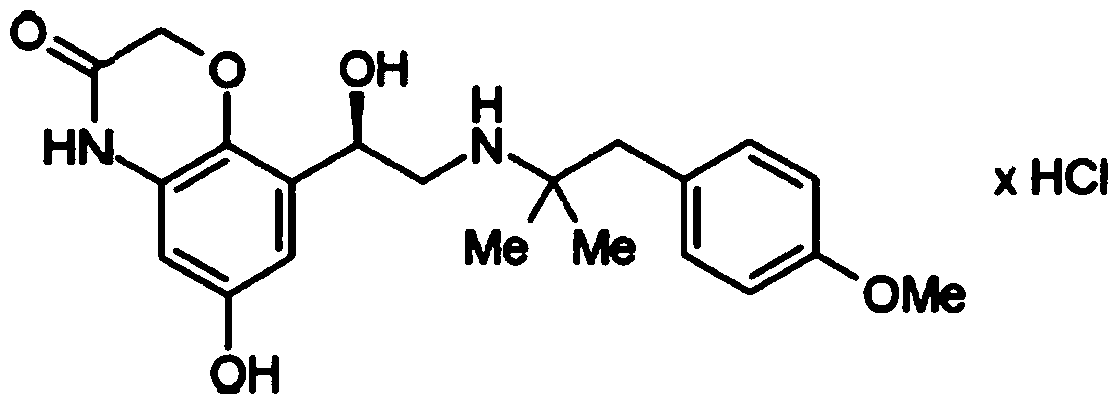
Crystal form C of olodaterol hydrochloride and preparation method thereof
A technology of olodaterol hydrochloride and crystal form, which is applied in the field of medicine, can solve the problems of inability to obtain stable crystalline products, affect the efficacy of drugs, and difficulties in quality control and preparation work, and achieve improved stability, improved drug efficacy, and improved solubility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Example 1 Preparation of olodaterol hydrochloride crystal form C
[0064] 1) Preparation of 6-benzyloxy-8-(R)-oxiranyl-4H-benzo[1,4]oxazin-3-one
[0065] 100.6g (0.3mol) of 6-benzyloxy-8-((R)-2-chloro-1-hydroxyl-ethyl-4H-benzo[1,4]-oxazin-3-one and 2L of Add DMF into the reaction flask, stir, cool down in an ice bath, and drop to 0°C, add 400ml of 2N aqueous sodium hydroxide solution, keep it at 0-5°C for four hours, pour the reaction solution into ice water, and stir at 0-5°C After 1 hour, filter and vacuum-dry the filter cake at 50° C. to obtain 86 g of off-white solid. Yield 96%, purity HPLC: 96.5%.
[0066] 2) 6-Benzyloxy-8-{(R)-1-hydroxy-2-[2-(4-methoxy-phenyl)-1,1-dimethyl-ethylamino]-ethyl The preparation of base}-4H-benzo[1,4]oxazin-3-one
[0067] 52.5g (0.178mol) of 6-benzyloxy-8-(R)-oxiranyl-4H-benzo[1,4]oxazin-3-one prepared in step 1), 63g (0.351 mol) 2-(4-methoxy-phenyl)-1,1-dimethyl-ethylamine and 500ml of isopropanol were added to the reaction ...
Embodiment 2
[0078] Example 2 Preparation of olodaterol hydrochloride crystal form C
[0079] Add 6 g of the crude product of olodaterol hydrochloride obtained in Example 1, 45 ml of isopropanol and 3 ml of methanol into the reaction flask, stir, heat up to 45-50° C. to dissolve, keep stirring for half an hour, cool down to 20° C., 20° C. Stir at ~25°C for 24 hours, filter, and vacuum-dry the filter cake at 50°C to obtain 5.4 g of off-white solid, yield: 90%, purity: 99.4%.
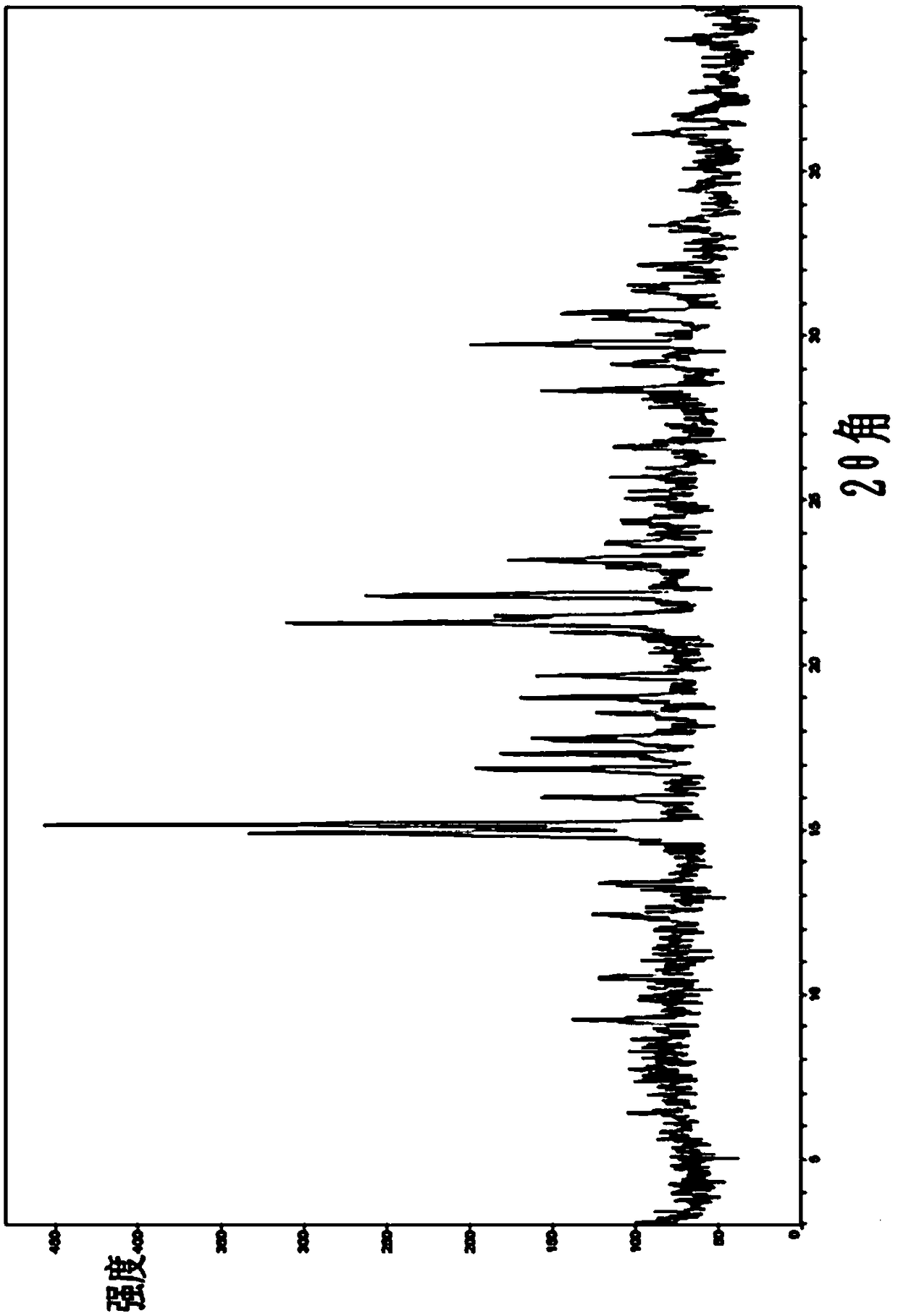
[0080] Adopt Bruker D8Advance X-ray diffractometer, the product of present embodiment obtains and figure 1 Basically consistent X-ray diffraction pattern (pattern omitted).
[0081] According to the "Chinese Pharmacopoeia" (2005 edition) two appendix XIX J drug hygroscopicity test guidelines, the hygroscopicity of the olodaterol hydrochloride crystal form C prepared in this example was measured, and the hygroscopicity weight gain was 0.23%. Measured at room temperature, the solubility of crystal C in water is 1050...
Embodiment 3
[0082] Example 3 Preparation of olodaterol hydrochloride crystal form C
[0083] Add 5.3 g of the crude product of olodaterol hydrochloride obtained in Example 1, 70 ml of isopropanol and 10 ml of methanol into the reaction flask, stir, heat up to 45-50° C. to dissolve, keep stirring for half an hour, and cool down to 25° C. Stir at 20-25°C for 15 hours, filter, and vacuum-dry the filter cake at 50°C to obtain 4.8 g of off-white solid, yield: 91%, purity: 99.3%.
[0084] Adopt Bruker D8Advance X-ray diffractometer, the product of present embodiment obtains and figure 1 Basically consistent X-ray diffraction pattern (pattern omitted).
[0085] According to the "Chinese Pharmacopoeia" (2005 edition) two appendix XIX J drug hygroscopicity test guidelines, the hygroscopicity of the olodaterol hydrochloride crystal form C prepared in this example was measured, and the hygroscopicity weight gain was 0.21%. Measured at room temperature, the solubility of crystal C in water is 105...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com