Oral pharmaceutical composition containing teriparatide and preparation method thereof
A technology of teriparatide and its composition, which is applied in the field of oral pharmaceutical composition containing teriparatide and its preparation, can solve the problems of side effects, difficulty in exerting the effect of absorption promotion, etc., so as to improve patient compliance and increase penetration rate and the effect on bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
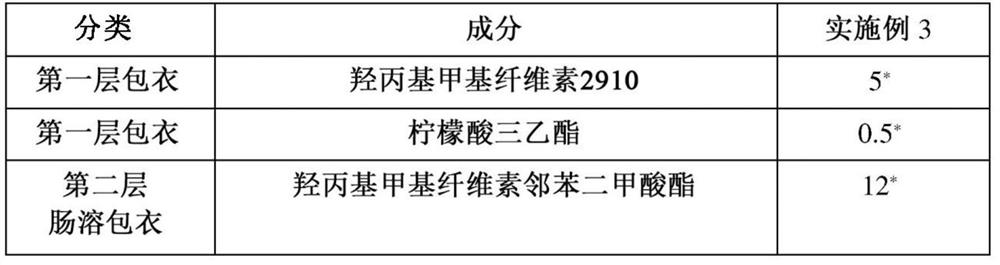
Examples
Embodiment 1
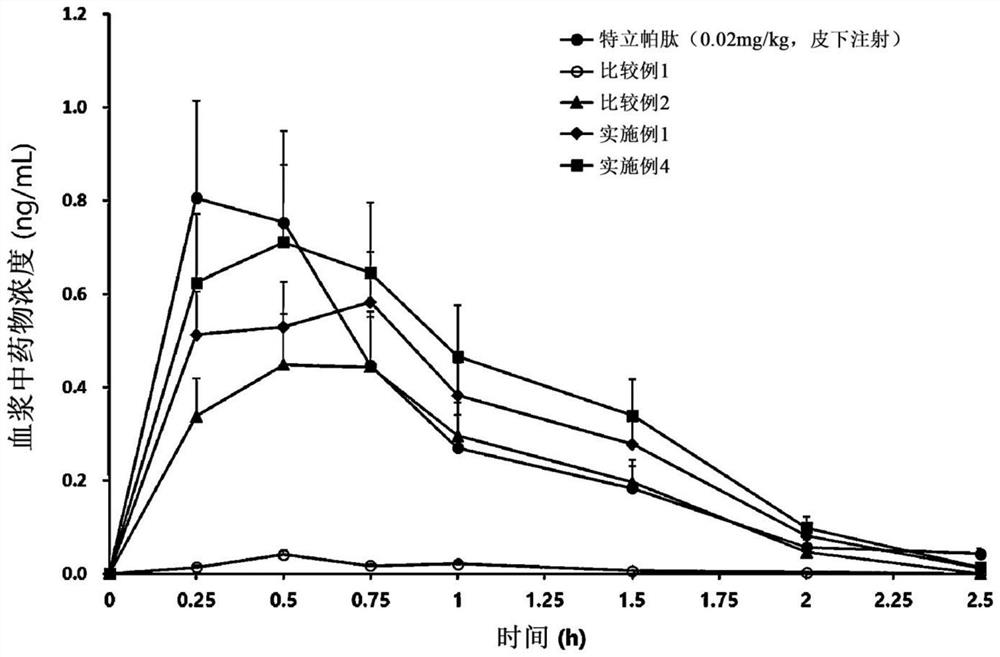
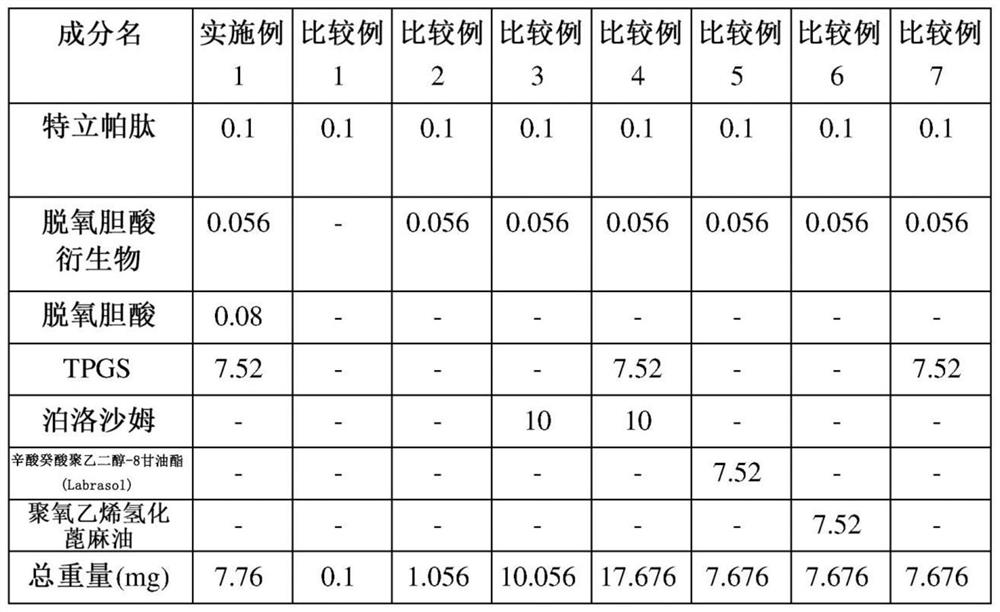
[0057] Example 1 and Comparative Example: Ionic complexation of teriparatide, deoxycholic acid, deoxycholic acid derivatives and solubilizers preparation of
[0058] Teriparatide and D-α-tocopherol polyethylene glycol 1000 succinate (TPGS) as a solubilizer were dissolved in pure water, and then separately prepared Aqueous solutions of deoxycholic acid derivatives to prepare ionically bonded complexes. At this time, an aqueous solution of a deoxycholic acid derivative was slowly added, so that teriparatide and N, which is a deoxycholic acid derivative, were α -Deoxycholyl-L-lysinyl methyl ester (N α -deoxycholyl-L-lysyl-methylester, DCK) in a molar ratio of 1:2 or 1:4. Then, a separately prepared aqueous solution of sodium deoxycholate was slowly added while stirring the above-mentioned complex solution to prepare an ionomer complex. At this time, an aqueous solution of deoxycholic acid was slowly added so that the molar ratio of teriparatide to deoxycholic acid was 1:4 ...
experiment example 1
[0062] Experimental Example 1: Synthesis of a complex consisting of teriparatide, deoxycholic acid, a deoxycholic acid derivative and a solubilizer Confirmation of intestinal mucosal permeability
[0063] The artificial intestinal mucosal permeability evaluation system (parallel artificial membranepermeability assay, PAMPA) was used to evaluate the effective permeability (effective permeability, P e ). First, the samples of Example 1 and Comparative Examples 1 to 7 were dissolved in phosphate buffered saline (PBS, pH 6.8) to a concentration of 200 μg / mL of teriparatide, and then 200 μL were added to the PAMPA system. The supply part and the receiving part of the PAMPA system were respectively filled with 300 μL of phosphate buffered saline (PBS, pH 6.8), and then the supply part and the receiving part were combined and left at room temperature for 5 hours. Then, the solution in each well of the receiving part and the supplying part was taken out, filtered through a membra...
experiment example 2
[0078] Experimental Example 2: Permeation of a complex consisting of teriparatide, deoxycholic acid, a deoxycholic acid derivative, and a solubilizer Confirmation of the permeability of intestinal cell membranes
[0079] The apparent permeability of the complexes prepared as in Example 1 and Comparative Examples 1 to 7 was evaluated as follows with respect to the Caco-2 cell membrane, which is an intestinal cell membrane. Caco-2 cells were individually treated to a concentration of 1 x 10 in a 24-well transfer chamber (Transwell). 5 After cells / mL, cells were cultured for 14-16 days, and then the resistance across the Caco-2 cell membrane (transmembrane resistance, TEER) value was >350 Ω cm 2 The cell monolayer was used for the experiments. First, the medium in the transfer chamber was removed, and then the supply part and the receiving part were filled with HBSS, and after culturing at 37° C. for 20 minutes, the TEER value was measured again, and then the HBSS was remove...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com