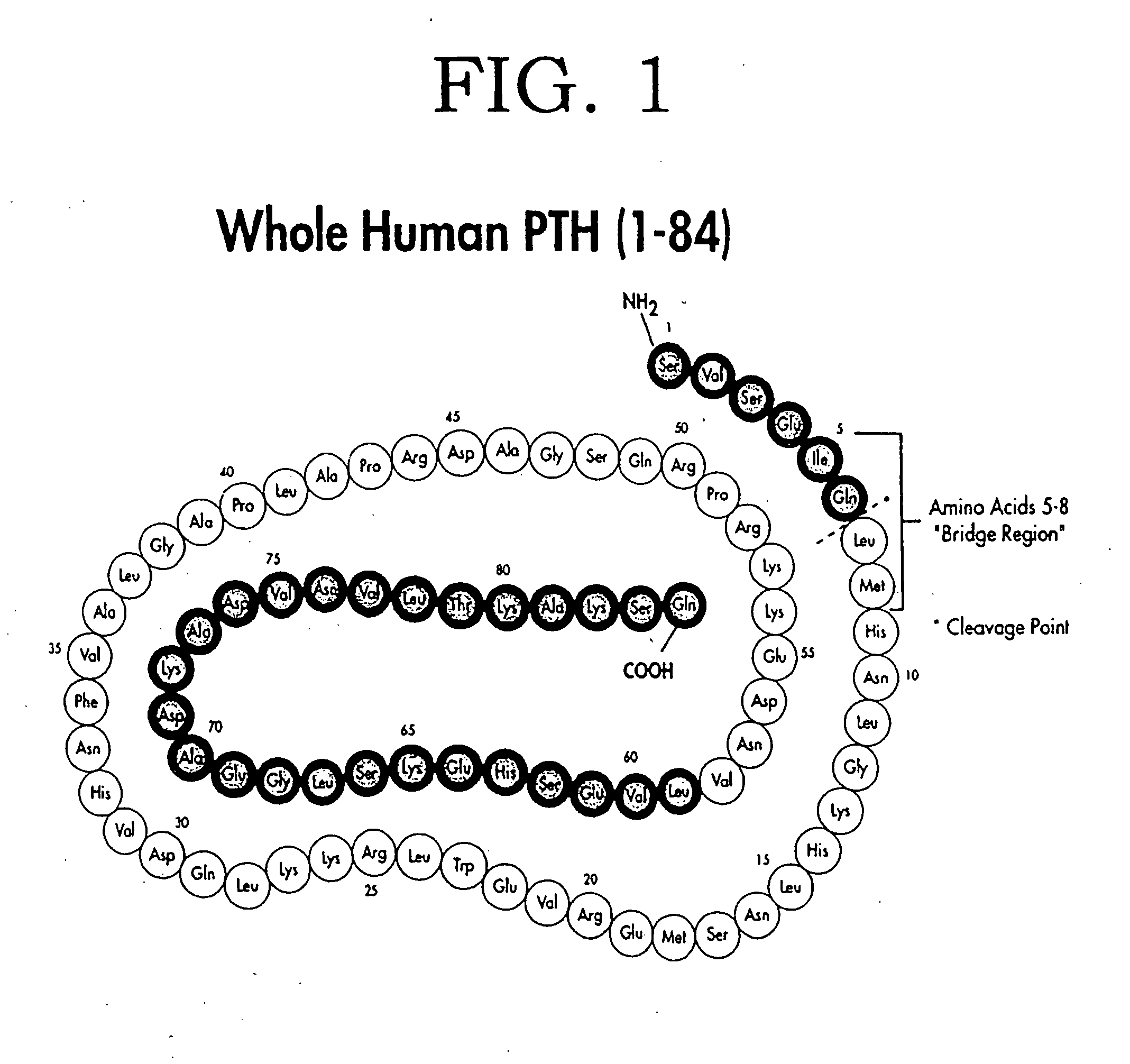
Methods for monitoring and guiding therapeutic suppression of parathyroid hormone in renal patients having secondary hyperparathyroidism
a technology of parathyroid hormone and renal patients, which is applied in the field of monitoring and guiding therapeutic suppression of parathyroid hormone in renal patients having secondary hyperparathyroidism, can solve the problems of increasing calcium intake, reducing and affecting the function of the kidney, so as to reduce the level of pth antagonist and minimize the effect of pth antagonis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
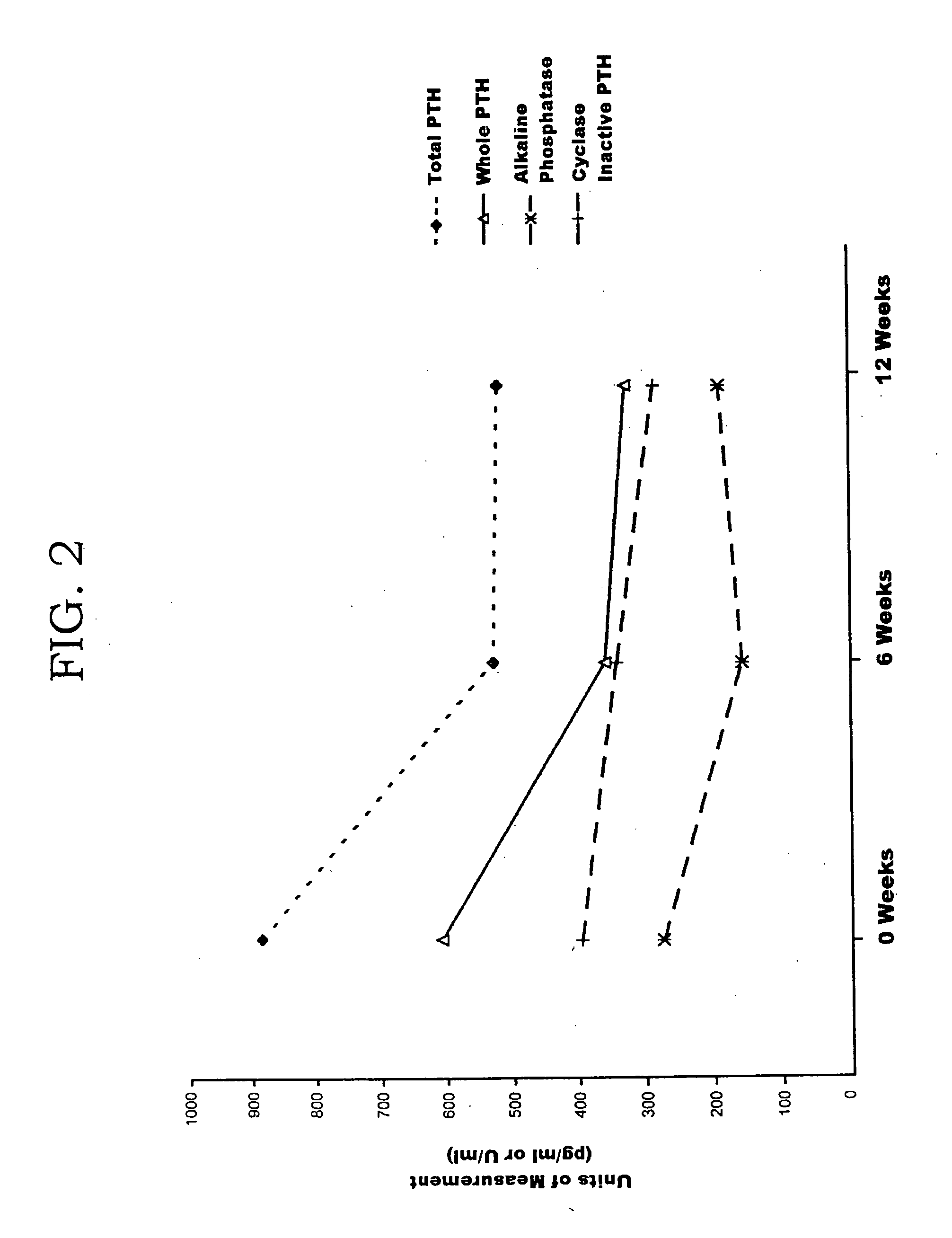
[0064] A clinical trial was held for ninety ESRD patients. Each patient had been receiving vitamin D suppressant therapy in accordance with the manufacturer's guidelines. Each patient was removed from the therapy for a washout period of four weeks, and this was confirmed by a rise in PTH measurements after removal of the therapeutic. PTH maxacalcitol (made by Chugai Pharmaceutical Corporation of Tokyo, Japan) suppressant therapy was started after the washout at a constant administration of 5.5 μg intravenously every three days. Blood samples were obtained from each patient after the washout (week 0), six weeks after therapy restart (week 6), and twelve weeks after therapy restart (week 12). The samples were assayed for PTH agonist levels and PTH antagonist levels using the PTH agonist assay and total PTH assay made by Scantibodies Laboratory, Inc. The samples were assayed for bone specific alkaline phosphatase using a commercially available immunoassay from Hybritech, Inc. of San Di...
example 2
[0068] To verify that PTH agonist and PTH antagonist concentrations and the PTH agonist / antagonist ratio accurately discriminate between high and low bone turnover in renal patients, bone biopsy data is obtained from renal patients having secondary hyperparathyroidism. See Faugere, M-C, et. al., Kidney Int'l. 2001; 60:1460-68. Bone biopsy data will also verify that calculation and evaluation of both PTH agonist and PTH antagonist level data in renal patients provides a more useful therapeutic and prognostic indicator than evaluation of PTH agonist data alone.
[0069] Experimental Design
[0070] Patients with a total PTH greater than 200 pg / ml (as measured by an Intact PTH assay), will have PTH agonist and PTH antagonist levels and the PTH agonist / antagonist ratio determined by Scantibodies® CAP PTH assay(PTH agonist), Scantibodies® Whole PTH assay (PTH agonist), Scantibodies® total intact PTH assay (total PTH) and / or Scantibodies® intact PTH assay (total PTH). Those with a total PTH l...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com