Resolution method for chiral Fenoldopam
A chirality, compound technology, applied in organic chemistry methods, chemical instruments and methods, separation of optically active compounds, etc., can solve the problems of hypokalemia intraocular pressure, side effects, increase, etc., to reduce side effects, improve drug efficacy, and reduce drugs. effect of dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
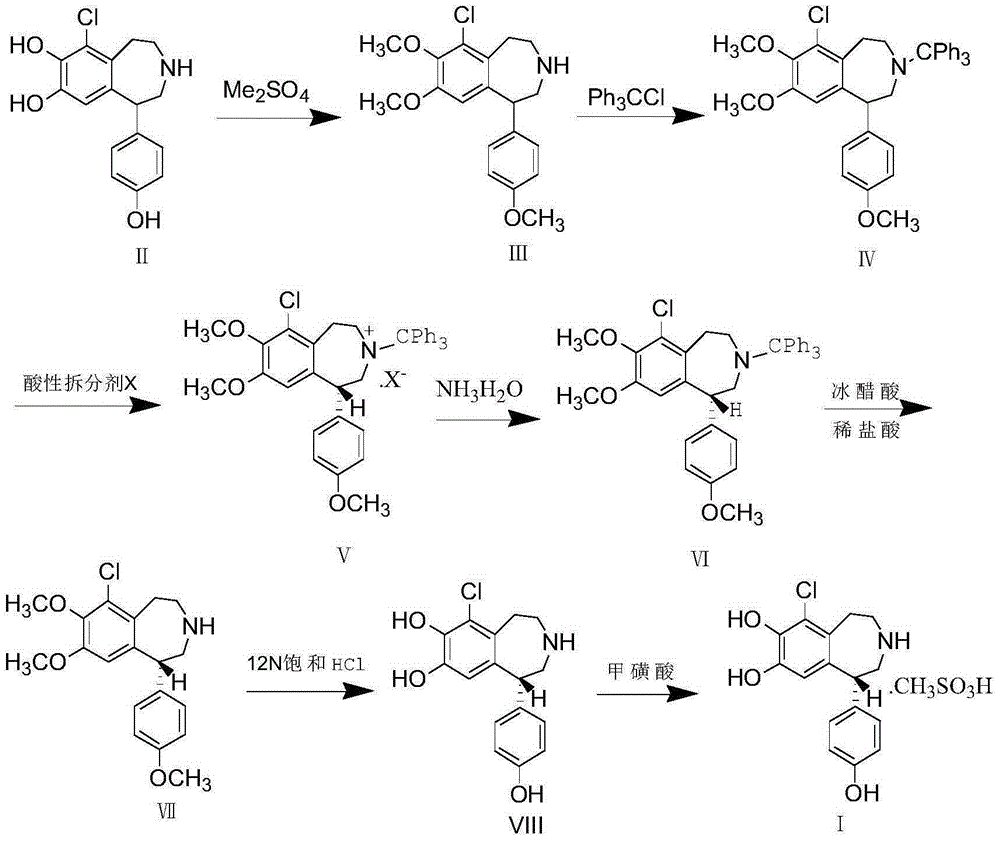
[0035] Experimental Example 1: Synthesis and resolution method of chiral fenoldopam of the present invention
[0036]A. Hydroxyl protection of fenodopam free base is dissolved in absolute ethanol, and Me is added dropwise under stirring at room temperature 2 SO 4 , drop in 2 hours, then continue to stir and react for 0.8-1.2h, add the base shown in claim 5 to adjust the pH to 7.5-8.5; the resulting mixture is extracted with CH2Cl2, the extract is washed with water, then washed with brine, and finally washed with Anhydrous MgSO 4 Drying; the dried extract was filtered, and the filtrate was concentrated in vacuo to obtain a light yellow oil; the crude oil was dried in vacuo overnight to obtain compound III;
[0037] B. The protection compound III of amine group was dissolved in dichloromethane, added triethylamine, added TrCl under stirring at room temperature, and continued the reaction for 4-5hr; filtered, washed the mother liquor three times, and anhydrous MgSO 4 Drying, r...
experiment example 2
[0040] Experimental example 2: Structural characterization and identification of the product
[0041] The structure and atomic number of the obtained product are as follows:
[0042]
[0043] First carry out structural identification with nuclear magnetic resonance spectroscopy, the method is as follows: Weigh an appropriate amount (10-20 mg) of the product sample, put it into a nuclear magnetic tube, and add the deuterated solvent DMSO-d 6 Dissolved, measured with a Bruker400 nuclear magnetic resonance spectrometer at room temperature, the obtained spectrogram is shown below, and the data list and the corresponding hydrogen atoms and structural analysis of each chemical shift value are shown in the following table:
[0044] Chemical shift (ppm)
proton number
Coupled state
Coupling constant (Hz)
attribution
2.33
3
s
CH 2
2.89
1
t
J=11.7
H2
3.19
1
t
J=12.5
3.37-3.52
...
Embodiment 1
[0051] A. Hydroxyl protection
[0052] 125g (410mmol) of fenodopam free base (compound II) was dissolved in 1L of absolute ethanol, and Me2SO4116ml (155g, 1.23mol) was added dropwise under stirring at room temperature. 3 h 2 O was adjusted to pH 8; the resulting mixture was treated with CH 2 Cl 2 extraction, the extract was washed with water, then with brine, and finally with anhydrous MgSO 4 dry. The dried extract was filtered, and the filtrate was concentrated to dryness in vacuo to obtain 139 g of light yellow oil; the crude oil was dried in vacuo overnight to obtain 135 g (95% yield) of compound III;
[0053] B. Protection of amine groups
[0054] Dissolve 135g (about 388mmol) of compound III in 600ml of dichloromethane, add 70ml (500mmol) of triethylamine, add 112g (402mmol) of TrCl under stirring at room temperature, and continue the reaction for 4-5hr; filter, wash the solid with dichloromethane, combine the filtrates, Washed three times with water, anhydrous MgSO...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com