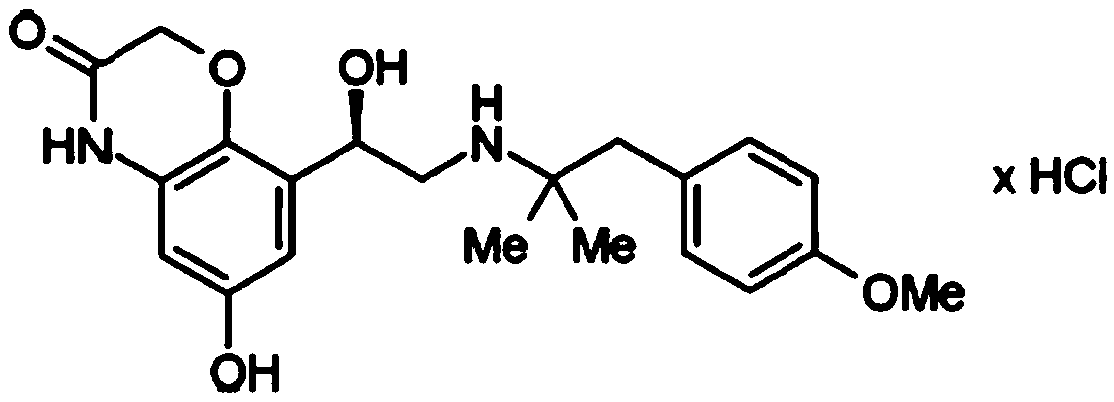
Olodaterol hydrochloride crystalline form B and preparation method thereof
A technology of olodaterol hydrochloride and crystal form, which is applied in the field of medicine, can solve the problems of affecting the efficacy of drugs, difficulties in quality control and preparation work, and inability to obtain stable crystalline products, so as to improve bioavailability, stability, and solubility Improved effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] The preparation of embodiment 1 olodaterol hydrochloride crystal form B
[0066] 1) Preparation of 6-benzyloxy-8-(R)-oxiranyl-4H-benzo[1,4]oxazin-3-one
[0067] 100.6 g (0.3 mol) of 6-benzyloxy-8-((R)-2-chloro-1-hydroxy-ethyl-4H-benzo[1,4]-oxazin-3-one and 2LDMF were added Put it into the reaction bottle, stir, cool down in an ice bath, and drop to 0°C, add 400ml of 2N sodium hydroxide aqueous solution, keep it at 0-5°C for four hours, pour the reaction solution into ice water, stir at 0-5°C for 1 hour, Filtration and vacuum drying of the filter cake at 50°C yielded 86g of off-white solid. Yield 96%, purity HPLC: 96.5%.
[0068] 2) 6-Benzyloxy-8-{(R)-1-hydroxy-2-[2-(4-methoxy-phenyl)-1,1-dimethyl-ethylamino]-ethyl The preparation of base}-4H-benzo[1,4]oxazin-3-one
[0069] 52.5g (0.178mol) of 6-benzyloxy-8-(R)-oxiranyl-4H-benzo[1,4]oxazin-3-one prepared in step 1), 63g (0.351 mol) 2-(4-methoxy-phenyl)-1,1-dimethyl-ethylamine and 500ml of isopropanol were adde...
Embodiment 2
[0083] The preparation of embodiment 2 olodaterol hydrochloride crystal form B
[0084] Add 5 g of the crude product of olodaterol hydrochloride obtained in Example 1, 50 ml of isopropanol and 5 ml of methanol into the reaction flask, stir, heat up to 50-55 ° C, dissolve, keep stirring for half an hour, cool down to 30 ° C, add The seed crystals prepared in Example 1 were incubated and stirred at 25-30°C for 2 hours, filtered, and the filter cake was vacuum-dried at 50°C to obtain 4.6 g of off-white solid, yield: 92%, purity: 99.7%.
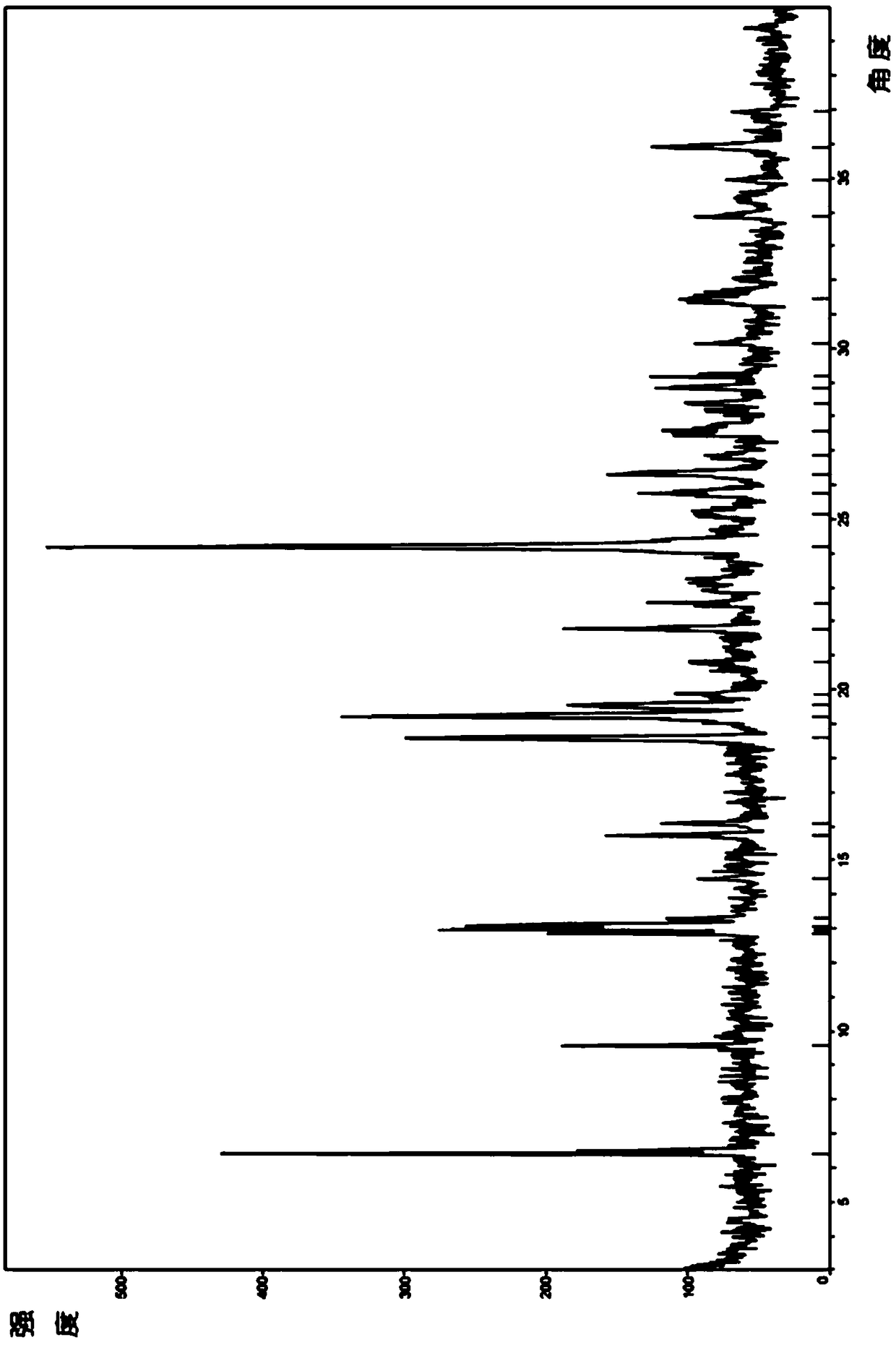
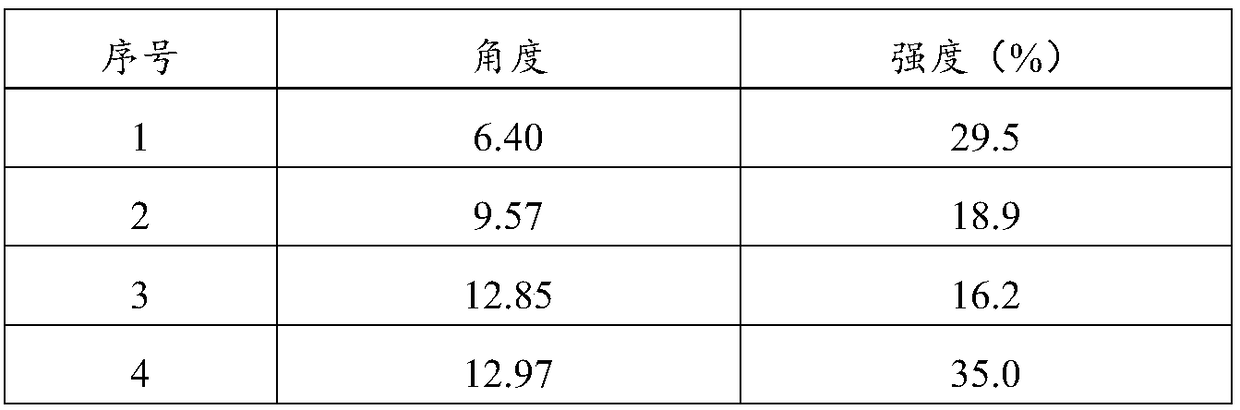
[0085] Adopt Bruker D8 Advance X-ray diffractometer, the product of this embodiment obtains and figure 1 Basically consistent X-ray diffraction pattern (pattern omitted).
[0086] According to the "Chinese Pharmacopoeia" (2005 edition) two appendix XIX J drug hygroscopicity test guidelines to test, measure the hygroscopicity of the olodaterol hydrochloride crystal form B prepared in this example, the result hygroscopicity weight gain 0.33%. M...
Embodiment 3
[0087] The preparation of embodiment 3 olodaterol hydrochloride crystal form B
[0088] Add 4.3 g of the crude product of olodaterol hydrochloride obtained in Example 1, 60 ml of isopropanol and 4 ml of methanol into the reaction flask, stir, heat up to 50-55° C. to dissolve, keep stirring for half an hour, and cool down to 30° C. Add seed crystals, keep stirring at 25-30°C for 2 hours, filter, and vacuum-dry the filter cake at 50°C to obtain 3.8g of off-white solid, yield: 88%, purity: 99.3%.
[0089] Adopt Bruker D8 Advance X-ray diffractometer, the product of this embodiment obtains and figure 1 Basically consistent X-ray diffraction pattern (pattern omitted).
[0090] According to the "Chinese Pharmacopoeia" (2005 edition) two appendix XIX J drug hygroscopicity test guidelines to test, measure the hygroscopicity of the olodaterol hydrochloride crystal form B prepared in this example, the result hygroscopicity weight gain 0.25%. Measured at room temperature, the solubil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com