Synthesis method of D,L-danshensu isopropyl ester
A technology of danshensu isopropyl ester and scrambled danshensu, which is applied in the field of drug synthesis, can solve the problems such as the yield to be improved, and achieve the effects of being easy to handle or recover, having little environmental pollution and reducing production costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
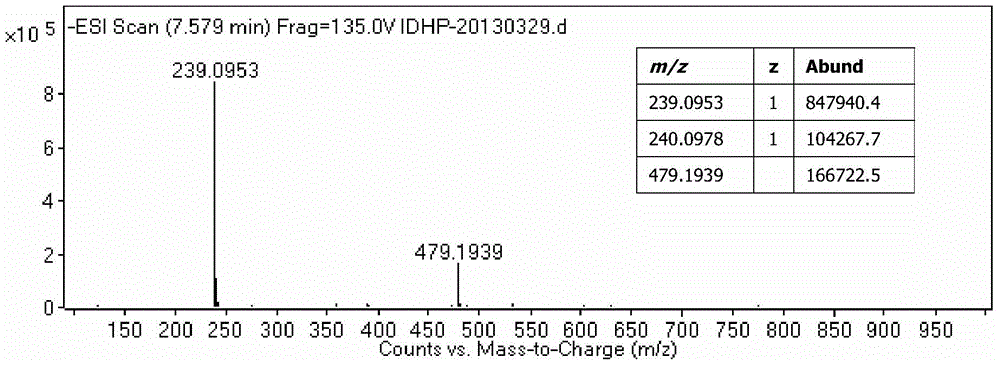
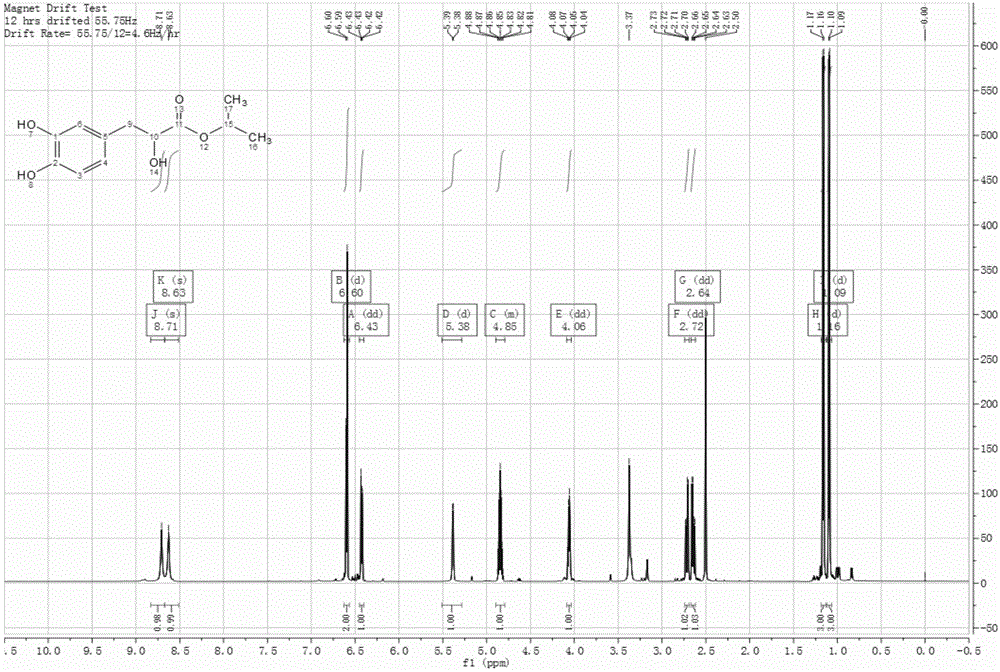
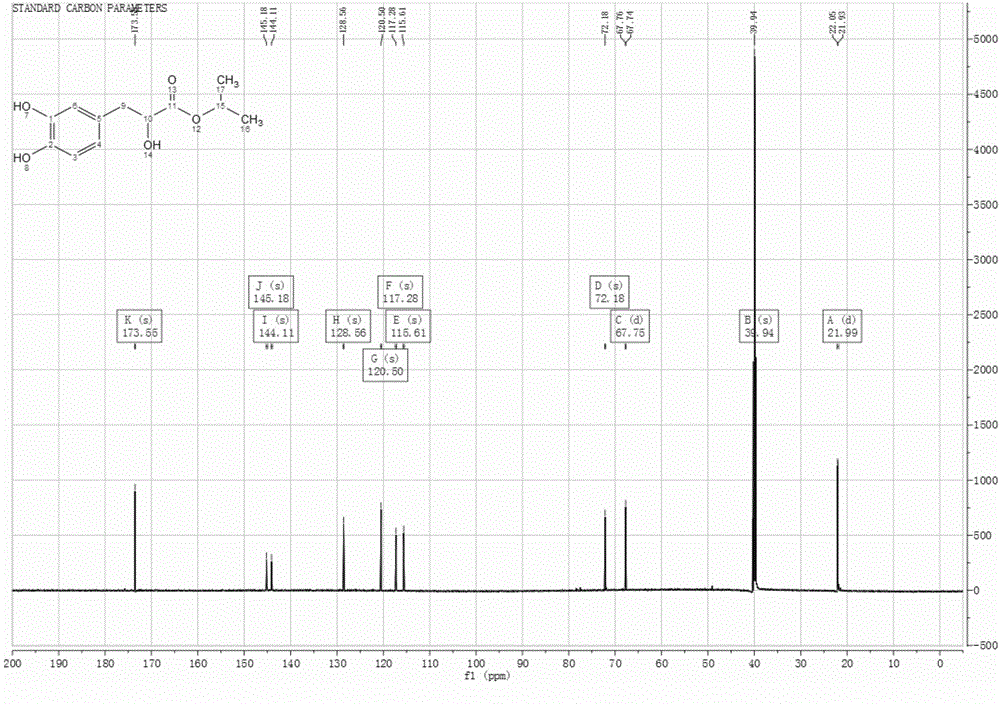
Image
Examples
Embodiment 1
[0056] (1) Synthesis of 3,4-dibenzyloxybenzaldehyde:
[0057] Dissolve 55.2g (0.40mol) of protocatechualdehyde with 600ml N,N-dimethylformamide, slowly add 116.0g (0.92mol) of benzyl chloride to it, and then add anhydrous K 2 CO 3 165.6g (1.2mol), stirring reaction at room temperature for 2 hours, then adding K 2 CO 3 55.2g (0.40mol), heated at 120°C for 2 hours, cooled to room temperature, filtered with suction, the filtrate was added to ice water, acidified with dilute hydrochloric acid, a yellow precipitate was produced, filtered with suction, washed with ice water to obtain compound 3,4-dibenzyl Oxybenzaldehyde 122.1g, the yield is 96%.
[0058] (2) Synthesis of isopropyl chloroacetate:
[0059] Add 56.4 g (0.5 mol) of chloroacetyl chloride to the mixed solution [the mixed solution is 400 mL of dichloromethane, 30.0 g (0.5 mol) of isopropanol and 50.5 g (0.5 mol) of triethylamine] at 0° C. Stir the reaction for 2 h, add 200 mL of water to the reaction solution, wash w...
Embodiment 2
[0073] This embodiment differs from Embodiment 1 in that:
[0074] (1) Synthesis of 3-(3,4-dibenzyloxyphenyl) isopropyl glycidate:
[0075] Dissolve 12.2 g (0.225 mol) of sodium methoxide in 150 mL of methanol to obtain solution 1;
[0076] Dissolve 47.7g (0.15mol) of 3,4-dibenzyloxybenzaldehyde and 20.5g (0.15mol) of isopropyl chloroacetate in 150mL of dioxane to obtain solution 2;
[0077] Drop solution 2 into solution 1 within 30 mm, stir overnight at room temperature, add the reaction solution to 100 mL of ice water, adjust to neutral with hydrochloric acid, extract with dichloromethane (200 mL×4), combine the organic phases, wash with anhydrous sodium sulfate After drying and concentration under reduced pressure, the crude product was recrystallized from isopropanol / petroleum ether to obtain 38.3 g of off-white solid with a yield of 61%.
[0078] (2) Synthesis of Danshensu Isopropyl Ester:
[0079] Dissolve 18g of 3-(3,4-dibenzyloxyphenyl)glycidic acid isopropyl ester ...
Embodiment 3
[0081] This embodiment differs from Embodiment 1 in that:
[0082] (1) Synthesis of 3-(3,4-dibenzyloxyphenyl) isopropyl glycidate:
[0083] Dissolve 21.6 g (0.225 mol) of sodium tert-butoxide in 150 mL of tert-butanol to obtain solution 1;
[0084] Dissolve 47.7g (0.15mol) of 3,4-dibenzyloxybenzaldehyde and 20.5g (0.15mol) of isopropyl chloroacetate in 150mL of dioxane to obtain solution 2;
[0085] Drop solution 2 into solution 1 within 30 mm, stir overnight at room temperature, add the reaction solution to 100 mL of ice water, adjust to neutral with hydrochloric acid, extract with dichloromethane (200 mL×4), combine the organic phases, wash with anhydrous sodium sulfate After drying and concentration under reduced pressure, the crude product was recrystallized from isopropanol / petroleum ether to obtain 41.4 g of off-white solid with a yield of 66%.
[0086] (2) Synthesis of Danshensu Isopropyl Ester:
[0087] Dissolve 6g of 3-(3,4-dibenzyloxyphenyl)glycidyl isopropyl in 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com