A method for broad-spectrum identification of β-receptor agonist drugs
A receptor agonist and drug technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of increased detection workload and difficulty, difficulty in obtaining, high price, etc., to ensure people's food safety, good social benefits and Economic benefits, wide linear range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
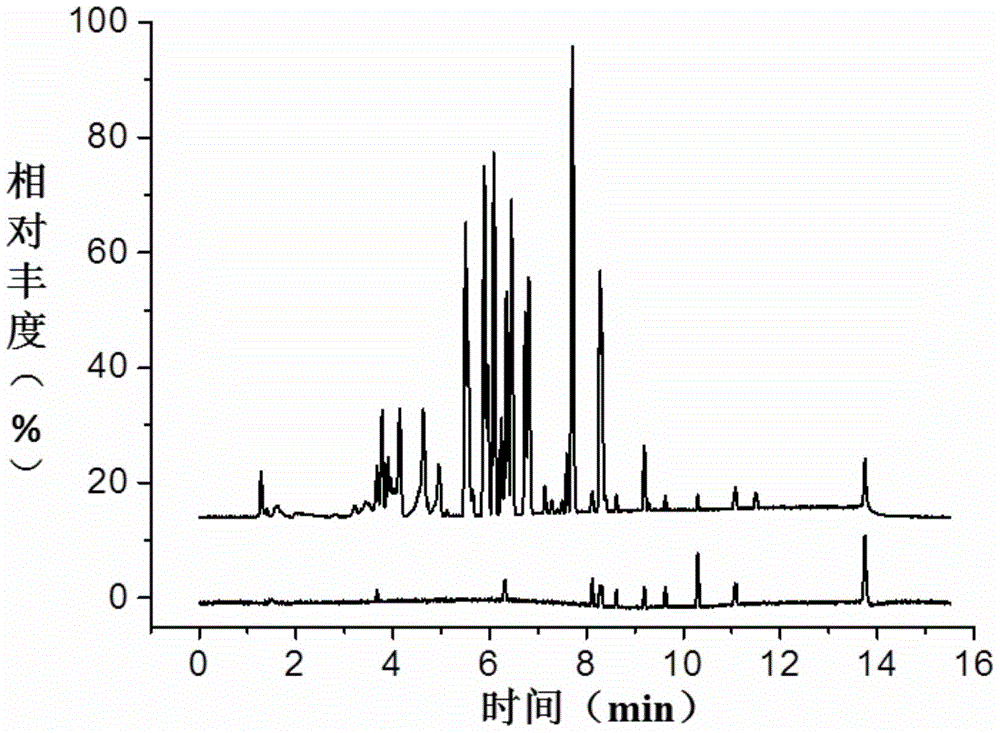
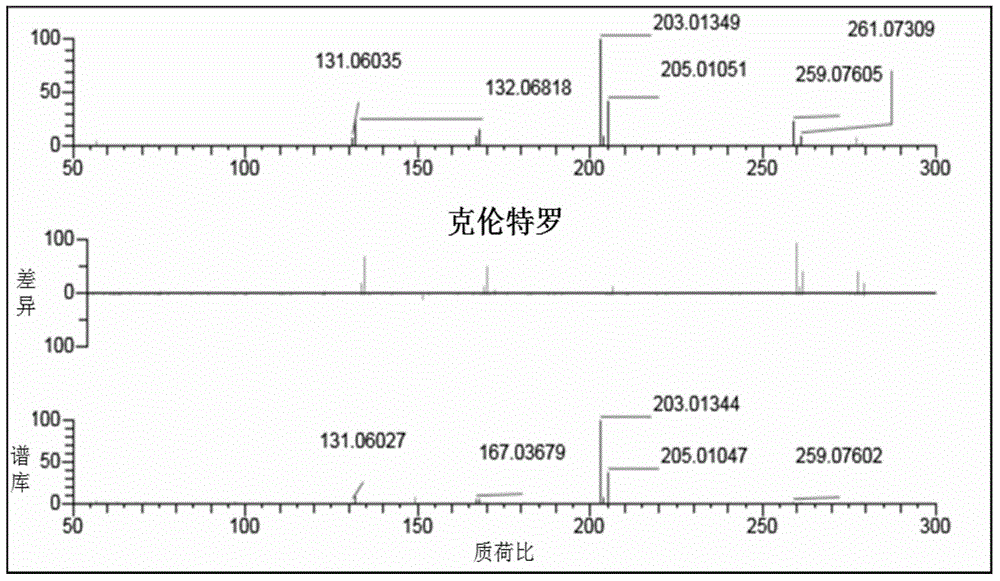
[0070] Example 1 Construction of mass spectral library of β-receptor agonist drugs
[0071] (1) Use the Q-E mass spectrometer to perform primary and secondary mass spectrometry scanning on each standard sample.
[0072] Using 32 existing standard products, including commonly used clenbuterol, ractopamine, albuterol and other β1, β2, β3-receptor agonist drugs, high-resolution mass spectrometry UPLC-Q-Extractivesystem (UPLC, WatersACQUITYUPLCH-ClassBioSystem ; MS, ThermoFisher Q-Exactive).
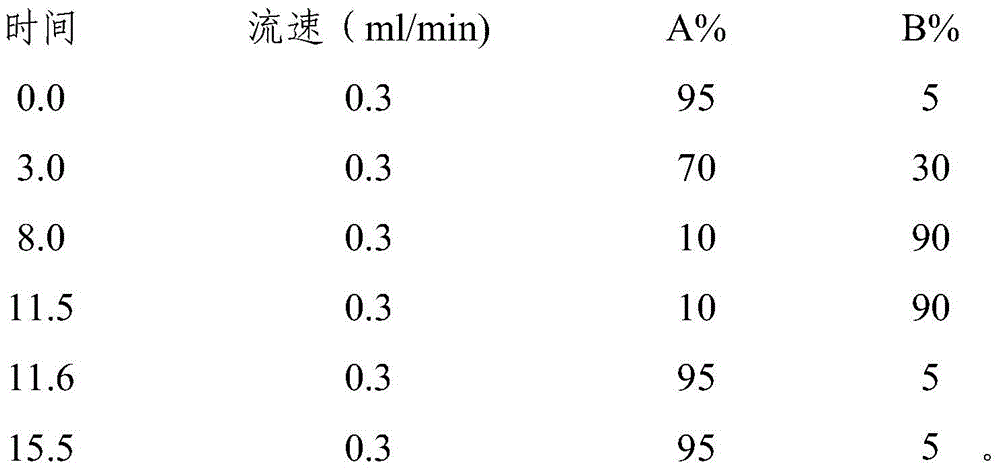
[0073] Optimization of its pretreatment method, liquid phase conditions, and mass spectrometry conditions: the optimization of the pretreatment method mainly includes two aspects, the first is the determination of the feed extract solution, using 1.0% formic acid water and hydrochloric acid methanol to extract feed samples respectively, and it was found that 1.0% The extraction efficiency of % formic acid water is 60%-70%, while the extraction efficiency of hydrochloric acid methanol is gre...
Embodiment 2
[0114] Example 2 Identification of β-receptor agonist drugs in actual samples
[0115] 1. Pretreatment of the sample to be tested; ①Extraction: a. Accurately take 1 volume of urine sample and place it in a 10ml centrifuge tube, add 3 volumes of ammonium acetate buffer (pH5.2), vortex and mix, 8,000 Centrifuge at rpm for 10min, and take the supernatant for later use;
[0116] b. Accurately take 1 volume of plasma sample and place it in a 10ml centrifuge tube, add 3 times the volume (6ml ammonium acetate buffer (pH5.2), mix well, add 6% perchloric acid (precipitated protein) with the same volume of plasma, Centrifuge at 8,000rpm for 10min, and take the supernatant for later use;
[0117] c. Accurately weigh 2g of tissue samples (biceps femoris, liver, kidney) into a 50ml centrifuge tube, add 3mL of ammonium acetate buffer (pH5.2), add 150ul of glucuronic acid / sulfatase (first use acetic acid Ammonium buffer diluted 5 times), homogenized by a tissue homogenizer, oscillating at ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Collision energy | aaaaa | aaaaa |
| Collision energy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com