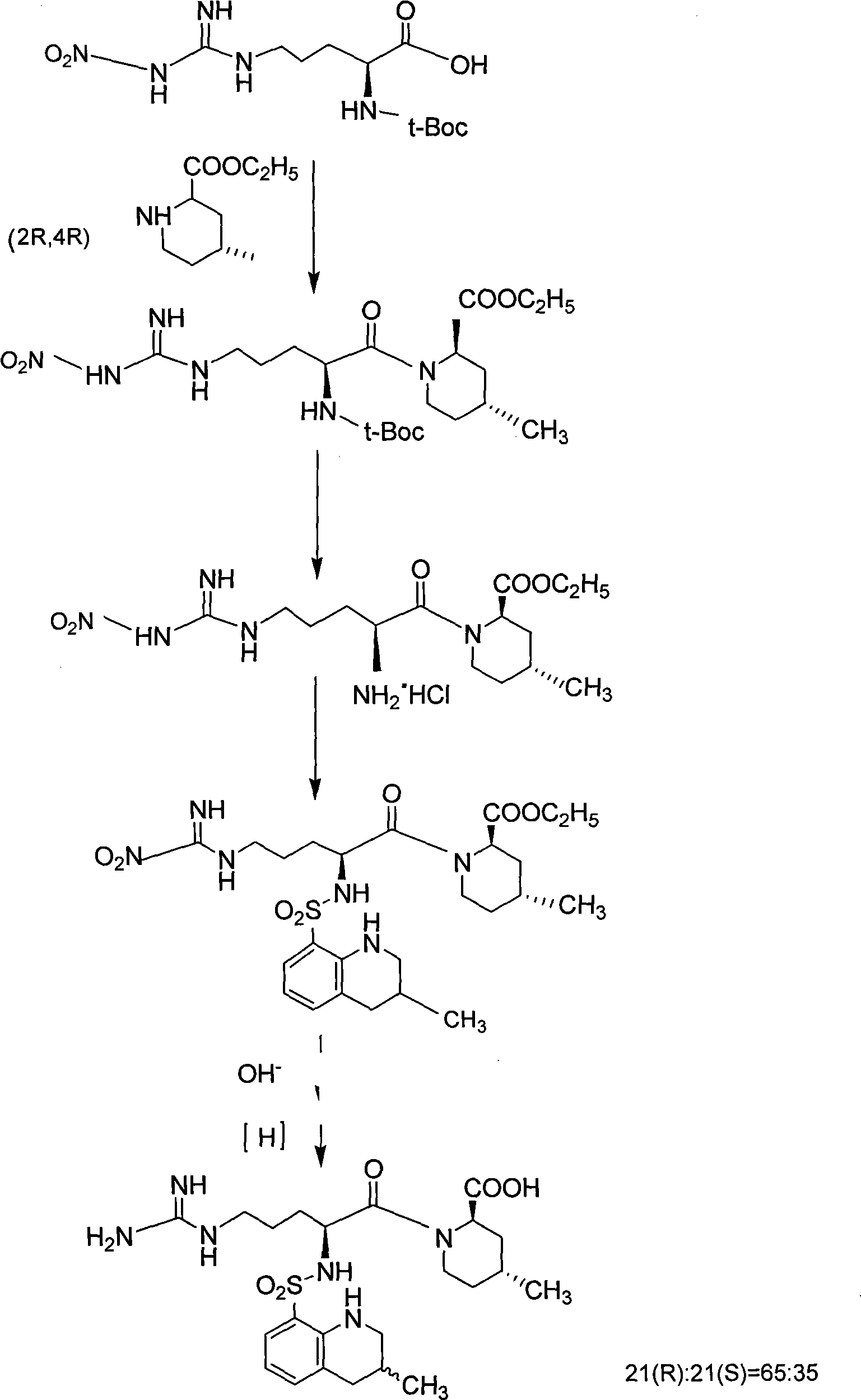
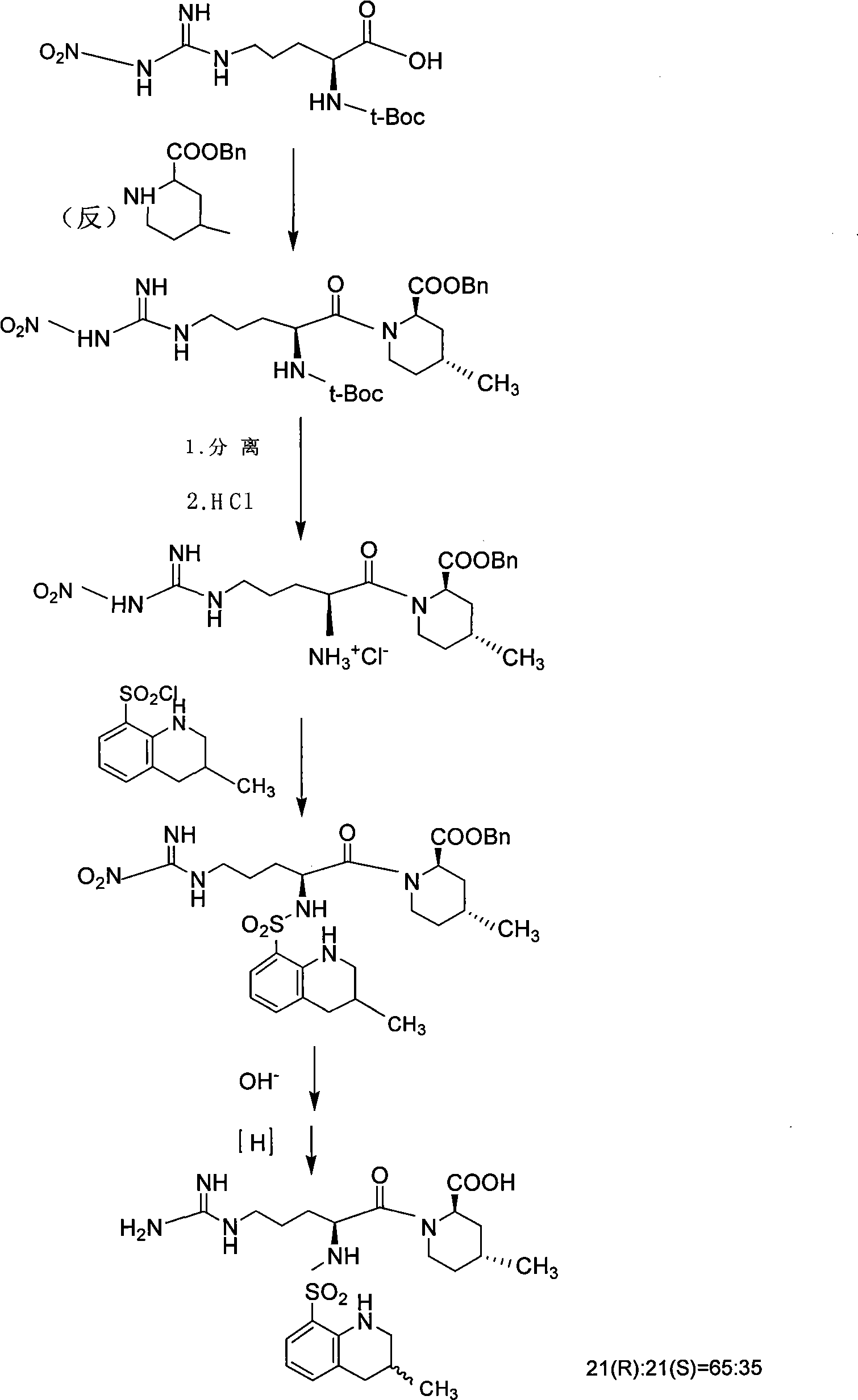
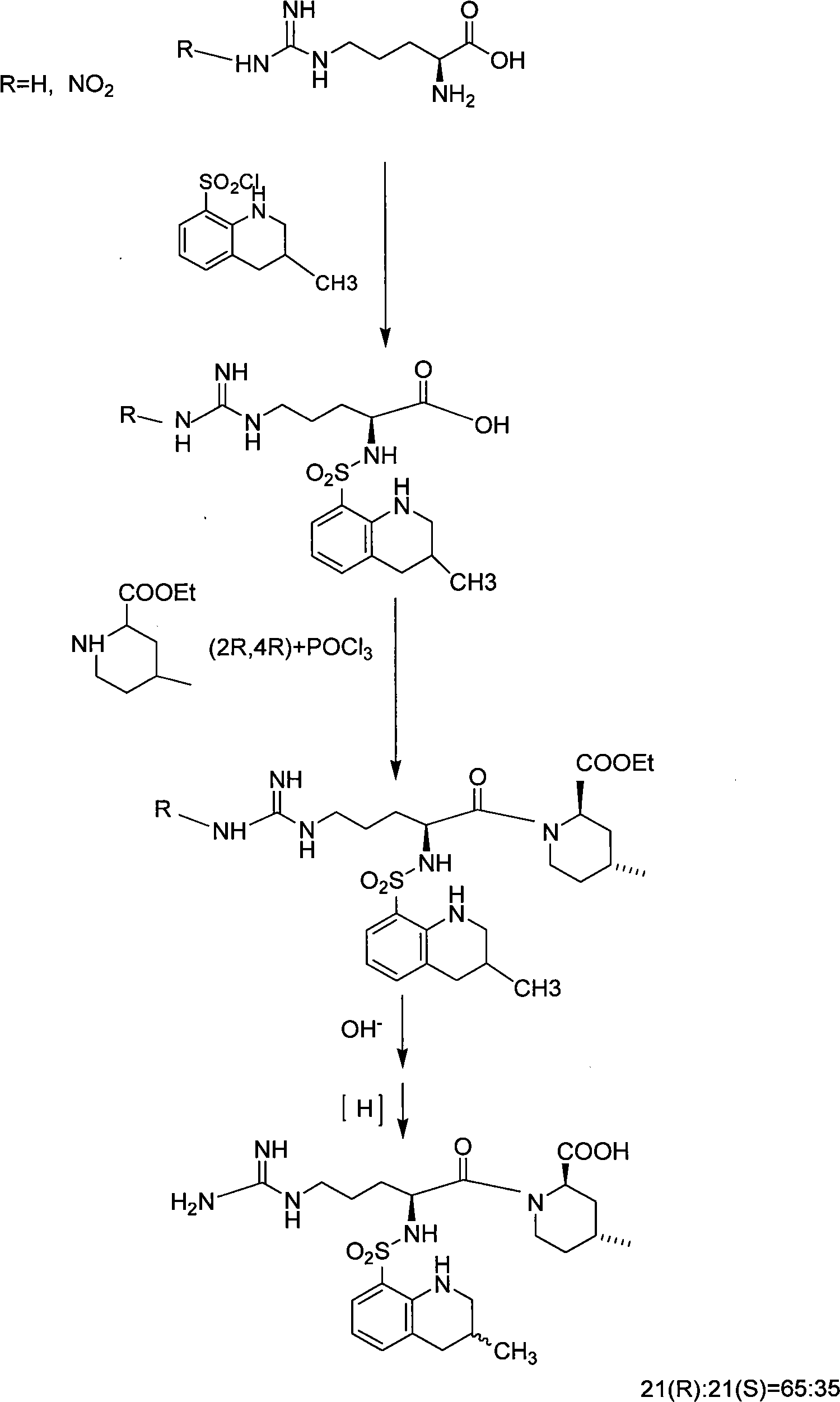
Solid phase method for synchronizing Argatroban
A technology of argatroban and solid-phase method, applied in the field of solid-phase synthesis of argatroban, which can solve the problem of low yield in liquid phase reaction, increased post-reaction treatment work, and difficult purification and separation of intermediates and target products And other problems, to achieve considerable economic and practical value, low cost, wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: Preparation of (2R, 4R)-N-Fmoc-4-methyl-2-piperidinecarboxylic acid-chloro resin or hydroxyl resin
[0042] Add 200.0 g of chlorine resin or hydroxyl resin (substitution degree: 0.5 mmol / g) into a 1000 ml round bottom flask, add DMF 600 ml, and stir vigorously. In an ice-water bath, 36g (2R, 4R)-N-Fmoc-4-methyl-2-piperidinecarboxylic acid and equal molar ratios of DIC, HOBt, and DIPEA were added to the above round-bottomed flask filled with resin to react for 2 hours. After the reaction is completed, filter, wash 3 times with DMF and 3 times with DCM, then shrink with methanol for 30 minutes to obtain (2R, 4R)-N-Fmoc-4-methyl-2-piperidinecarboxylic acid-chloro resin or hydroxyl resin, and detect The degree of substitution is 0.48mmol / g, and the yield is 96%.
Embodiment 2
[0043] Embodiment 2: Preparation of (2R, 4R)-4-methyl-2-piperidinecarboxylic acid-chloro resin or hydroxyl resin
[0044]Add (2R,4R)-N-Fmoc-4-methyl-2-piperidinecarboxylic acid and chlorine resin prepared above into a 1000ml round bottom flask, add 600ml of 20% DBLK and stir vigorously for 30min. After the reaction was completed, filter, wash with DMF for 3 times, and DCM for 3 times, and set aside for use.
Embodiment 3
[0045] Embodiment 3: Preparation of Fmoc-Arg (X)-(2R, 4R)-4-methyl-2-piperidinecarboxylic acid-chloro resin or hydroxyl resin
[0046] Put the (2R,4R)-4-methyl-2-piperidinecarboxylic acid-chloro resin or hydroxyl resin to be used into a 1000ml round bottom flask, add 700ml tetrahydrofuran and stir vigorously. In an ice-water bath, 65g of Fmoc-Arg(X)-OH and equal molar ratios of DIC, HOBt, and DIPEA were added to the above round-bottomed flask filled with resin and reacted for 2 hours. After the reaction is completed, filter, wash 3 times with DMF, wash 3 times with DCM, and shrink with methanol for 30 minutes to obtain Fmoc-Arg(X)-(2R,4R)-4-methyl-2-piperidinecarboxylic acid-chloro resin or hydroxyl Resin, the detected substitution degree is 0.45mmol / g, and the yield is 94%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com