Method for preparing cyclodipeptide cyclo(L-Asp-L-Pro)
A technology of aspartame and cyclic dipeptide, applied in the direction of organic chemistry, etc., can solve the problem of no synthesis method of aspartame cyclic dipeptide, achieve considerable economic and practical value, simple reaction operation, and easy post-processing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1, L-L type aspartame dipeptide
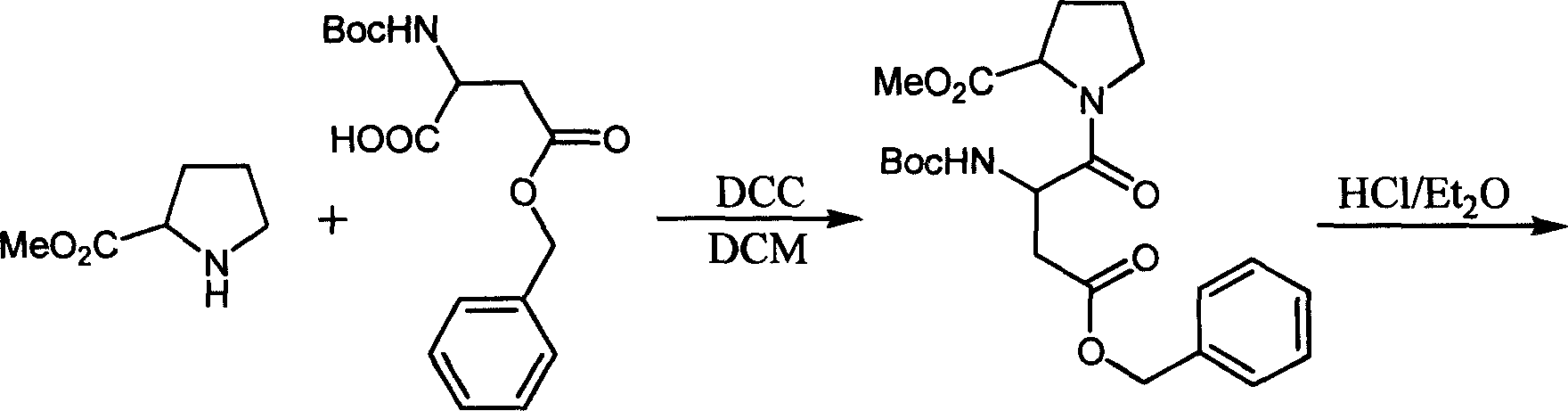
[0032] 1. Add L-Pro-OMe (450mg, 3.4mmol), Boc-L-Asp(OBzl)-OH (904mg, 2.8mmol), DCC (803mg, 3.9mmol) into a 100ml round bottom flask, mix all , add DCM (15ml), stir at room temperature for 20min, TLC traces the completion of the reaction, evaporate the solvent on the rotary evaporator, dissolve with ethyl acetate (15ml), filter, spin dry to obtain a yellow gum, and then use ethyl acetate The ester (15ml) was dissolved, and there was a small amount of insoluble matter, filtered, and finally washed with 0.1N HCl, saturated NaHCO 3 Wash with saturated NaCl for 2-3 times to obtain 1.20 g of oil, yield: 99%. Electrospray mass spectrometry (ESI-MS) and nuclear magnetic resonance (1HNMR 13 C NMR) measurement, proves that the obtained product is correct.
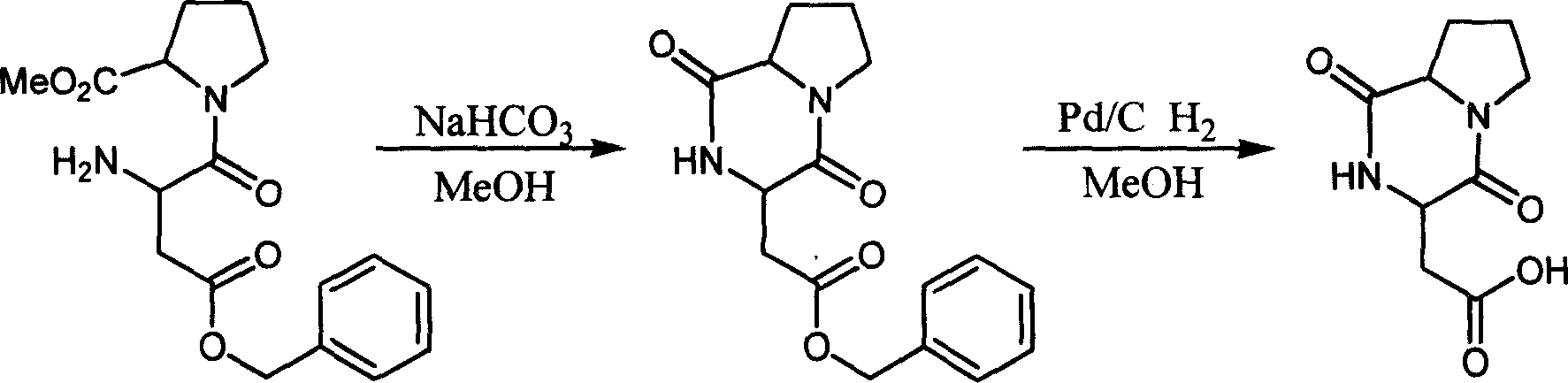
[0033] 2. Add 200 mg (0.46 mmol) of N-tert-butoxycarbonyl-β-benzyl aspartame in a 50 ml round-bottomed flask, add 10 mg of Pd / C, and take out the air in the three-necked flask. ...
Embodiment 2
[0036] Embodiment 2, L-L type aspartame dipeptide
[0037] 1. Add L-Pro-OMe (450mg, 3.4mmol), Boc-L-Asp(OBzl)-OH (904mg, 2.8mmol), DCC (803mg, 3.9mmol) into a 100ml round bottom flask, mix all , add DCM (15ml), stir at room temperature for 20min, TLC traces the completion of the reaction, evaporate the solvent on the rotary evaporator, dissolve with ethyl acetate (15ml), filter, spin dry to obtain a yellow gum, and then use ethyl acetate The ester (15ml) was dissolved, and there was a small amount of insoluble matter, filtered, and finally washed with 0.1N HCl, saturated NaHCO 3 Wash with saturated NaCl for 2-3 times to obtain 1.20 g of oil, yield: 99%. electrospray mass spectrometry (ESI-MS) and nuclear magnetic resonance ( 1 HNMR 13 C NMR) measurement, proves that the obtained product is correct.
[0038] 2. Add 200 mg (0.46 mmol) of N-tert-butoxycarbonyl-β-benzyl aspartame in a 50 ml round-bottomed flask, add 2 ml of saturated HCI ether solution, then add 3 ml of ether,...
Embodiment 3
[0041] Embodiment 3, D-L type aspartame dipeptide
[0042] 1. Add L-Pro-OMe (450mg, 3.4mmol), Boc-D-Asp(OBzl)-OH (904mg, 2.8mmol), DCC (803mg, 3.9mmol) into a 100ml round bottom flask, and mix them all , add DCM (15ml), stir at room temperature for 20min, TLC traces the completion of the reaction, evaporate the solvent on the rotary evaporator, dissolve with ethyl acetate (15ml), filter, spin dry to obtain a yellow gum, and then use ethyl acetate The ester (15ml) was dissolved, and there was a small amount of insoluble matter, filtered, and finally washed with 0.1N HCl, saturated NaHCO 3 Wash with saturated NaCl for 2-3 times to obtain 1.19 g of oil, yield: 98%. Electrospray mass spectrometry (ESI-MS) and nuclear magnetic resonance (1HNMR 13 C NMR) measurement, proves that the obtained product is correct.
[0043] 2. Add 200 mg (0.46 mmol) of N-tert-butoxycarbonyl-β-benzyl aspartame in a 50 ml round-bottomed flask, add 2 ml of saturated HCl in ether, then add 3 ml of ether,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com