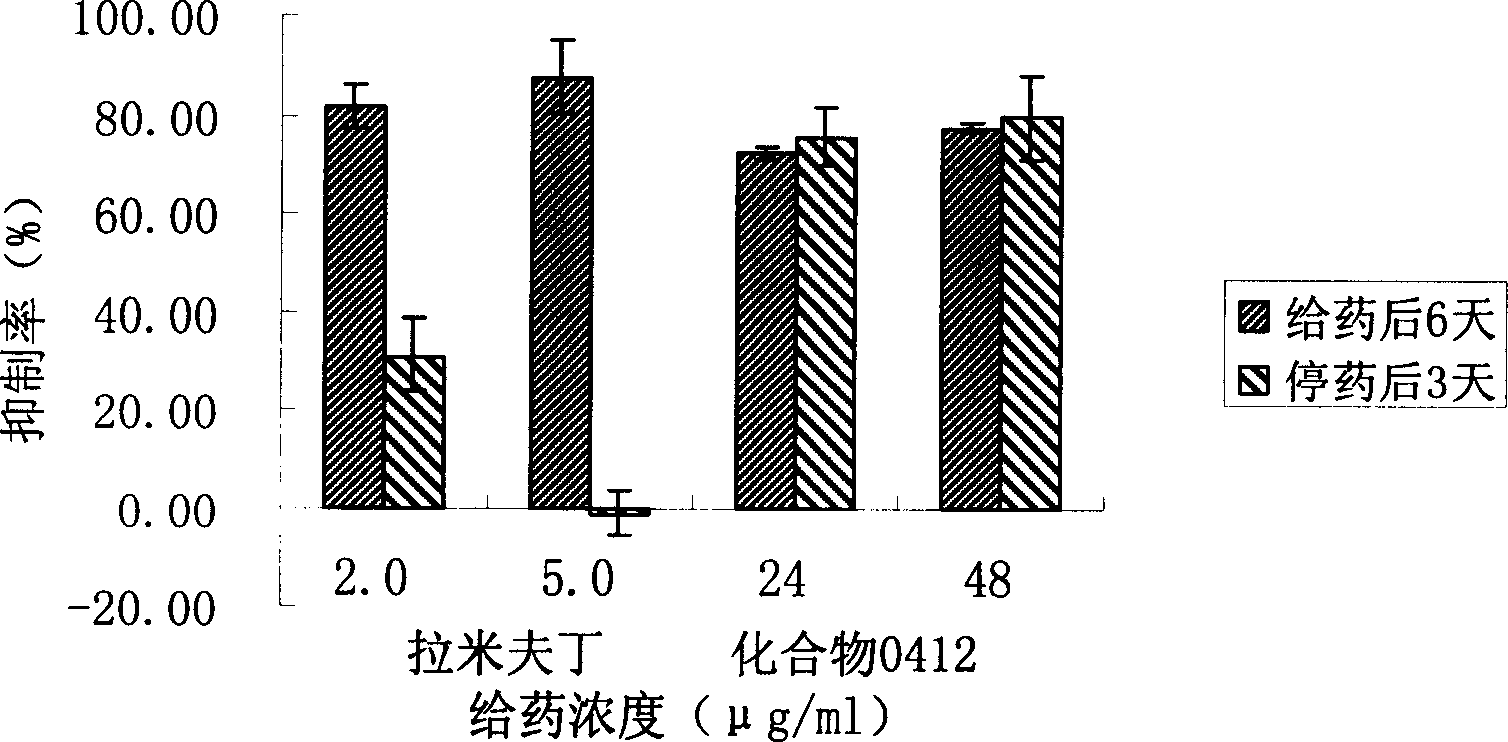
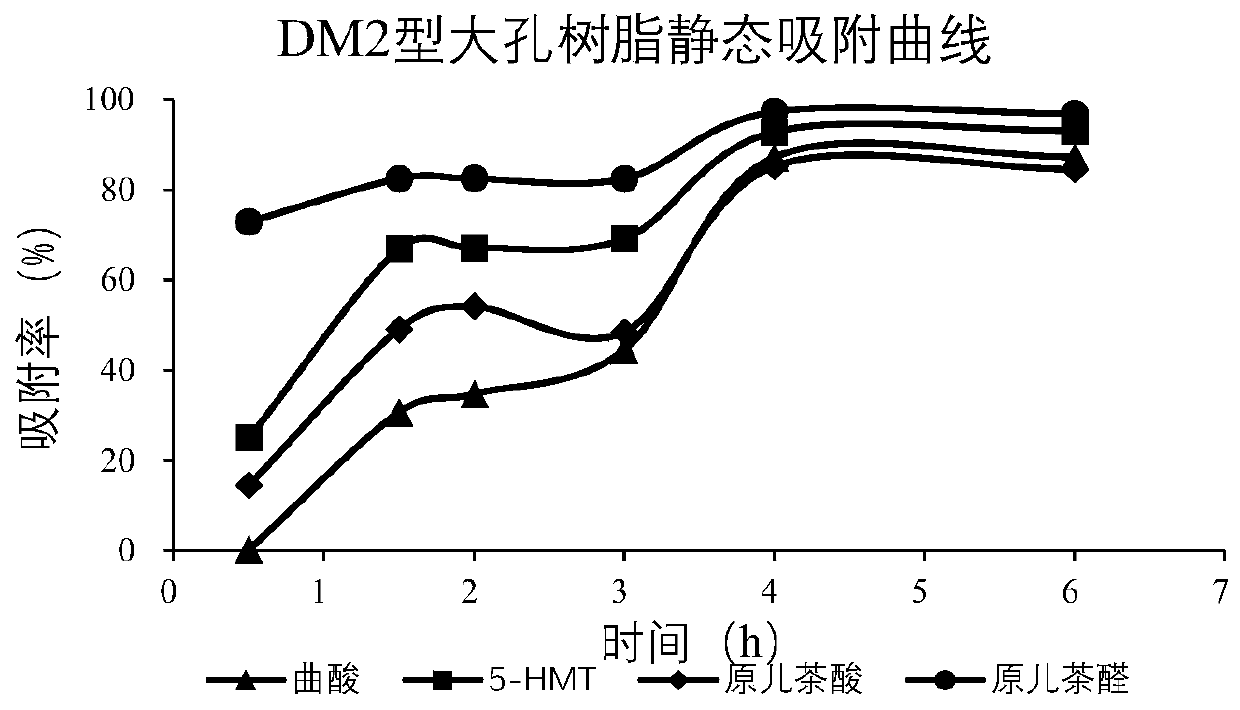
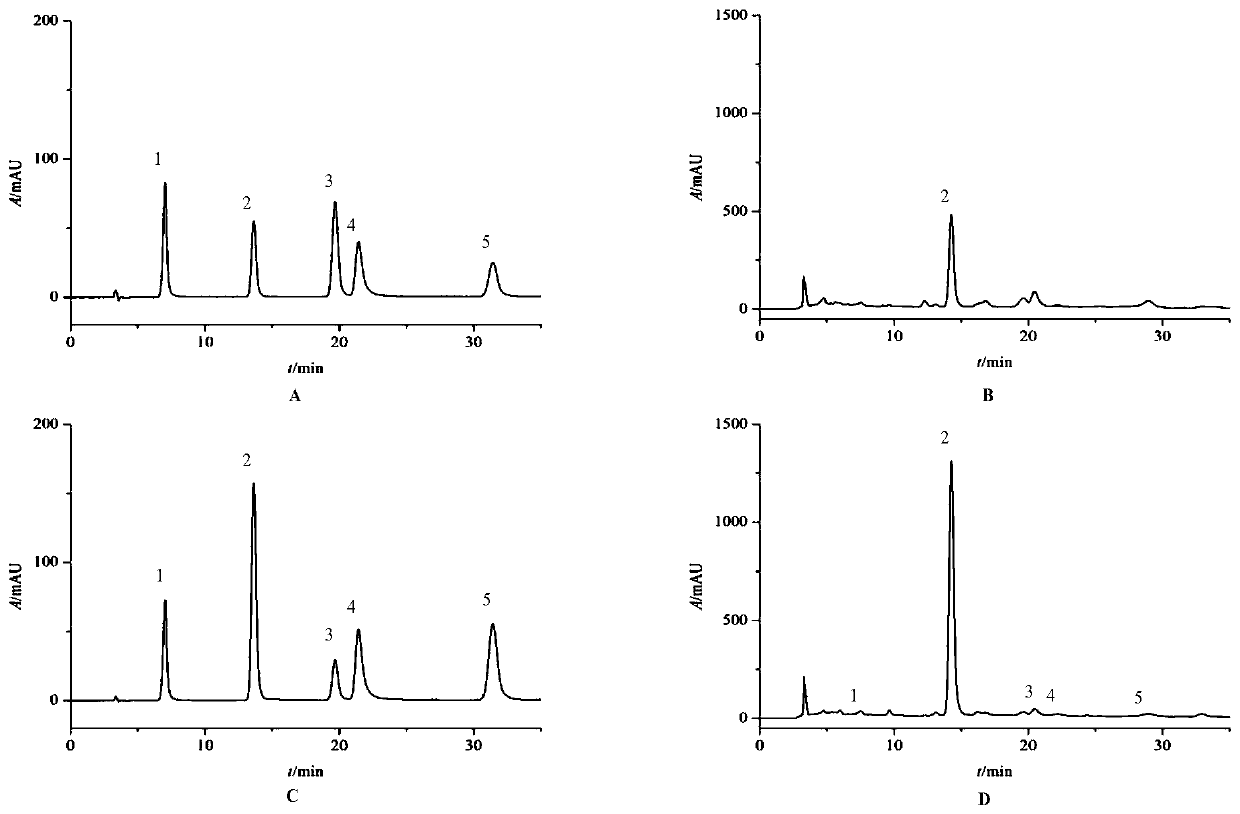
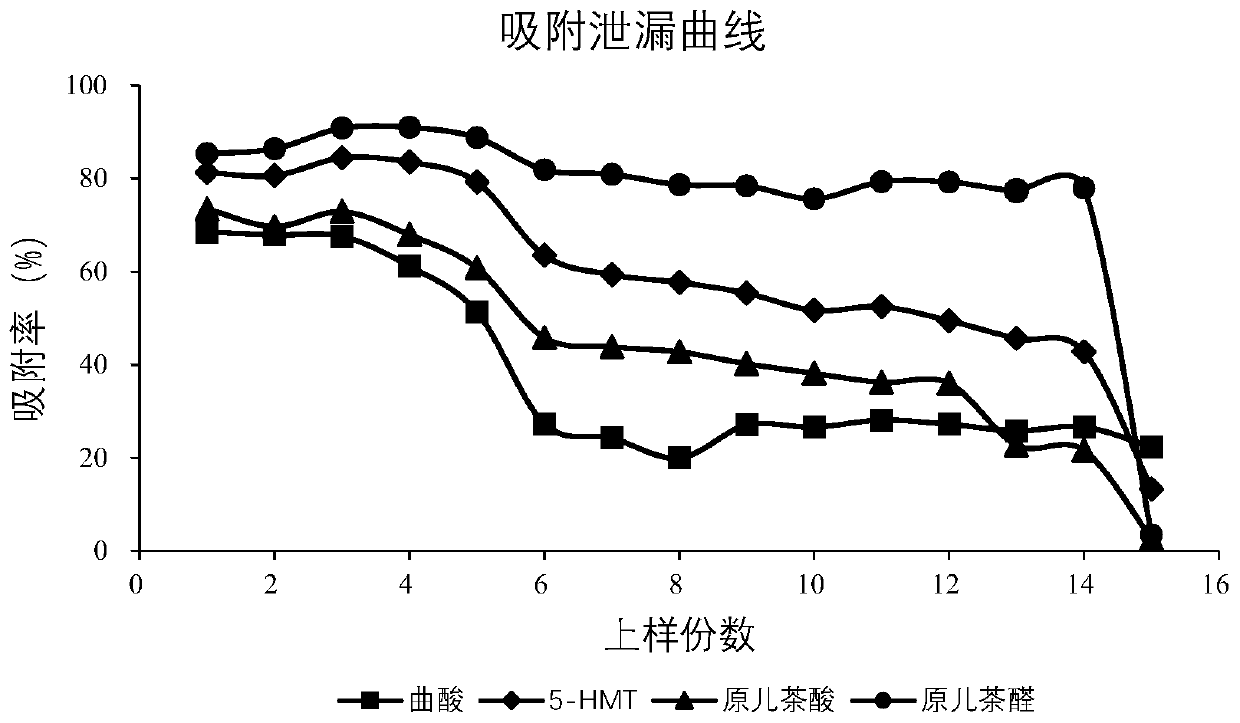
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
68 results about "Protocatechuic aldehyde" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Protocatechuic aldehyde is a phenolic aldehyde, a compound released from cork stoppers into wine. This molecule can be used as a precursor in the vanillin synthesis by biotransformation by cell cultures of Capsicum frutescens, a type of Chili pepper. It is also found in the mushroom Phellinus linteus.
Synthesis method of D,L-danshensu isopropyl ester
ActiveCN103980120ARaw materials are cheap and easy to getShort reaction stepsPreparation from carboxylic acid halidesOrganic compound preparationHydrogenSynthesis methods
The invention relates to a synthesis method of D,L-danshensu isopropyl ester. The method comprises taking protocatechuic aldehyde as an initial raw material, protecting phenolic hydroxyl by benzyl, carrying out Darzens epoxidation, followed by carrying out catalytic reduction with palladium catalyst / hydrogen or Raney nickel / hydrogen, and thus obtaining the D,L-danshensu isopropyl ester. The purity of the product synthesized by the method can reach 98%, and the yield can reach 55%. Moreover, the synthesis method has the advantages of simple and easily obtained raw materials, short routes and high yield, and is suitable for large-scale industrialized production.
Owner:NORTHWEST UNIV
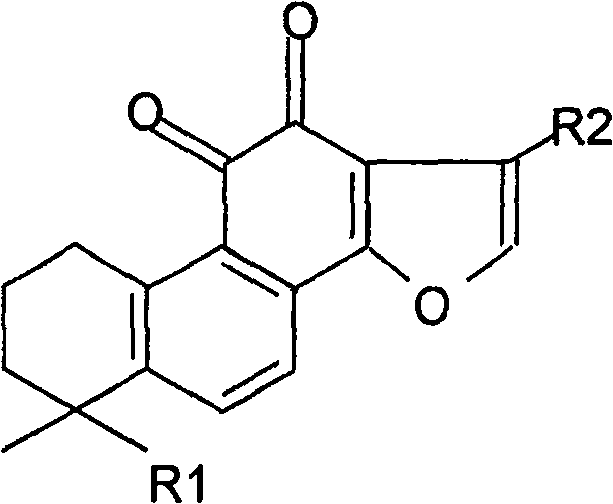
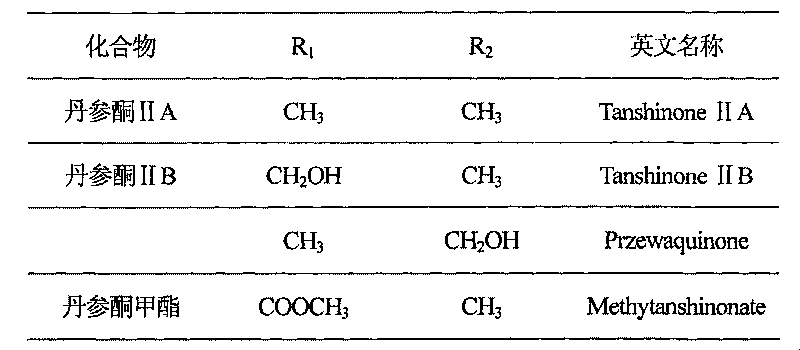
Total tanshinone and total phenolic acid extract in red-rooted salvia root and its production
The invention is concerned with a kind of extract of total ketone of salviae miltiorrhizae and total phenolic acid and its produce method form radix Salviae Miltiorrhizae. The extract of total ketone of salviae miltiorrhizae has cryptotanshinone, tanshinone I, tanshinone IIA, methyl Tanshinon, dihydrotanshinon I and ramification. The extract of total phenolic acid has salvianolic acid A, salvianolic acid B, protocatechuic aldehyde and ramification. The extract can be got form one or arbitrary compound of extraction with solvent method, macroporous resin method, column chromatography and liquid-liquid counter-current chromatography. The summation of the content to each total ketone of salviae miltiorrhizae is 20 to 100 percnte (w / w) of the extract of total ketone of salviae miltiorrhizae, the contene of cryptotanshinone, tanshinone I and tanshinone IIA is 5 to 100 percent (w / w) of whole content of total ketone of salviae miltiorrhizae. The summation of the content to each total phenolic acid is 5 to 100 percent (w / w) of the extract of the radix salviae miltiorrhizae total phenolic acid. The content of salvianolic acid B is the 5 to 100 percent (w / w) of the whole salvianolic acid.
Owner:石任兵 +1
Quality detection method for compound prepn of red sage and notoginseng
InactiveCN1772041ASimple methodGood precisionComponent separationBlood disorderHplc dadSalvianolic acid B
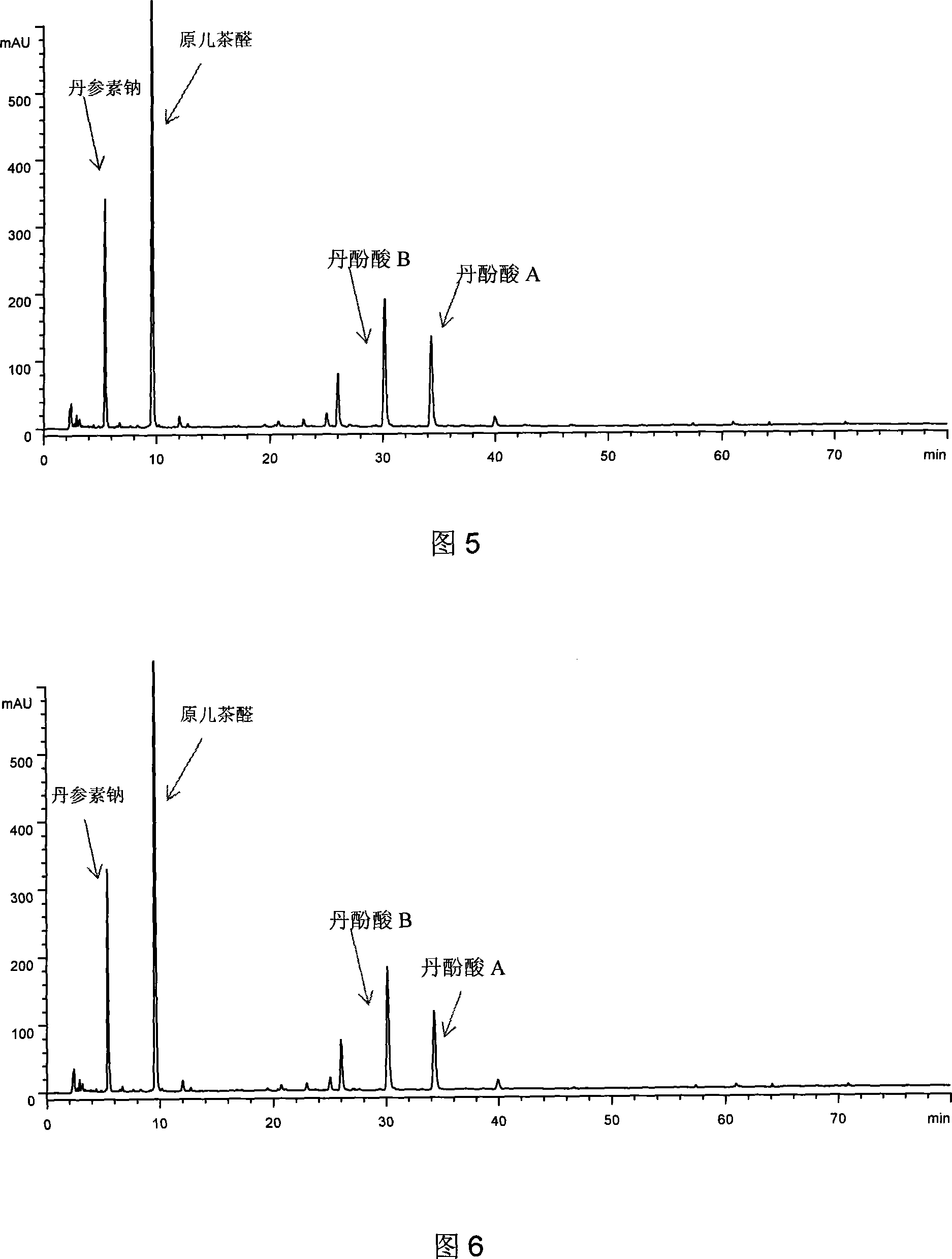
The present invention relates to Chinese medicine quality detecting technology, and is especially the quality detection method for compound preparation of red sage and notoginseng. HPLC-DAD process is adopted to measure the contents of protocatechuic aldehyde, salvianolic acid B, cryptotanshinone, neotanshinone IIA, arasaponin R1, ginsenoside Rg1 and ginsenoside Rb1 in compound red sage preparation simultaneously. The process is simple, precise, repeatable and reliable, and may be used in the quality control of compound red sage preparation.
Owner:CHINA PHARM UNIV
Pharmaceutical composition for treating senile dementia
ActiveCN1879697AOrganic active ingredientsNervous disorderSalvianolic acid BHigh volume manufacturing
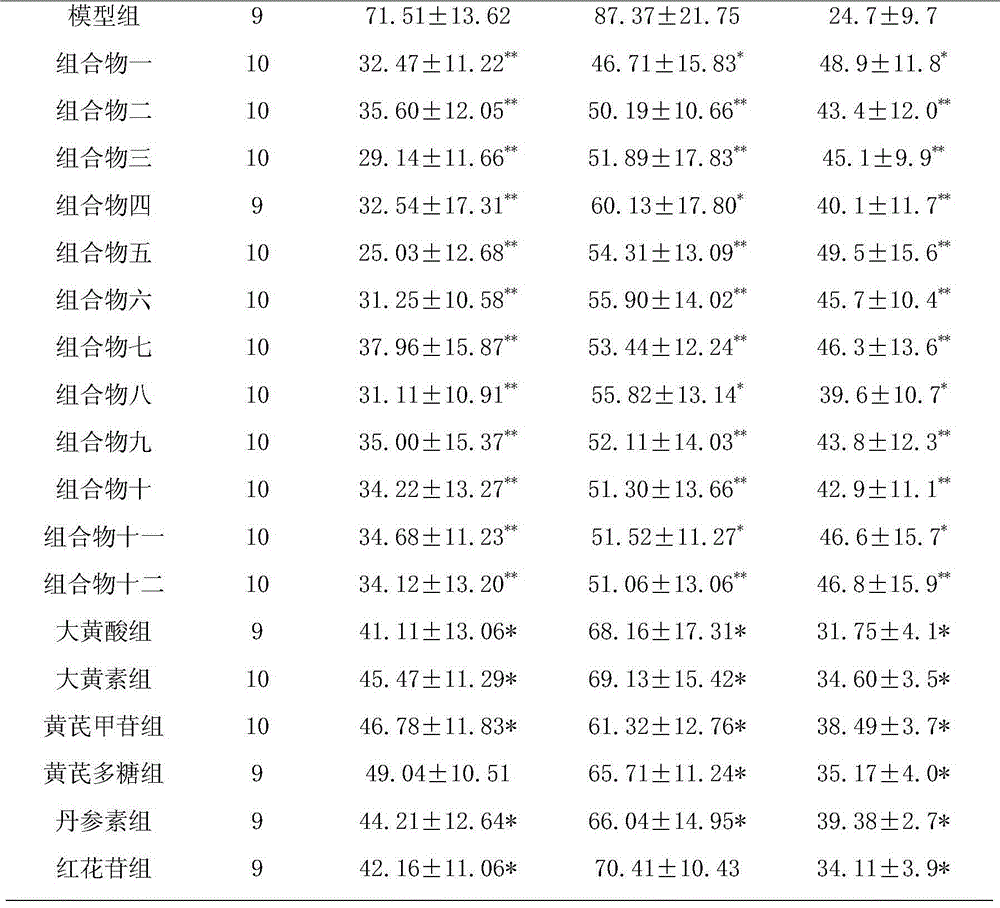
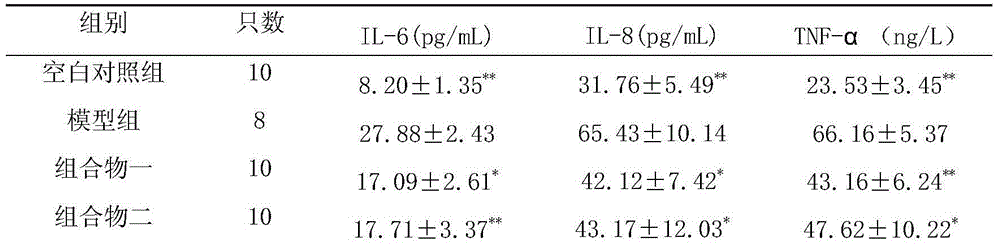
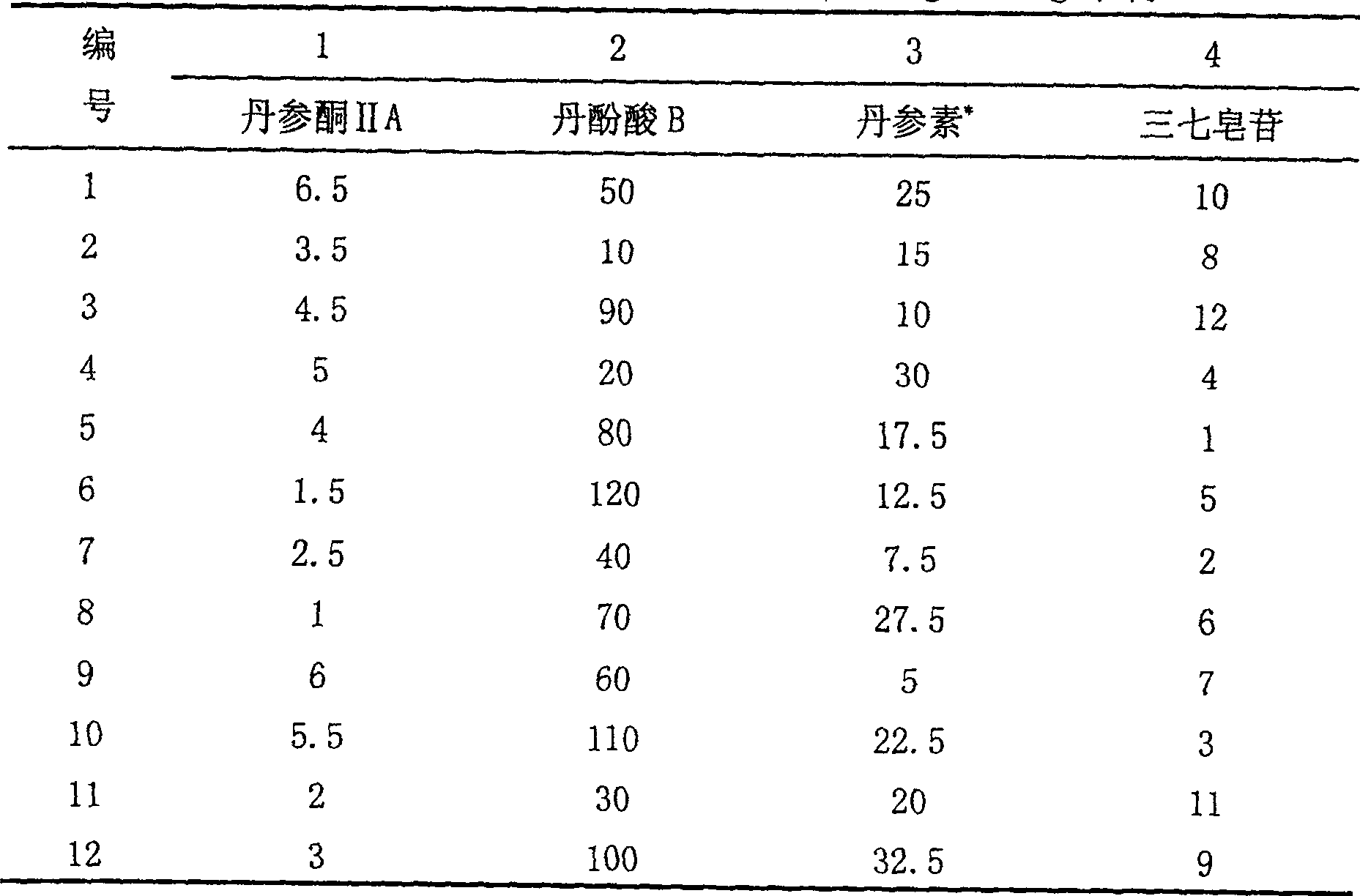
Disclosed is a medicinal composition for treating senile dementia, wherein 1g of the dried active sites comprises tanshinone IIA 1.79-9.01mg, salvianolic acid B 35.87-225.23mg, danshensu 4.93-45.05mg, protocatechuic aldehyde 0.10-4.50mg, and notoginseng saponin R 13.14-22.50mg.
Owner:GUANGZHOU BAIYUSN HUTCHISON WHAMPOA CHINESE MEDICINE
Method of preparing red sage root injection and quality control method thereof
The invention discloses a making method of radix salvia miltiorrhizae injection and quality controlling method, which is characterized by the following: controlling the condensing temperature in the controlling technical course based on traditional technique; utilizing soluble salviol acid as main effective component of tanner and salvia miltiorrhizae to remove residual tanner according to the differential molecular weight. The quality controlling method is characterized by the following: controlling the effective content of alviol acid A, alviol acid B, sodium tanshinone and protocatechualdehyde as quality controlling index; elevating the quality of radix salvia miltiorrhizae injection effectively. The invention reaches the full-new level of safely and effectively controllable quality, which has little imperfect reaction, high security, exact curative effect and controllable quality.
Owner:CHIATAI QINGCHUNBAO PHARMA
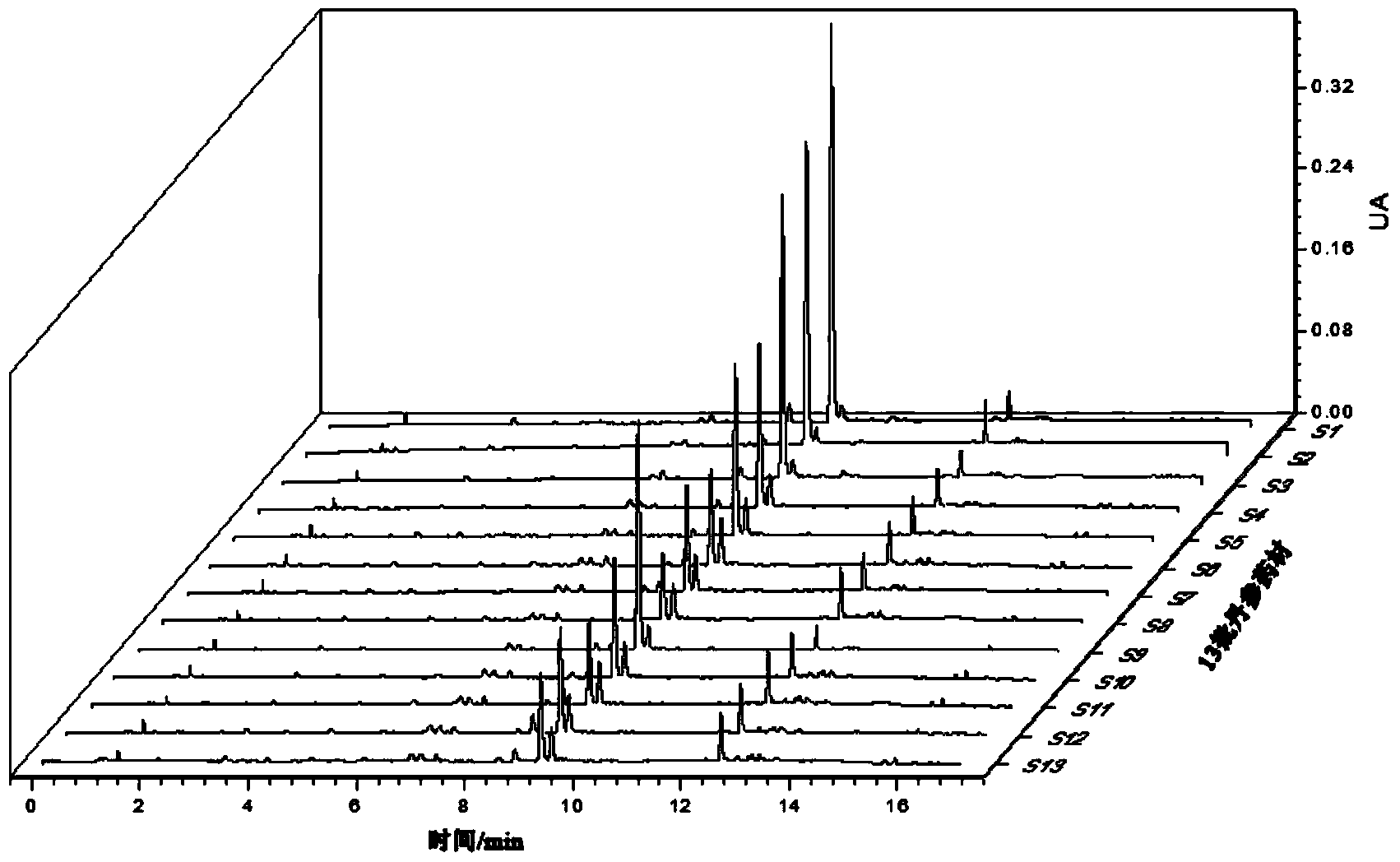
Method for establishing HPLC fingerprint spectrum of Zhuang medicinal material Blumea riparia (Bl.) DC
ActiveCN104458993AFully react chemical compositionInformativeComponent separationHplc fingerprintChemical composition
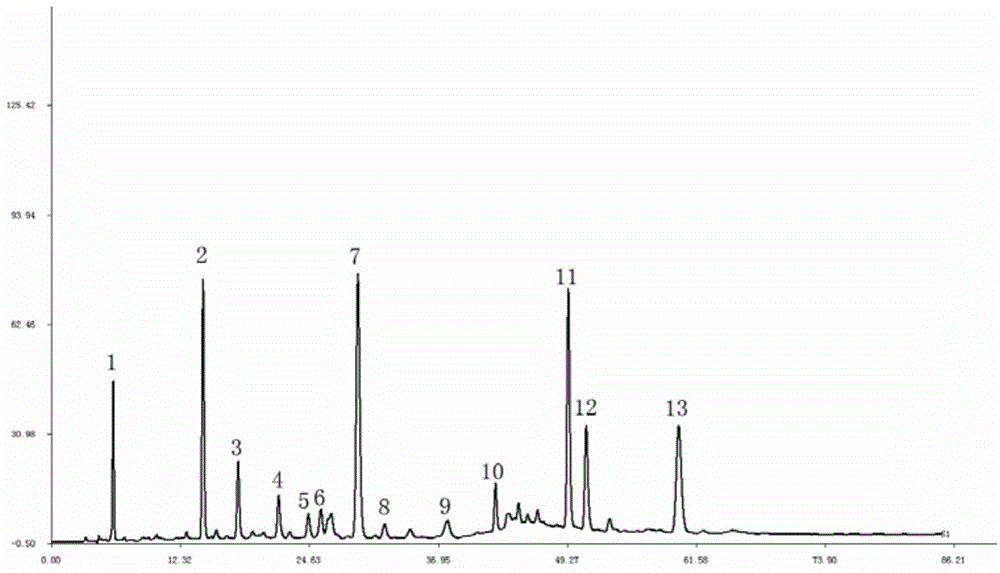
The invention discloses a method for establishing an HPLC fingerprint spectrum of a Zhuang medicinal material Blumea riparia (Bl.) DC. By adopting a high-performance liquid chromatography, and using main ingredients such as protocatechuic acid and protocatechuic aldehyde of Blumea riparia (Bl.) DC as reference substances, a fingerprint spectrum of a common pattern of the Zhuang medicine Blumea riparia (Bl.) DC medicinal material is obtained. The fingerprint spectrum has 13 chromatographic peaks, can fully reflect the chemical compositions of the Blumea riparia (Bl.) DC medicinal material, and is rich in information amount, the method has good reproducibility, a more powerful theoretical basis is provided for controlling the quality of the medicinal material and identifying the advantages and disadvantages of the medicinal material, the quality control and the level of true and false identification of the Zhuang medicine Blumea riparia (Bl.) DC medicinal material are improved, and the authenticity and the advantages and disadvantages of the Blumea riparia (Bl.) DC can be quickly and accurately identified.
Owner:广西万寿堂药业有限公司
Medicine for treating ischemic brain injury stroke and sequela of ischemic brain injury stroke and preparation method for medicine
The invention discloses a structural general formula of a compound LQC-T and the synthesis and the application of the compound LQC-T. Pharmacological experiments prove that the compound has an obvious effect of promoting new vessels of chick chorioallantoic membranes to grow; a compound LQC-T4 has an obvious medicinal effect of treating ischemic brain injury stroke and the sequela of the ischemic brain injury stroke; the maximal LQC-T4 single-day dose of mice is 5,400mg / kg; and the compound is extremely high in safety and can be used for preparing a medicine for treating the ischemic brain injury stroke and the sequela of the ischemic brain injury stroke after toxic responses do not occur after continuous observation within 1 to 4 days. According to the structural formula of the compound LQC-T, R is protocatechuic acid, protocatechuic aldehyde, vanillic acid, gallic acid, caffeic acid, ferulic acid and other aromatic organic acids or phenols or structural analogues of the organic acids or phenols.
Owner:薪火炙药(北京)科技有限公司
Method for detecting content of water-soluble ingredients in salvia miltiorrhiza medicinal material and application of method
InactiveCN104374844AEasy to separateEasy to identifyComponent separationSalvianolic acid BAdditive ingredient
The invention discloses a method for detecting the content of water-soluble ingredients in a salvia miltiorrhiza medicinal material and an application of the method. The method is an ultra-performance liquid chromatography method by using reference substances of tanshinol, protocatechualdehyde, rosmarinic acid, salvianolic acid B and salvianolic acid A as reference and 0.026% phosphoric acid aqueous solution-acetonitrile in a ratio being (85-10):(15-90) as a moving phase for gradient elution. The method for detecting the content of water-soluble ingredients in a salvia miltiorrhiza medicinal material can be used for simultaneously detecting the content of multiple water-soluble ingredients in a salvia miltiorrhiza medicinal material, and can be applied to detection of salvia miltiorrhiza medicinal material fingerprint spectrum. The method has a good effect for separating water-soluble ingredients in the salvia miltiorrhiza medicinal material, and has the characteristics of quickness, accuracy, high reproducibility and high recovery rate, and has strong specificity, high precision, good repeatability, high stability and accurate measurement result. By applying the method, multiple peaks of the fingerprint spectrum can be detected, and reflected information is relatively complete; and the peaks have good shapes, can be easily identified, have high similarity, and are accurate and reliable.
Owner:GUIZHOU XINBANG PHARMACEUTICAL CO LTD
Method for preparing freeze-dried powder of Danhong injection for treating cardiovascular, cerebrovascular diseases, and test method
A freeze-dried powder injection for treating cardiovascular and cerebrovascular diseases is prepared from red sage root and safflower through immersing safflower in worm water, decocting red sage root, mixing the immersing water with decoction, concentrating, depositing in alcohol, examining and freeze drying. Its test method is to measure the contents of danshinolic acid, danshensu sodium, protocatechuic aldehyde, and safflor yellow.
Owner:HAINAN GENERAL & KANGLI PHARMA
Chinese-medicinal preparation for treating eyeground bleeding and method for inspecting vision-improving prescription quality
InactiveCN101028487ABest preparation methodQualitative identification is validSenses disorderComponent separationOrganic solventMedicine
A Chinese medicine 'Xuemingmu' for treating eyeground hemorrhage is disclosed. Its quality test method features that the scutelloside, emodin, chrysophanol and protocatechuic aldehyde are qualitatively discriminated, and the content of danshensu is measured by high-effect liquid-phase chromatography.
Owner:XIAN BEILIN PHARMA
Natural pharmaceutical composition as well as traditional Chinese medicine composition containing same and application of natural pharmaceutical composition
ActiveCN108743600AStrong medicineGood effectAntimycoticsAldehyde active ingredientsAnti fungalRaw material
The invention relates to a natural pharmaceutical composition as well as a traditional Chinese medicine composition containing the same and application of the natural pharmaceutical composition. The natural composition comprises the following raw materials in weight ratio: 40 to 200 parts of paeoniflorin, 1 to 20 parts of ferulic acid, 10 to 50 parts of hydroxysafflor yellow and 1 to 20 parts of protocatechuic aldehyde. The traditional Chinese medicine composition is a composition containing the natural pharmaceutical. The natural pharmaceutical composition and the traditional Chinese medicinecomposition have anti-aGvHD and anti-fungal infection effects, and are of great significance to improve the success rate of transplantation, improve the prognosis of patients, and increase the long-term survival rate of patients with hematological diseases, recipients of allogeneic hematopoietic stem cells and patients with fungal sepsis.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
Establishment method of hplc fingerprint of Zhuang medicinal material Diangui Ainaxiang
ActiveCN104458993BLarge amount of informationRealize evaluationComponent separationHplc fingerprintChemical composition
The invention discloses a method for establishing an HPLC fingerprint spectrum of a Zhuang medicinal material Blumea riparia (Bl.) DC. By adopting a high-performance liquid chromatography, and using main ingredients such as protocatechuic acid and protocatechuic aldehyde of Blumea riparia (Bl.) DC as reference substances, a fingerprint spectrum of a common pattern of the Zhuang medicine Blumea riparia (Bl.) DC medicinal material is obtained. The fingerprint spectrum has 13 chromatographic peaks, can fully reflect the chemical compositions of the Blumea riparia (Bl.) DC medicinal material, and is rich in information amount, the method has good reproducibility, a more powerful theoretical basis is provided for controlling the quality of the medicinal material and identifying the advantages and disadvantages of the medicinal material, the quality control and the level of true and false identification of the Zhuang medicine Blumea riparia (Bl.) DC medicinal material are improved, and the authenticity and the advantages and disadvantages of the Blumea riparia (Bl.) DC can be quickly and accurately identified.
Owner:广西万寿堂药业有限公司
Method for preparing high-purity protocatechualdehyde from salvia miltiorrhiza
ActiveCN102241574ASolve residual problemsScale up productionCarbonyl compound separation/purificationSalvia miltiorrhizaAlcohol
The invention belongs to field of medicines and relates to a method for preparing high-purity protocatechualdehyde. The preparation method comprises the following steps: 1, preparing salvia miltiorrhiza extract by alkaline extraction and alcohol precipitation; 2, removing salvia miltiorrhiza components with relatively weak polarity by chromatography; 3, separating protocatechualdehyde by chromatography; 4, removing acid and decoloring; 5, refining and purifying; and 6, drying.
Owner:TIANJIN TASLY PHARMA CO LTD
Medicinal composition for treating renal diseases and pharmaceutical use of medicinal composition
InactiveCN105012334AEffective treatmentOrganic active ingredientsUrinary disorderDiseaseAstragaloside
The invention relates to a medicinal composition for treating renal diseases and the pharmaceutical use of the medicinal composition. The composition contains rhein, physcion, emodin glucoside, chrysophanol glucoside, salvianic acid, salvianolic acid B, hydroxysafflor yellow A, astragaloside, astragalus polysaccharide, salvianolic acid A, calycosin-7-glucoside, physcion glucoside, rosmarinic acid, carthamin yellow A, salvianolic acid D, salvianolic acid H, protocatechuic aldehyde, caffeic acid and gallic acid, wherein the chrysophanol glucoside is chrysophanol monoglucoside, the emodin glucoside is emodin monoglucoside, and the physcion glucoside is physcion monoglucoside. The composition can also contain C11H9N6O4, C17H14O4, C21H20O9, C19H32O12, C30H36O15 and C30H44O15. The composition is a preparation in a solid form, a liquid form, a gas form or a semisolid form, and a great number of experiments show that the composition can be used for treating renal diseases, renal failure, nephrotic syndromes, cardiovascular and cerebrovascular diseases, neoplastic diseases and the like.
Owner:XIAN SHIJISHENGKANG PHARMA IND
Total tanshinone and total phenolic acid extract in red-rooted salvia root and its production
Owner:石任兵 +1
Phellinus igniarius traditional Chinese medicine composition as well as extraction method thereof and application thereof in preparation of anti-tumor drugs
InactiveCN107519327ASignificant improvementEnhanced inhibitory effectFungi medical ingredientsNatural extract food ingredientsAdditive ingredientPhellinus igniarius
The invention relates to a phellinus igniarius traditional Chinese medicine composition as well as an extraction method thereof and application thereof in preparation of anti-tumor drugs. The phellinus igniarius traditional Chinese medicine composition is prepared from the following components by mass percent: 30-50% of ganoderma lucidum, 10-20% of phellinus igniarius and 30-50% of radix tetrastigme. The extraction method adopts ultrasonic-assisted extraction; the prepared composition extract contains the following main ingredients: polysaccharides, triterpenes, protocatechuic aldehyde, inoscavin, naringenin, 7-methoxy dihydrocamphene, phellinus igniarius flavone, dehydrovomifoliol, sakuranetin, methyl phellinus igniarius flavone, ganodenic acid, quercetin, kaempferol, and the like. The composition extract has an obvious improvement effect for cyclophosphamide-induced immune loss mice, and has a remarkable inhibiting effect for stomach cancer strains SGC7901 and BGC823, liver cancer strains HepG2 and SMMC7721, colon cancer strain HT-29, and the like. Compared with the singly used phellinus igniarius and radix tetrastigme, the composition has an obvious synergistic effect.
Owner:ZHEJIANG ACAD OF TRADITIONAL CHINESE MEDICINE
Detection method of phenolic acid compounds in compound radix salviae miltiorrhizae tablet
InactiveCN103776908AFully reflect the qualitySimple methodComponent separationSalvianolic acid BResource saving
The invention provides a detection method of phenolic acid compounds in a compound radix salviae miltiorrhizae tablet, and the detection method comprises the following steps: preparing a standard solution, a reference solution and a to-be-tested product solution, passing through a high performance liquid chromatography for detection; then calculating a relative correction factor of the phenolic acid compounds relative to salvianic acid A sodium; and using a formula IV shown in the specification too obtain the phenolic acid compound content in the compound radix salviae miltiorrhizae tablet. According to the detection method, by detection of one compound (salvianic acid A sodium), simultaneous determination of multiple compounds (salvianic acid A, protocatechuic aldehyde, rosmarinic acid, lithospermic acid and salvianolic acid B) can be realized, and the detection method is simple, rapid, accurate, good in universality, labor, and material and financial resource saving, and can more fully reflect the quality of the compound radix salviae miltiorrhizae tablet.
Owner:GUANGZHOU BAIYUSN HUTCHISON WHAMPOA CHINESE MEDICINE
Method for preparing protocatechuic aldehyde from vanillin
InactiveCN102241575ARaise the reaction temperatureLow reaction temperatureOrganic compound preparationCarbonyl compound preparationReaction temperatureEthyl acetate
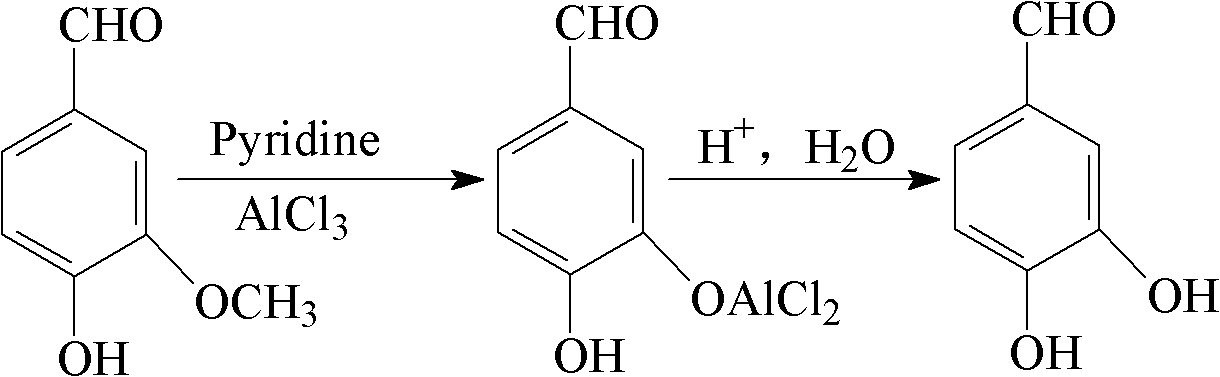
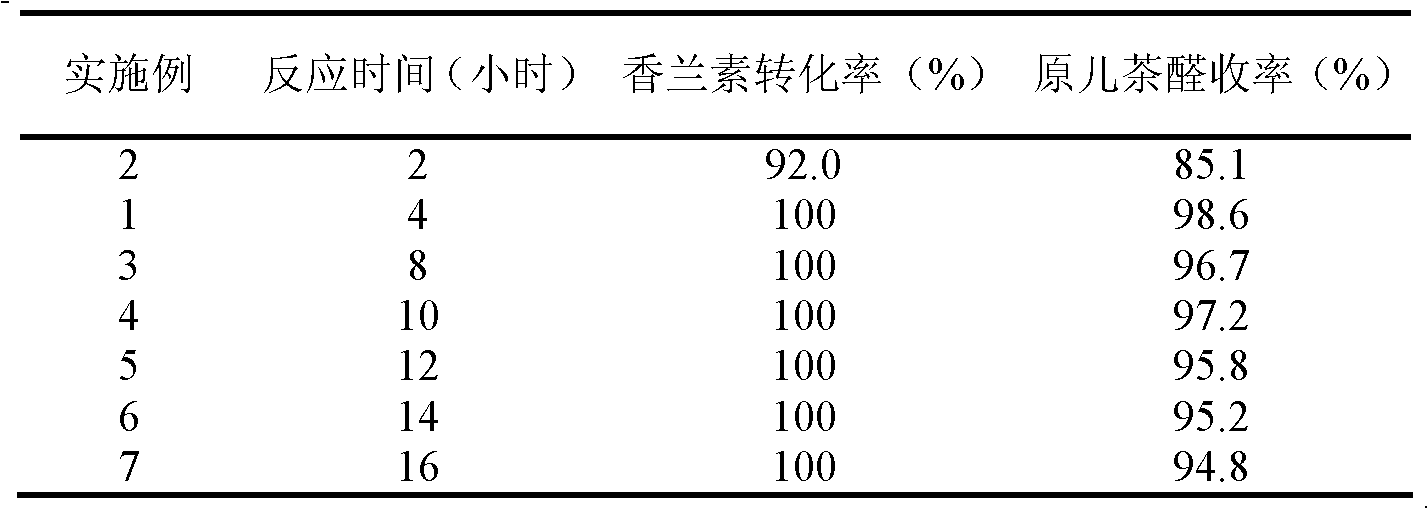
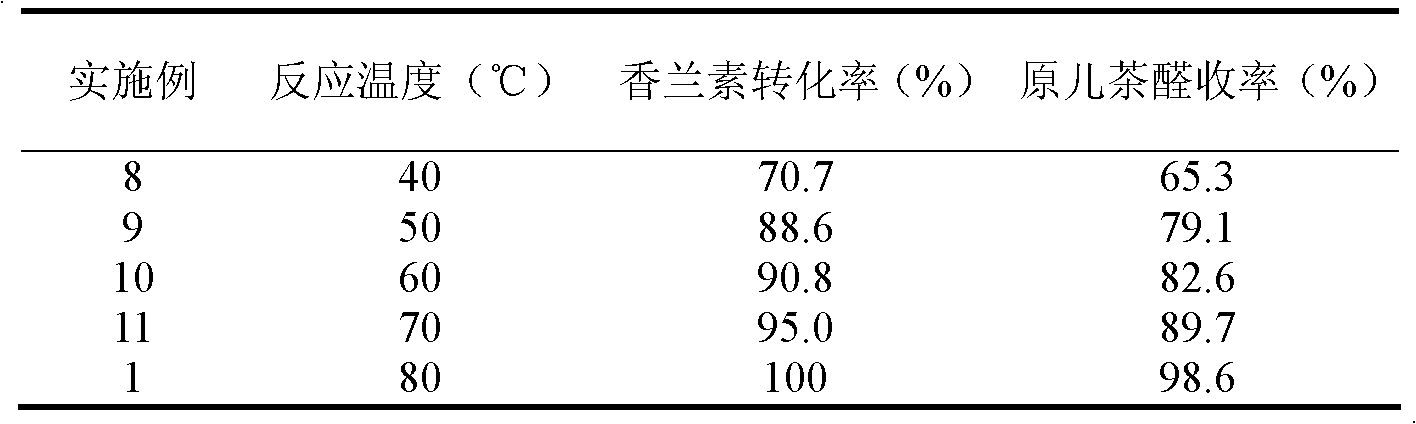
The invention relates to a method for preparing protocatechuic aldehyde from vanillin. The method comprises the following steps: (1) adding vanillin and a solvent into a reactor, wherein the use amount of the solvent is 1-10 ml / g vanillin; fully stirring, and adding a demethylation reagent, wherein the molar ratio of the vanillin to the demethylation reagent is 1:(0.6-1.6); (2) maintaining the reactor temperature at 0-10 DEG C, and adding pyridine, wherein the use amount of the pyridine is 0.5-2.6 ml / g vanillin; (3) raising the reaction system temperature to 30-90 DEG C, and reacting for 2-16 hours; (4) adding 12wt% diluted hydrochloric acid under stirring condition for acidifying to pH 2-4, continuously stirring for 0.5-1.0 hour, and stopping reaction; (5) extracting the reaction liquid with dichloromethane; and (6) then extracting the raffinate phase with ethyl acetate, drying, distilling and purifying to obtain protocatechuic aldehyde. In the method provided by the invention, an environmentally-friendly carbonate solvent is used to substitute for the dichloromethane solvent, the reaction temperature is increased and the reaction time is greatly shortened; and when ZnCl2 is used as the demethylation reagent, the equipment corrosion problem caused by the use of AlCl3 can be reduced.
Owner:HEBEI UNIV OF TECH
GPR35 receptor stimulant in salviae miltiorrhizae, inhibitor of Ca2+-ATPase and application
InactiveCN105521016AExpand the scope of clinical applicationNervous disorderAntipyreticATPaseSalvianolic acid B
The invention relates to the discovering of the action targets of traditional Chinese medicine salviae miltiorrhizae and widening of clinical application range, in particular to discovering of the new action targets of phenolic acid compounds in the salviae miltiorrhizae and new clinical application of the phenolic acid compounds. The phenolic acid compounds include similar-structured compounds such as protocatechuic aldehyde, tanshinol, caffeic acid, alkannic acid, salvianolic acid A, salvianolic acid B, salvianolic acid C and salvianolic acid D, the derivatives of the similar-structured compounds and the pharmaceutically-acceptable salt-forming compounds of the similar-structured compounds. In vitro cell experiments show that the compounds act on the orphan receptor GPR35 and current researches show that the orphan receptor GPR35 is related to diseases such as cardiac failure, hypertension, coronary heart disease, metabolic syndrome, asthma, pain, inflammation and cancer, so that the clinical application range of the compounds can be widened. In addition, the fact that the compounds have a double-target effect is discovered, and certain guidance is provided for the new clinical application.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for determining components of common wedgelet fern herb by adopting quantitative analysis multi-components by single marker
The invention provides a method for determining components of common wedgelet fern herb by adopting quantitative analysis multi-components by a single marker. The method is characterized in that the quantitative analysis multi-components by the single marker is carried out by taking protocatechuic acid which is cheap and easy to obtain as an internal reference object, and relative correction factors between protocatechuic acid, and protocatechuic acid, protocatechuic aldehyde, orientoside and vitexin in common wedgelet fern herb are established; the content of protocatechuic acid, protocatechuic aldehyde, orientoside and vitexin in common wedgelet fern herb is calculated through the correction factors; and a liquid chromatography is used for determining. The detection method is high in practicability and simple to operate and the cost is saved; and the content of four components including protocatechuic acid, protocatechuic aldehyde, orientoside and vitexin in common wedgelet fern herb can be effectively detected.
Owner:GUANGXI UNIV OF CHINESE MEDICINE
Freeze dried injection powder preparation for treating coronary heart disease and its preparing method
InactiveCN1857396AImprove ischemic stateImprove rheologyPowder deliveryLyophilised deliveryCoronary artery diseaseFreeze-drying
The present invention relates to Chinese medicine preparation, and is especially freeze dried injection powder preparation for treating coronary heart disease and its preparation process. The freeze dried injection powder preparation is prepared with red sage and Chuanxiong rhizome as main material, and through the steps of water extraction, precipitating to eliminate impurity, refining with macroporous resin, filtering, compounding the refined extract and freeze drying excipient with injection water to form solution, refining, packing and low temperature freeze drying. Each injection contains protocatechuic aldehyde 10-15mg, total phenolic acid 300-350mg and ligustrazine 10-15mg. The present invention has the advantages of simple preparation process, low cost, high stability, obvious curative effect, etc.
Owner:耿海波
Method for measuring content of active ingredients in Chinese honeylocust fruit
ActiveCN114674958AEasy to operateHigh sensitivityComponent separationAgainst vector-borne diseasesBiotechnologyApigenin
The invention provides a method for determining the content of active ingredients in Chinese honeylocust fruit, which adopts ultra-high performance liquid chromatography-mass spectrometry. The content of L-malic acid, protocatechuic aldehyde, protocatechuic acid, caffeic acid, scopoletin, liquiritigenin, apigenin, luteolin, eriodictyol, citrus aurantium, fustin, cryptochlorogenic acid, neochlorogenic acid, chlorogenic acid, vitexin, isovitexin, quercitrin, orientin and isoorientin in the Chinese honeylocust fruit can be rapidly detected. The method provided by the invention has the advantages of simple operation, high sensitivity, fast analysis speed and strong specificity, and can be used for quality control of Chinese honeylocust fruit.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
Purpose of 3,4-dihydroxy phenyl substituted phenol for preparing an ALDH2 activating agent and medicine for preventing and treating cardio-cerebral ischemia injury
The invention discloses a purpose of 3,4-dihydroxy phenyl substituted phenol for preparing an ALDH2 activating agent and medicine for preventing and treating cardio-cerebral ischemia injury. The invention provides a purpose of a 3,4-dihydroxy phenyl substituted phenol compound including but not limited to protocatechuic acid, tanshinol, danshinolic acid B and danshinolic acid A for preparing the ALDH2 activating agent, and further provides a purpose of the 3,4-dihydroxy phenyl substituted phenol compound for preparing medicine for preventing and treating cardio-cerebral ischemia injury; a medicine composition for preventing and controlling myocardial infarction or cerebral apoplexy by 3,4-dihydroxy phenyl substituted phenol or pharmaceutical salts of the 3,4-dihydroxy phenyl substituted phenol. The 3,4-dihydroxy phenyl substituted phenol or pharmaceutical salts of the 3,4-dihydroxy phenyl substituted phenol can be used for preparing medicine for preventing and controlling diseases relevant to ALDH2.
Owner:CHINA PHARM UNIV
Method for simultaneously and rapidly determining various phenolic acid and tanshinone components in compound salvia milliorrhiza tablets
InactiveCN108333282AShort preparation timeReduce consumptionComponent separationSalvianolic acid BCaffeic acid
The invention discloses a method for simultaneously and rapidly determining various phenolic acid and tanshinone components in compound salvia milliorrhiza tablets. The method comprises the followingsteps: mixing a product to be detected and an extracting solvent; after carrying out microwave assisted extraction, detecting by a high performance liquid chromatography; quantifying salvianic acid, protocatechuic aldehyde, caffeic acid, rosmarinic acid, lithospermic acid, salvianolic acid A, salvianolic acid B, dihydrotanshinone I, cryptotanshinone, tanshinone I and tanshinone IIA quantitation byadopting an external standard method. Under the condition that the compound salvia milliorrhiza tablets contain various complicated components, the method can be used for simultaneously determining 7phenolic acid components and 4 tanshinone components in the compound salvia milliorrhiza tablets; the quality analysis efficiency of the compound salvia milliorrhiza tablets and the quality control level of a product can be remarkably improved; the method has the advantages that a few of organic solvents are consumed, energy saving and environmental protection are saved, and a green and chemicaldevelopment concept is met.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Guanxinshutong capsule content detection method using quantitative analysis of multi-components by single-marker
ActiveCN106950289AGuaranteed stabilityGuaranteed uniformityComponent separationInjection volumeGallic acid ester
The invention provides a Guanxinshutong capsule content detection method using quantitative analysis of multi-components by single-marker (QAMS). According to the present invention, the chromatographic conditions comprise that the chromatographic column uses an octadecyl-bonded silica gel column as a filler, methanol is adopted as a mobile phase A, a 0.4% formic acid aqueous solution is adopted as a mobile phase B, a volume ratio of the mobile phase A to the mobile phase B is 3-90:97-10, linear gradient elution is performed, the detection wavelength is 280 nm, the column temperature is 35 DEG C, and the sample injection volume is 5 [mu]L; the content detection method can simultaneously determine the contents of gallic acid, danshensu sodium, protocatechuic acid, protocatechuic aldehyde, vanillin, rosmarinic acid, salvianolic acid B, eugenol, cryptotanshinone and tanshinone IIA in the Guanxinshutong capsule; and the detection method has characteristics of simpleness, accuracy, efficiency, good reproducibility, and good stability.
Owner:SHAANXI BUCHANG PHARMA
Pharmaceutical composition for treating senile dementia
The pharmaceutical composition for treating senile dementia relates to a medicinal product using plants as raw materials. It is an oral preparation made of medicinal active parts and conventional pharmaceutical excipients according to the conventional traditional Chinese medicine preparation process. The medicinal active parts are mainly prepared from Danshen and Panax notoginseng. Contains tanshinone II A 1.79~9.01mg, salvianolic acid B 35.87~225.23mg, danshensu 4.93~45.05mg, protocatechualdehyde 0.10~4.50mg, notoginseng saponin R13.14~22.50mg; tanshinone IIA: salvianolic acid B: The weight ratio of danshensu: protocatechualdehyde: notoginseng saponin R1 is 4-20:80-500:11-100:0.2-10:7-50. The medicament provided by the invention is effective in preventing and treating senile dementia; the active ingredient composition, content and ratio of the product are clear; the product quality is controllable and operable; and it can be mass-produced.
Owner:GUANGZHOU BAIYUSN HUTCHISON WHAMPOA CHINESE MEDICINE
Danshen root extract, production, medicine preparation and inspection thereof
ActiveCN1315495CIncreased content of effective partsHigh yieldComponent separationCardiovascular disorderAngor pectorisPharmaceutical formulation
An extract of red sage root for preparing the injection to treat coronary heart disease and angina pectoris contains danshensu, protocathechuic aldehyde and danshinolic acid. Its preparing process and test method are also disclosed.
Owner:葛磊
Novel use of 3, 4-dihydroxy-benzene formaldehyde and its derivatives in treating hypatitis B and virus infectious diseases
The present invention relates to the application of substituted derivative of protocatechuic aldehyde, ester derivative of protocatechuic aldehyde, acetal derivative of protocatechuic aldehyde, amide derivative of protocatechuic aldehyde, and Schiff base derivative of protocatechuic aldehyde in treating hepatitis B and other viral infectious diseases; and the said medicine compound or their composition may be used through combination with antiviral neucleoside analog medicine in treating hepatitis B and other viral infectious diseases.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
Prepared cibotium barometz tuber effective parts and preparation method thereof
InactiveCN109846911AImprove securityThe preparation method is simple and environmentally friendlySkeletal disorderPteridophyta/filicophyta medical ingredientsMedicinal herbsMethanol water
The invention provides prepared cibotium barometz tuber effective parts and a preparation method thereof. The preparation method comprises the following steps of taking 40g of prepared cibotium barometz tuber medicinal material fine powder, adding 800mL of methanol-water in the volume ratio being 70 to 30, performing reflux extraction twice, wherein the reflux extraction time each time is 2h, performing filtration, and taking filtrate for standby application; additionally taking 3g of activated DM2 type resins, and performing loading into a column; taking 15mL of a cibotium barometz tuber extracting solution, conveying samples to a detection instrument, performing adsorption for 4h, performing eluting with 45mL of methanol-water in the volume ratio being 20 to 80, collecting an eluent, andperforming evaporation to dryness so as to obtain the prepared cibotium barometz tuber effective parts. According to the cibotium barometz tuber effective parts prepared by the method, the kojic acidcontent in percentage by mass is more than 0.4%, the 5-HMF content in percentage by mass is more than 13.5%, the protocatechuic acid content in percentage by mass is more than 0.6%, and the protocatechuic aldehyde content in percentage by mass is more than 0.5%. The preparation method is simple and environmental-friendly and is suitable for large-scale industrial production.
Owner:SHANDONG UNIV
Determination method of capsule fingerprint spectrum for dredging collaterals and reducing phlegm
ActiveCN110927302AImprove stabilityGood reproducibilityComponent separationCholic acidSalvianolic acid B
The invention relates to a determination method of a capsule fingerprint spectrum for dredging collaterals and reducing phlegm, which comprises the following steps: (1) test solution prepartion: taking capsule contents for dredging collaterals and reducing phlegm, adding methanol, carrying out ultrasonic extraction, filtering, and taking the subsequent filtrate to obtain the test solution; (2) preparation of reference substance solutions: taking tauroursodeoxycholic acid, gastrodin, sodium tanshinol, tanshinone IIA, salvianolic acid B, protocatechuic aldehyde, ginsenoside Rb1, ginsenoside Rg1,notoginsenoside R1, rheum emodin and rheinic acid reference substances which are dried under reduced pressure to constant weight, and respectively adding methanol to prepare the reference substance solutions; (3) determination: precisely measuring the test solution and the reference solution respectively, injecting the solutions into a high performance liquid chromatograph for determination, andrecording chromatograms; and (4) performing comparison according to the chromatogram and the standard comparison fingerprint, and determining that the product is qualified if the chromatogram and thestandard comparison fingerprint are consistent.
Owner:SHANDONG WOHUA PHARMACEUTICALS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com