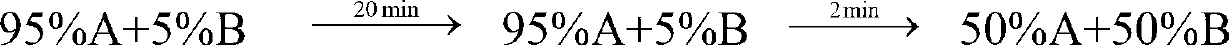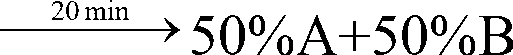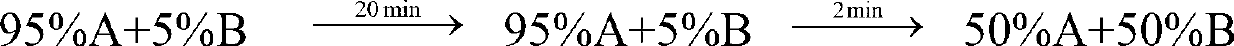Purification method of ganirelix acetate
A ganirelix and purification method technology, applied in the field of medicinal chemistry, can solve the problems of uneven salt formation of refined peptides, toxicity of trifluoroacetic acid, unfavorable purification, etc., and achieves good elution peak shape, improved purification scale, and easy The effect of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: the purification of ganirelix acetate
[0030] (1) Purification of ganirelix crude peptide:
[0031] Dissolve 2.0 g of crude ganirelix peptide in 100 ml of purified water and acetonitrile (v:v=3:1), filter, and collect the filtrate for later use.
[0032] Purification chromatography conditions:
[0033] Chromatographic column: 50×250mm, filled with octadecylsilane bonded silica gel as stationary phase filler.
[0034] Flow rate: 80ml / min.
[0035] Monitoring wavelength: 280nm.
[0036]Mobile phase A phase: 20mM sodium perchlorate solution, adjust pH to 1.5 with phosphoric acid.
[0037] Mobile Phase B: Acetonitrile.
[0038] gradient:
[0039] Sample loading: 2.0g (100ml).
[0040] After equilibrating the chromatographic column with 75%A+25%B for 5 minutes, load the sample, run gradient purification, monitor and collect the target peak fractions in three sections: before the peak, at the top of the peak, and after the peak. The pre-peak and post-...
Embodiment 2
[0052] Embodiment 2: the purification of ganirelix acetate
[0053] (1) Purification of ganirelix crude peptide:
[0054] Dissolve 2.0 g of crude ganirelix peptide in 100 ml of purified water and acetonitrile (v:v=3:1), filter, and collect the filtrate for later use.
[0055] Purification chromatography conditions:
[0056] Chromatographic column: 50×250mm, filled with octadecylsilane bonded silica gel as stationary phase filler.
[0057] Flow rate: 80ml / min.
[0058] Monitoring wavelength: 280nm.
[0059] Mobile phase A phase: 50mM sodium perchlorate solution, adjust the pH to 2.0 with phosphoric acid.
[0060] Mobile Phase B: Acetonitrile.
[0061] gradient:
[0062] Sample loading: 2.0g (100ml).
[0063] Equilibrate the chromatographic column with 75%A+25%B for 5 minutes, load the sample, run gradient purification, monitor and collect the target peak fractions in three sections: before the peak, at the top of the peak, and after the peak. The pre-peak and post-pea...
Embodiment 3
[0075] Embodiment 3: the purification of ganirelix acetate
[0076] (1) Purification of ganirelix crude peptide:
[0077] Dissolve 2.0 g of crude ganirelix peptide in 100 ml of purified water and acetonitrile (v:v=3:1), filter, and collect the filtrate for later use.
[0078] Purification chromatography conditions:
[0079] Chromatographic column: 50×250mm, filled with octadecylsilane bonded silica gel as stationary phase filler.
[0080] Flow rate: 80ml / min.
[0081] Monitoring wavelength: 280nm.
[0082] Mobile phase A phase: 100mM sodium perchlorate solution, adjust the pH to 2.5 with phosphoric acid.
[0083] Mobile Phase B: Acetonitrile.
[0084] gradient:
[0085] Sample loading: 2.0g (100ml).
[0086] Equilibrate the chromatographic column with 75%A+25%B for 5 minutes, load the sample, run gradient purification, monitor and collect the target peak fractions in three sections: before the peak, at the top of the peak, and after the peak. The pre-peak and post-pe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com