Method for preparing Leuprorelin by combination of solid phase method and liquid phase method
A technology of leuprolide and solid-phase method is applied in the field of preparing leuprolide by combining solid-phase method and liquid-phase method, which can solve problems such as low application value, complicated operation and production cycle, and achieves low cost and considerable Economical and practical value, less environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
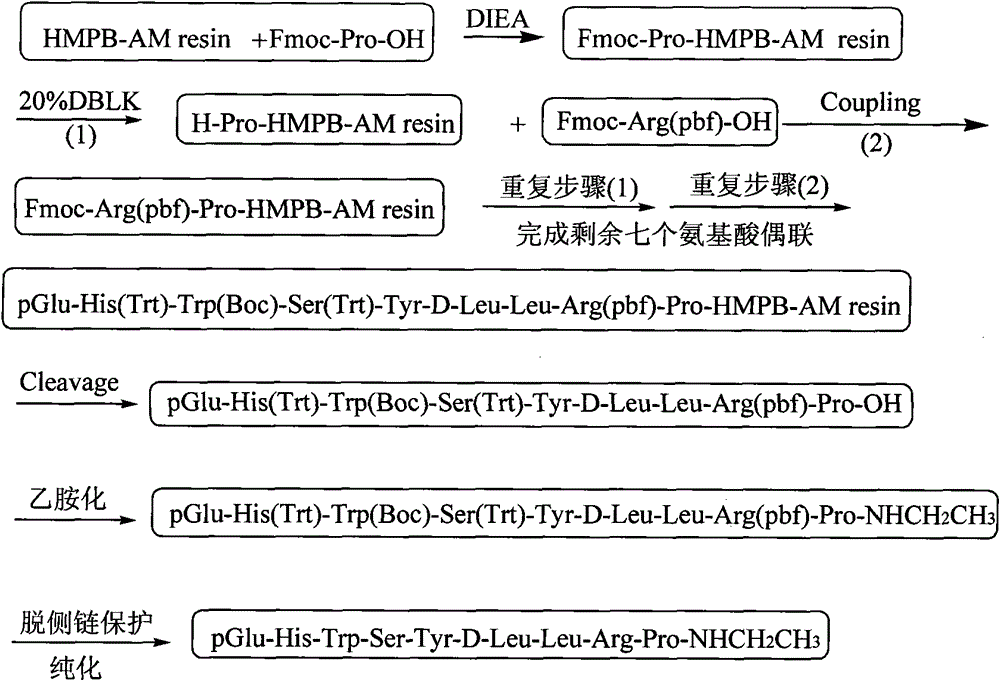
[0044] The preparation of embodiment 1 Fmoc-Pro-HMPB-AM resin
[0045] Using HMPB-AM resin with a substitution degree of 0.2mmol / g for the reaction, the obtained Fmoc-Pro-HMPB-AM resin has a detection substitution degree of 0.15mmol / g, and the production process of the same molar amount consumes a large amount of resin and a large amount of solvent , uneconomical and not conducive to environmental protection, should not be used; the HMPB-AM resin with a substitution degree of 1.2mmol / g is used for the reaction, and the detection substitution degree of the obtained Fmoc-Pro-HMPB-AM resin can reach 0.87mmol / g, although the same The molar production process consumes a small amount of resin, but the purity of the obtained crude peptide is reduced by 30%, so that the downstream purification yield is reduced by 60-70%.
[0046] The more preferred scheme of this embodiment is to add 111.0 g of HMPB-AM resin, with a substitution degree of 0.9 mmol / g, into the solid-phase reaction colu...
Embodiment 2
[0047] Example 2 Preparation of Leuprolide Precursor Peptide-HMPB-AM Resin with Fully Protected Side Chains
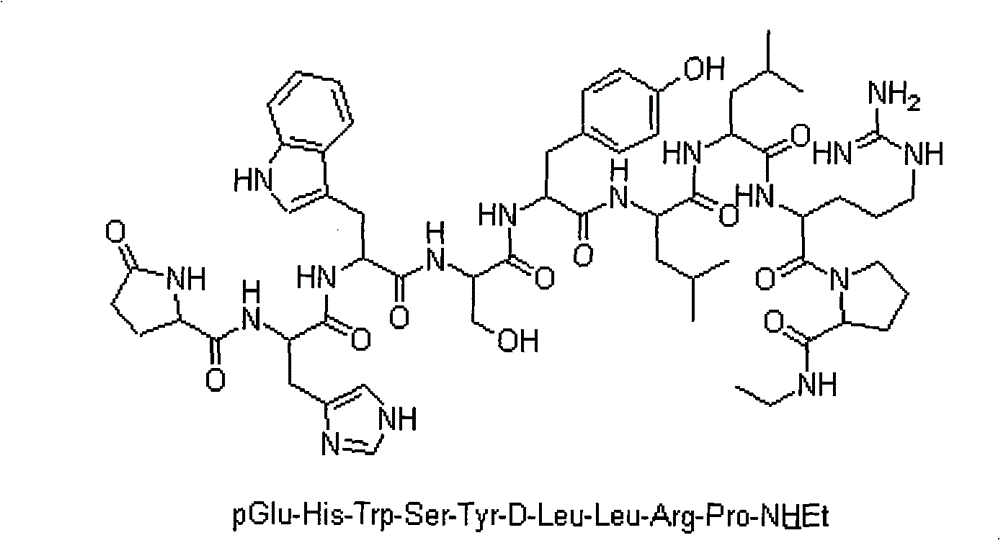
[0048] Weigh 100 g of Fmoc-Pro-HMPB-AM resin (0.6 mmol / g, 60 mmol) into the reactor, wash once with DMF, and swell with DCM for 0.5 hours. After swelling, 20% DBLK was used to remove Fmoc protection, and then washed 4 times with DMF and 2 times with DCM. 77.76g Fmoc-Arg(pbf)-OH (120mmol), 24.315g HOBt (180mmol), 37.8g DCC were dissolved in DCM (a small amount of DMF can be added to aid dissolution), added to a solid phase reactor, and reacted at room temperature for 2h (reaction The end point was determined by the ninhydrin method). Repeat the above steps to complete Fmoc-Leu-OH, Fmoc-D-Leu-OH, Fmoc-Tyr-OH, Fmoc-Ser(Trt)-OH, Fmoc-Trp(Boc)-OH, Fmoc-His(Trt) )-OH and the linkage of remaining amino acids such as pGlu. After the pGlu coupling was completed, the resin was washed 3 times with DMF and 3 times with DCM, then shrunk with methanol, and dried overnight in a va...
Embodiment 3
[0049] Example 3 Preparation of Leuprolide Precursor Peptide-HMPB-AM Resin with Fully Protected Side Chains
[0050] Weigh 1111.1 g of Fmoc-Pro-HMPB-AM resin (0.45 mmol / g, 500 mmol) into the reactor, wash once with DMF, and swell with DCM for 0.5 hours. After swelling, 20% DBLK was used to remove Fmoc protection, and then washed 4 times with DMF and 2 times with DCM. With 648.8g Fmoc-Arg (pbf)-OH (1000mmol), 202.7g HOBt (1500mmol), 568.8g HBTU (1500mmol) are dissolved in DCM (can add a small amount of DMF to aid dissolution), add in the solid phase reactor, then 523.0ml (3000mmol) DIPEA was introduced into the reaction column, and reacted at room temperature for 2h (the end point of the reaction was determined by the ninhydrin method). Repeat the above steps to complete Fmoc-Leu-OH, Fmoc-D-Leu-OH, Fmoc-Tyr-OH, Fmoc-Ser(Trt)-OH, Fmoc-Trp(Boc)-OH, Fmoc-His(Trt) )-OH and the linkage of remaining amino acids such as pGlu. After the pGlu coupling was completed, the resin was was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com