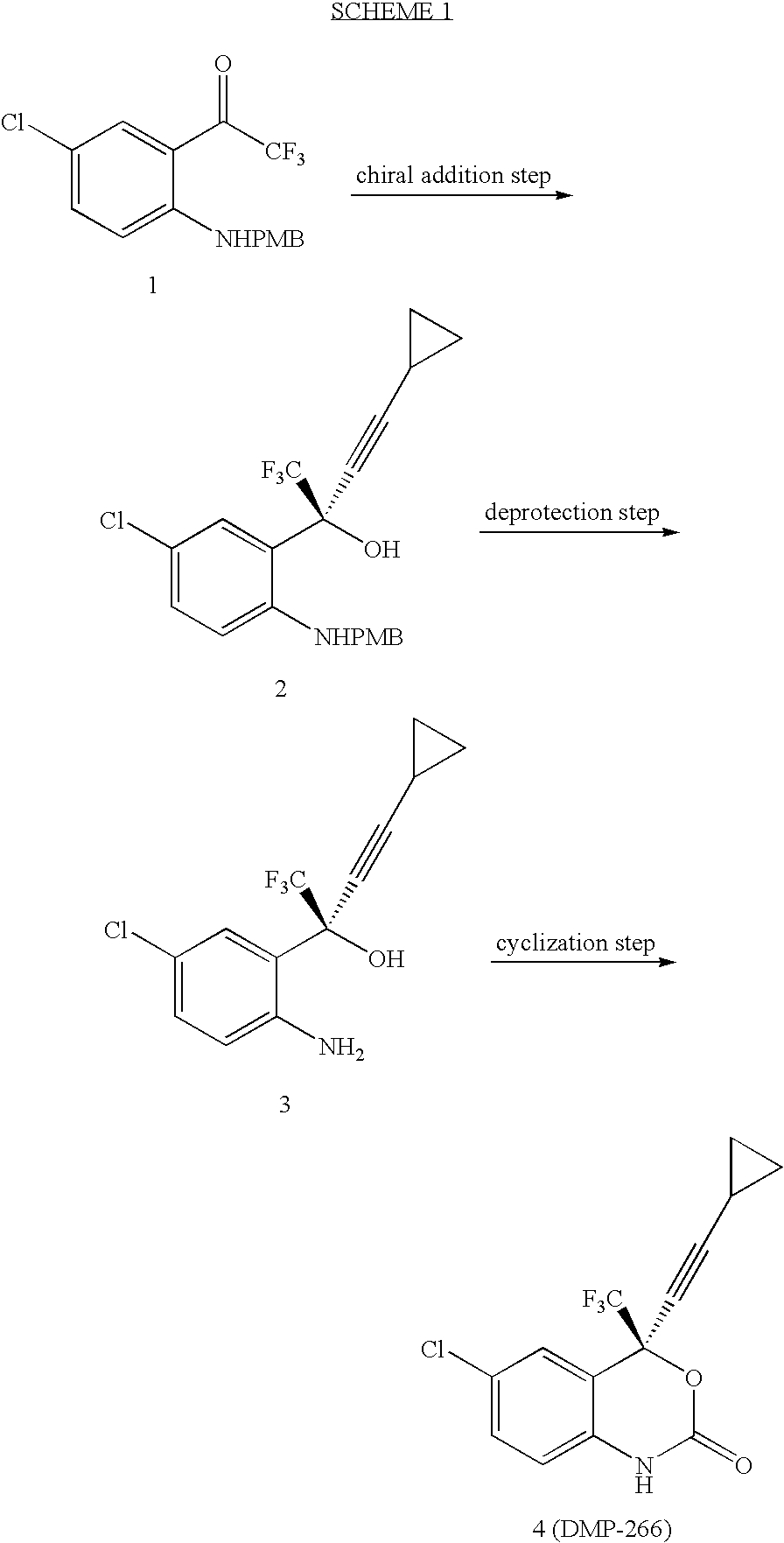
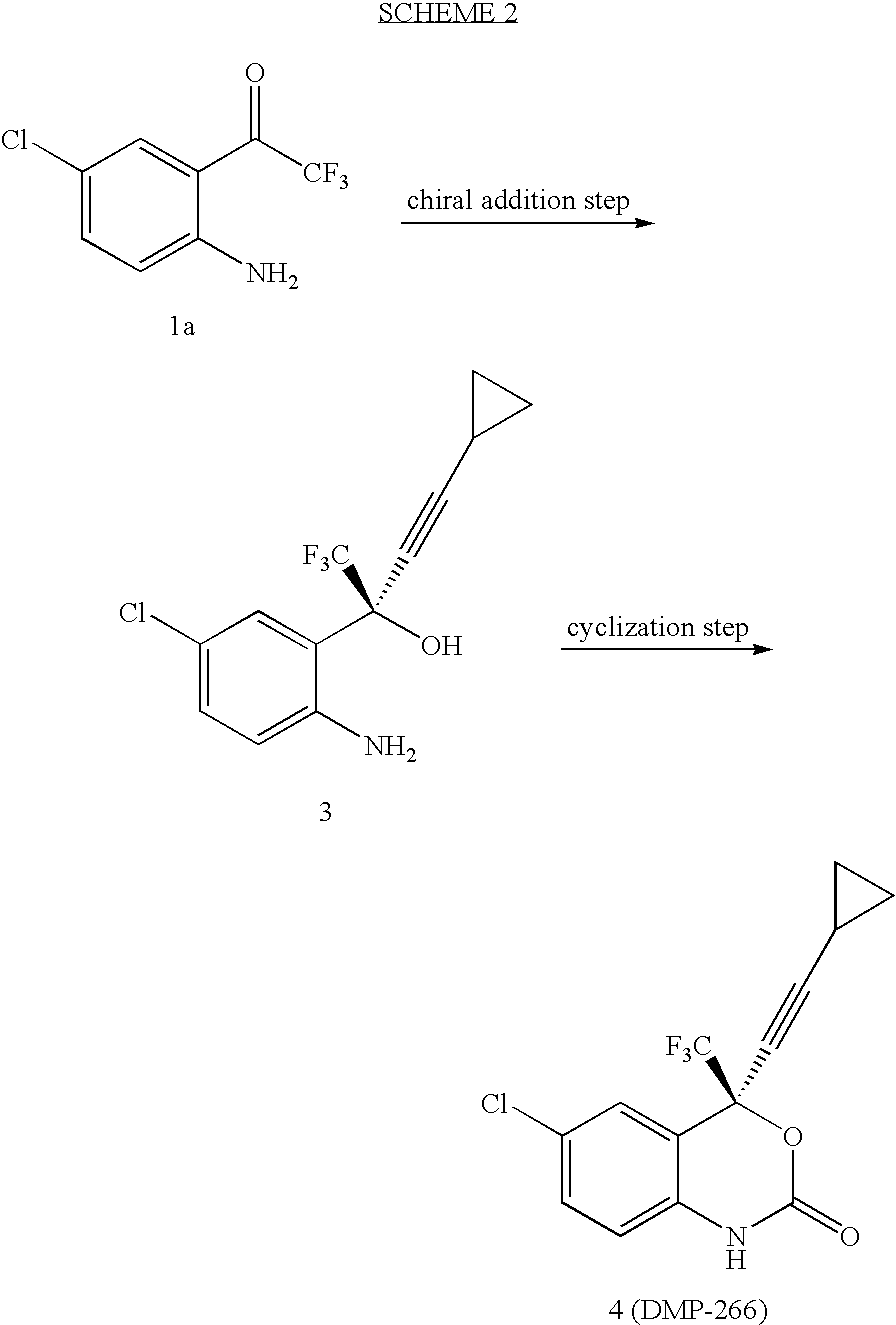
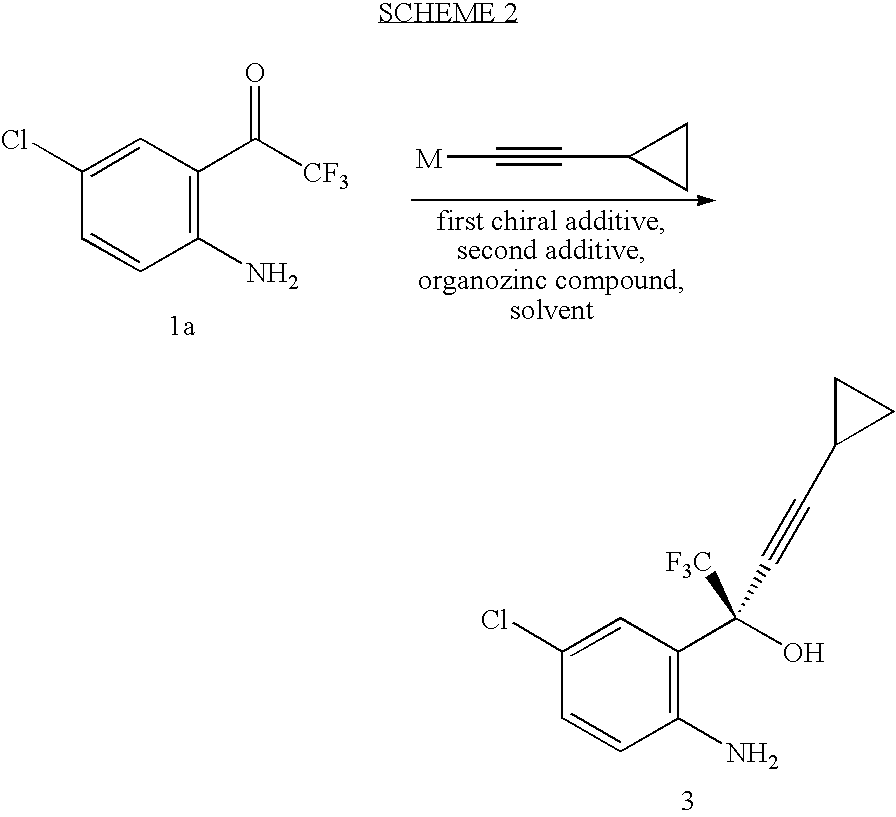
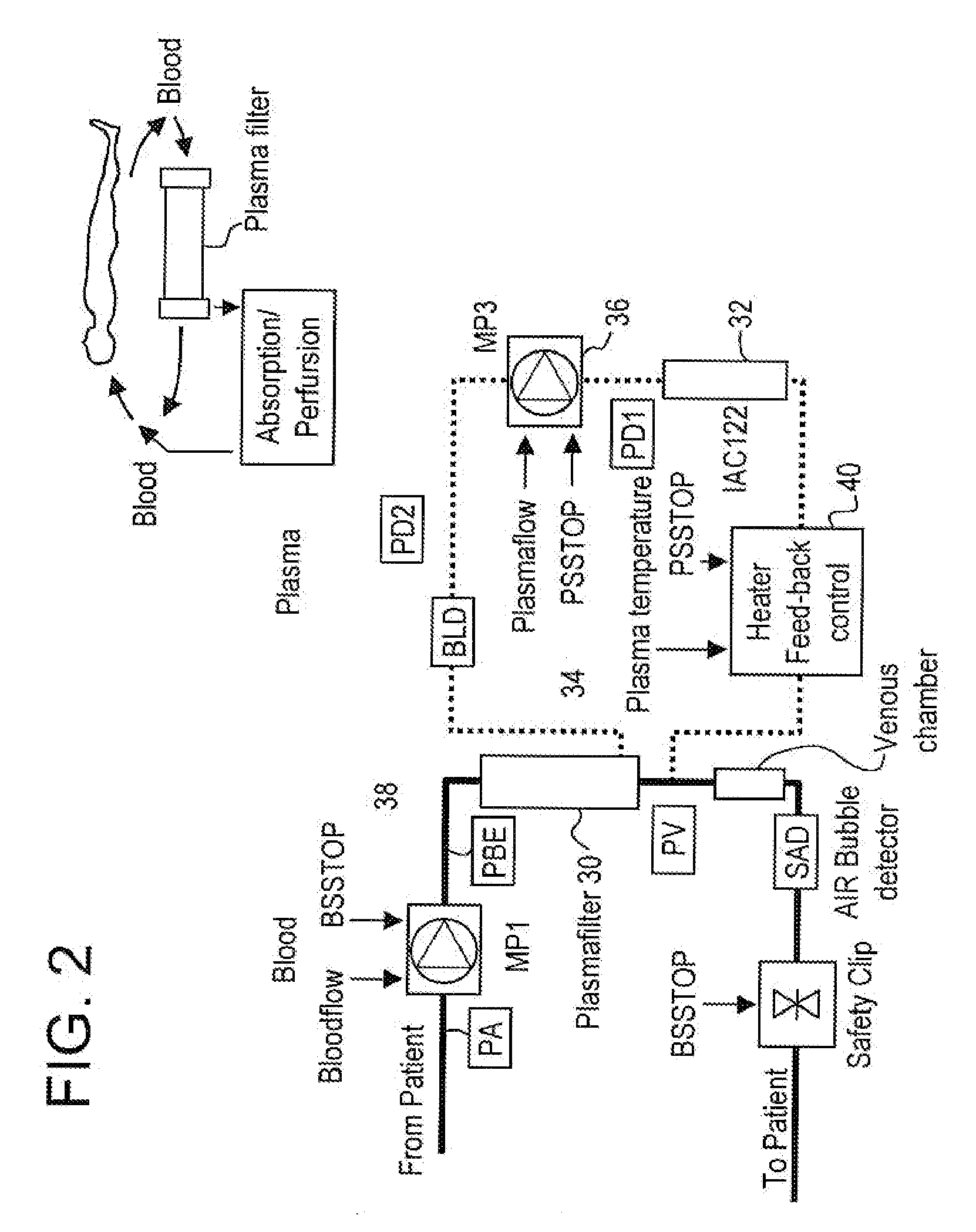
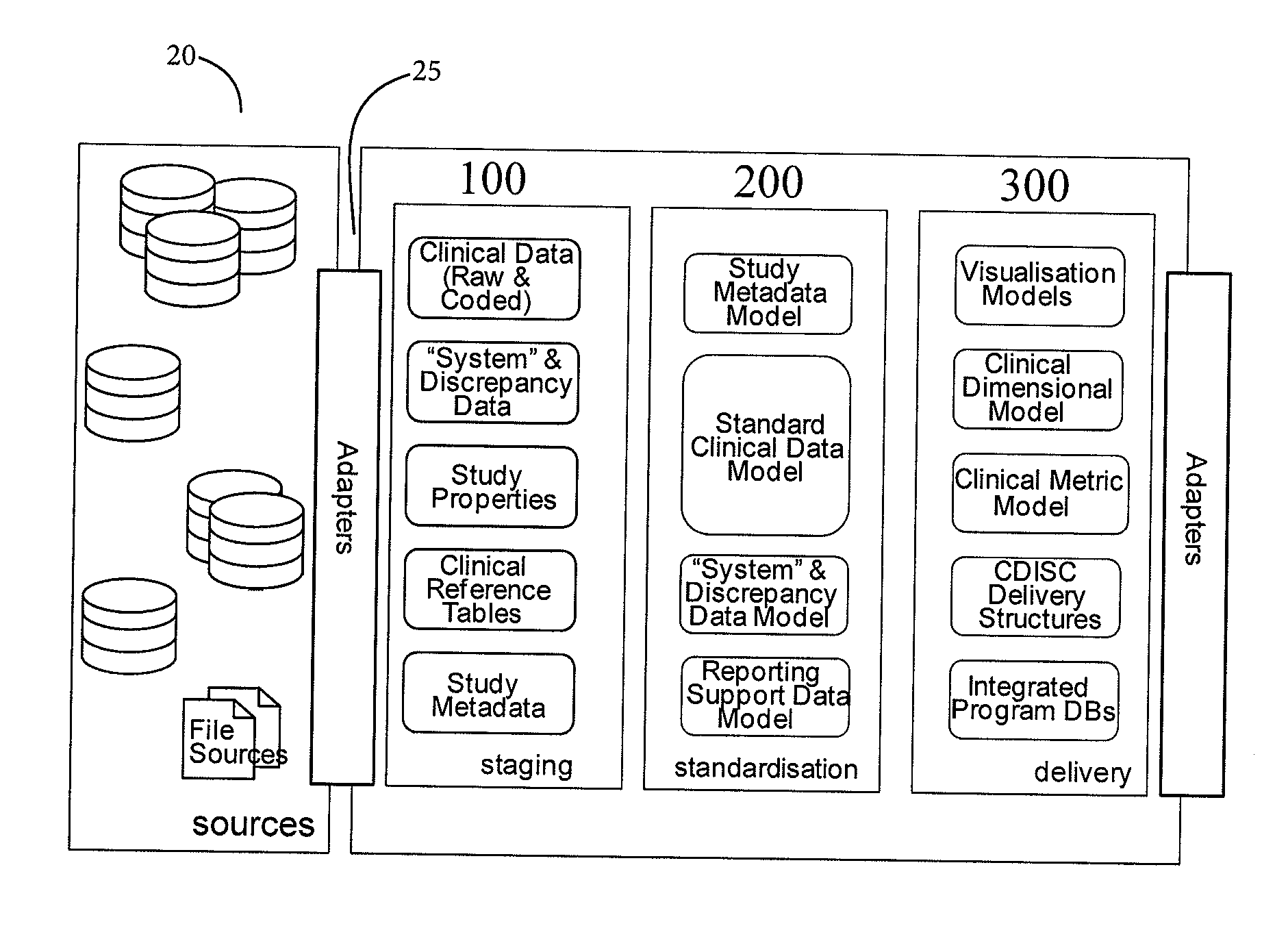
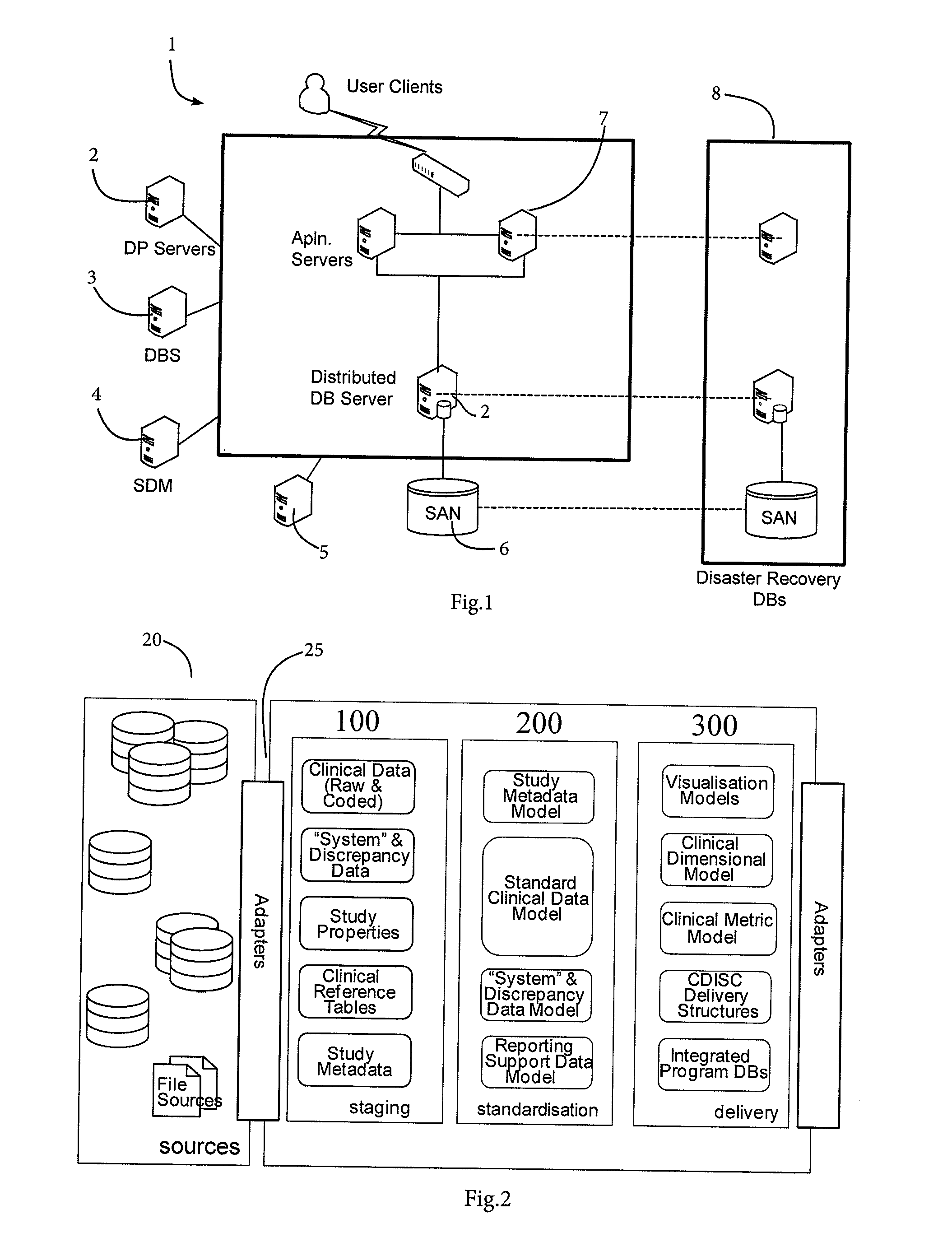
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
306 results about "Clinical study" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Soft contact lenses displaying superior on-eye comfort
The present invention relates to soft contact lenses having an overall comfort preference of at least about 2 to 1 as compared to an Acuvue® contact lens and measured after one week of daily wear. The present invention further relates to a soft contact lens comprising an oxygen transmissibility greater than about 70 barrers / mm and physical properties suitable to provide wearer comfort over at least about 9 hours in at least about 80% of wearers, as measured in a randomized, double masked clinical study.
Owner:JOHNSON & JOHNSON VISION CARE INC
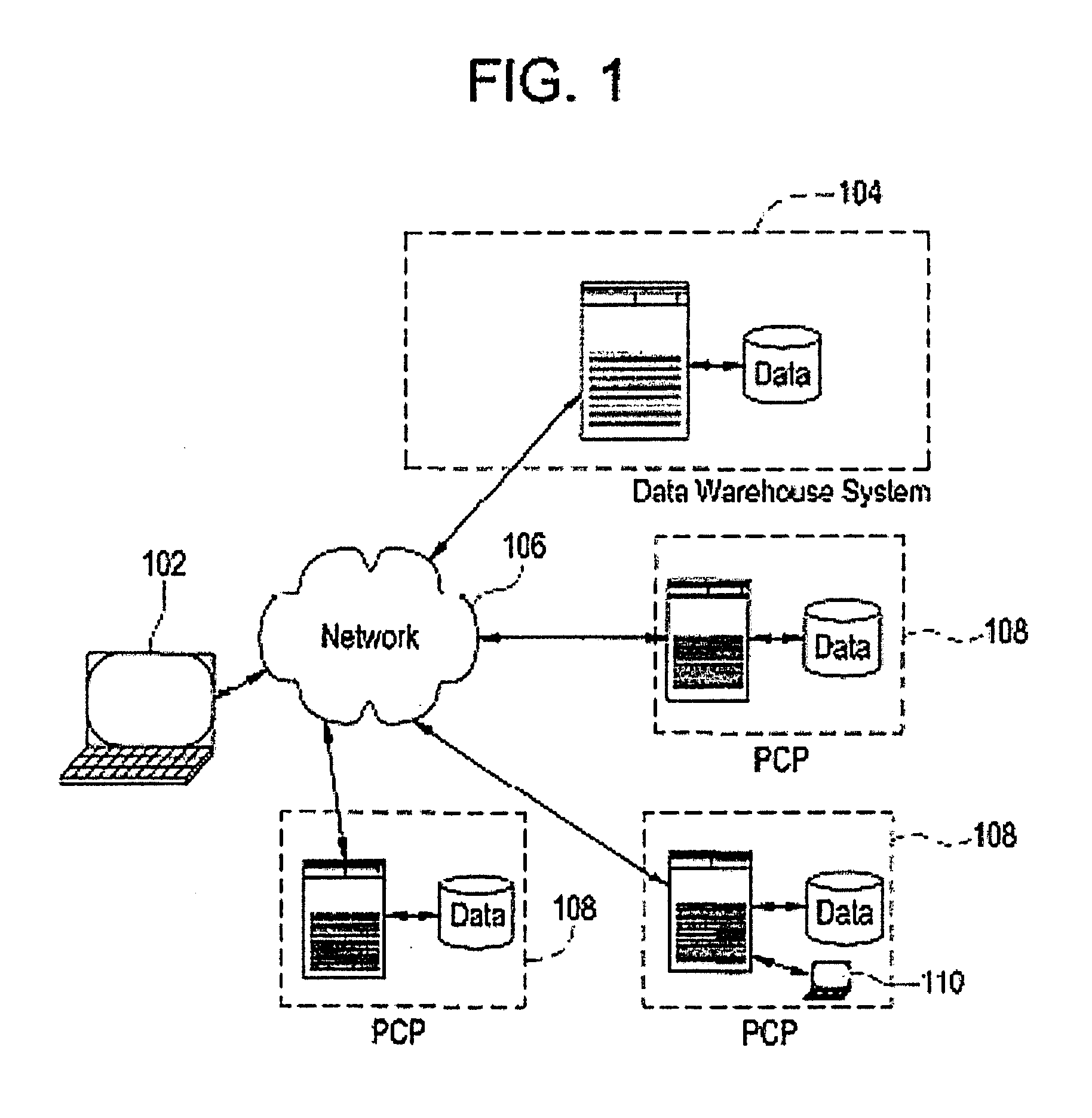
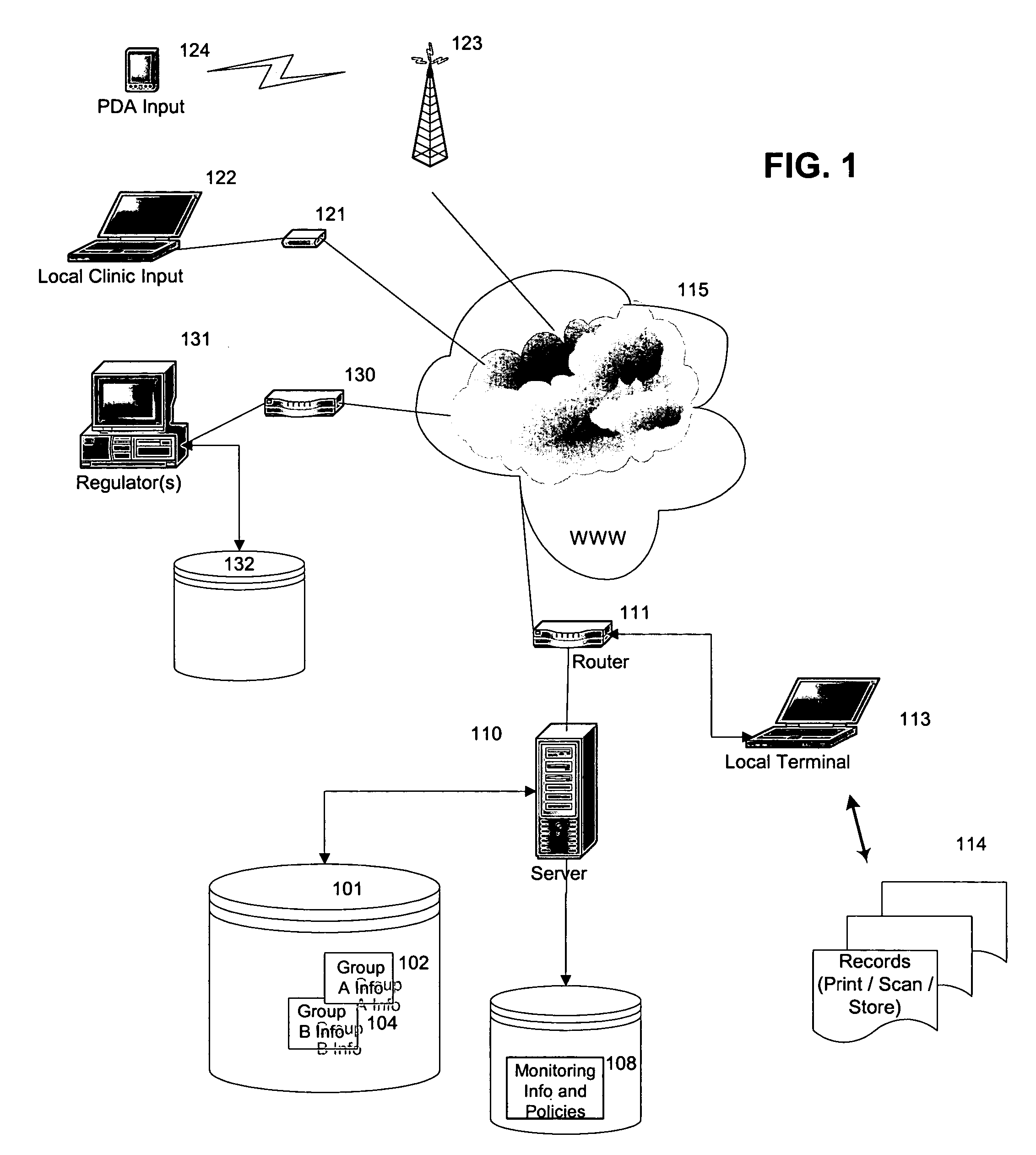
Method of monitoring patient participation in a clinical study
InactiveUS20050182664A1Strengthen incentivesSpeed up the processComputer-assisted medical data acquisitionOffice automationClinical trialClinical study
A method is proposed for monitoring participation of a patient in at least one clinical trial. The method includes collecting data regarding at least one clinical trial for the patient. Using a computer device, the collected data is then compared to at least one threshold. Finally, the patient is rewarded upon the collected data at least meeting at least one threshold.
Owner:SIEMENS AG
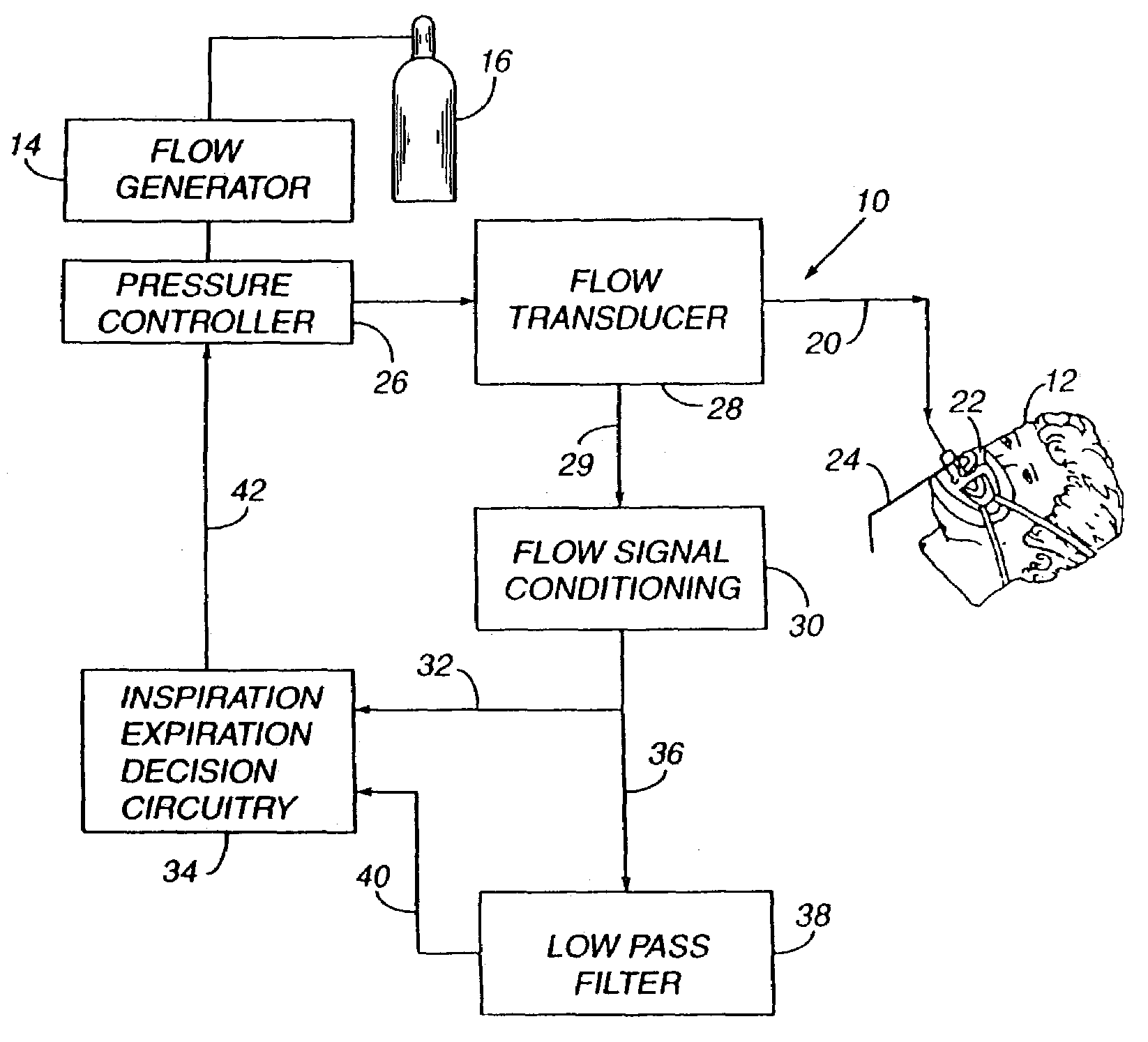
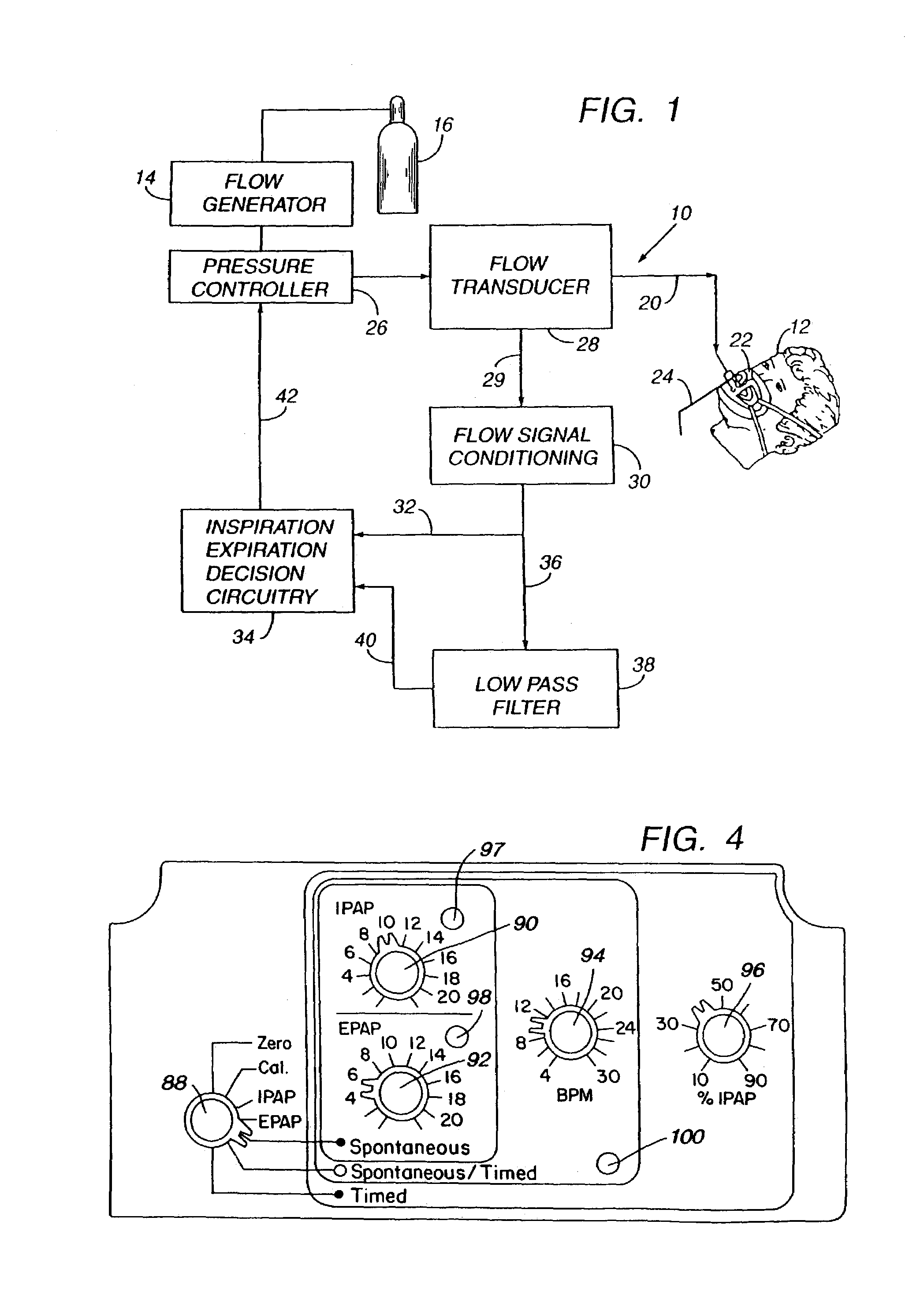
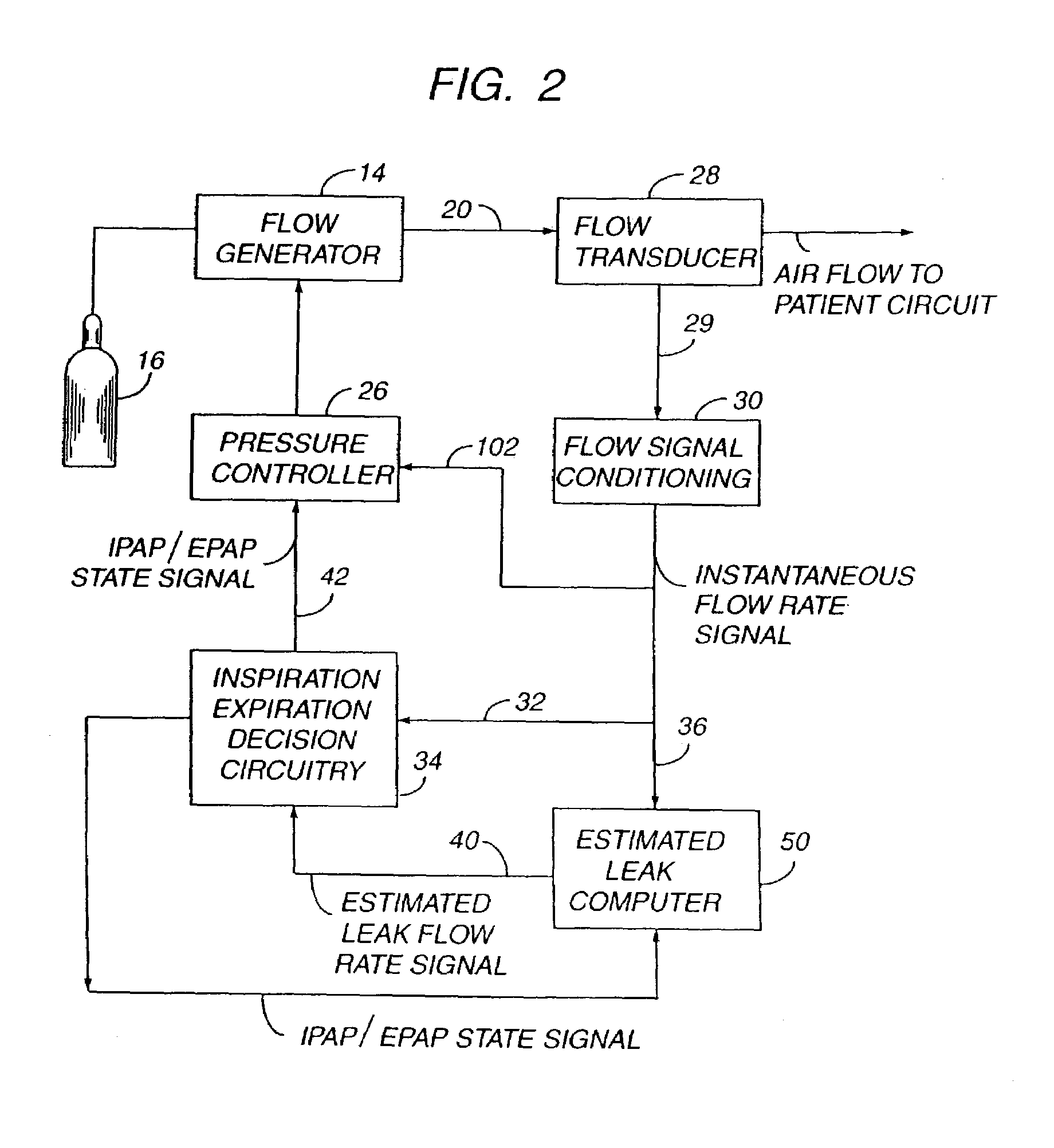
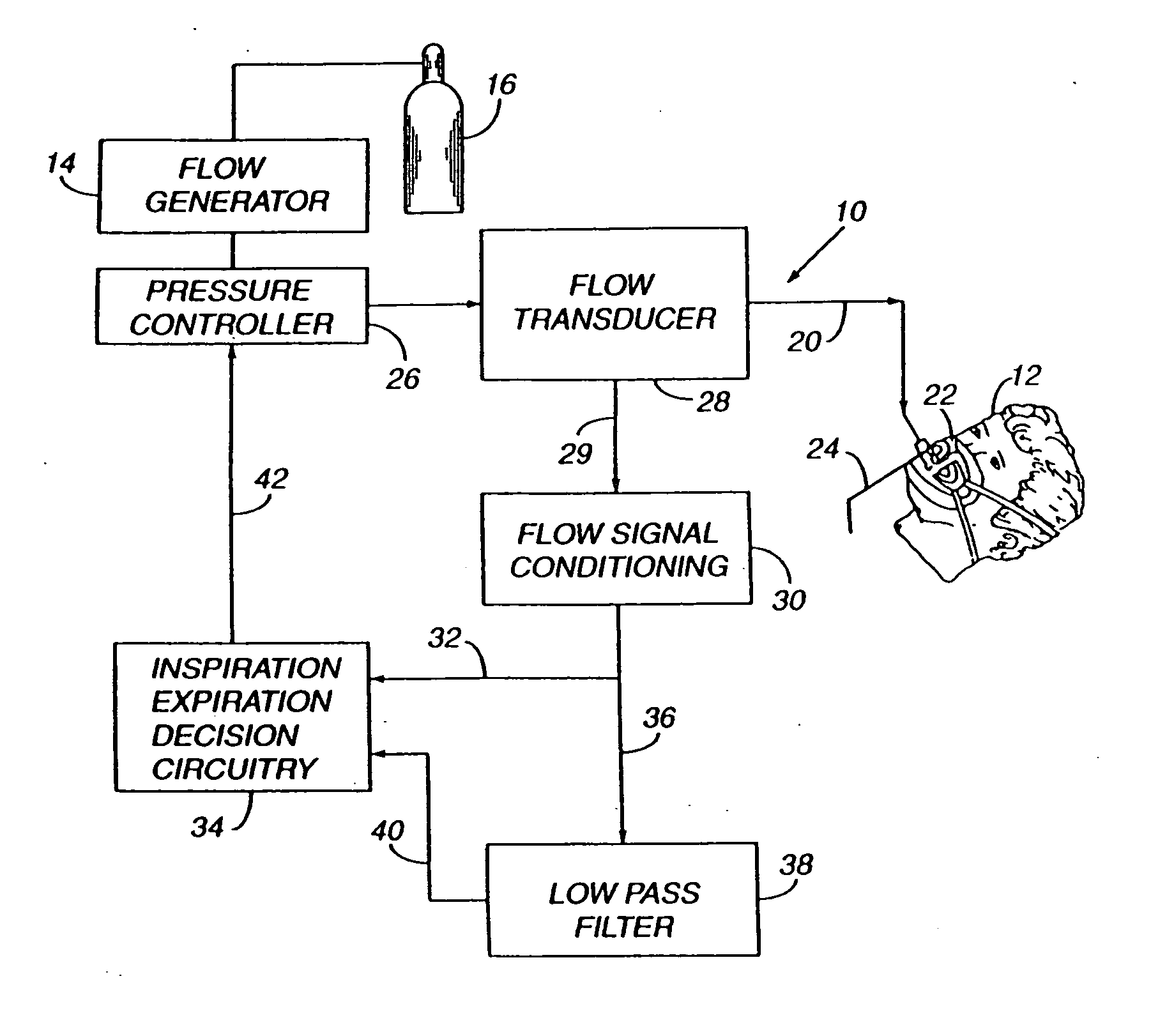
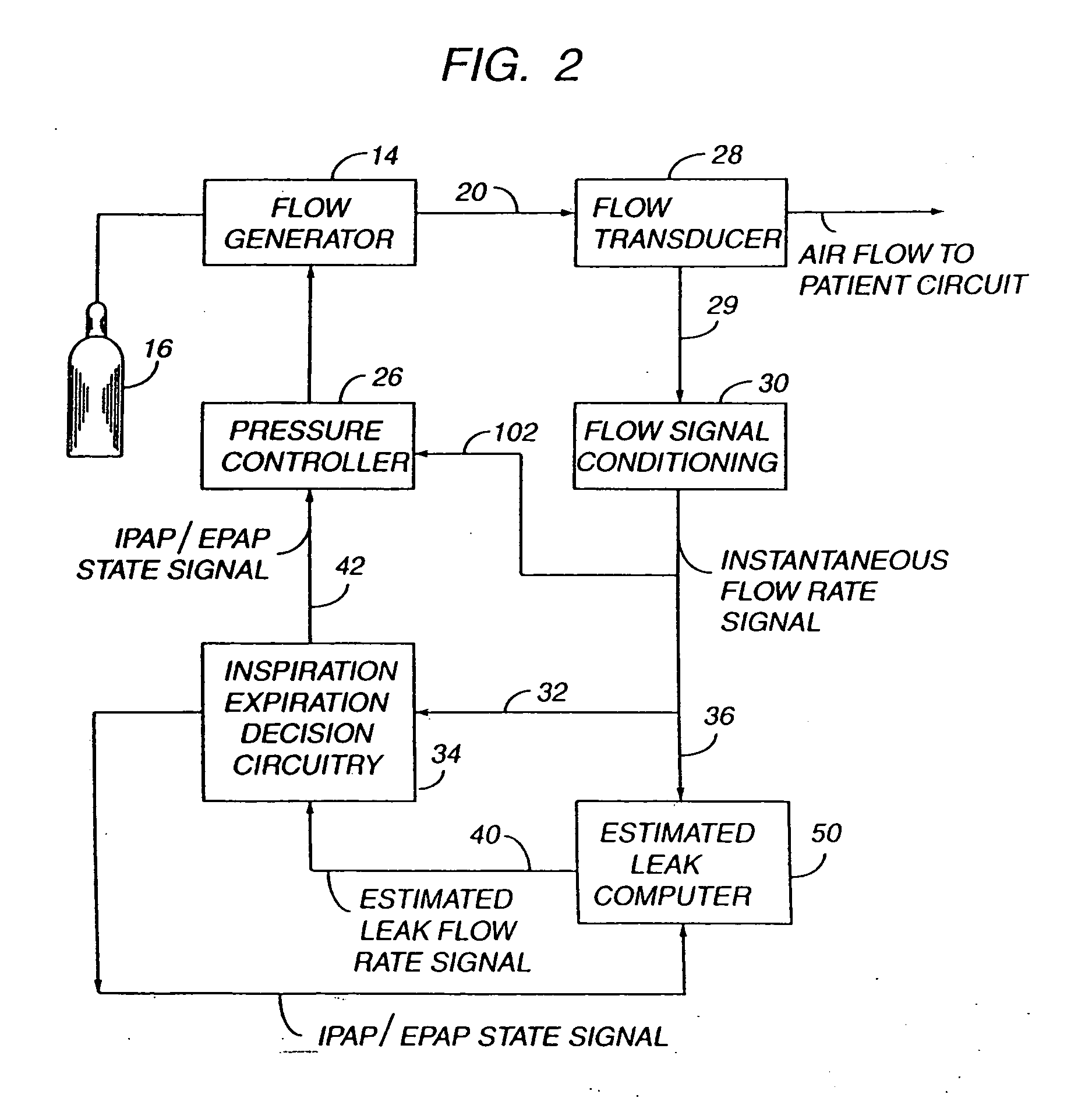
Sleep apnea treatment apparatus
InactiveUS7013892B2Reduce pressureEasy to operateRespiratorsOperating means/releasing devices for valvesSleep researchClinical study
Improved methodology and apparatus for the clinical study and treatment of sleep apnea which incorporates one or more of the following features: (1) application of mono-level, alternating high and low level, or variable positive airway pressure generally within the airway of the patient with the mono-level, high and low level, or variable airway pressure generally being coordinated with and / or responsive to the spontaneous respiration of the patient, (2) usage of adjustably programmable pressure ramp circuitry capable of producing multiple pressure ramp cycles of predetermined duration and pattern whereby the ramp cycles may be customized to accommodate the specific needs of an individual sleep apnea patient so as to ease the patient's transition from wakefulness to sleep, (3) remote control or patient-sensed operation of the apparatus, (4) employment of safety circuitry, reset circuitry and minimum system leak assurance circuitry, controls and methods, and (5) utilization of clinical control circuitry whereby sleep disorder data may be compiled and appropriate therapy implemented during a one-night sleep study.
Owner:RIC INVESTMENTS LLC
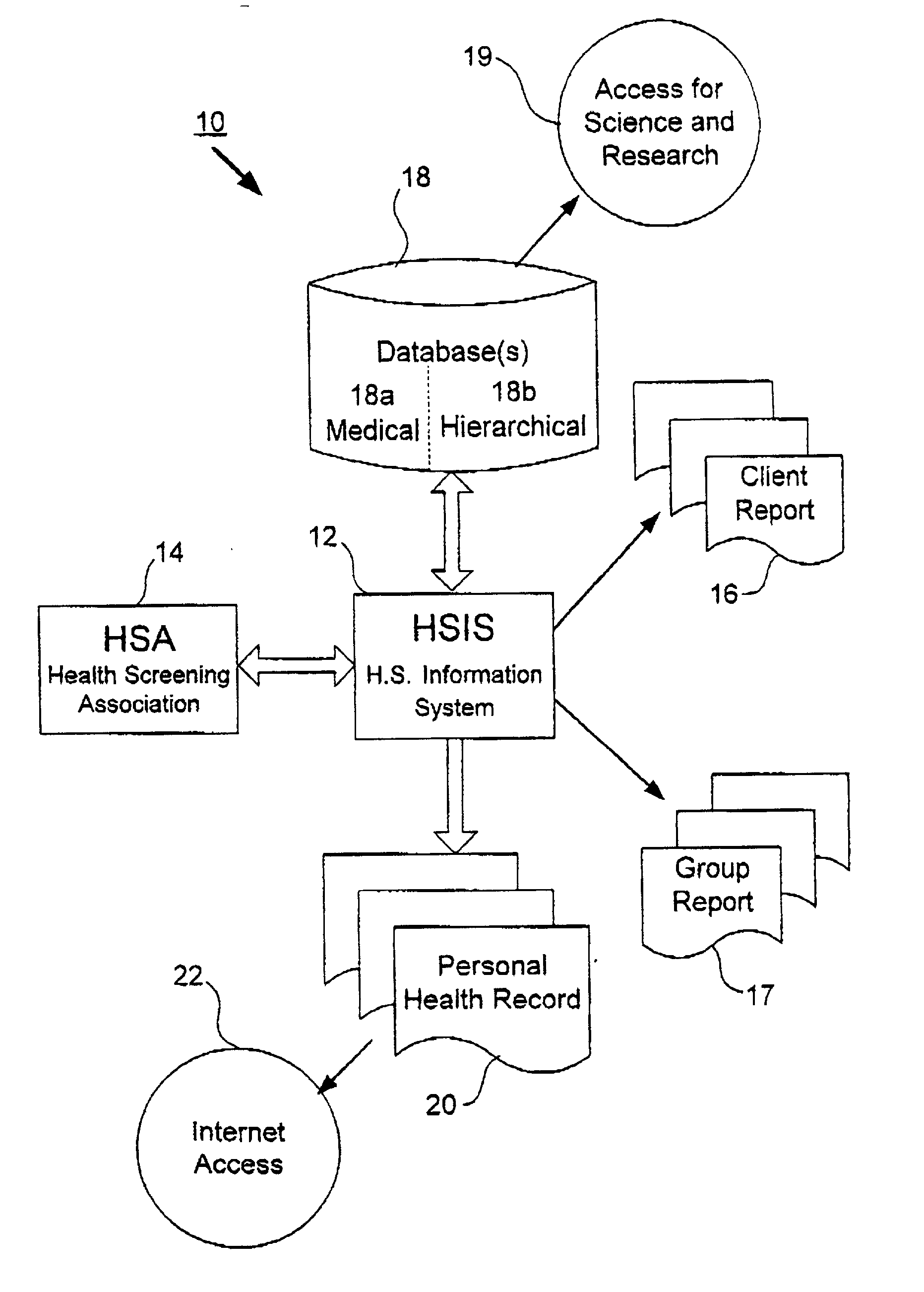
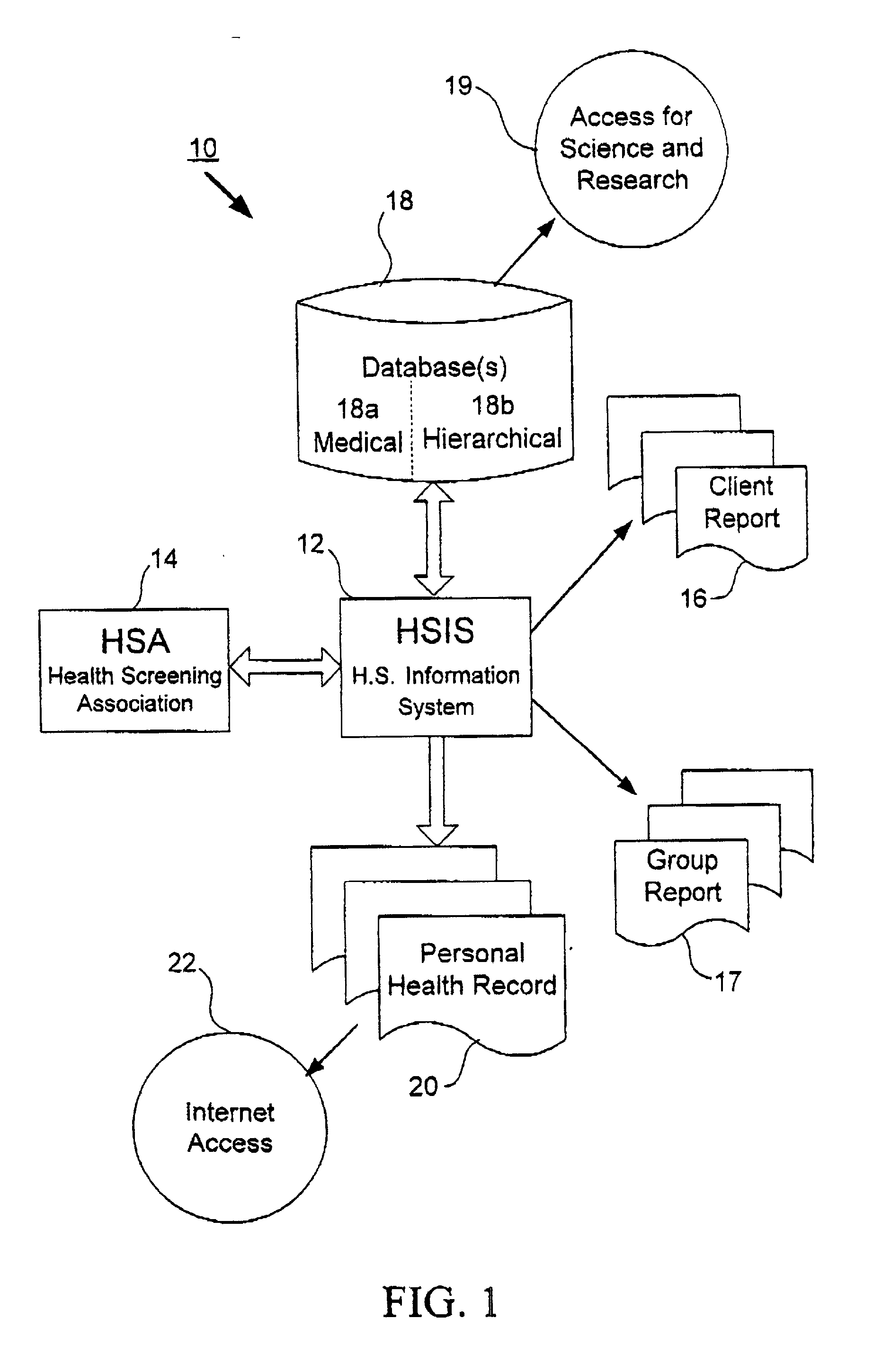
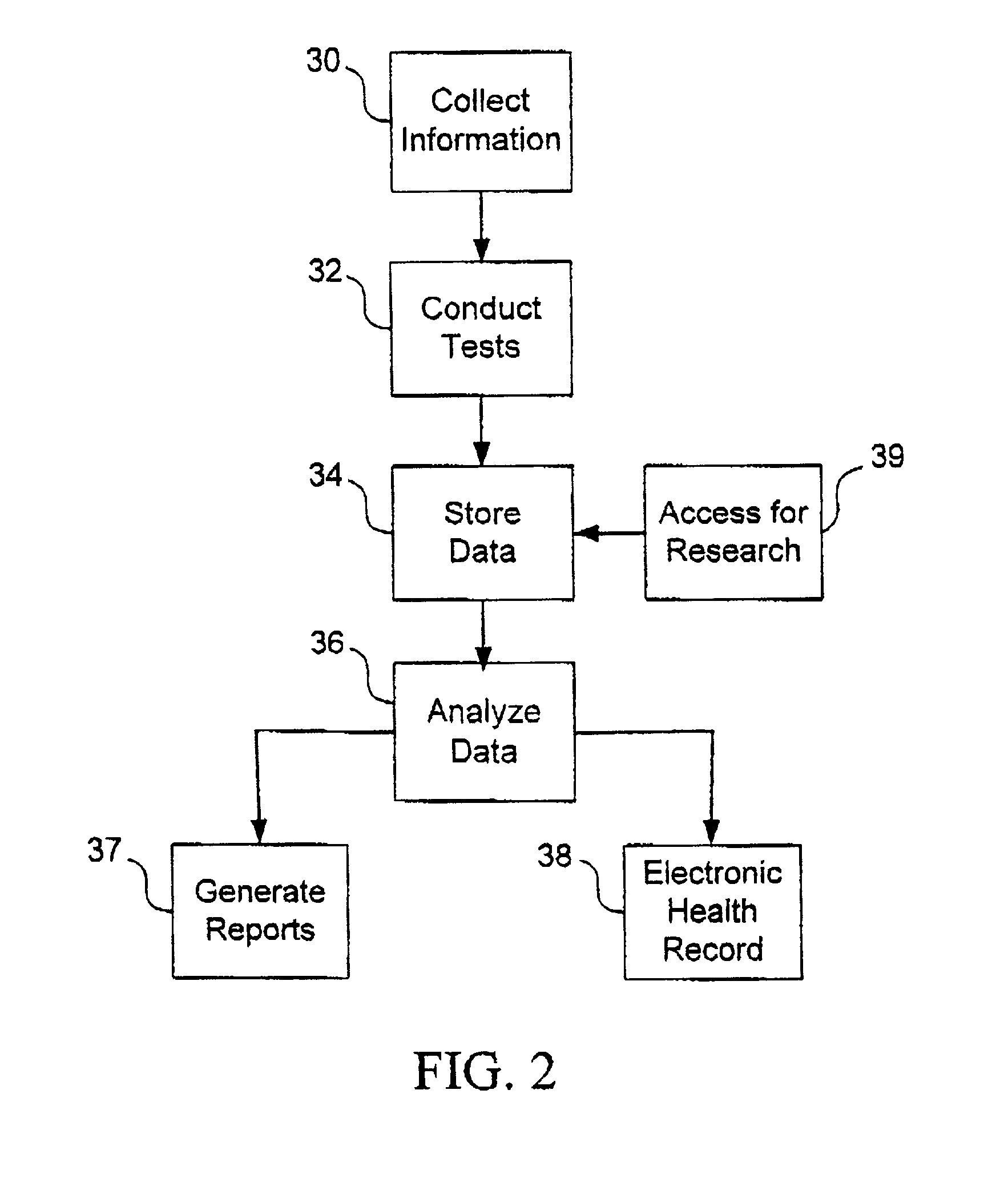
Method, system and computer program for health data collection, analysis, report generation and access
InactiveUS20030187688A1Easy maintenanceData processing applicationsHealth-index calculationClinical studyDemographic data
A health data management system is provided. Specifically, the invention includes a system and method for collecting screening, diagnostic, and demographic data from clients, processing and analyzing health data from health risk assessments and screening tests, generating custom reports, maintaining heath data, pre-populating data into user accessible personal health records and aggregate data for scientific research and clinical studies. The invention can be implemented in numerous ways, including as a system, a device, a method, or a computer readable medium.
Owner:HEALTHSCREEN INT
Compressed tablet formulation
This invention relates to a 50% drug loaded compressed tablet formulation for efavirenz. Efavirenz is a non-nucleoside reverse trancriptase inhibitor being studied clinically for use in the treatment of HIV infections and AIDS.
Owner:MERCK SHARP & DOHME CORP
Systems and methods for enrollment of clinical study candidates and investigators
ActiveUS20080010254A1Easy to identifyMedical communicationMedical data miningClinical studyPatient data
Certain embodiments of the present invention provide systems and methods for clinical study notification. Certain embodiments provide a method for clinical study notification. The method includes accessing a pool of potential study participants generated from electronic patient data. The method also includes identifying one or more healthcare providers associated with the pool of potential study participants. The method further includes electronically communicating with the one or more healthcare providers and / or the pool of potential study participants regarding participation in a clinical study. Additionally, the method includes allowing the one or more healthcare providers and / or the pool of potential study participants to join the clinical study.
Owner:GENERAL ELECTRIC CO
Compressed tablet formulation
InactiveUS7060294B2Organic active ingredientsCapsule deliveryNucleoside Reverse Transcriptase InhibitorClinical study
This invention relates to a 50% drug loaded compressed tablet formulation for efavirenz. Efavirenz is a non-nucleoside reverse trancriptase inhibitor being studied clinically for use in the treatment of HIV infections and AIDS.
Owner:MERCK SHARP & DOHME CORP
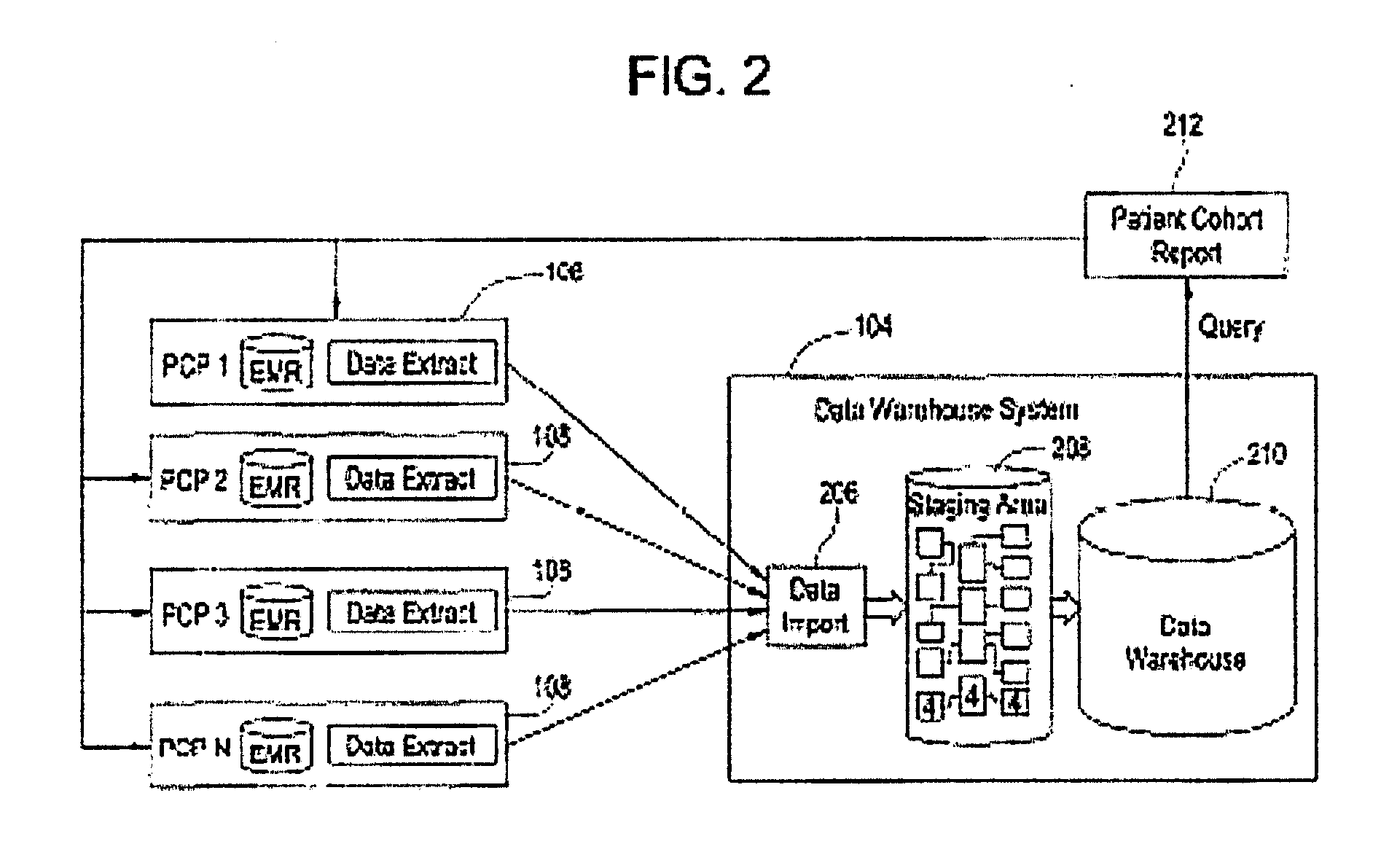
Integrated data collection and analysis for clinical study
A data collection system includes remote, implantable sensors for monitoring one or more patient parameters, collecting and processing data from those sensors and utilizing that data in the performance of a clinical study of a drug or other pharmacological agent. The system assists with preparation of a protocol for a clinical trial; presentation of that protocol; assuring compliance with the protocol; and generating useful results from data collected via the system and externally for presentation to an approval forum.
Owner:MEDTRONIC INC
Coenzyme-binding glucose dehydrogenase
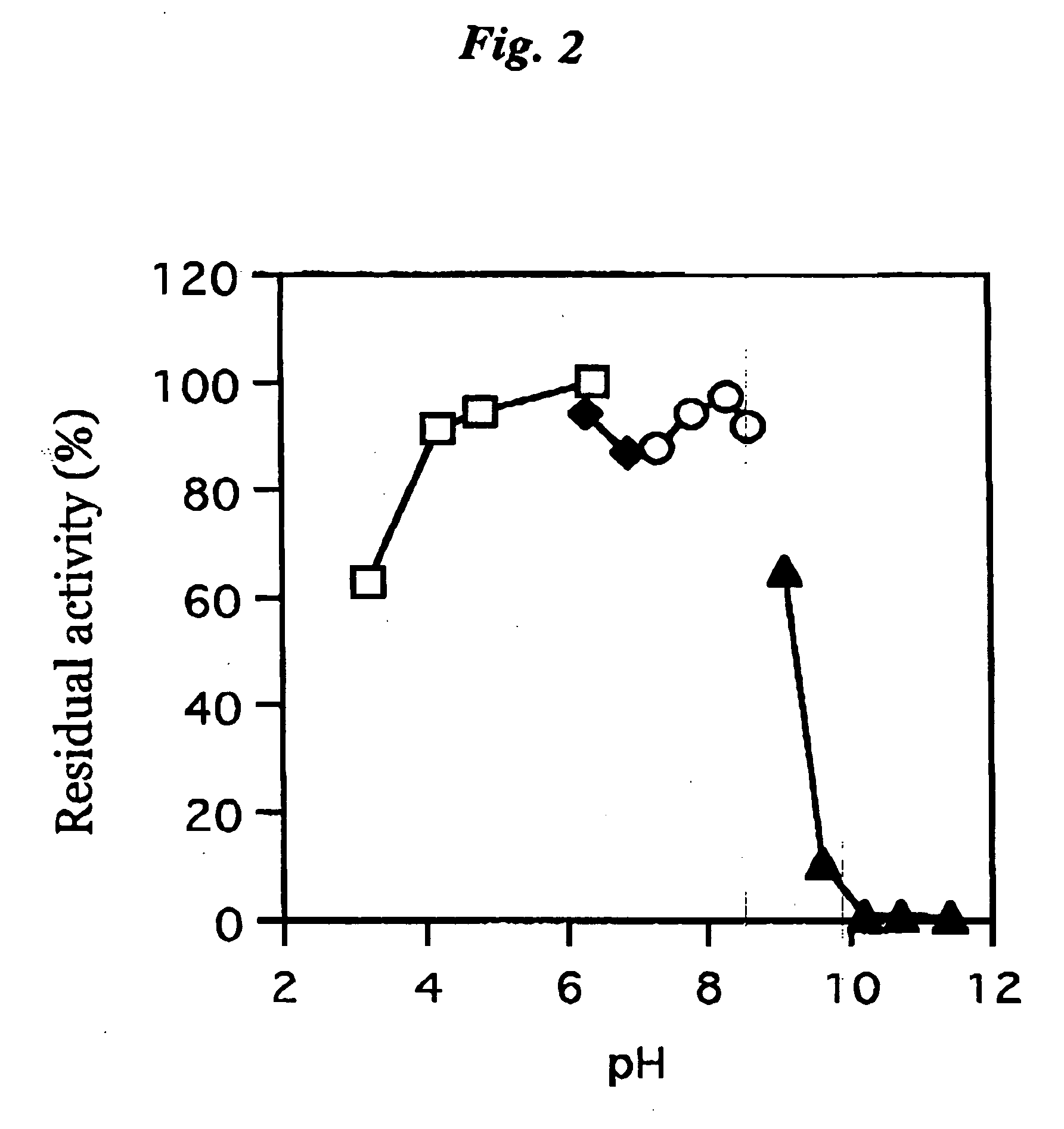
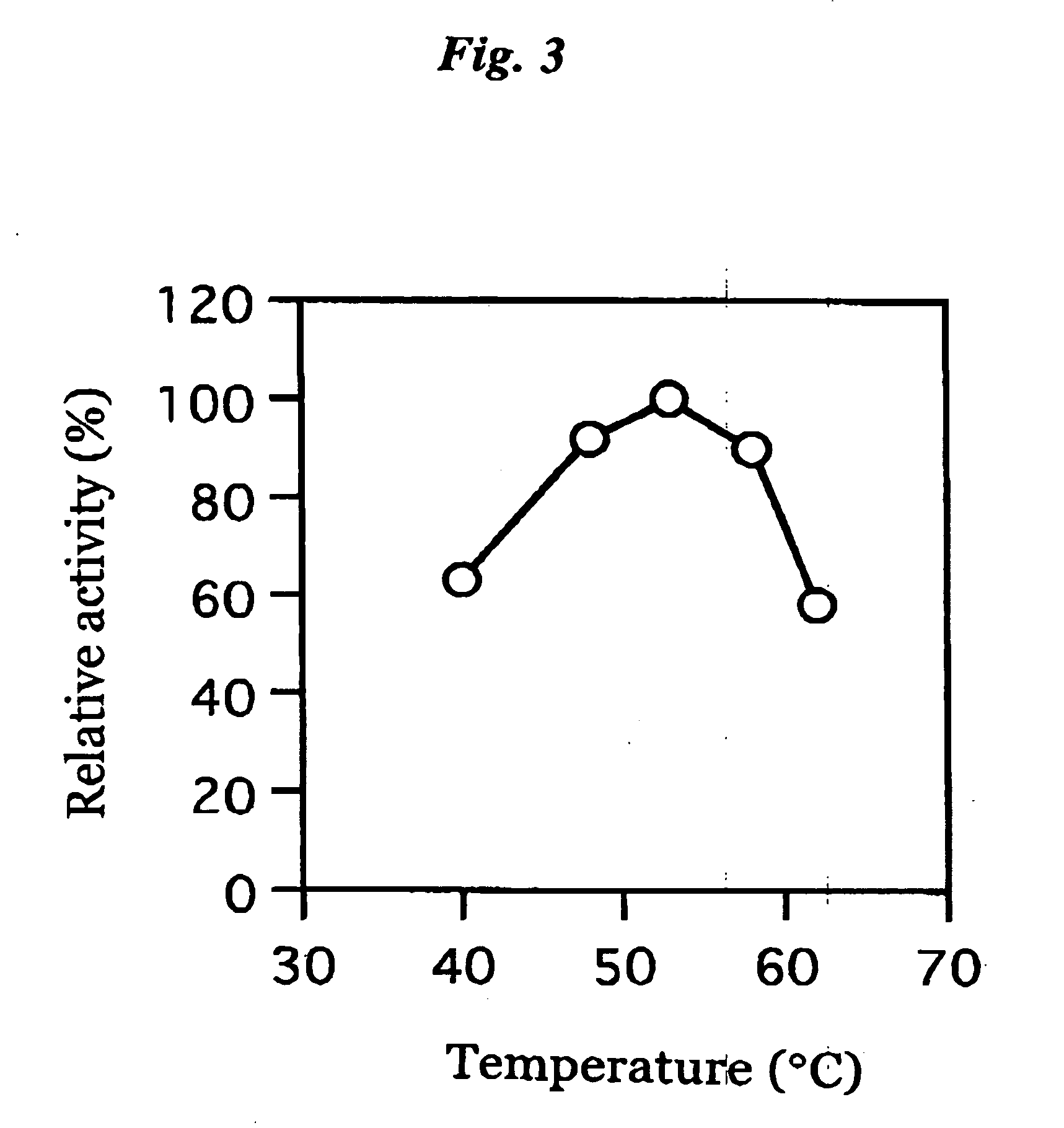
ActiveUS20060063217A1The process is convenient and fastImprove accuracyFungiMicrobiological testing/measurementGlucose sensorsPhenanthroline
The present invention provides a microorganism-derived soluble coenzyme-binding glucose dehydrogenase which catalyzes a reaction for oxidizing glucose in the presence of an electron acceptor, has an activity to maltose as low as 5% or less, and is inhibited by 1,10-phenanthroline. The invention also provides a method for producing the coenzyme-binding glucose dehydrogenase, and a method and a reagent for measuring employing the coenzyme-binding glucose dehydrogenase. According to the invention, the coenzyme-binding glucose dehydrogenase can be applied to an industrial field, and a use becomes possible also in a material production or analysis including a method for measuring or eliminating glucose in a sample using the coenzyme-binding glucose dehydrogenase as well as a method for producing an organic compound. It became also possible to provide a glucose sensor capable of accurately measuring a blood sugar level. Therefore, it became possible to provide an enzyme having a high utility, such as an ability of being used for modifying a material in the fields of pharmaceuticals, clinical studies and food products.
Owner:PHC CORP
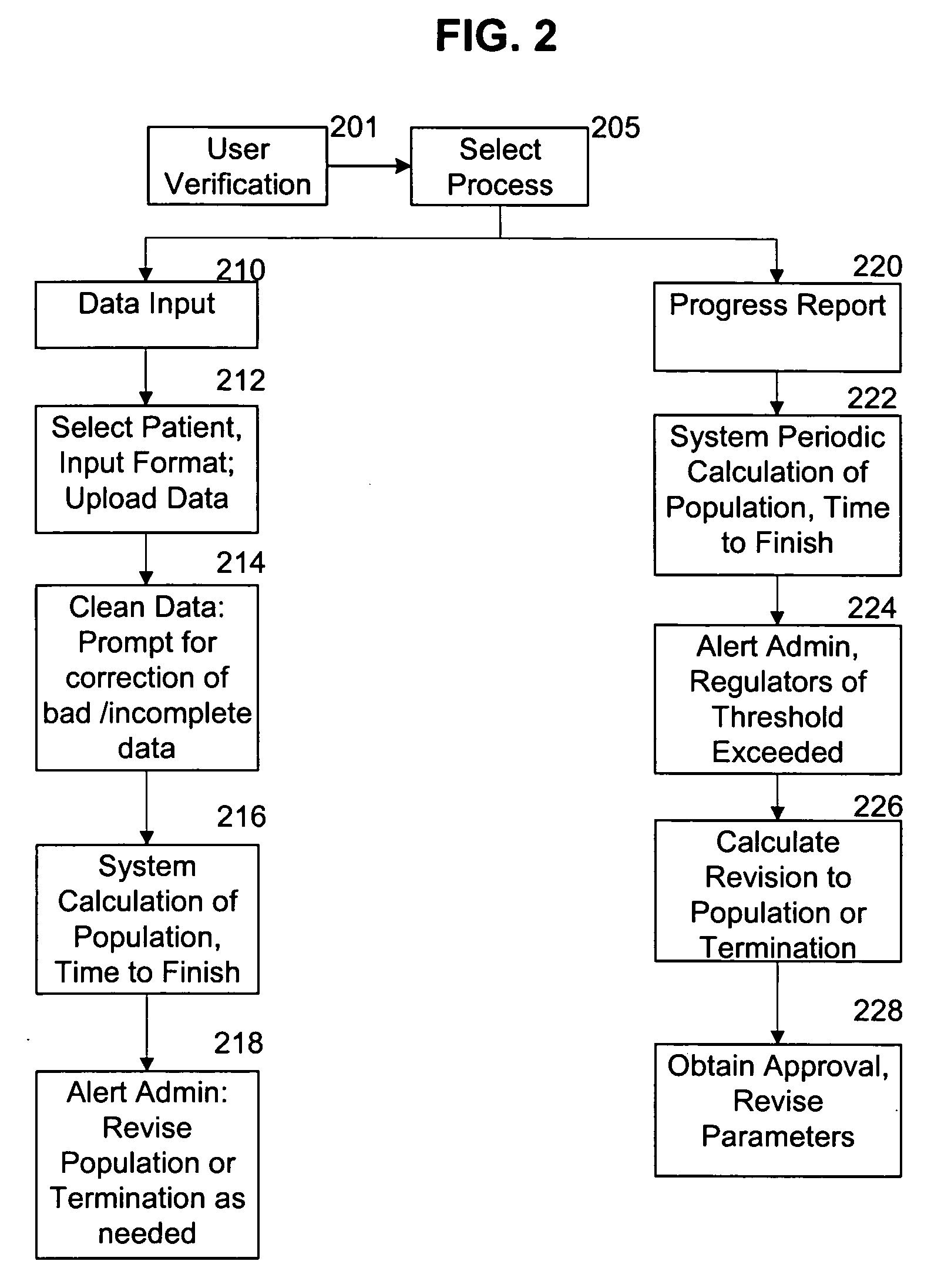
System for continuous outcome prediction during a clinical trial
InactiveUS20060129326A1Easy to controlData processing applicationsComputer-assisted medical data acquisitionClinical efficacyClinical psychology
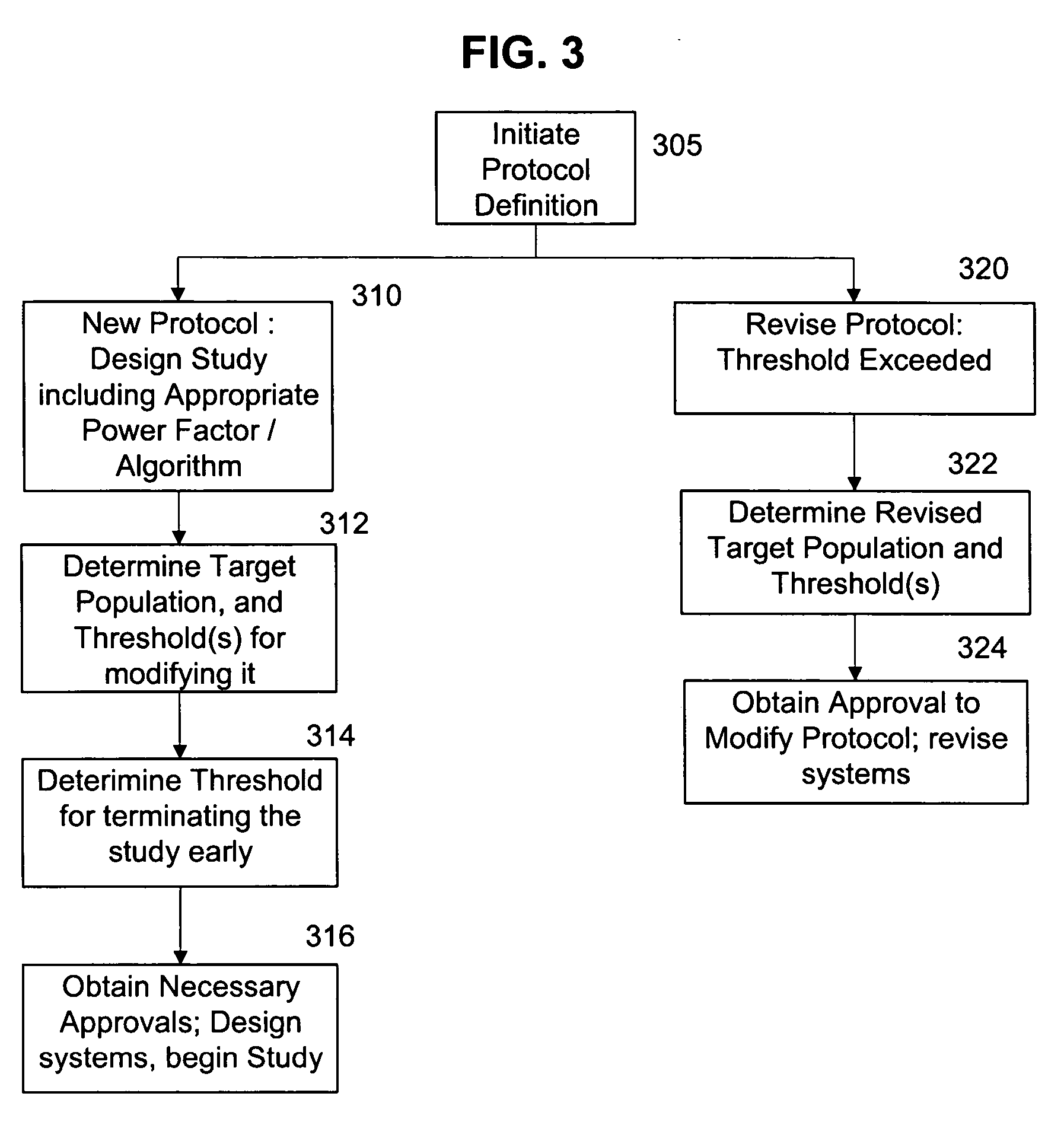
The present invention provides a method, apparatus, and computer instructions for improved control of clinical trials. In a preferred embodiment, after a clinical trial is initiated, data is regularly cleaned and processed to statistically analyze the data. The outcome includes a predictive measure of the timing and level by which the study will achieve one or more statistically significant levels, allowing mid-course modifications to the study (e.g., in population size, termination, etc.). Modification can be planned as part of the initial protocol, using thresholds or other appropriate criteria relating to the statistical outcome, making possible pre-approved protocol changes based on the statistical findings. This process has significant implications for the management of clinical studies, including ensuring the minimum possible time and number of patients are used in clinical studies to either prove (or disprove) the clinical efficacy of drugs or treatments.
Owner:AZERA RES
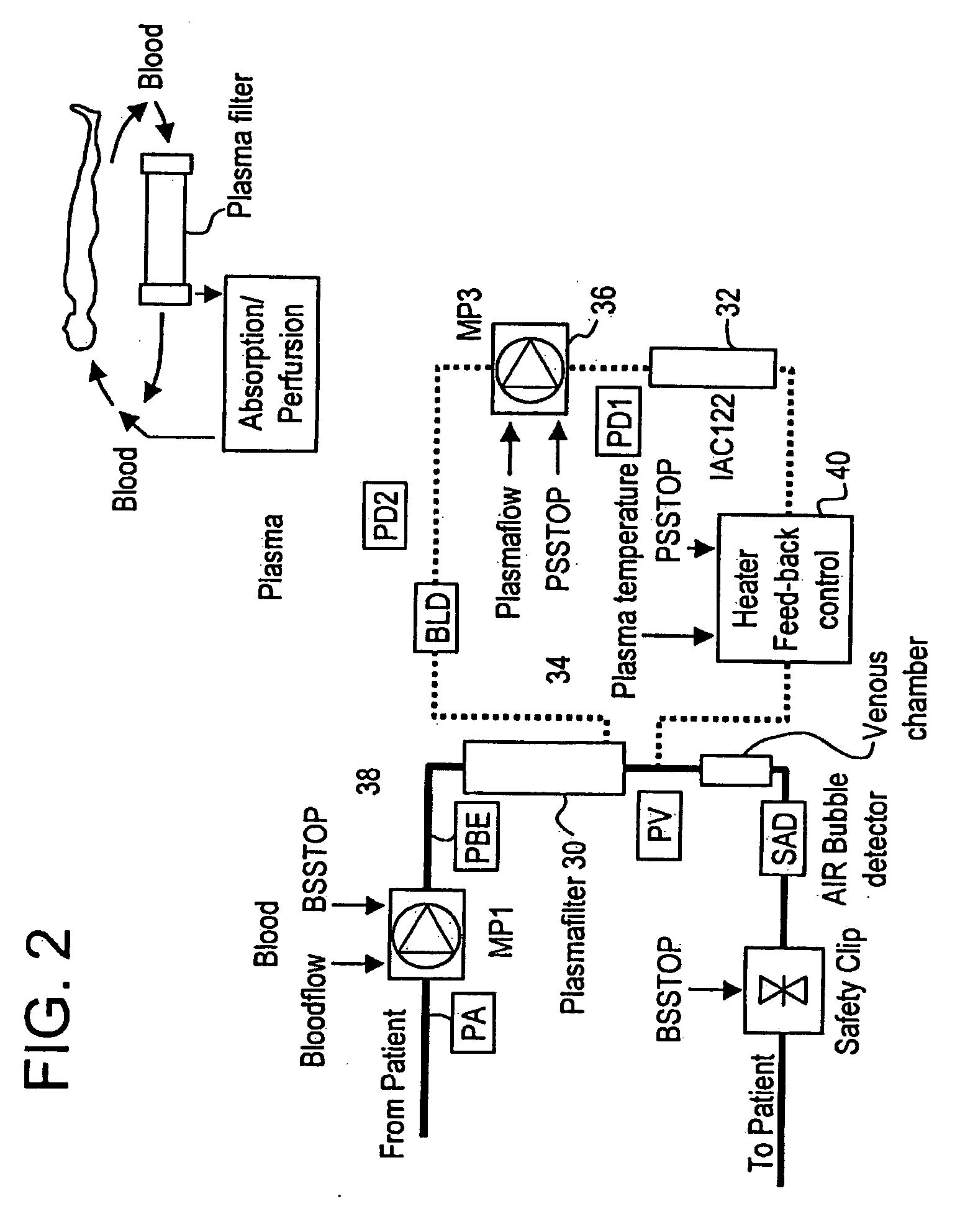
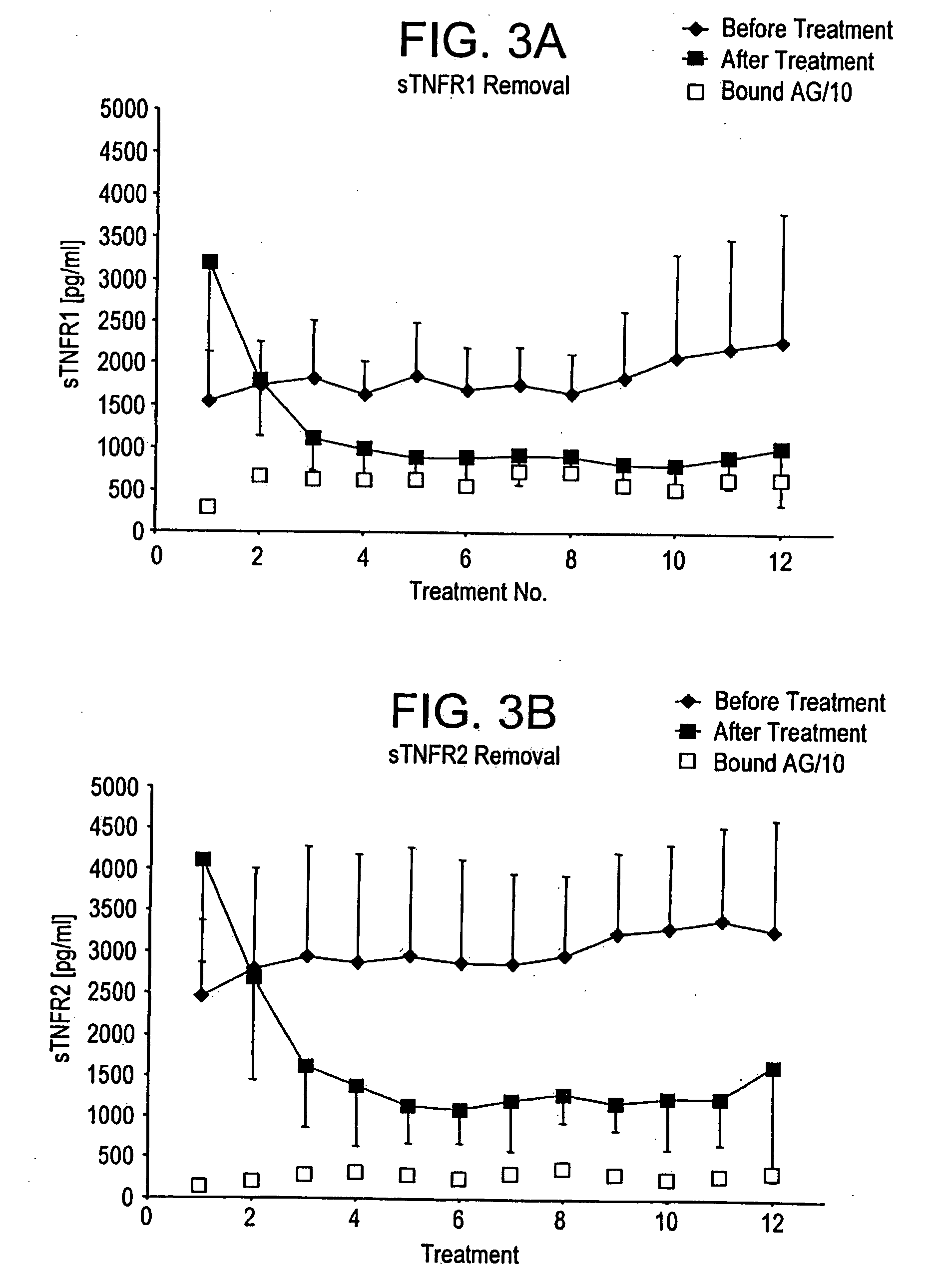
Method and system to remove soluble TNFR1, TNFR2, and IL2 in patients
InactiveUS20050265996A1Induce remissionPeptide/protein ingredientsHaemofiltrationDiseaseAntiendomysial antibodies
A method, and system, to induce remission in diseases characterized by excess production of sTNR and interleukin 2 has been developed. In the most preferred embodiment, the system consists of antibodies to sTNFR1, sTNFR2 and sIL2R immobilized in a column containing a material such as SEPHAROSE™. The patient is connected to a pheresis machine which separates the blood into the plasma and red cells, and the plasma is circulated through the column until the desired reduction in levels of sTNFR1, sTNFR2, and IL2 is achieved, preferably to less than normal levels. In the preferred method, patients are treated three times a week for four weeks. This process can be repeated after a period of time. Clinical studies showed reduction in tumor burden in patients having failed conventional chemotherapy and radiation treatments.
Owner:INNATUS CORP
Soft contact lenses displaying superior on-eye comfort
The present invention relates to soft contact lenses having an overall comfort preference of at least about 2 to 1 as compared to an Acuvue® contact lens and measured after one week of daily wear. The present invention further relates to a soft contact lens comprising an oxygen transmissibility greater than about 70 barrers / mm and physical properties suitable to provide wearer comfort over at least about 9 hours in at least about 80% of wearers, as measured in a randomized, double masked clinical study.
Owner:JOHNSON & JOHNSON VISION CARE INC
Integrated data collection and analysis for clinical study
InactiveUS20060224326A1Data processing applicationsComputer-assisted medical data acquisitionMedicineClinical study
A data collection system includes remote, implantable sensors for monitoring one or more patient parameters, collecting and processing data from those sensors and utilizing that data in the performance of a clinical study of a drug or other pharmacological agent. The system assists with preparation of a protocol for a clinical trial; presentation of that protocol; assuring compliance with the protocol; and generating useful results from data collected via the system and externally for presentation to an approval forum.
Owner:MEDTRONIC INC
Apparatus and Method for Analyzing Skin Using L*a*b* Colorspace
An imaging system and method has digital image capture and analysis capability. The digital images may be taken in a variety of illumination conditions, with the skin response indicating skin condition. The digital images may be converted from RGB format to L*a*b* format and analyzed quantitatively to assess color and brightness. The color / brightness information from the digital images may be used to assess skin condition and changes thereof, as well as selecting cosmetics provided in a range of colors. The color information gleaned from the digital images of a population may be utilized to identify a palette of colors for cosmetics or to aid in conducting clinical studies.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
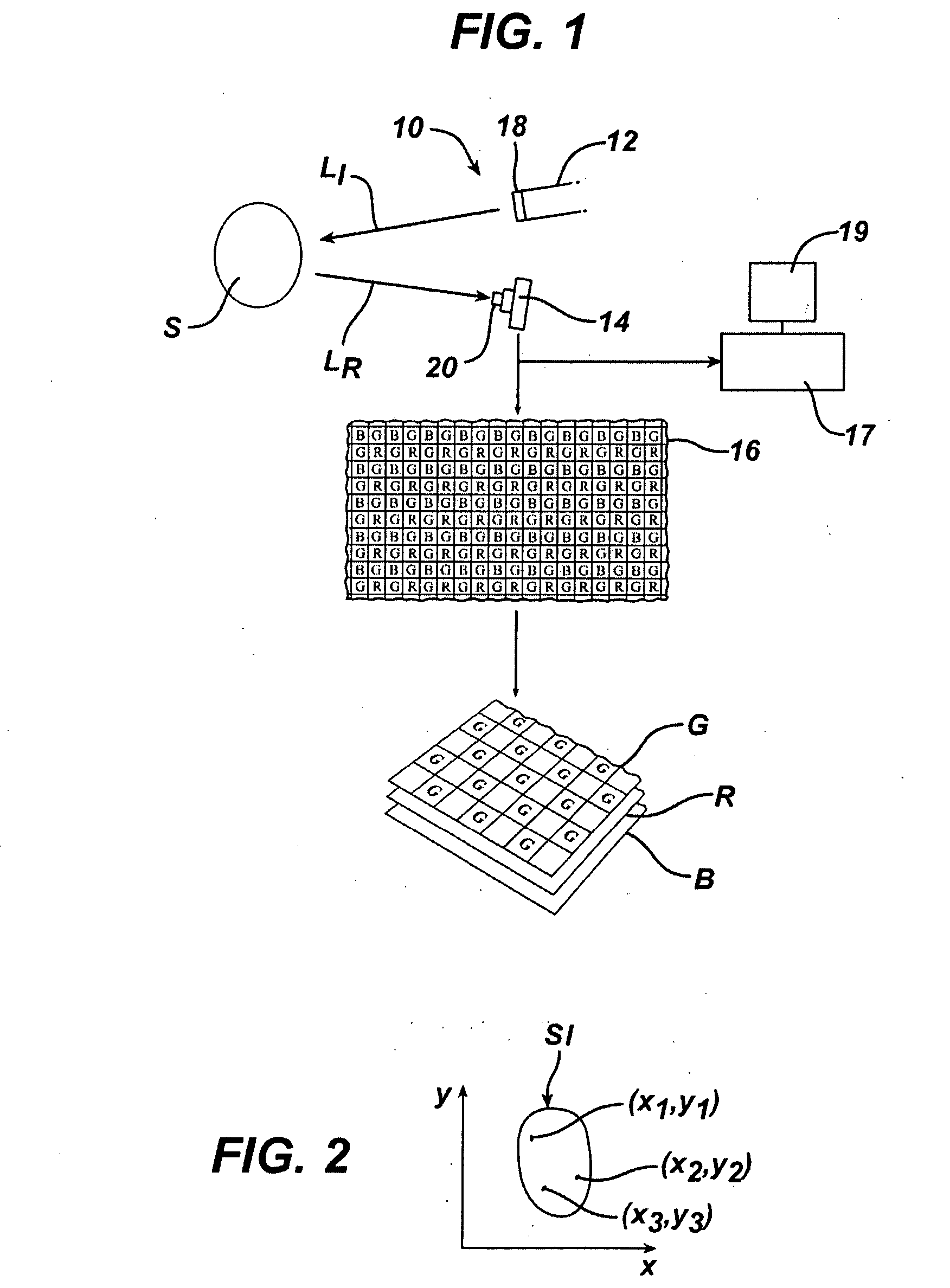
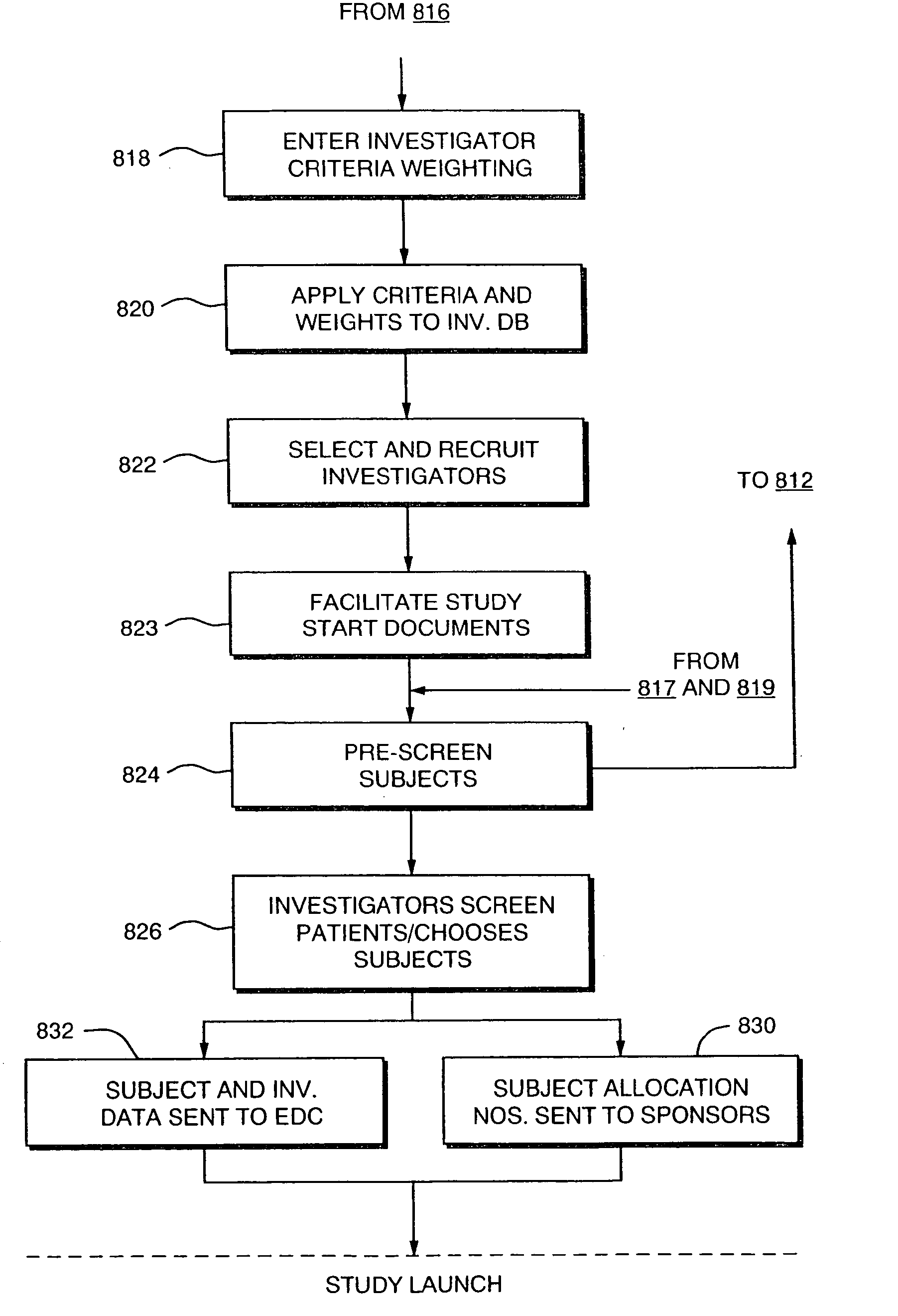
Systems and Methods for Selecting and Recruiting Investigators and Subjects for Clinical Studies
InactiveUS20080133270A1Computer-assisted medical data acquisitionOffice automationMedicineSubject matter
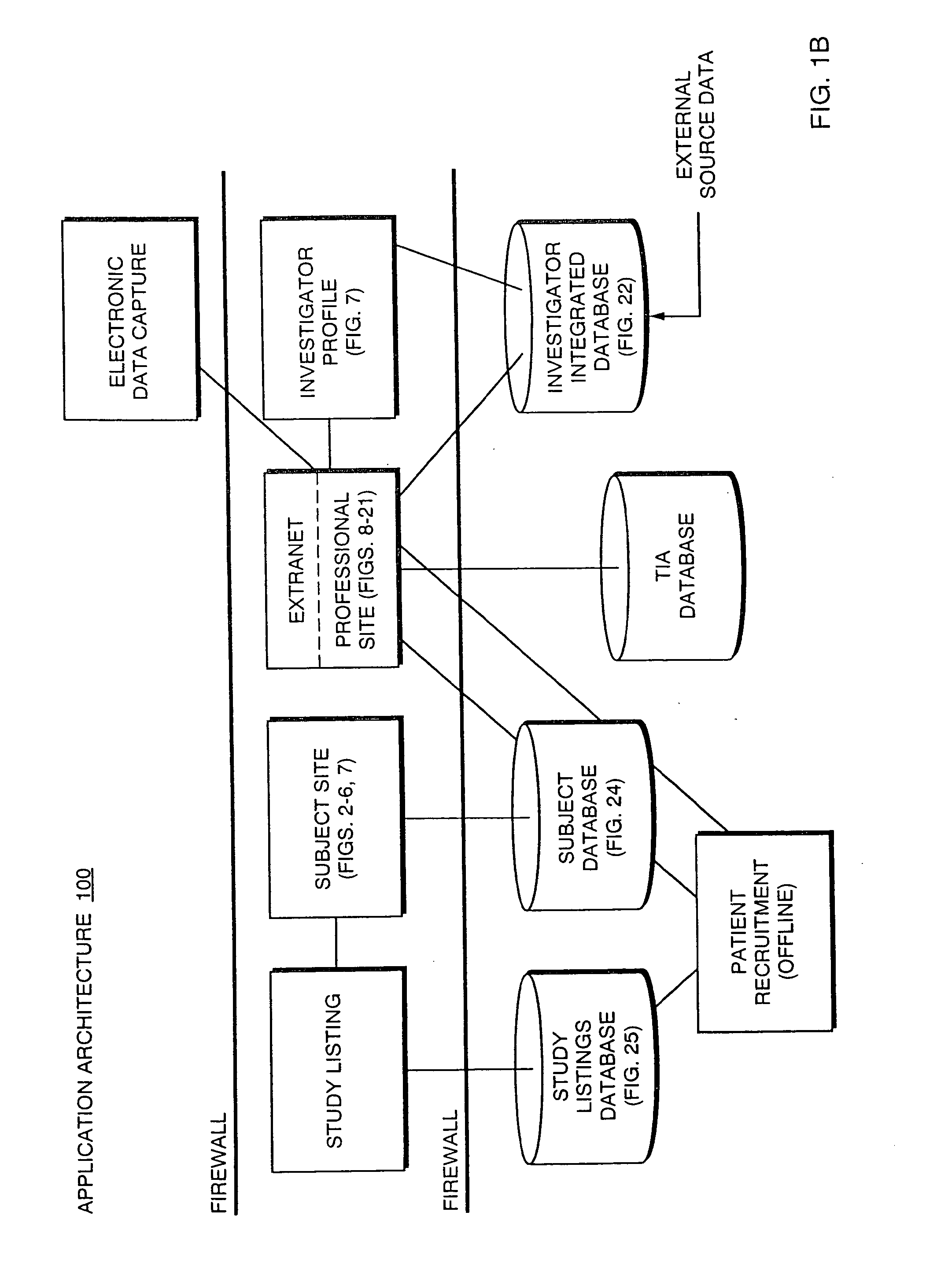
The present invention is directed to an integrated on-line interactive forum that promotes exchange of information among clinical study sponsors, clinical study investigators, and potential clinical study subjects. The forum includes an investigator database that contains information suitable for identification of qualified investigators for clinical studies and a subject database that contains information suitable for identification of eligible subjects for clinical studies. An extranet is coupled to the investigator database and the subject database. The extranet allows sponsors and investigators to exchange securely documents required to start a clinical study. The forum also optionally includes one or more web pages that provide information describing clinical studies to potential clinical study subjects and permit potential clinical study subjects to register for inclusion in the subject database. A therapeutic incidence area database is also optionally integrated into the forum
Owner:MICHELSON LESLIE DENNIS +2
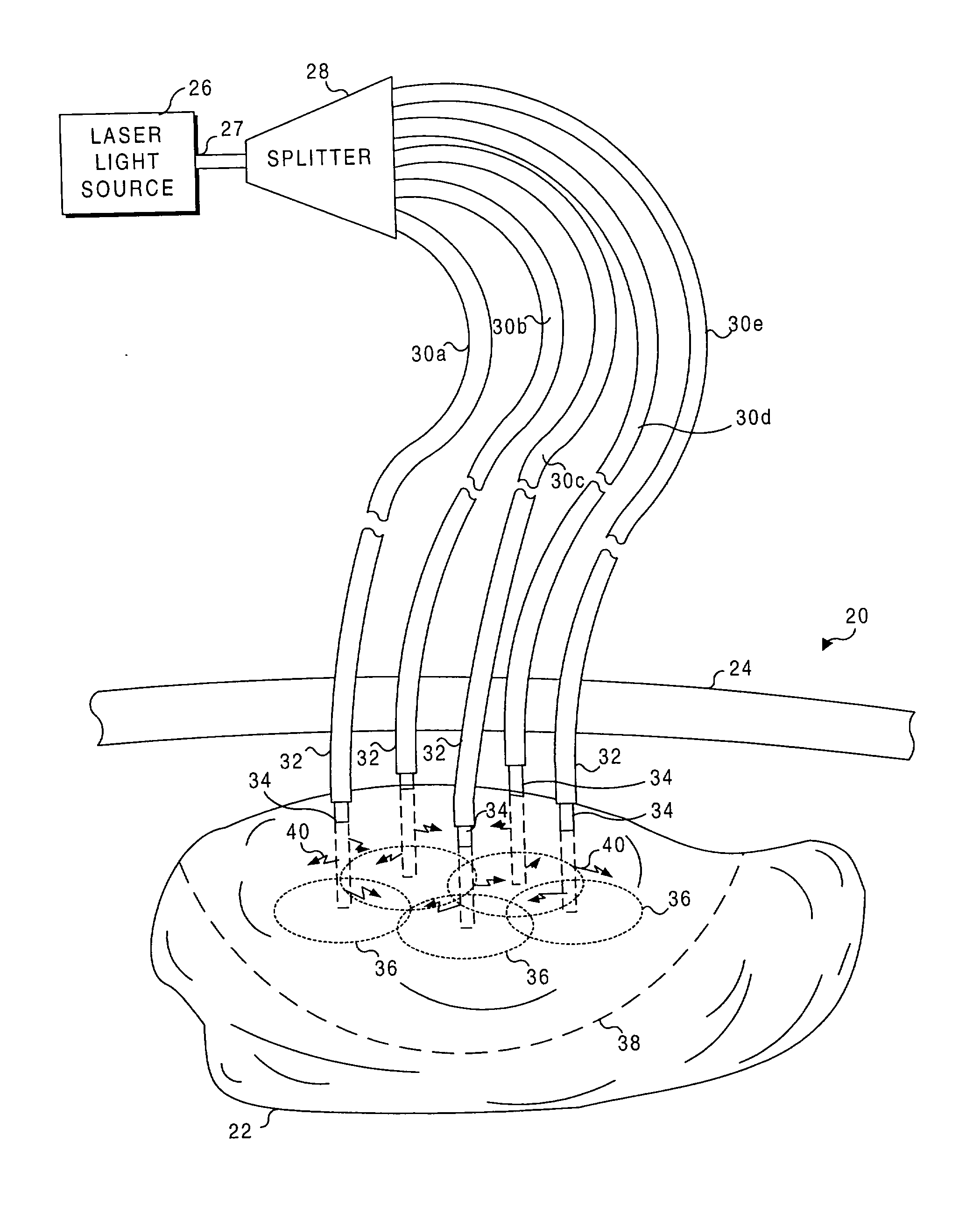
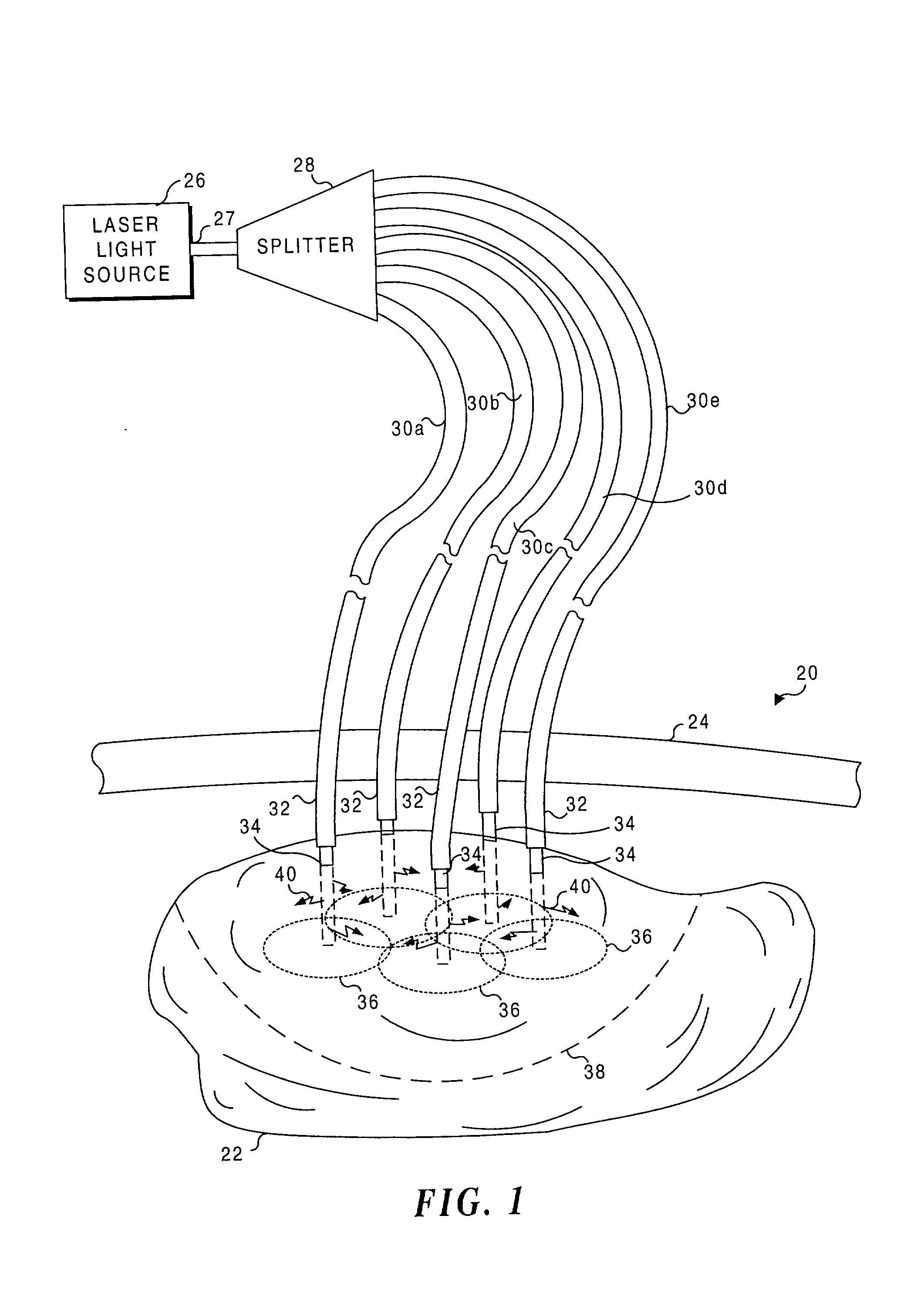
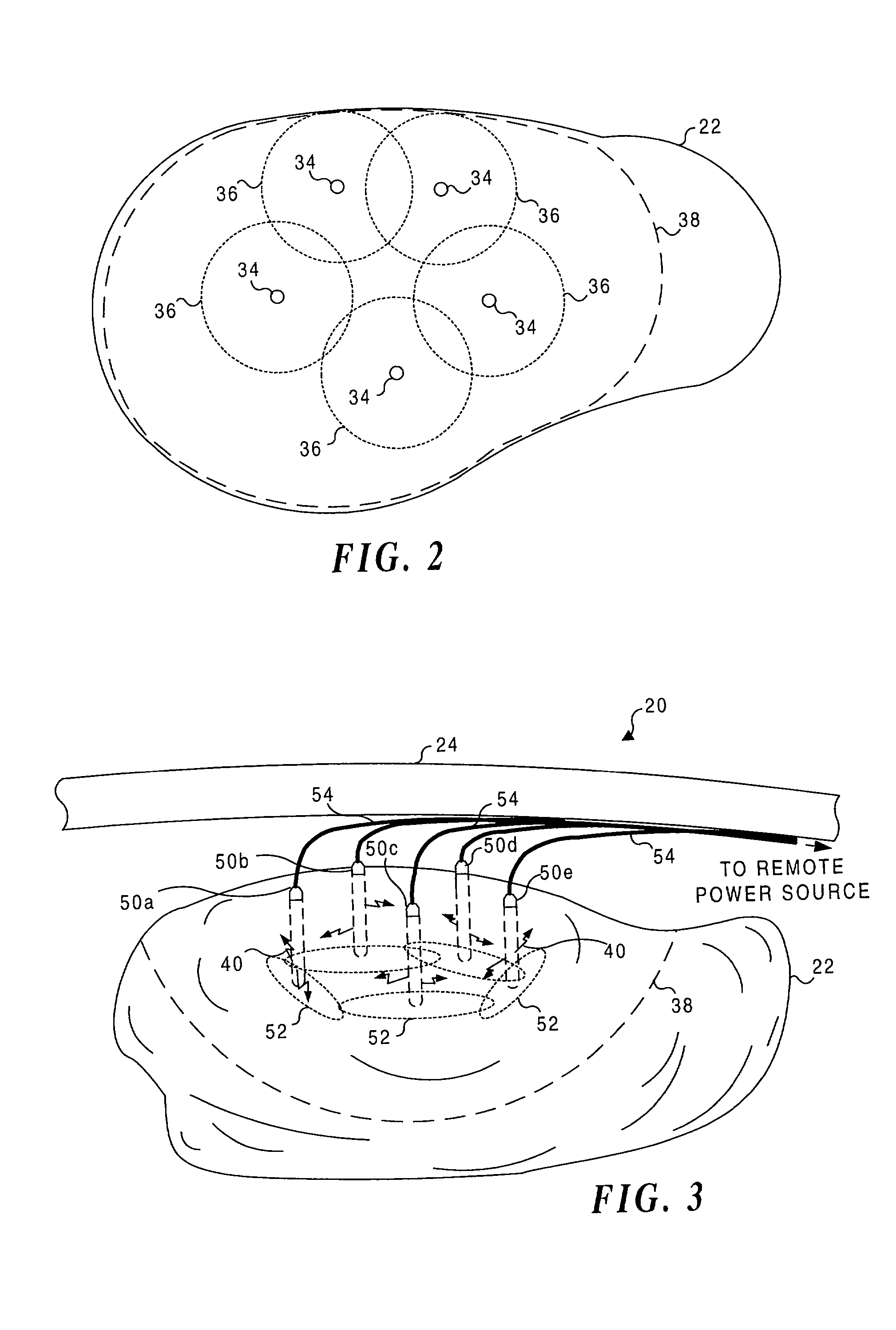
Application of light at plural treatment sites within a tumor to increase the efficacy of light therapy
Light is administered during photodynamic therapy (PDT) for an extended period of time at a plurality of sites distributed within the abnormal tissue of a tumor. A clinical study has shown that a substantially greater volume of abnormal tissue in a tumor is destroyed by the extended administration of light therapy from a plurality of probes than would have been expected based upon the teaching of the prior art. In this process, a plurality of light emitting optical fibers or probes are deployed in a spaced-apart array. After a photoreactive agent is absorbed by the abnormal tissue, the light therapy is administered for at least three hours. The greater volume of necrosis in the tumor is achieved due to one or more concomitant effects, including: the inflammation of damaged abnormal tissue and resultant immunological response of the patient's body; the diffusion and circulation of activated photoreactive agent outside the expected fluence zone, which is believed to destroy the abnormal tissue; a retrograde thrombosis or vascular occlusion outside of the expected fluence zone; and, the collapse of the vascular system that provides oxygenated blood to portions of the tumor outside the expected fluence zone. In addition, is possible that molecular oxygen diffusing and circulating into the expected fluence zone is converted to singlet oxygen during the extended light therapy, causing a gradient of hypoxia and anoxia that destroys the abnormal tissue outside the expected fluence zone.
Owner:LIGHT SCI ONCOLOGY
Inflammatory eye disorders
ActiveUS20130336557A1Improve reflectivityIncrease in sizeOrganic active ingredientsSenses disorderClinical studyOcular disease
Provided herein are methods of evaluating efficacy of a treatment in a subject having eye inflammation (e.g., a subject having dry eye syndrome) and selecting a subject for participation in a clinical study. Also provided are methods of treating a subject having eye inflammation (e.g., a subject having dry eye syndrome).
Owner:MASSACHUSETTS EYE & EAR INFARY
Sleep apnea treatment apparatus
InactiveUS20060118112A1Easy to operateReduce pressureRespiratorsOperating means/releasing devices for valvesPositive airway pressureRemote control
Improved methodology and apparatus for the clinical study and treatment of sleep apnea which incorporates one or more of the following features: (1) application of mono-level, alternating high and low level, or variable positive airway pressure generally within the airway of the patient with the mono-level, high and low level, or variable airway pressure generally being coordinated with and / or responsive to the spontaneous respiration of the patient, (2) usage of adjustably programmable pressure ramp circuitry capable of producing multiple pressure ramp cycles of predetermined duration and pattern whereby the ramp cycles may be customized to accommodate the specific needs of an individual sleep apnea patient so as to ease the patient's transition from wakefulness to sleep, (3) remote control or patient-sensed operation of the apparatus, (4) employment of safety circuitry, reset circuitry and minimum system leak assurance circuitry, controls and methods, and (5) utilization of clinical control circuitry whereby sleep disorder data may be compiled and appropriate therapy implemented during a one-night sleep study.
Owner:RIC INVESTMENTS LLC
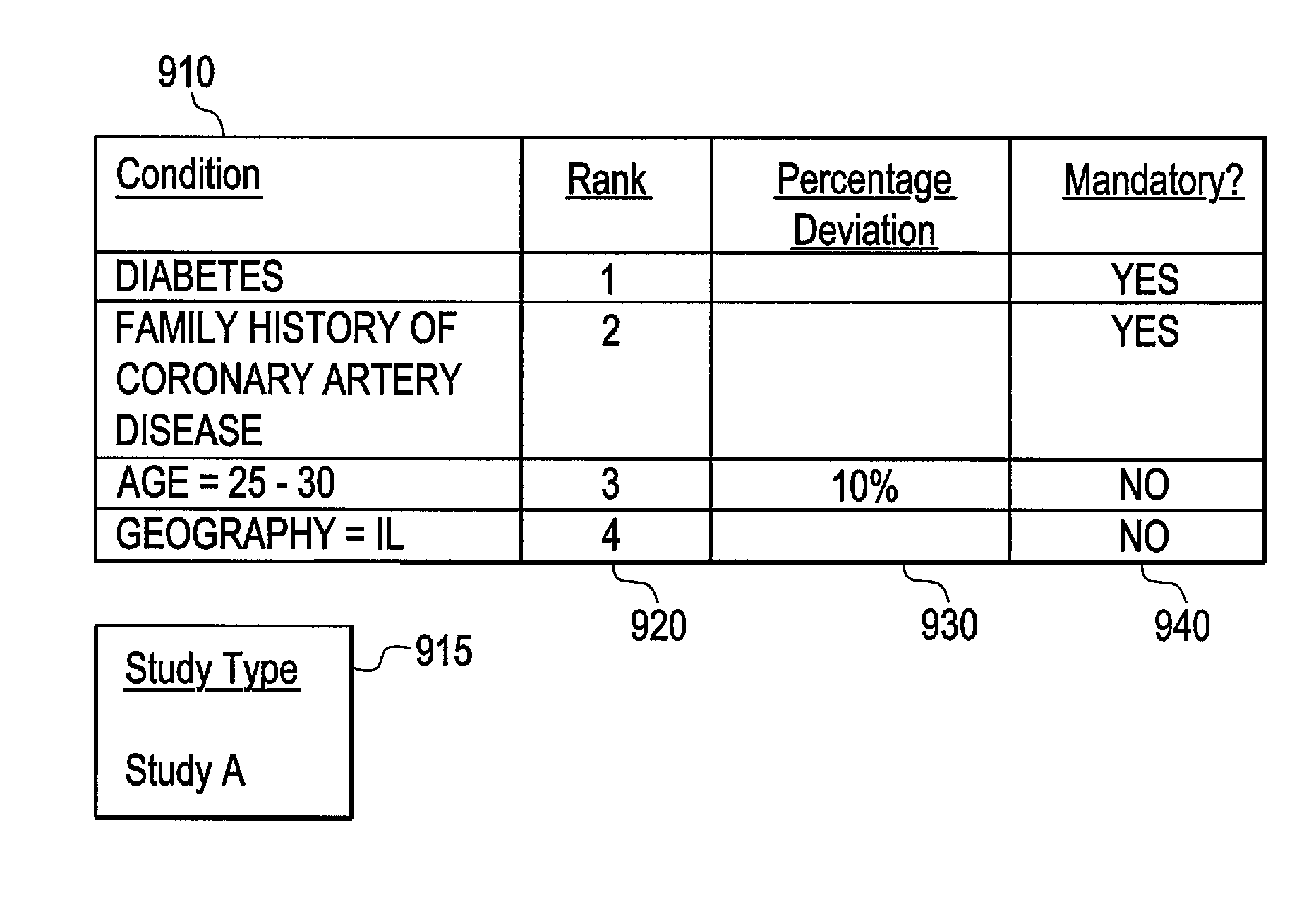
Systems and methods for refining identification of clinical study candidates
ActiveUS20070294110A1Medical data miningDigital data processing detailsNumerical rangeClinical study
Certain embodiments of the present invention provide systems and methods for refining the identification of study candidates. In an embodiment, the method may include receiving a plurality of clinical conditions at a user interface. The conditions may generally be tied to codified terms in electronic medical patient records. The method may also include receiving parameters for tailoring a search of electronic medical data for the clinical conditions. For example, the parameters may weight the conditions and may include ranking the conditions, determining whether the conditions are mandatory for the pool, and determining a percentage deviation for numerical ranges. The method may also include executing an optimization function to create a pool according to the specified conditions and parameters. The method may also include displaying the pool in an order from the most optimal results to the least optimal results.
Owner:GENERAL ELECTRIC CO
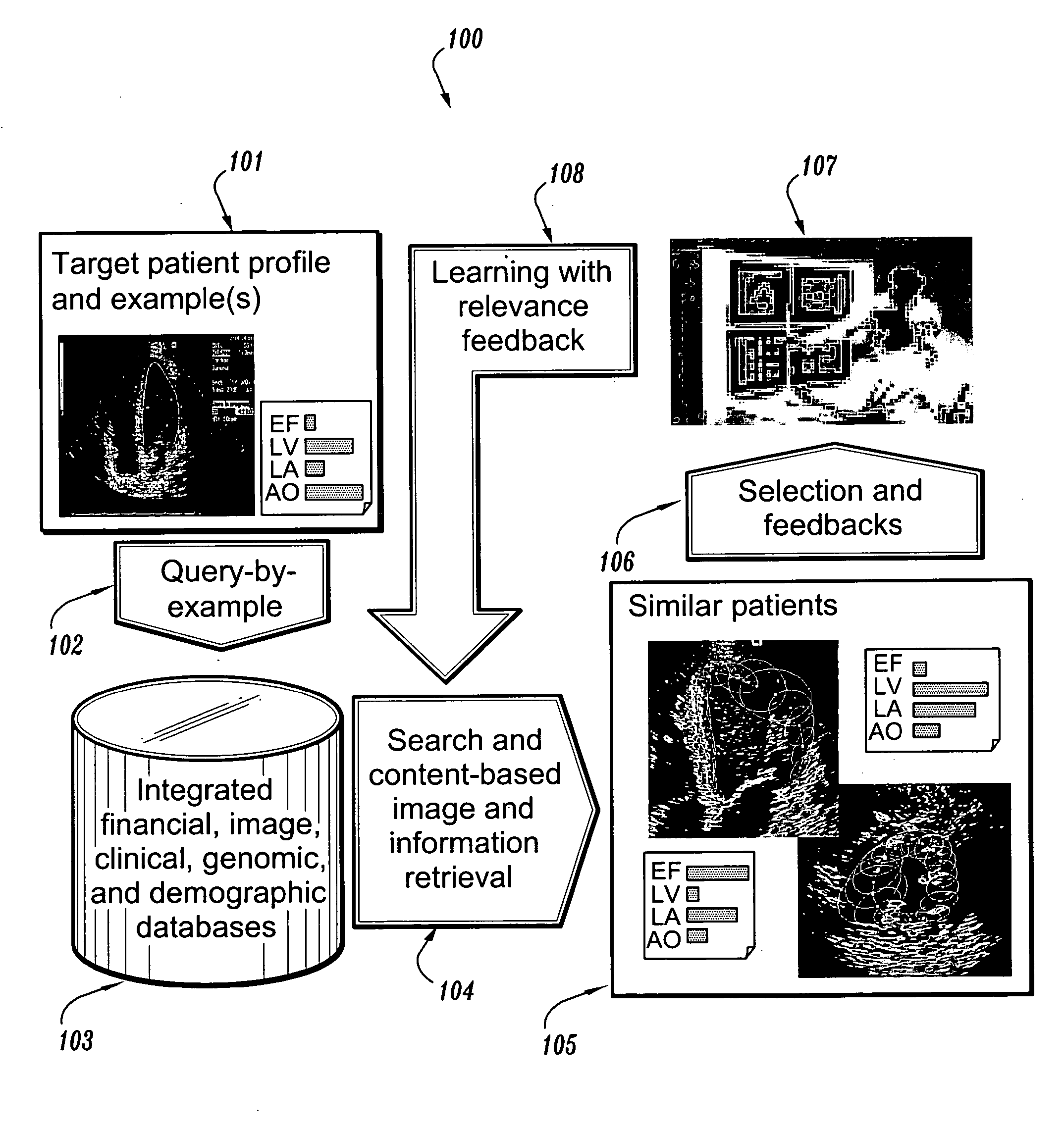
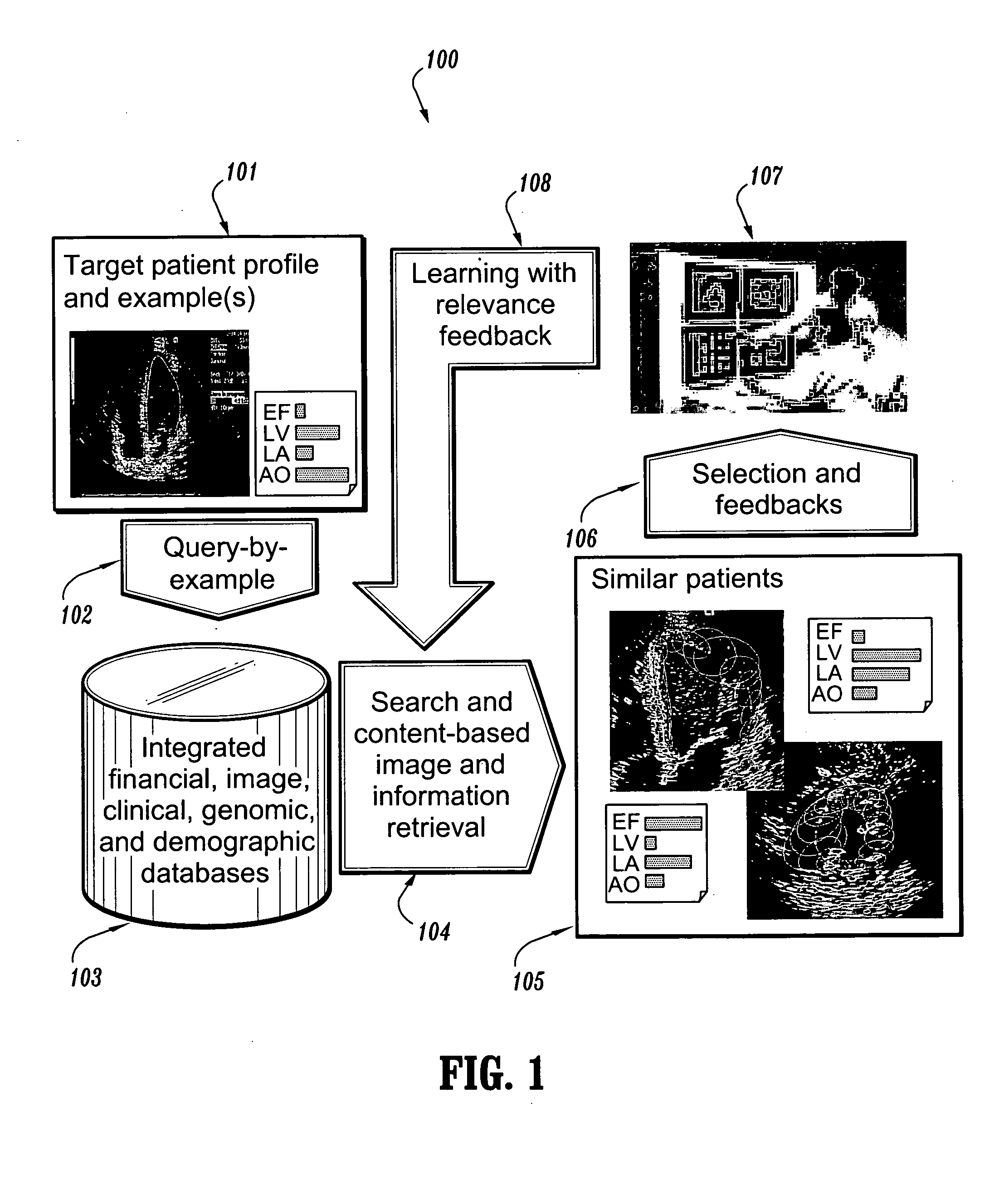
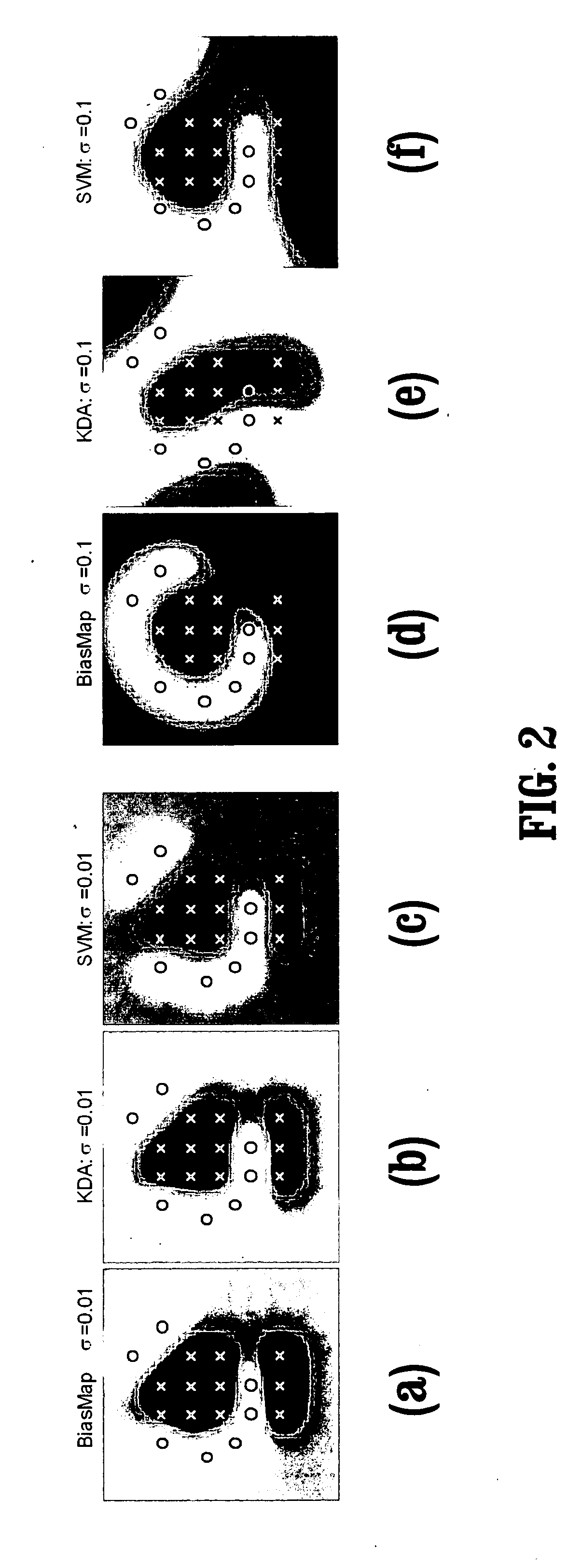
System and method for patient identification for clinical trials using content-based retrieval and learning
InactiveUS20050210015A1Avoid difficult choicesMedical data miningDigital data processing detailsSubject matterClinical trial
A method for selecting a subject for a clinical study includes providing a criteria for selecting one or more subjects from a database, performing a content based similarity search of the database to retrieve subjects who meet the selection criteria, presenting the selected subjects to a user, and receiving user feedback regarding the selected subjects. The feedback can concern whether each of the selected subjects presented to the user is suitable for the clinical study. The method also includes learning from the feedback to improve the content based similarity search, performing an improved content based similarity search of the database to retrieve additional subjects who meet the selection criteria, and presenting the additional subjects to the user.
Owner:SIEMENS MEDICAL SOLUTIONS USA INC
Methods of determining acute myeloid leukemia response to treatment with farnesyltransferase
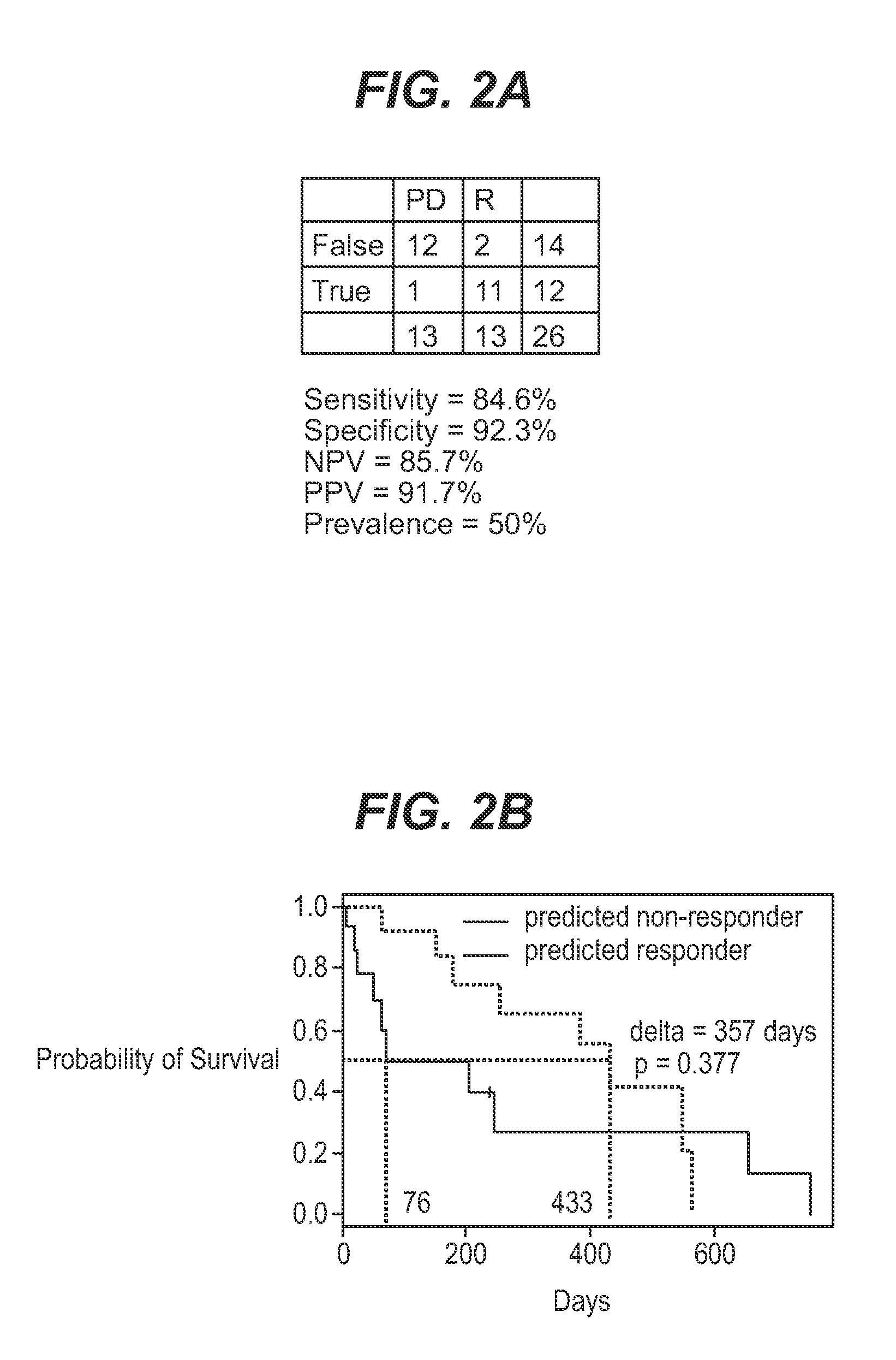
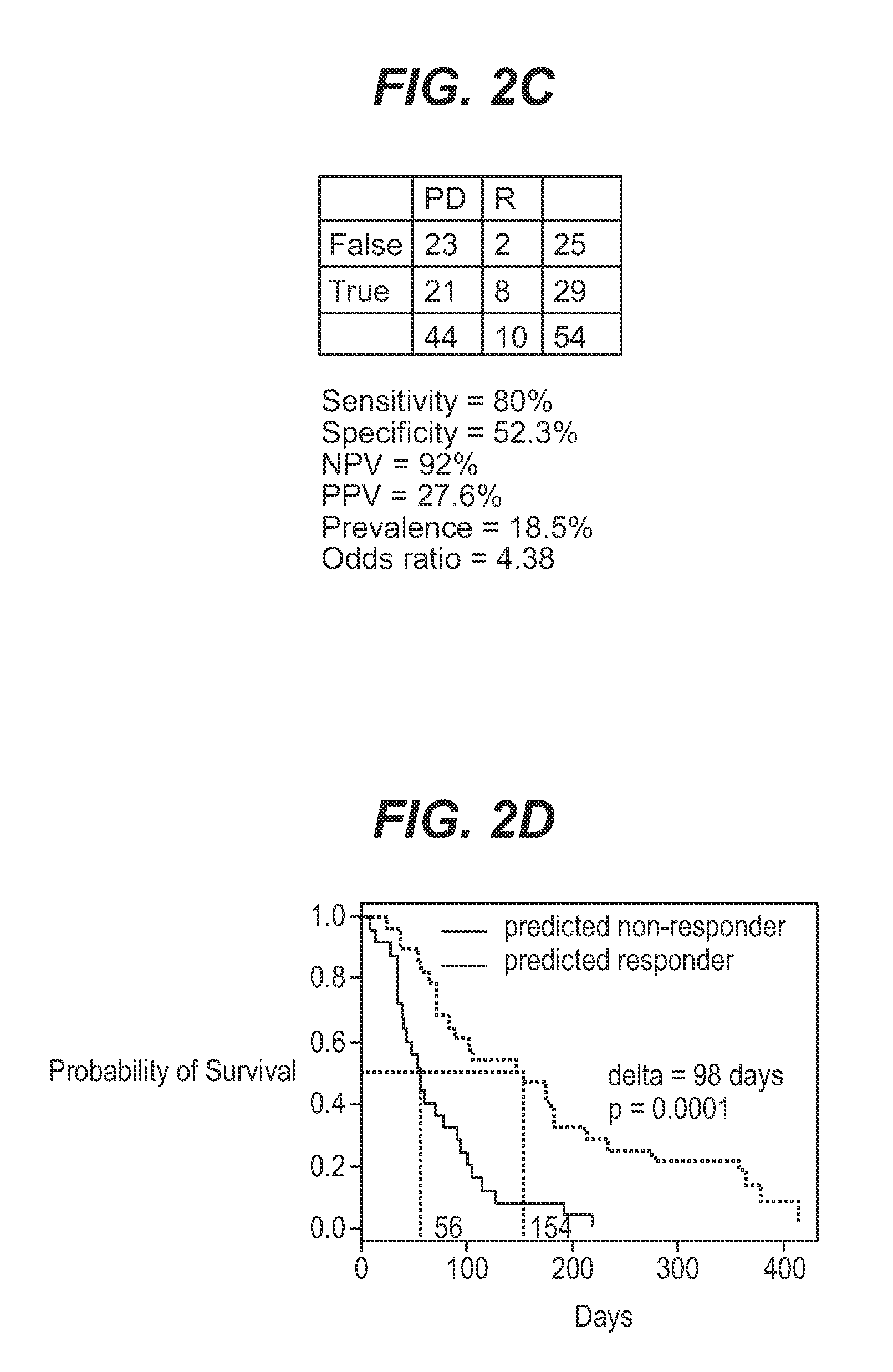
InactiveUS7932036B1Improve accuracyHigh response rateSugar derivativesMicrobiological testing/measurementNewly diagnosedClinical study
We analyzed bone marrow from 67 patients from a phase 2 study of farnesyltransferase inhibition with tipifarnib (R115777, ZARNESTRA®), in older adults with previously untreated, poor-risk acute myeloid leukemia (AML) for N-Ras mutations, global gene expression, and / or quantitative PCR (qPCR) of specific genes. Microarray profiling identified a two-gene expression ratio (RASGRP1:APTX) which provided the greatest accuracy for predicting response to tipifarnib. We demonstrated that this classifier could predict response to tipifarnib in an independent set of 54 samples from relapsed or refractory AML, with a NPV and PPV of 92% and 28%, respectively (odds ratio of 4.4). Therefore, in both newly diagnosed and relapsed or refractory AML, this classifier improves the overall response rate by approximately 50% while maintaining a high NPV, and significantly improves patient overall survival. The two-gene classifier was also validated by qPCR in thirty AML samples from the same clinical study demonstrating a negative predictive value (NPV) and positive predictive value (PPV) of 81% and 50%, respectively (odds ratio of 4.3). These data indicate that a simple two-gene expression assay may have utility in diagnosing a population of AML patients who are more likely to respond to tipifarnib.
Owner:JANSSEN DIAGNOSTICS LLC
Method of improving a clinical study
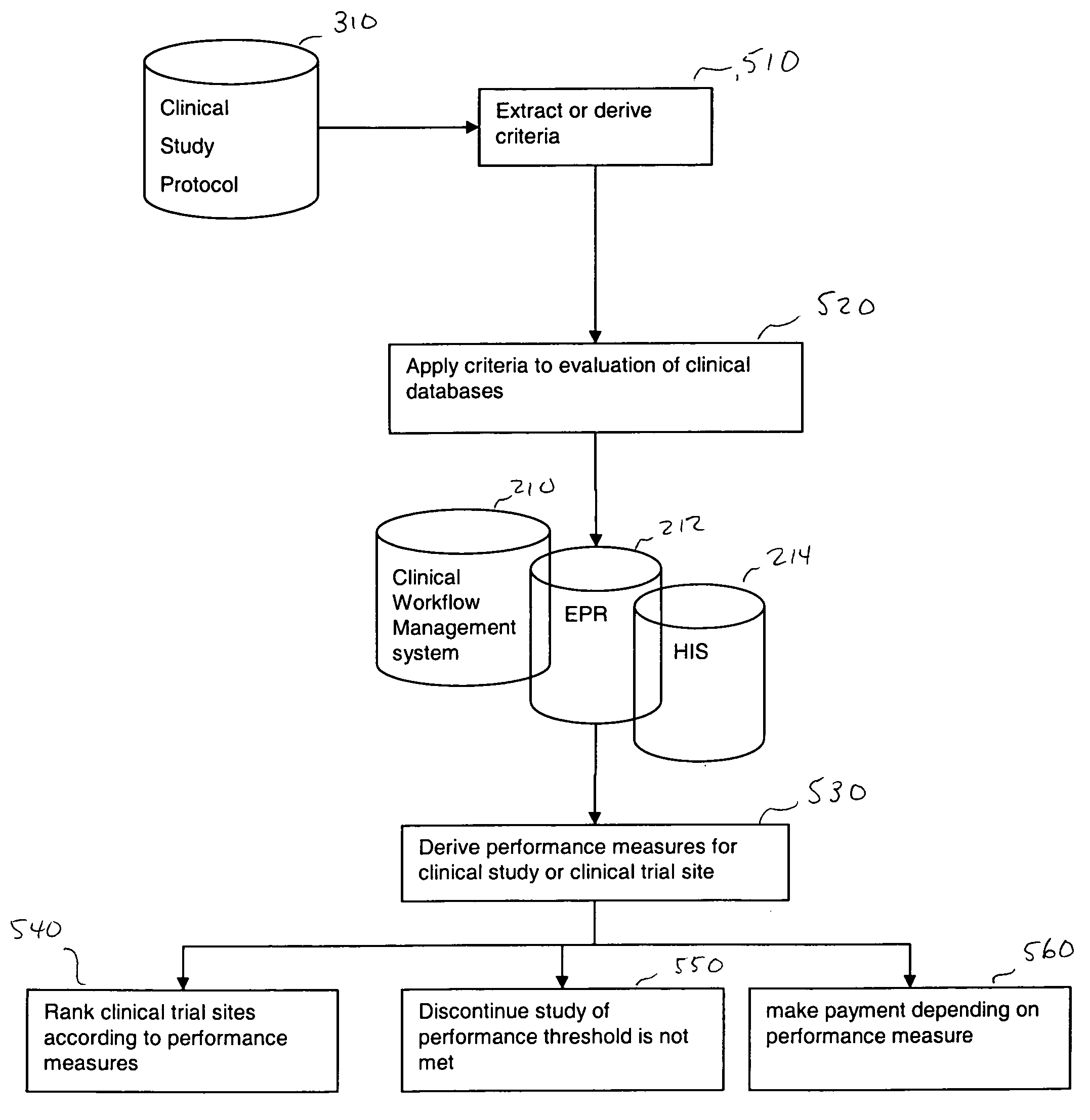
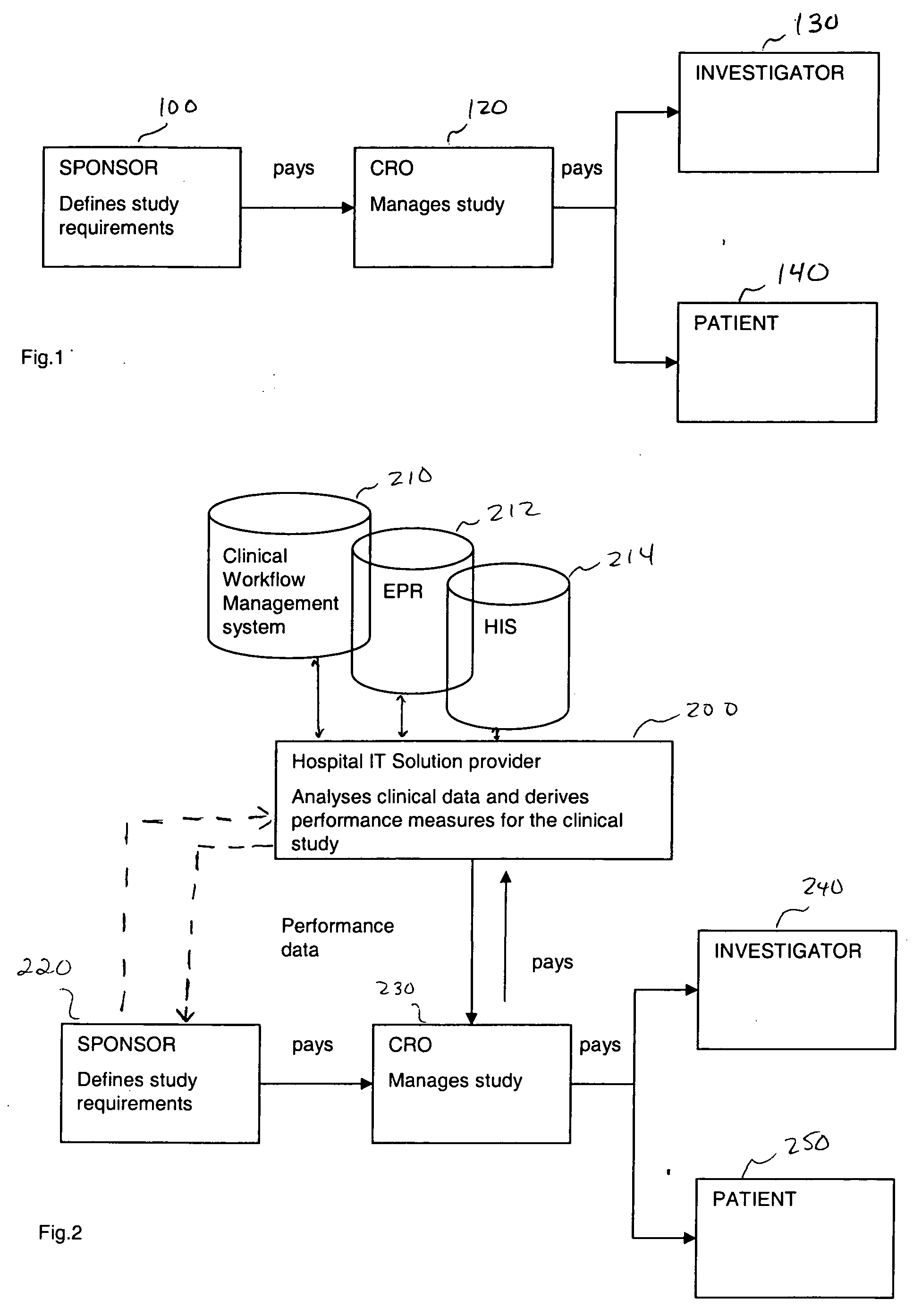
InactiveUS20050182658A1Increased cost-effectivenessSpeed up the processData processing applicationsComputer-assisted medical data acquisitionClinical studyFamily medicine
A method is proposed for improving a clinical study. The method includes obtaining criteria for the clinical study, and comparing clinical data to the obtained criteria using a computer device. Thereafter, using the computer device, performance measures are derived based upon the comparisons. These derived performance measures are then usable to improve the clinical study. For example, these performance measures may be used for ranking, and consequently improving, the clinical study.
Owner:SIEMENS AG
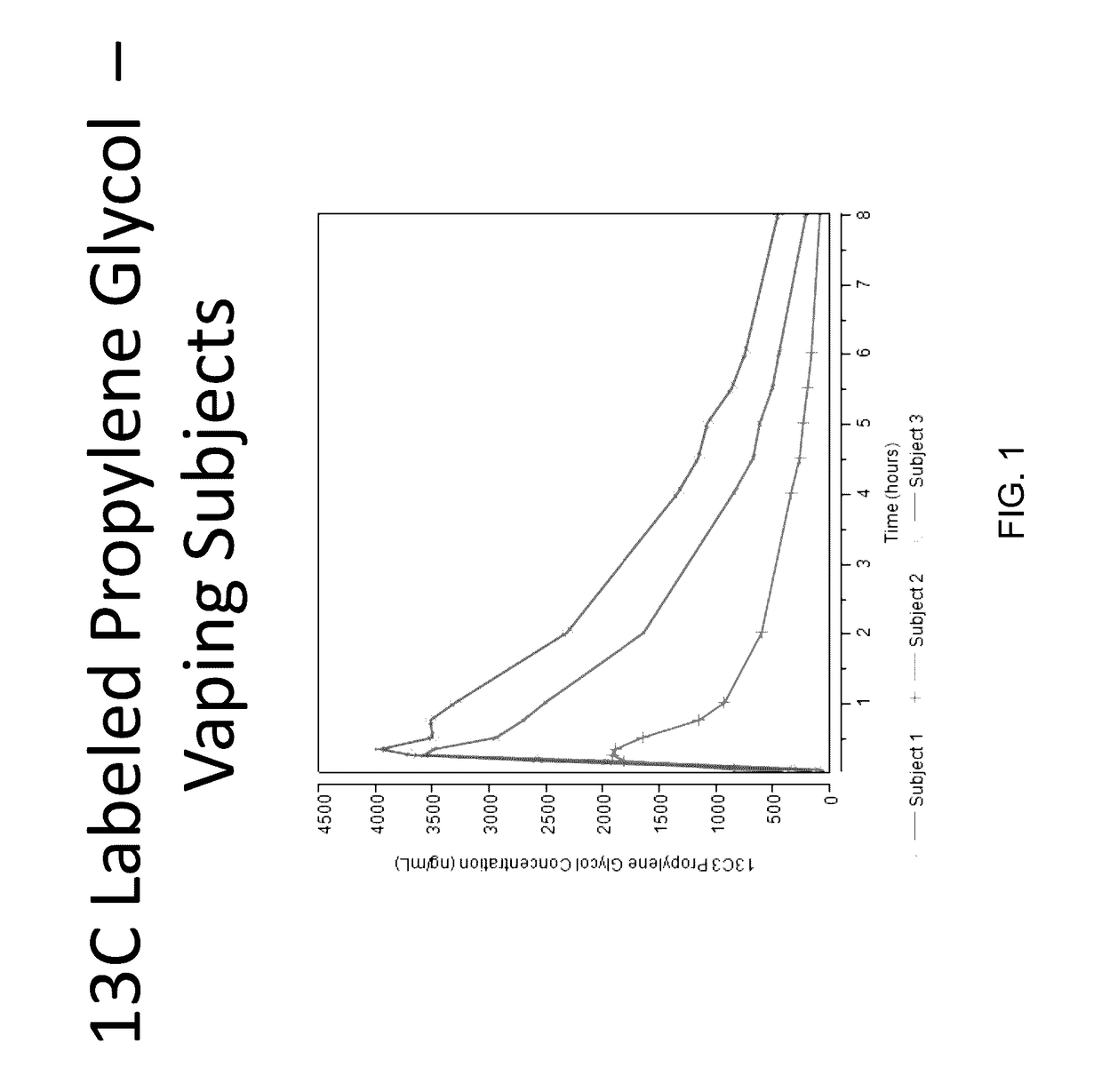
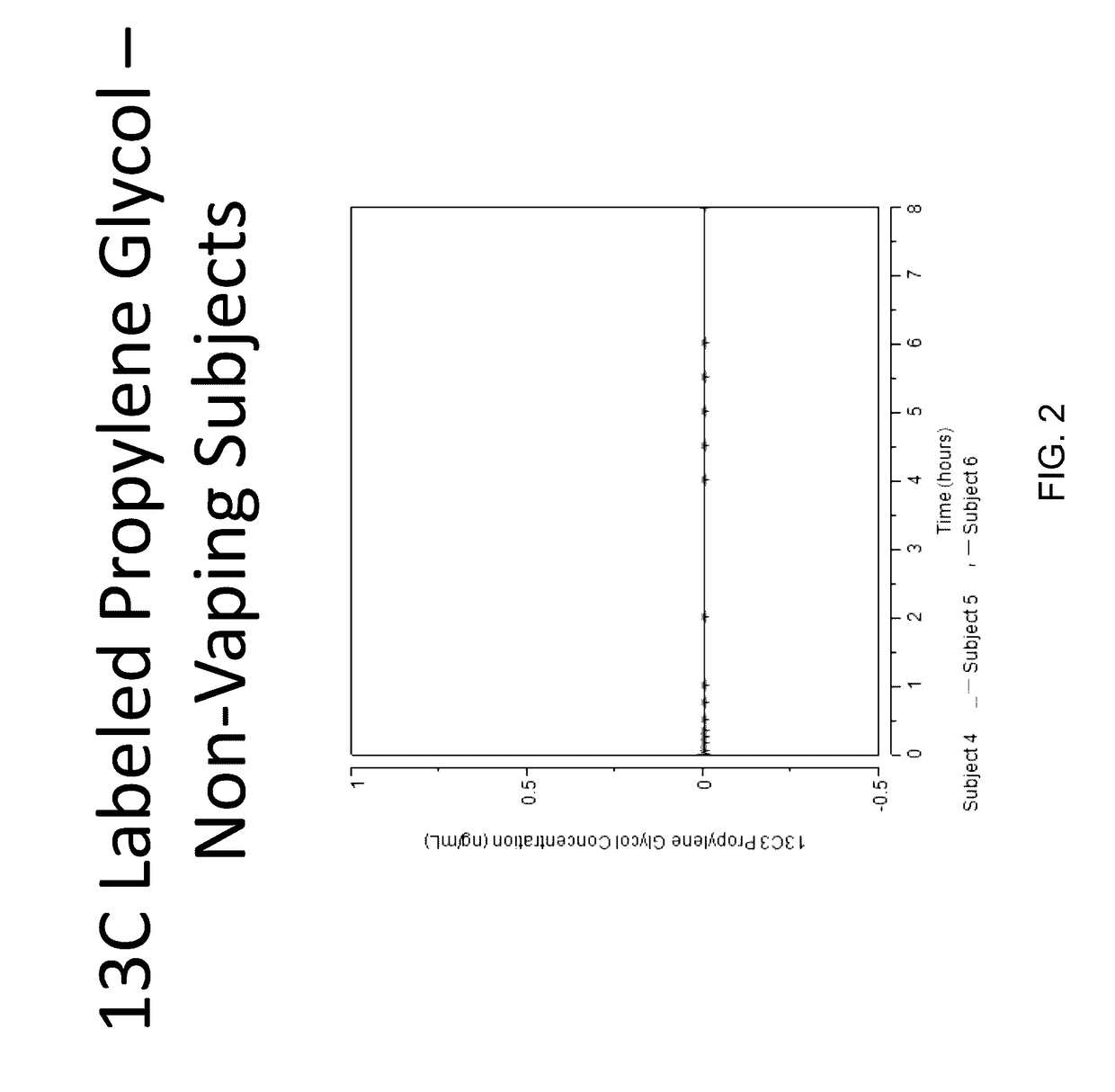
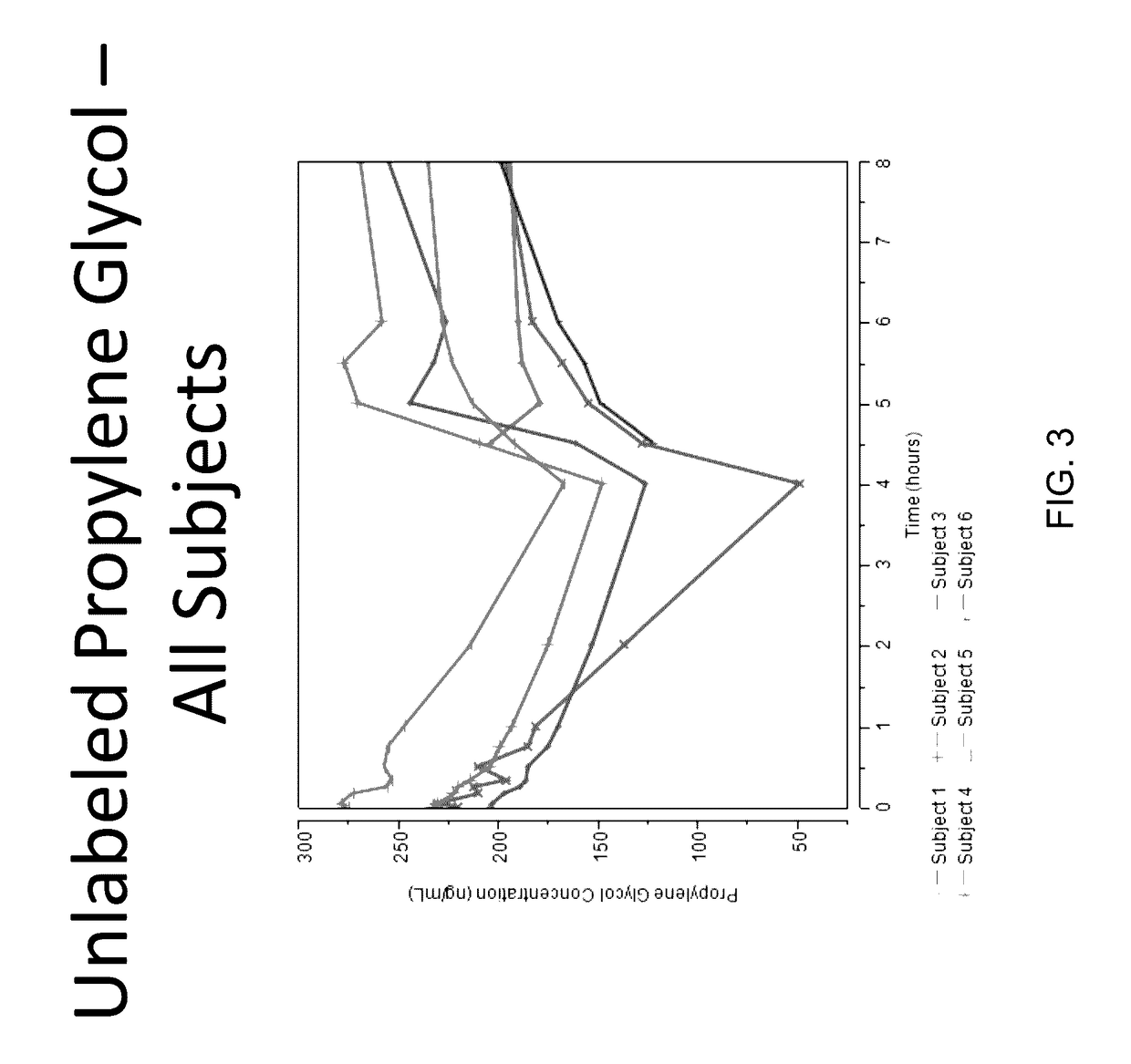
Isotopically-labeled solvents and the use of same in testing e-cigarettes
Isotopically-labeled species of propylene glycol and glycerol are provided as solvents for use in electronic nicotine delivery systems (e.g., as disposed in a cartridge in an e-cigarette). The isotopically-labeled species are distinguishable from the non-isotopically-labeled species (e.g., by mass spectrometry). Thus, methods are provided for the measurement of the quantity of solvent or of a solvent heating by-product (e.g., formaldehyde, acetaldehyde), or of a metabolite of the drug, delivered to a user by analysis of blood samples taken subsequent to dosing by use of the electronic nicotine delivery system. An example of an isotopically-labeled species of propylene glycol for use in such measurement methods is [13C]3H8O2. A clinical study of e-cigarettes loaded with this isotopically-labeled species resulted in the following blood plasma concentration profiles in “vaping” subjects.
Owner:CELERION INC
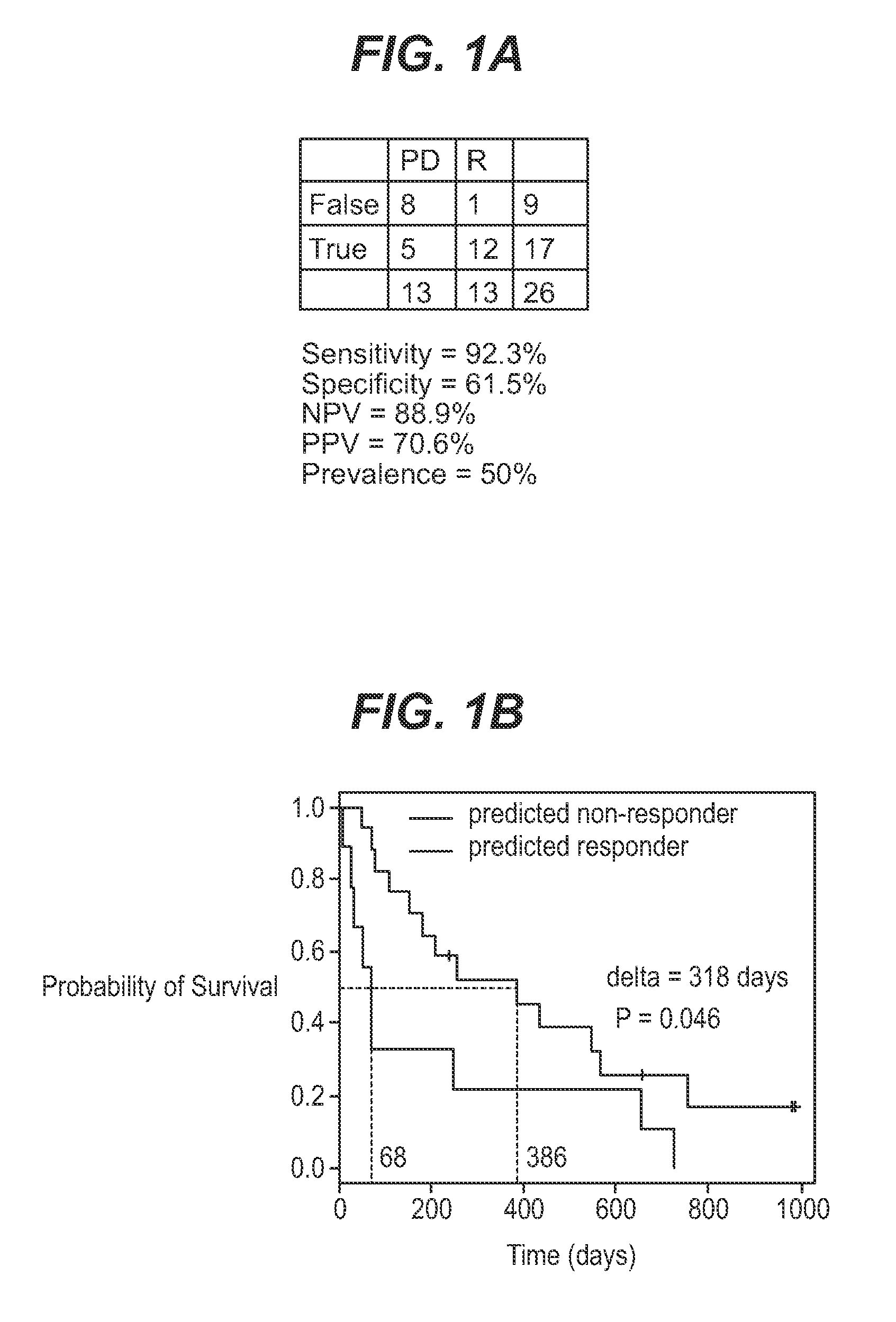
Methods for treating or predicting risk of a ventricular tachyarrhythmia event
InactiveUS20130345805A1Slow heart beat more regularlyElectrotherapyDisease diagnosisVentricular TachyarrhythmiasClinical study
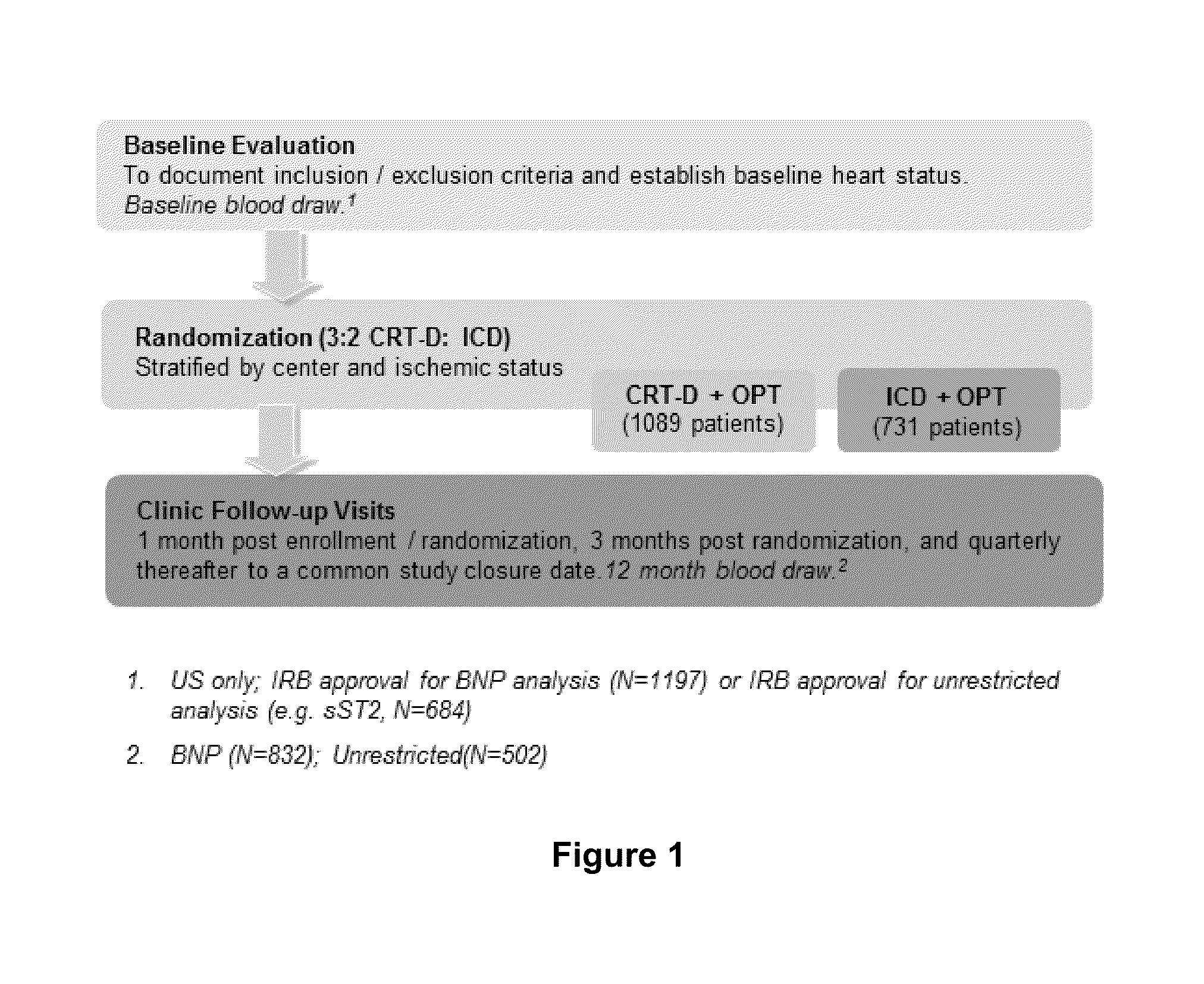
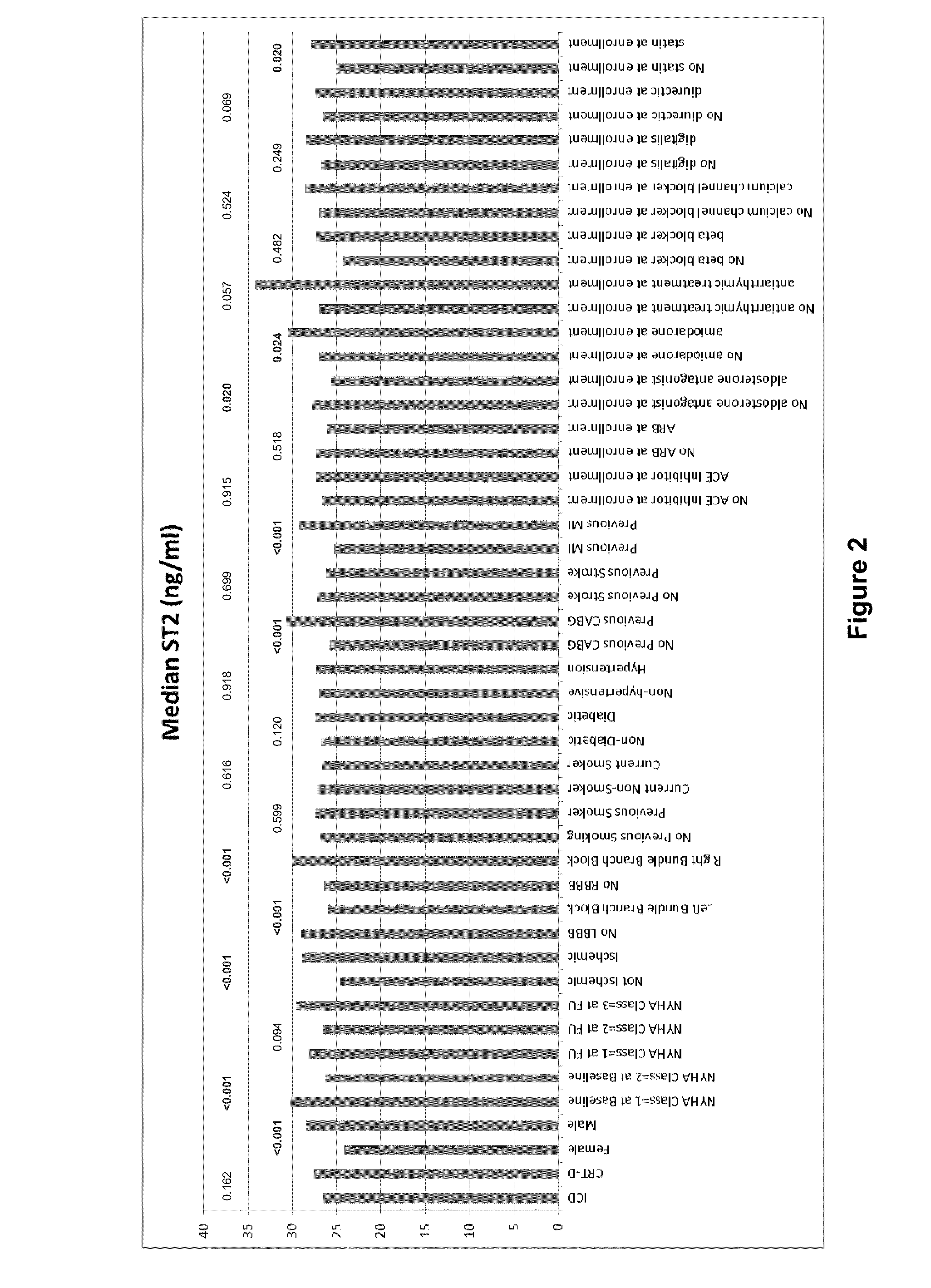
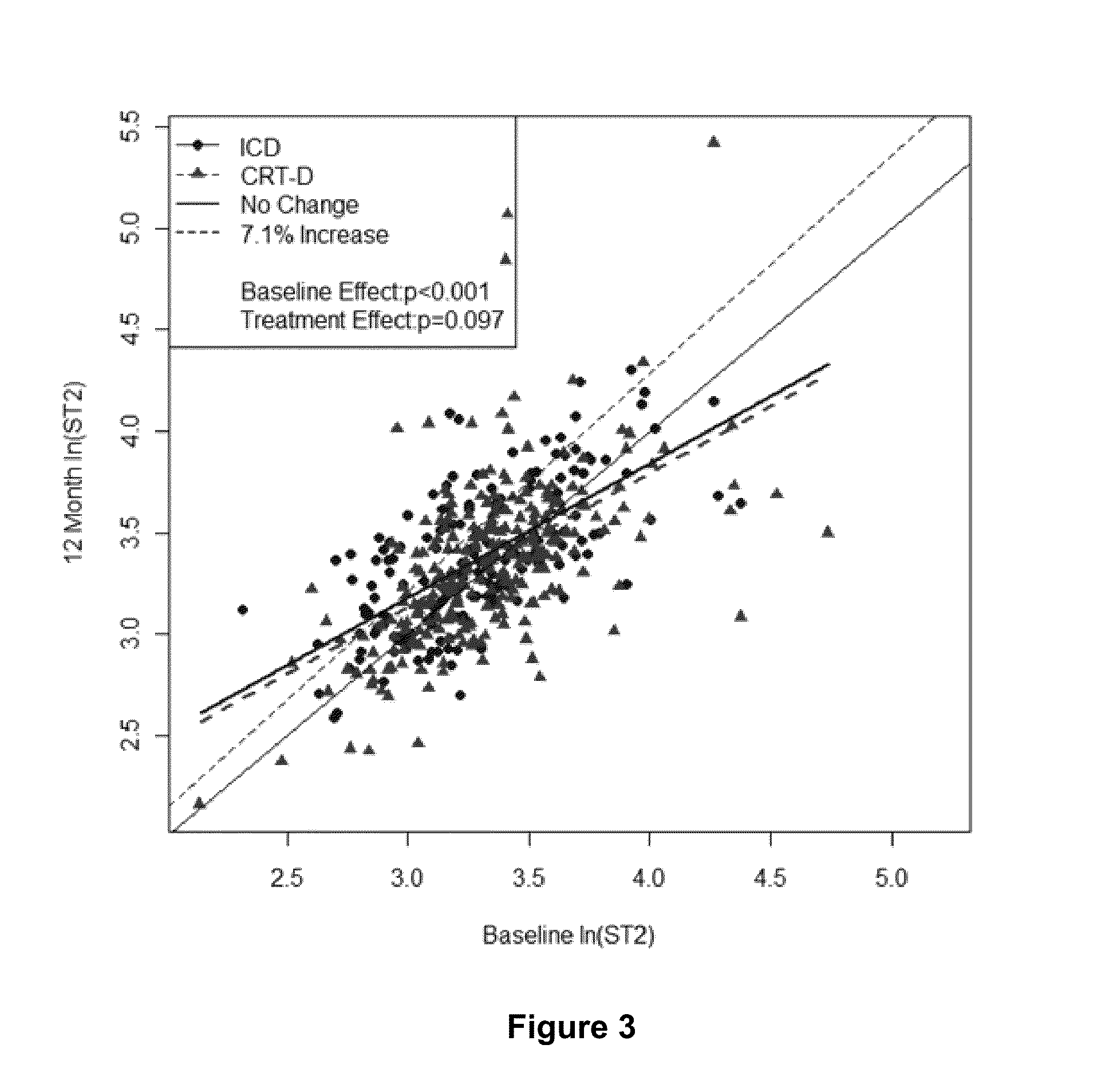
Provided herein are methods that include (i) determining a level of soluble ST2 in a biological sample from a subject, (i) comparing the level of soluble ST2 in the biological sample to a reference level of soluble ST2 (e.g., a level of soluble ST2 in the subject at an earlier time point), and (iii) selecting, implanting, replacing, or reprogramming an implanted cardiac device, e.g., an ICD, CRT, or CRT-D device, for a subject having an elevated level of soluble ST2 in the biological sample compared to the reference level of soluble ST2, or selecting a subject for participation in, or stratifying a subject participating in, a clinical study of a treatment for reducing the risk of a ventricular tachyarrhythmia (VTA) event. Also provided are methods for evaluating the risk of a VTA event in a subject. Also provided are kits for performing any of these methods.
Owner:CARDIAC PACEMAKERS INC +1
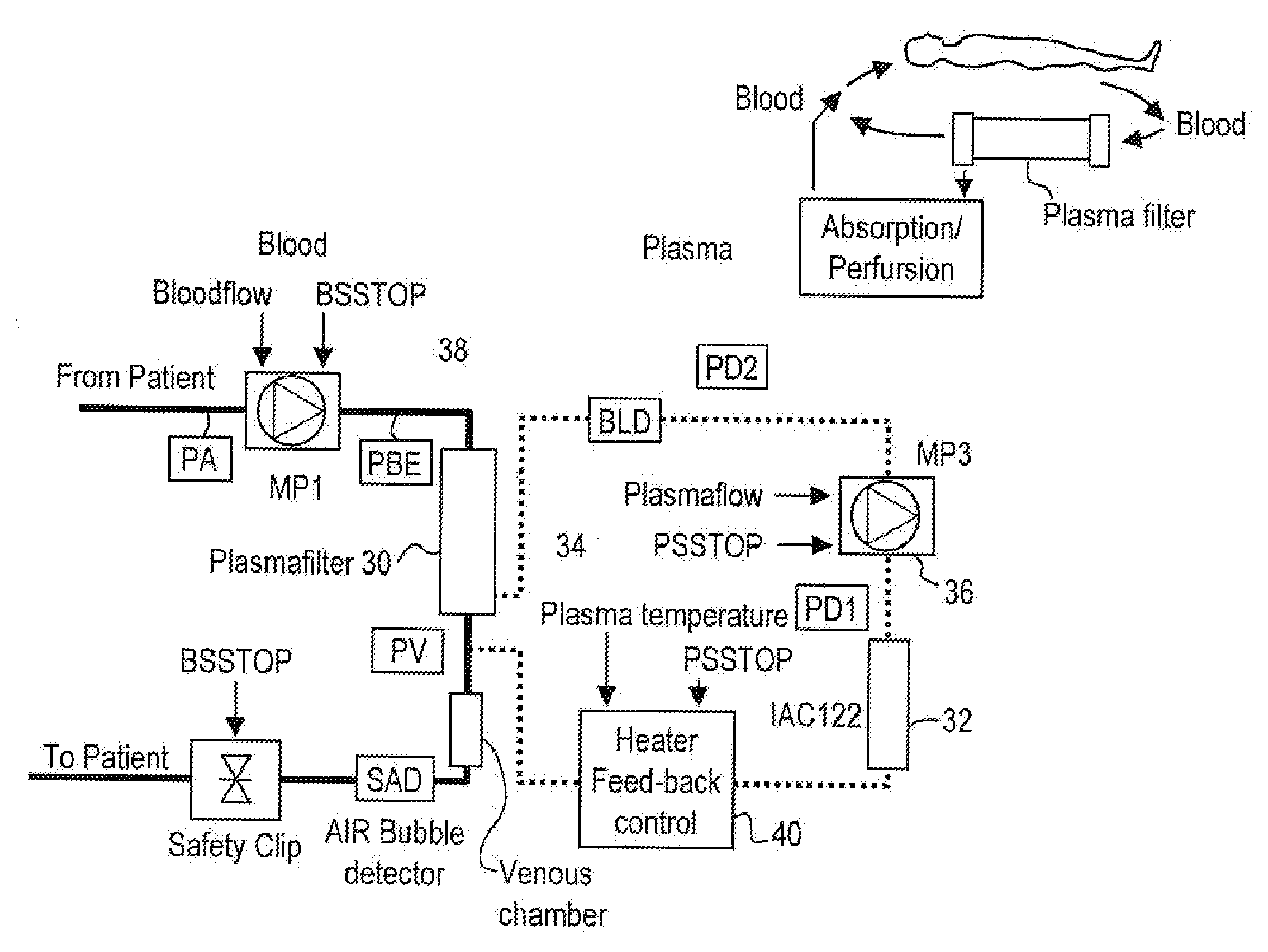
Method and system to remove soluble tnfr1, tnfr2, and il2 in patients
A method, and system, to induce remission in diseases characterized by excess production of sTNR and interleukin 2 has been developed. In the most preferred embodiment, the system consists of antibodies to sTNFR1, sTNFR2 and sIL2R immobilized in a column containing a material such as SEPHAROSE™. The patient is connected to a pheresis machine which separates the blood into the plasma and red cells, and the plasma is circulated through the column until the desired reduction in levels of sTNFR1, sTNFR2, and IL2 is achieved, preferably to less than normal levels. In the preferred method, patients are treated three times a week for four weeks. This process can be repeated after a period of time. Clinical studies showed reduction in tumor burden in patients having failed conventional chemotherapy and radiation treatments.
Owner:INNATUS CORP
Clinical data management system
ActiveUS20130238351A1Data processing applicationsComputer-assisted medical data acquisitionClinical studyData transport
A clinical data management system (1) has databases (20), processors in servers (2-4) which are programmed to process clinical data and communicate with user interfaces and external systems interfaces, and at least one database. The system imports source data from disparate clinical site sources into staging databases at refresh intervals, maintains data models, and maps data from the staging databases into the data models, and feeds data from the data models into data delivery databases. There is a uniform refresh frequency for the staging databases. The system output is regularly updated data for clinical site performance, quality and risk metrics to a clinical study team. The data mapper servers identify each of a plurality of source data stages, and transform data from each stage to one or more data models according to one or more mapsets, each mapset defining a transformation.
Owner:ICON CLINICAL RESERCH LTD
Method and system to remove soluble tnfr1, tnfr2, and il2 in patients
InactiveUS20110129441A1Peptide/protein ingredientsIon-exchanger regenerationDiseaseConventional chemotherapy
A method, and system, to induce remission in diseases characterized by excess production of sTNR and interleukin 2 has been developed. In the most preferred embodiment, the system consists of antibodies to sTNFR1, sTNFR2 and sIL2R immobilized in a column containing a material such as SEPHAROSE™. The patient is connected to a pheresis machine which separates the blood into the plasma and red cells, and the plasma is circulated through the column until the desired reduction in levels of sTNFR1, sTNFR2, and IL2 is achieved, preferably to less than normal levels. In the preferred method, patients are treated three times a week for four weeks. This process can be repeated after a period of time. Clinical studies showed reduction in tumor burden in patients having failed conventional chemotherapy and radiation treatments.
Owner:INNATUS CORP
Coenzyme-binding glucose dehydrogenase
Owner:PHC CORP
Systems and methods for refining identification of clinical study candidates
Certain embodiments of the present invention provide systems and methods for refining the identification of study candidates. In an embodiment, the method may include receiving a plurality of clinical conditions at a user interface. The conditions may generally be tied to codified terms in electronic medical patient records. The method may also include receiving parameters for tailoring a search of electronic medical data for the clinical conditions. For example, the parameters may weight the conditions and may include ranking the conditions, determining whether the conditions are mandatory for the pool, and determining a percentage deviation for numerical ranges. The method may also include executing an optimization function to create a pool according to the specified conditions and parameters. The method may also include displaying the pool in an order from the most optimal results to the least optimal results.
Owner:GENERAL ELECTRIC CO
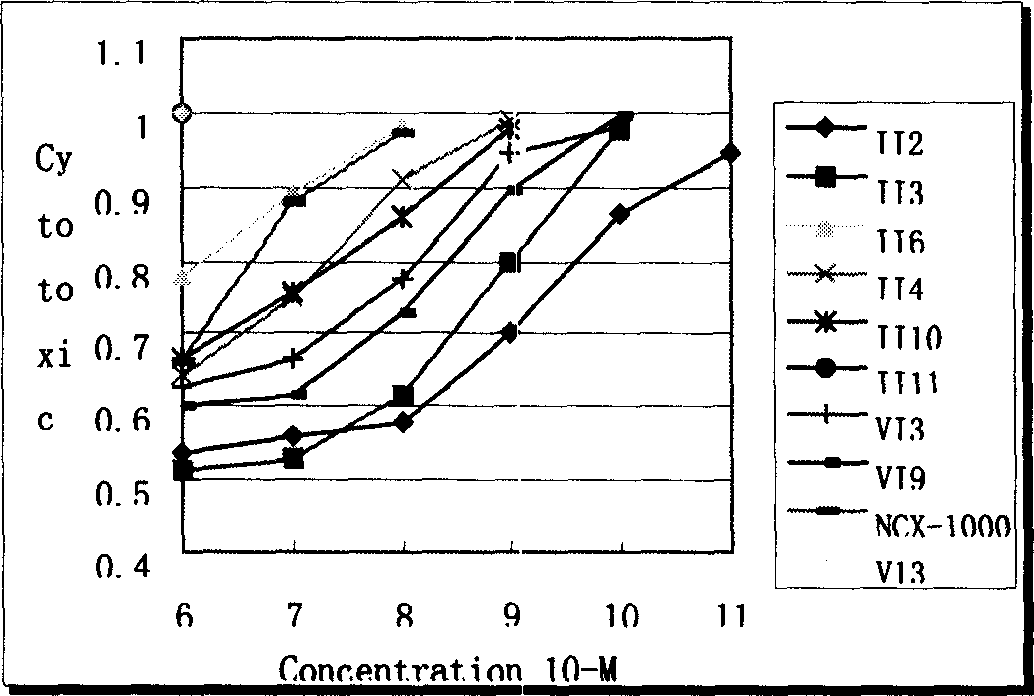
Oleanolic acid couple derivatives and their pharmaceutical use
The invention discloses an oleanolic acid couple, which is a nitric oxide donator type OLA derivative coupled by dissimilar NO donators with hepatic disease treating medicament oleanolic acid (OLA) through binding groups with ester bonds or amido bonds, wherein the suppressor cell apoptosis action of some of the compounds is stronger than that of the NCX-10000 that are undergone US stage I clinical research, thus may possibly be used for the treatment of hepatitis and cirrhosis.
Owner:CHINA PHARM UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com