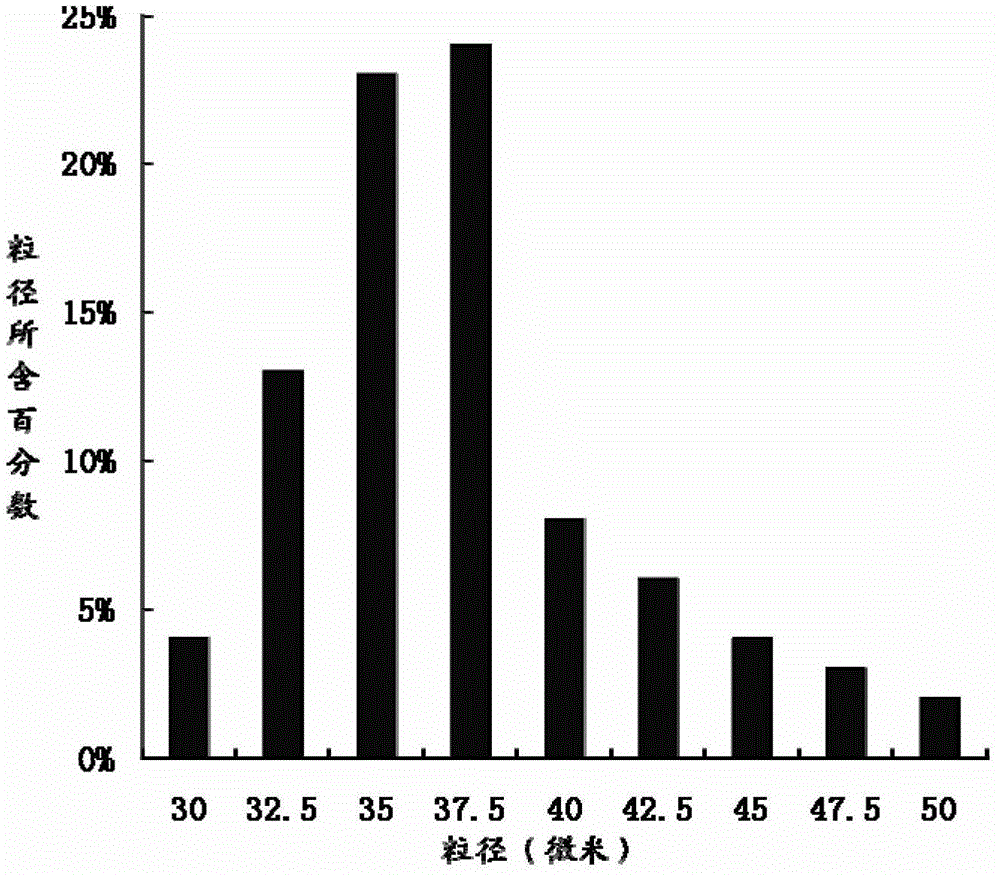
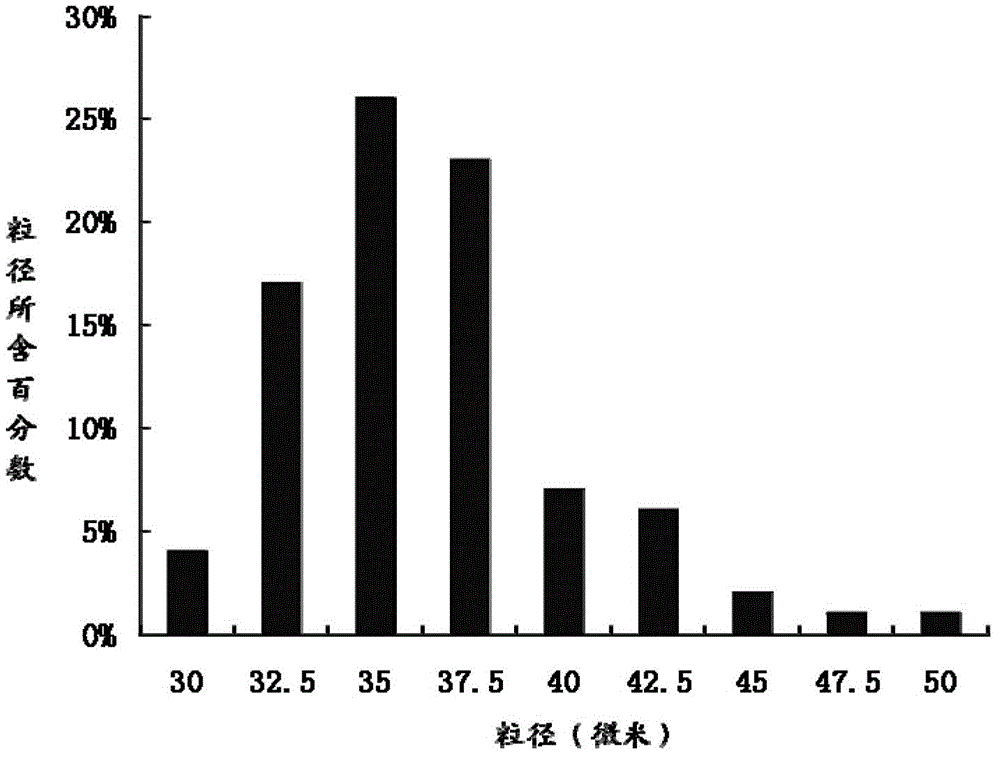
Teriparatide sustained-release microsphere and preparation method thereof
A technology of sustained-release microspheres and teriparatide, applied in the field of medicine, can solve the problems of injection site damage, lack of sustained-release, and low patient compliance, achieving high encapsulation rate, reducing the probability of infection, The effect of reducing the number of doses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0050] The preparation method of the teriparatide sustained-release microspheres provided by the present invention comprises the following steps: taking a carrier and teriparatide and dissolving them in an organic solvent, mixing them with an aqueous solution of a surfactant, and emulsifying to obtain an emulsion; taking the emulsion and surfactant The aqueous solution of the agent is mixed, the organic solvent is removed, purified and dried.
[0051] The organic solvent is one or a mixture of two or more of dichloromethane, acetone, ethyl acetate, acetonitrile, methyl acetate, 1,4-dioxane, ethanol, and dimethylformamide.
[0052] In the preparation method of the teriparatide sustained-release microspheres provided by the present invention, the mass volume concentration of the surfactant in the aqueous solution of the surfactant mixed with the organic solvent containing the carrier and the teriparatide is 1% to 10%.
[0053] The surfactant in the aqueous solution of the surfac...
Embodiment 1
[0080] Example 1 Preparation of Teriparatide Sustained-release Microspheres by Emulsion Solvent Evaporation Method
[0081] Accurately weigh 2g of lactic acid-glycolic acid copolymer (85:15, Mw=15000) and 200mg of teriparatide, dissolve in 40mL of dichloromethane as the dispersed phase; add the above dispersed phase slowly in a water bath at 10°C Put it into 200mL aqueous solution containing 1% polyvinyl alcohol, emulsify for 1 hour, the emulsification condition is 1000 rpm, stir while adding; add the obtained emulsion into 500mL aqueous solution containing 0.1% polyvinyl alcohol, the temperature is 25 ℃, stir The organic solvent was volatilized in 4 hours to form solidified microspheres; the solution obtained above was collected by centrifugation at 3000 rpm, washed three times with purified water to remove the drug and polyvinyl alcohol on the surface of the microspheres, and dried in vacuum for two days to obtain Teriparatide extended-release microspheres.
Embodiment 2
[0082] Example 2 Preparation of Teriparatide Sustained Release Microspheres by Emulsion Solvent Evaporation Method
[0083] Accurately weigh 10g of lactic acid-glycolic acid copolymer (75:25, Mw=15000) and 200mg of teriparatide, dissolve it in 40mL of dichloromethane as the dispersed phase; slowly add the above dispersed phase in a water bath at 10°C Put it into 200mL aqueous solution containing 2% polyvinyl alcohol, emulsify for 1 hour, the emulsification condition is 1000 rpm, stir while adding; add the obtained emulsion into 500mL aqueous solution containing 0.2% polyvinyl alcohol, the temperature is 25 ℃, stir The organic solvent was volatilized in 4 hours to form solidified microspheres; the solution obtained above was collected by centrifugation at 3000 rpm, washed three times with purified water to remove the drug and polyvinyl alcohol on the surface of the microspheres, and dried in vacuum for two days to obtain Teriparatide extended-release microspheres.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com