New process for preparing teriparatide
A technology of teriparatide and peptide resin, which is applied in the field of solid-phase synthesis technology to realize the synthesis process of teriparatide, which can solve the problems of many impurities, excessive waste liquid, cumbersome operation steps, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
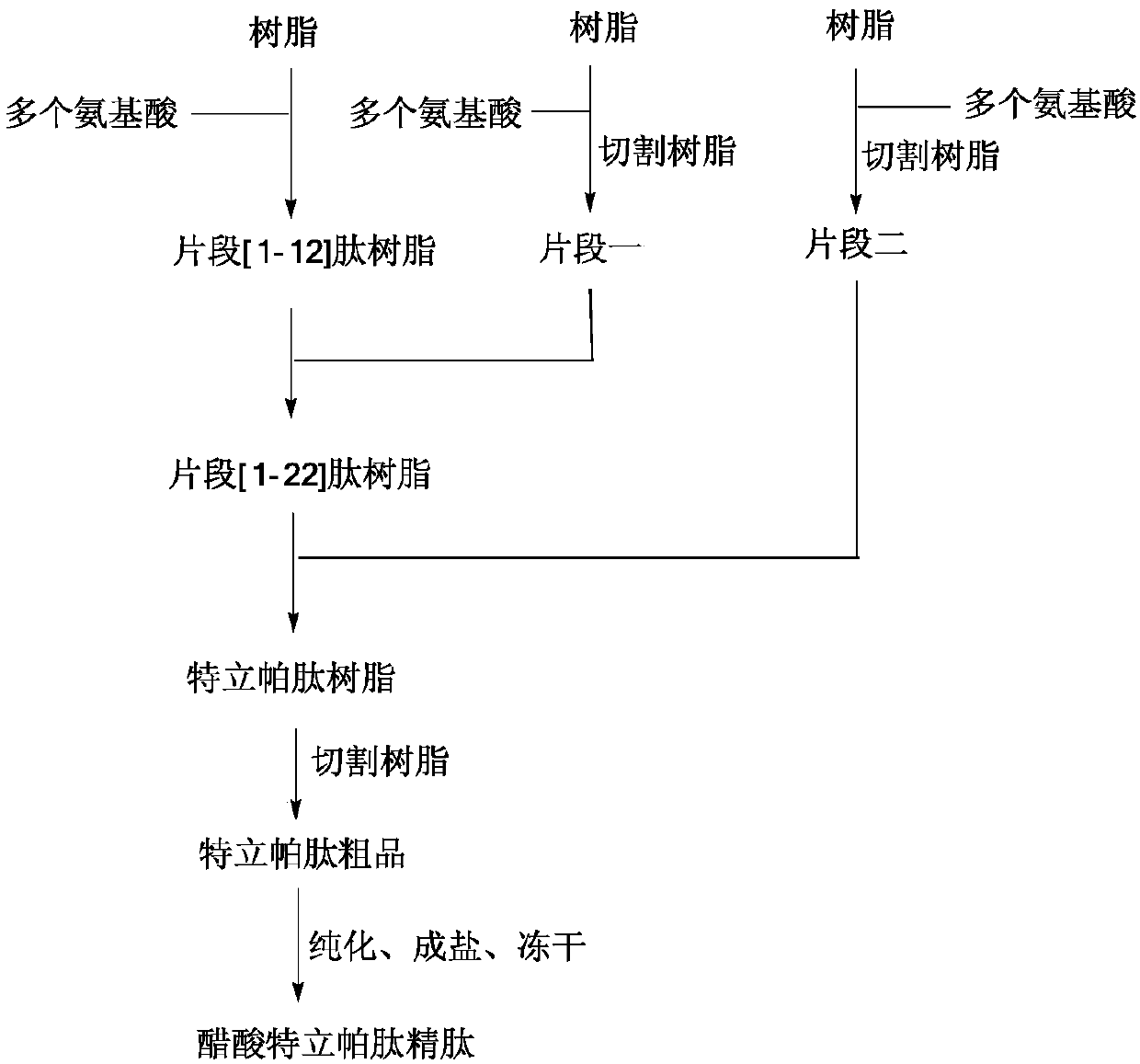
[0078] Example 1: Synthesis of Fragment [1-12] Peptide Resin
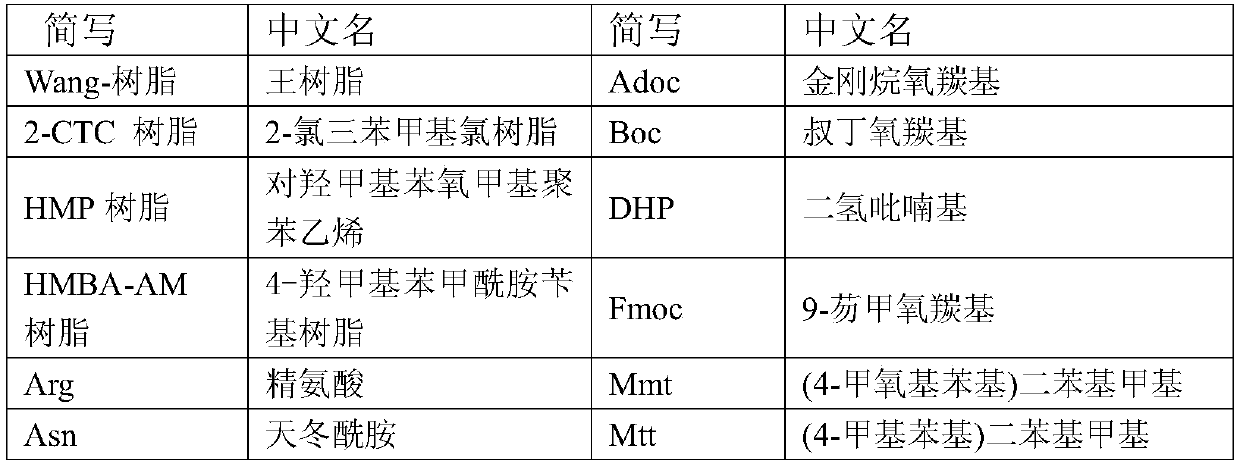
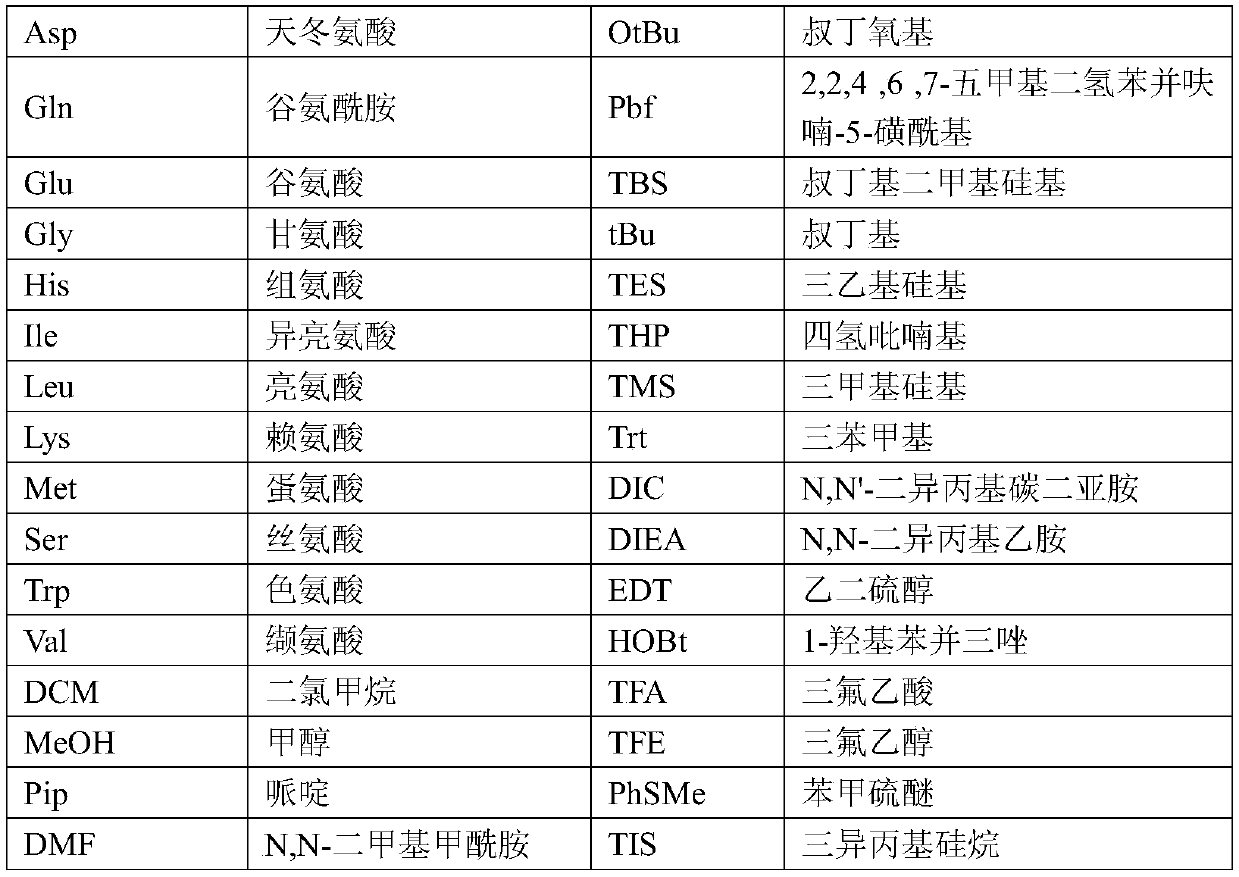
[0079] Add Fmoc-Phe-Wang resin (10.00g substitution degree 0.35mmol / g) into the solid-phase reaction bottle, swell with 200mL DCM and drain it. The filter cake was mixed with 300ml of 20% piperidine / DMF solution, stirred for 5 minutes and then drained; then 300mL of 20% piperidine / DMF solution was added, mixed and stirred for 15 minutes, and drained. Wash the filter cake with an appropriate amount of DMF, drain it, and store it in a solid-phase reaction bottle for use. In another dry reaction flask, add Fmoc-Asn(Trt)-OH (6.45g), HOBT (1.46g), 30mL DMF in sequence, stir, add DIC (1.36g) within 30min under ice bath, and stir to obtain an activated solution. The newly obtained solution was added to the above-mentioned solid-phase reaction flask, and stirred for 2 h under the protection of nitrogen until the coupling reaction was detected by ninhydrin to complete. Drained, the filter cake was washed with an appropria...
Embodiment 2
[0081] Embodiment two: the synthesis of fragment one
[0082] Add 2-CTC Resin (25.00g, degree of substitution 1.12mmol / g) and 400mL DCM in sequence to the solid-phase reaction bottle, and suction filter after swelling to obtain a filter cake. Fmoc-Glu(OtBu)-OH (8.51g) and 100mL DCM were added sequentially, stirred under nitrogen protection, and DIEA (about 9.53g) was added dropwise. After dropping, stir the reaction for 2h. MeOH (25 mL) was added and stirring was continued for 30 min. After draining, the filter cake was washed alternately with MeOH and DCM, drained, and vacuum-dried at room temperature to obtain the linker resin. After testing, the degree of substitution is 0.73mmol / g;
[0083] Add the above-mentioned resin and 200mL DCM sequentially into the solid-phase reaction bottle, drain it after swelling. Add 100mL of 20% piperidine / DMF solution, mix and stir for 5 minutes, and drain; then add 100mL of 20% piperidine / DMF solution, mix and stir for 15 minutes, and dr...
Embodiment 3
[0086] Embodiment three: the synthesis of fragment two
[0087] Add 2-CTC Resin (25.00g, degree of substitution 1.12mmol / g) and 400mL DCM sequentially into the solid-phase reaction flask, swell and drain to obtain a filter cake. Add 100 mL of DCM, Fmoc-Gly-OH (5.95 g) in sequence, stir under nitrogen protection, and add DIEA (9.53 g) dropwise. After dropping, the reaction was stirred for 2 h, MeOH (25 mL) was added, and the reaction was stirred for 30 min with nitrogen bubbling. Drained, and the filter cake was alternately washed with DCM and MeOH. Drained and vacuum-dried at room temperature to obtain joint resin. After testing, the degree of substitution was 0.74mmol / g.
[0088] Swell the above material with 200mL DCM and drain it. The filter cake was mixed with 100 mL of 20% piperidine / DMF solution, stirred for 5 minutes, and drained; then 100 mL of 20% piperidine / DMF solution was added, mixed and stirred for 15 minutes, and drained. An appropriate amount of DMF was us...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com