Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
37 results about "Systemic reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methods and systems to identify operational reaction pathways
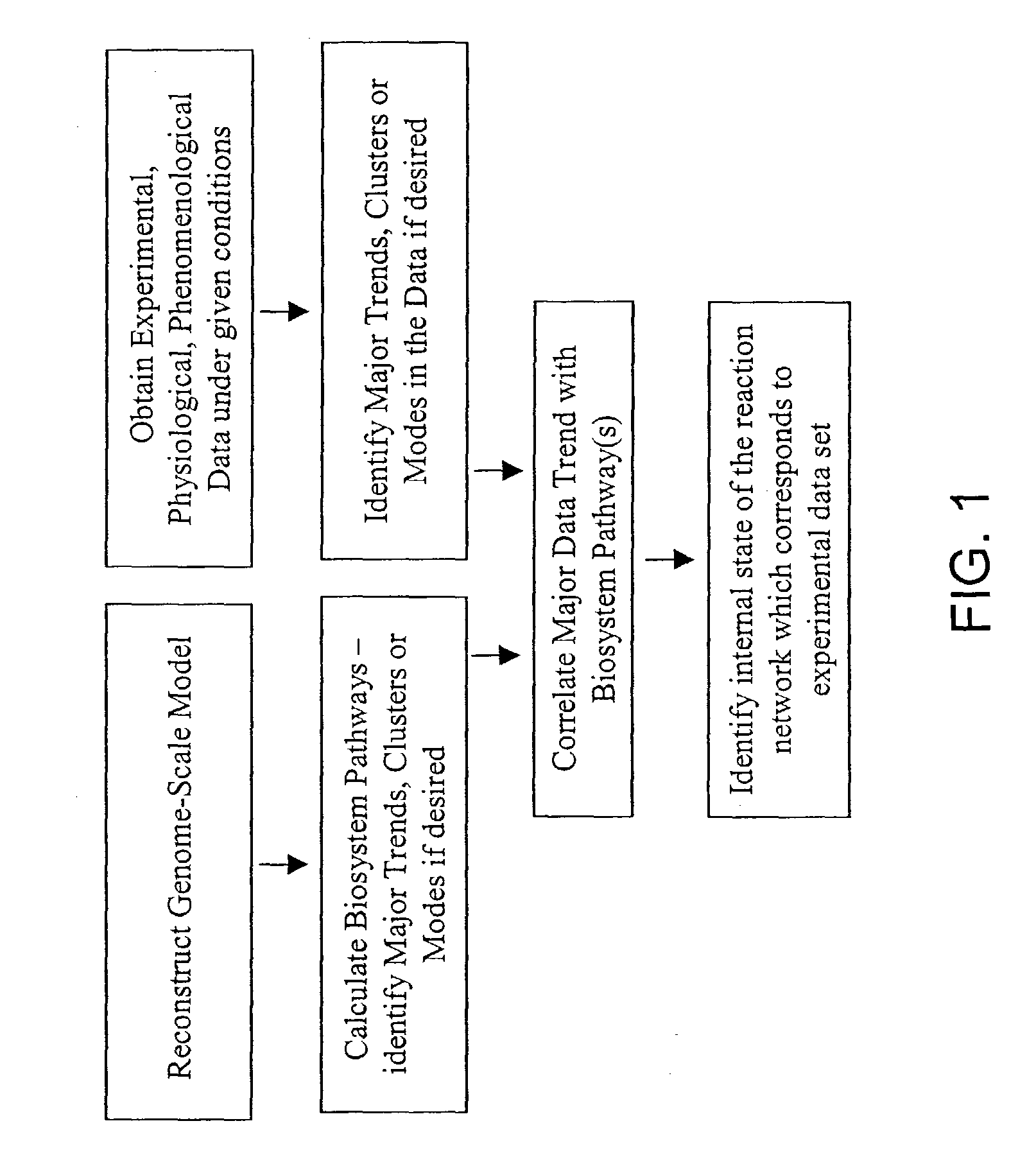
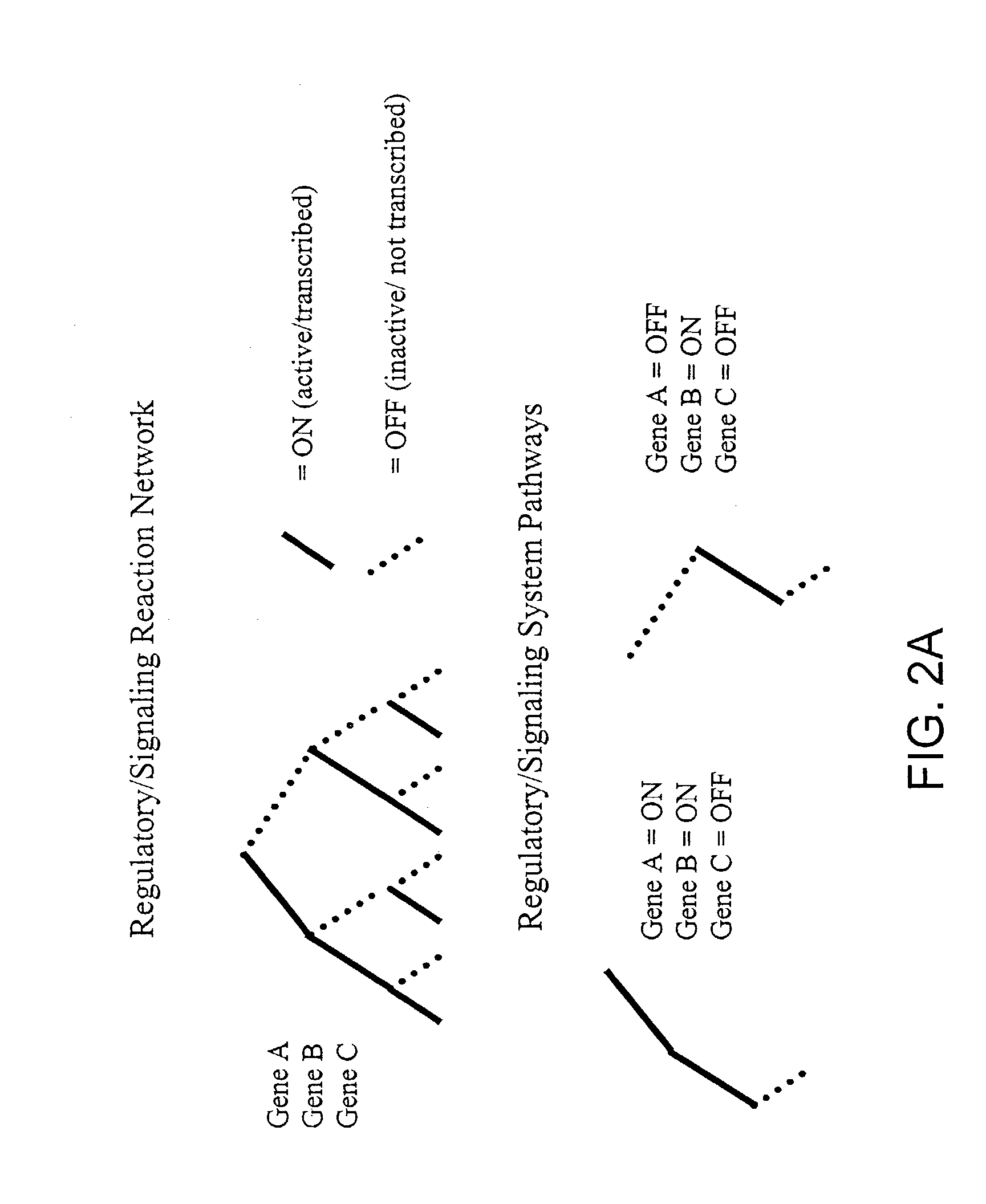
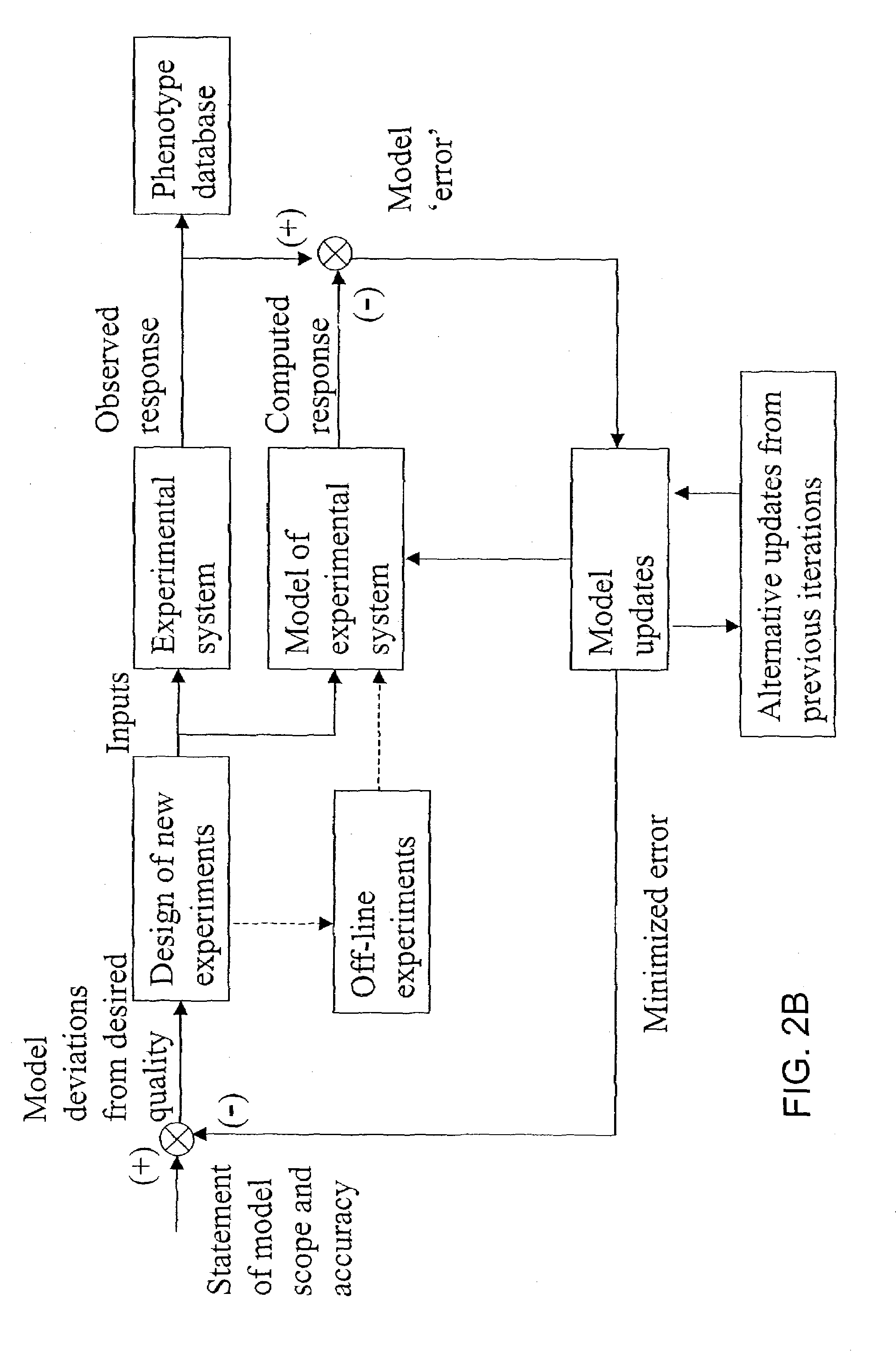
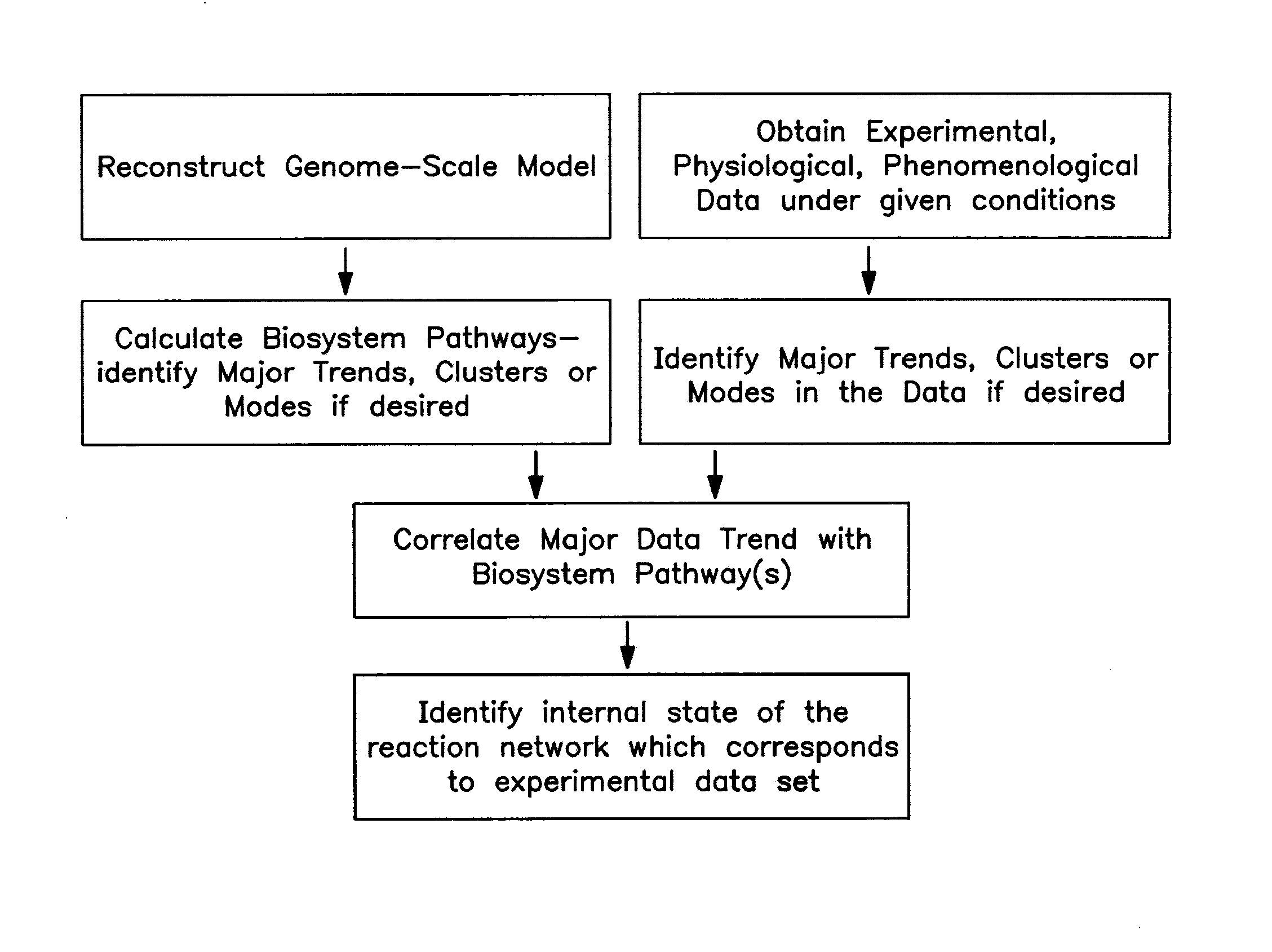
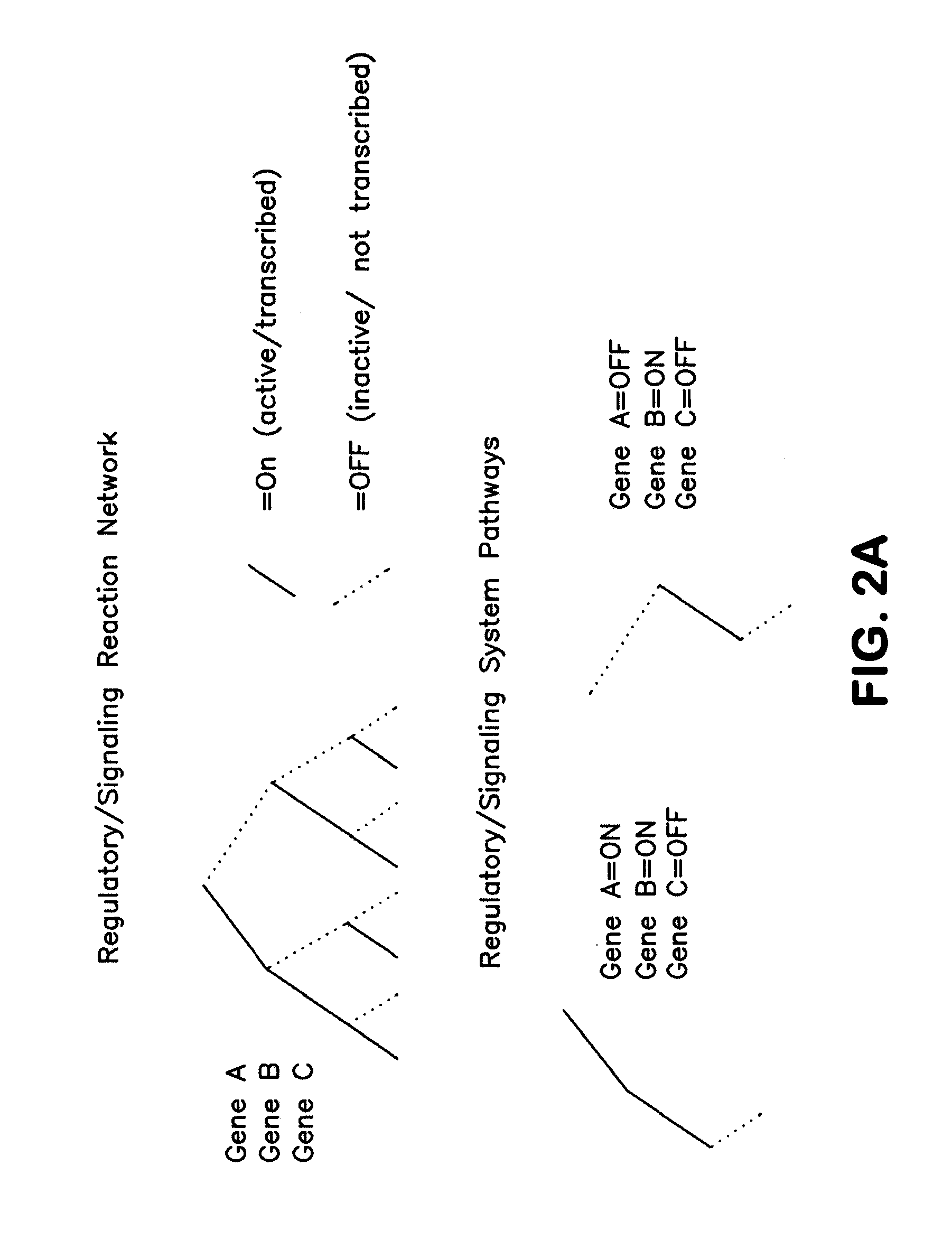
The present invention provides a method for identifying an operational reaction pathway of a biosystem. The method includes (a) providing a set of systemic reaction pathways through a reaction network representing said biosystem; (b) providing a set of phenomenological reaction pathways of said biosystem, and (c) comparing said set of systemic reaction pathways with said set of phenomenological reaction pathways, wherein a pathway common to said sets is an perational reaction pathway of said biosystem. Also described is a method of refining a biosystem reaction network; a method of reconciling biosystem data sets; a method of determining the effect of a genetic polymorphism on whole cell function; and a method of diagnosing a genetic polymorphism-mediated pathology.
Owner:RGT UNIV OF CALIFORNIA
Solid tumor treating medicine composition
The present invention is solid tumor treating medicine composition and belongs to the field of medicine technology. The medicine composition contains melphalan in effective anticancer amount and medicinal supplementary material. The medicinal supplementary material is mainly biocompatible, degradable and absorbable polymer, and during the degradation and absorption of the polymer, melphalan is released slowly to local tumor part to lower the systemic toxic reaction and to maintain local medicine concentration. The present invention has enhanced treating effect. The solid tumor includes cerebral tumor, liver cancer, lung cancer, esophagus cancer, gastric cancer, etc.
Owner:DASEN BIOLOGICAL PHARMA CO LTD
Methods of disease detection and characterization using computational analysis of urine raman spectra
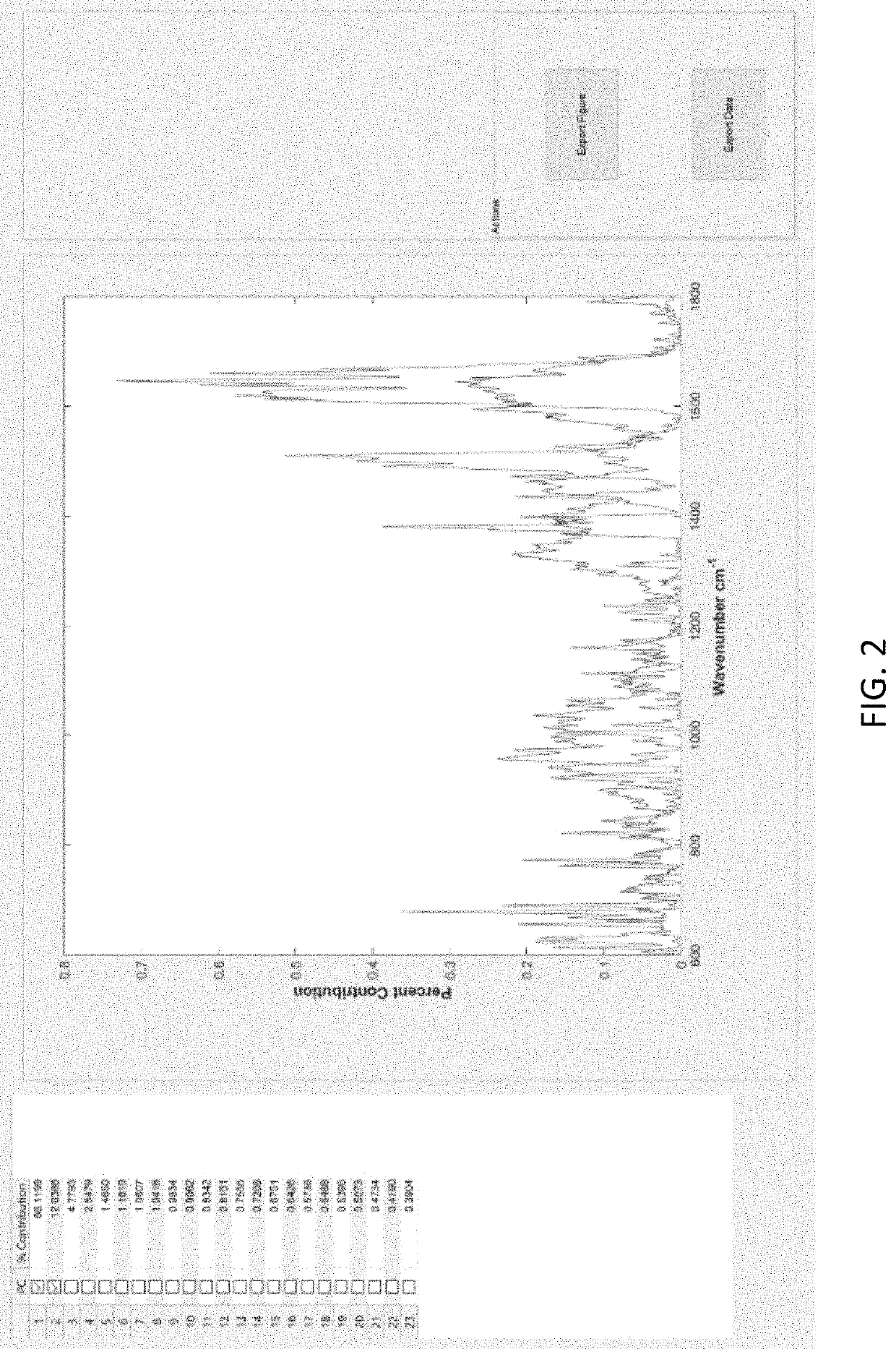
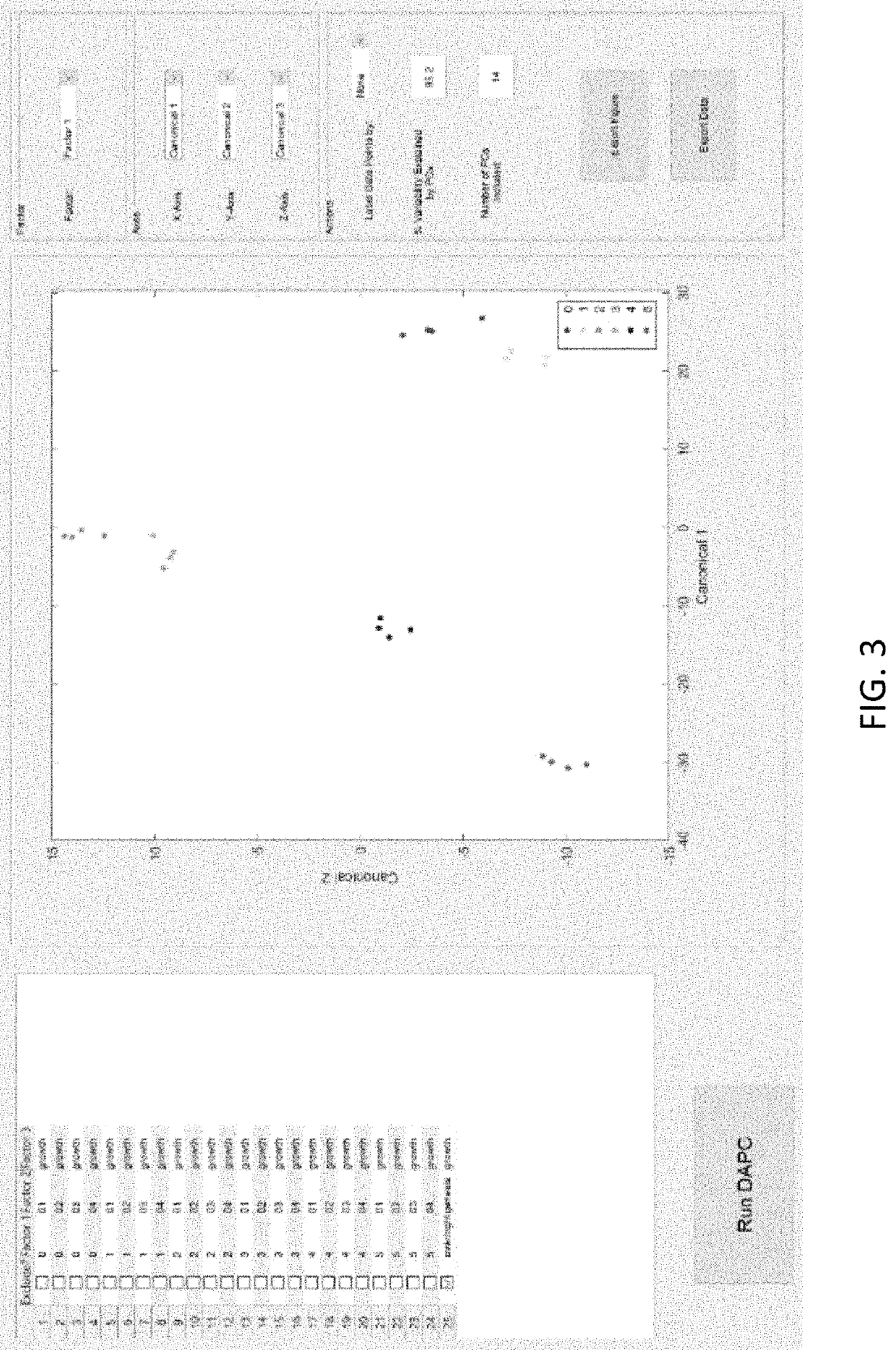
Disease detection and characterization using computational analysis of Raman spectra is used to detect disease-specific multi-molecular patterns “spectral fingerprint” associated with specific diseases, cellular physiologic derangements, or altered metabolism from systemic reactions to disease. Comparison of the Raman spectral fingerprint of urine from subjects with specific diseases and those not (healthy persons) provides the means to identify key disease-associated changes in urine molecular composition. Methods include applying baseline correction to spectra of a desired wavenumber range e.g., with the Goldindec algorithm, or with ISREA and StaBAL; vector or specific band normalization; and one or more of principal component analysis (PCA); discriminant analysis of principal components (DAPC); principal least squares (PLS) regression, machine learning with neural networks (NN); identification of wavenumber loadings; calculation of total canonical distance (TCD); total spectral distance (TSD), total principal component distance (TPD); ANOVA; pairwise comparisons; and performing leave-one-out or multi-fold cross-validation analysis of chemometric models (DAPC, PLS, NN) to report predictive capabilities in terms of accuracy, sensitivity (true-positives), and specificity (true-negatives), positive predictive value (PPV) and negative predictive value (NPV).
Owner:VIRGINIA TECH INTPROP INC
Medicine composition containing tyrosine kinase restraining agent
InactiveCN101081207APharmaceutical delivery mechanismPharmaceutical non-active ingredientsDocetaxelWhole body
The anticancer medicine composition containing tyrosine kinase inhibitor is slow released injection and slow released implant. The effective anticancer components include tyrosine kinase inhibitor selected from Erbitux, Iressa, Tarceva, Sunitinib, Trastuzumab, etc, and / or composition selected from Docetaxel, deacetyl taxol, taxol, etc. The slow releasing supplementary material is selected from p(LAEG-EOP), p(DAPG-EOP), etc. The released injection and slow released implant may be injected or set in tumor for slow releasing to maintain effective medicine concentration for over 50 days, and has obviously lowered systemic reaction on the medicine and capacity of enhancing the chemotherapeutic and radiotherapeutic effect.
Owner:JINAN KANGQUAN PHARMA TECH
Methods and Systems to Identify Operational Reaction Pathways
The present invention provides a method for identifying an operational reaction pathway of a biosystem. The method includes (a) providing a set of systemic reaction pathways through a reaction network representing said biosystem; (b) providing a set of phenomenological reaction pathways of said biosystem, and (c) comparing said set of systemic reaction pathways with said set of phenomenological reaction pathways, wherein a pathway common to said sets is an operational reaction pathway of said biosystem. Also described is a method of refining a biosystem reaction network; a method of reconciling biosystem data sets; a method of determining the effect of a genetic polymorphism on whole cell function; and a method of diagnosing a genetic polymorphism-mediated pathology.
Owner:RGT UNIV OF CALIFORNIA
Temperature controlled sustained-release injection comprising alkyl agent and method for preparing the same
InactiveCN101273962APharmaceutical delivery mechanismPharmaceutical non-active ingredientsWater insolubleWhole body
The invention relates to a temperature-controlled sustained-release injection containing an alkylating agent and a preparation method thereof, the temperature-controlled sustained-release injection comprises effective anti-cancer amount of the alkylating agent, an amphiphilic block copolymer, a solvent and a certain amount of drug release regulator, wherein, the mixture of the amphiphilic block copolymer and a solvent without organic solvent has the temperature-sensitive gelatinization feature, which is flowable liquid in the environment that is lower than the body temperature and can be automatically converted to the water-insoluble gel that can not flow and be biodegradable for absorption in an endotherm, and the water-insoluble gel can allow the contained angiogenesis inhibitor to have the local sustained release in a tumor and maintain the effective drug concentration for a plurality of weeks to a plurality of months. The sustained-release gel injection can be injected in the tumor or the tumor periphery or be arranged in the postoperative tumor cavity, thus significantly reducing the systemic reaction of the drug and being used for the treatment of the tumors in different stages. The alkylating agent is selected from cyclophosphamide, melphalan, leukeran, 4H-cyclophosphamide peroxide, norcantharidin, mannosulfan, treosulfan, ritrosulfan, ethoglucid, pipobroman, piposulfan, pumitepa, uredepa, azatepa and so on.
Owner:SHANDONG LANJIN PHARMA +1
Temperature controlled sustained-release injection containing anti-cancer medicine
InactiveCN101273965APharmaceutical delivery mechanismPharmaceutical non-active ingredientsTherapeutic effectVinorelbine
The invention relates to a temperature-controlled sustained-release injection containing an anti-cancer drug, which consists of the anti-cancer drug and an amphiphilic block copolymer hydrogel and has the temperature-sensitive gelatinization feature, the temperature-controlled sustained-release injection is flowable liquid in the environment that is lower than the body temperature and can be automatically converted to the water-insoluble gel that can not flow and be biodegradable for absorption in an endotherm, thus allowing the drug to have the local sustained release in a tumor and maintain the effective drug concentration for a plurality of weeks to a plurality of months; the temperature-controlled sustained-release injection can be injected in the tumor or the tumor periphery or be arranged in the postoperative tumor cavity, thus significantly reducing the systemic reaction of the drug, strengthening the treatment effects of chemotherapy, radiotherapy and other non-surgical therapies, and being used for the treatment of the tumors in different stages. The anti-cancer drug can be vincristine, vinorelbine, navelbine, vindesine, vinleurosine, vinrosidine, cephalotaxine, bleomycin, daunomycin, aclarubicin, epirubicin, idarubicin, pirarubicin, valrubicin, mitomycin C, actinomycin D, losoxantrone, mitoxantrone, mitozolomide, temozolomide and so on.
Owner:SHANDONG LANJIN PHARMA +1
Application of human RTN4B protein in preparation of medicine for healing wound
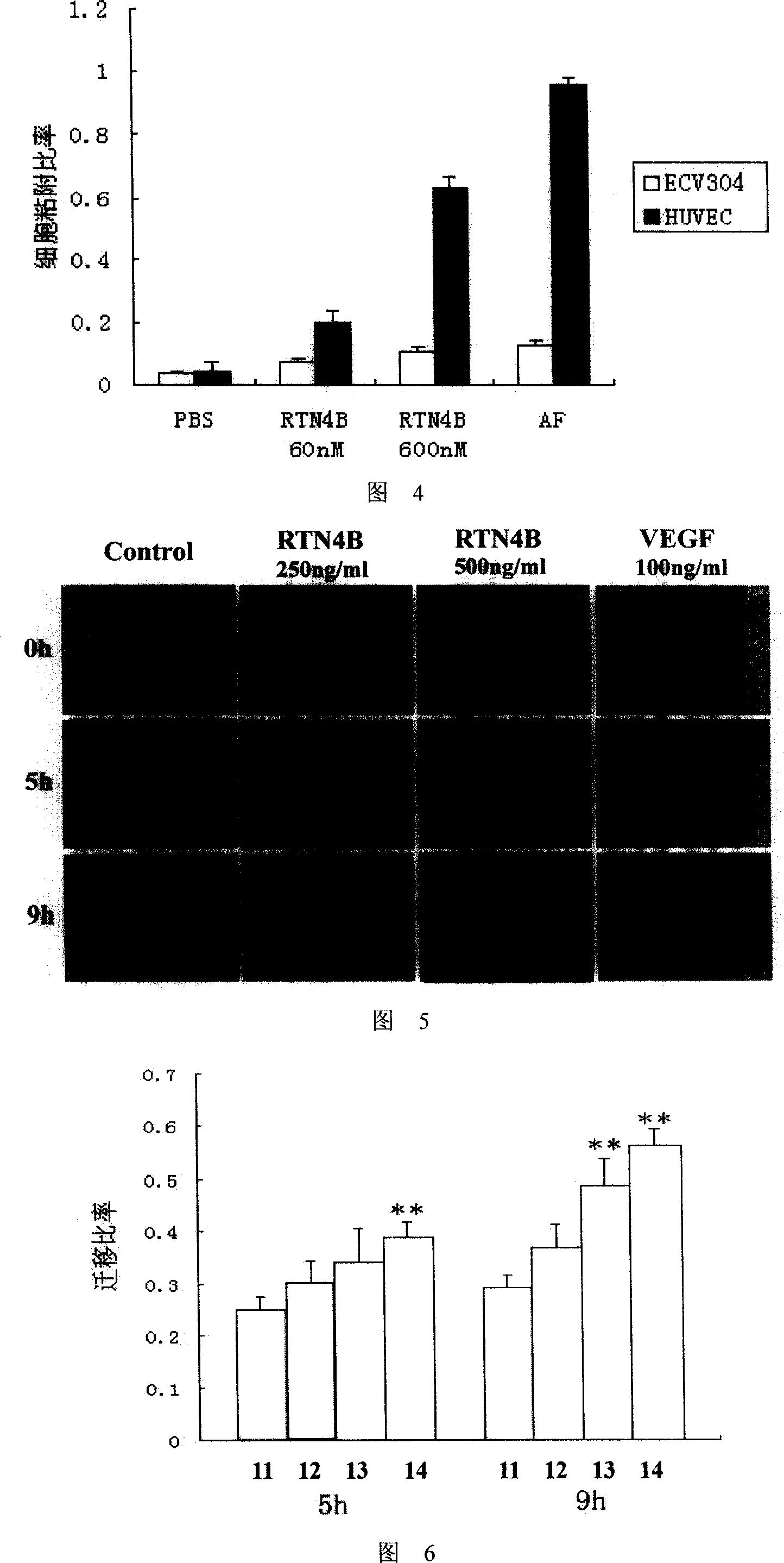
This invention relates to the field of gene engineering and medicament, specifically relates to the application of RTN 4B in preparing wound healing drugs. Healing of wound is a very complicated process. Wound not only changes part of organism, but also initiate systemic reaction to different extent, meanwhile, wound part will bring pain to patients. The inventive RTN 4B can promote angiogenesis, enhance adhesion and migration of blood vessel cell; and can be used for preparing medicament for promoting healing of wound to shorten period of treatment and relieve pain.
Owner:FUDAN UNIV
Adjuvanted vaccine which is substantially free of non-host albumin
InactiveUS20050232948A1Improve scalabilitySufficient protectionBiocideSsRNA viruses positive-senseAdjuvantSystemic reaction
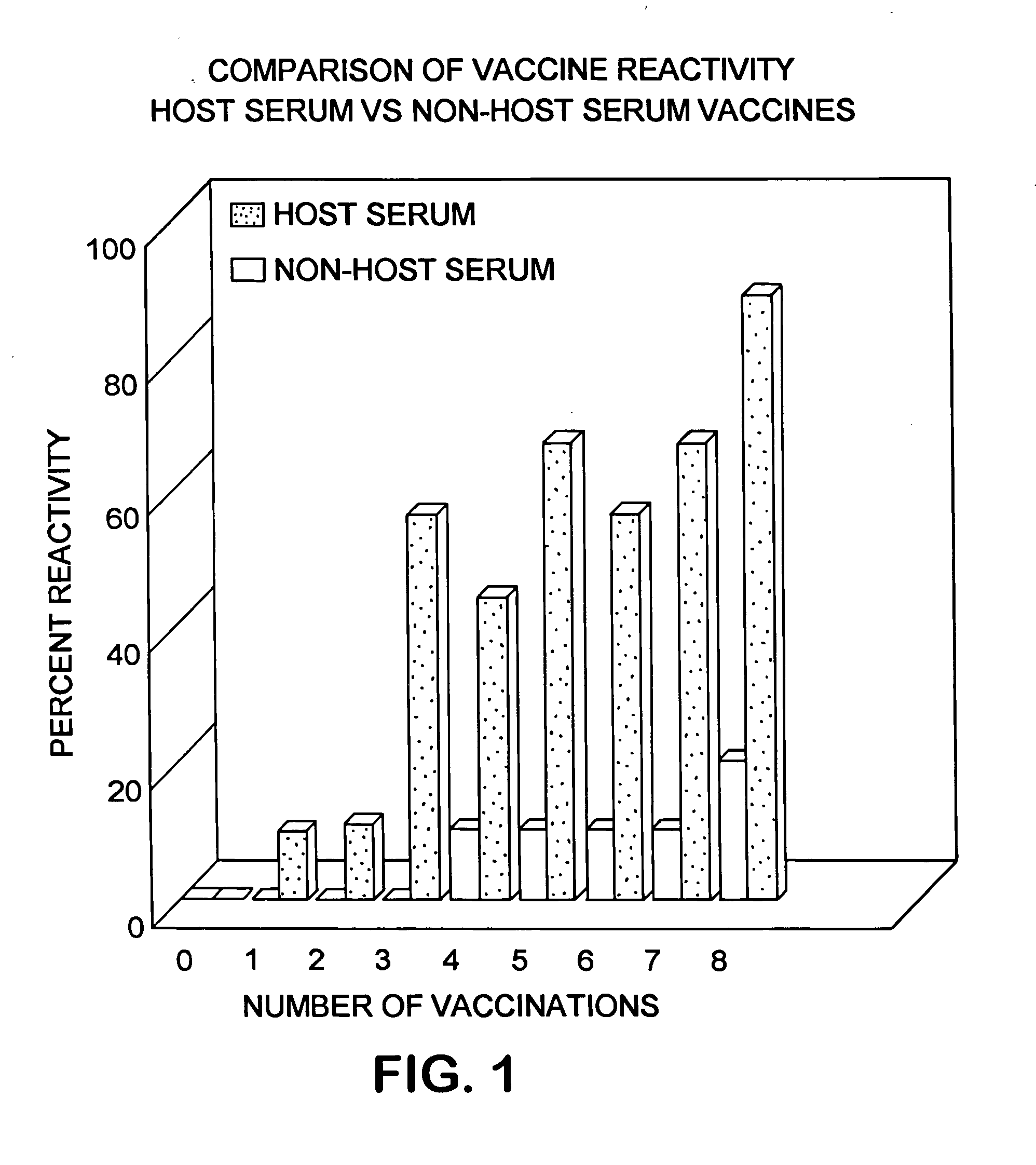
Disclosed herein is a serum-based adjuvanted vaccine which is substantially free of non-host albumin and the use thereof in reducing or preventing post-vaccination systemic reactions.
Owner:HENNESSY KRISTINA J +3
Sustained-release injection containing nitrosourea drugs
The invention provides a sustained-release injection containing nitrosourea drug (galamustine), which contains sustained-release microspheres and solvents. The sustained-release microspheres each comprise an anticancer-active component selected from nitrosourea drugs (such as nimustine and carmustine) and / or topoisomerase inhibitors, and a sustained-release agent. The solvents are common solvents or special solvents containing suspending agent. The viscosity of the suspending agent ranges from 100cp to 3000cp (at a temperature ranging from 20 DEG C to 30 DEG C). The suspending agent is selected from sodium carboxymethylcellulose and the like. The sustained-release agent is selected from p(LAEG-EOP) or p(DAPG-EOP) or other polyphosphate ester copolymers, or copolymer or blend of polyphosphate ester and PLA, polifeprosan, PLGA or poly(erucidic acid dipolymer-sebacic acid). The topoisomerase inhibitor is selected from camptothecin, hydroxycamptothecine, topotecan, lartotecan, irinotecan, etoposide or teniposide. The anticancer composition is also available in the dosage form of sustained-release implant, can retain the effective drug concentration for more than 60 days after intratumoral or local injection or implantation, can obviously reduce the systemic reaction to the drug, and can selectively enhance the curative effect of non-operative treatments such as radiotherapy and chemotherapy.
Owner:JINAN SHUAIHUA PHARMA TECH
Temperature controlled sustained-release injection containing steroids anti-cancer drugs
InactiveCN101273963APharmaceutical delivery mechanismPharmaceutical non-active ingredientsGoserelinTherapeutic effect
The invention relates to a temperature-controlled sustained-release injection containing a hormone anti-cancer drug, which comprises the anti-cancer drug, an amphiphilic block copolymer, a solvent and a certain amount of drug release regulator, wherein, the mixture of the amphiphilic block copolymer and a solvent without organic solvent has the temperature-sensitive gelatinization feature, which is flowable liquid in the environment that is lower than the body temperature and can be automatically converted to the water-insoluble gel that can not flow and be biodegradable for absorption in an endotherm, and the water-insoluble gel can allow the contained hormone anti-cancer drug to have the local sustained release in a tumor and maintain the effective drug concentration for a plurality of weeks to a plurality of months; the viscosity of the temperature-controlled sustained-release injection is 10cp to 3000cp ( at 5 DEG C to 30 DEG C ), and the gelatinization temperature is 35 DEG C to 37 DEG C. The sustained-release injection can be injected in the tumor or the tumor periphery or be arranged in the postoperative tumor cavity, thus significantly reducing the systemic reaction of the drug, selectively strengthening the treatment effects of chemotherapy, radiotherapy and other non-surgical therapies, and being used for the treatment of the tumors in different stages. The anti-cancer drug can be triptorelin, goserelin, leuprorelin, anastrozole, idoxifene, tamoxifen and other hormone anti-cancer drugs.
Owner:SHANDONG LANJIN PHARMA +1
Compositions containing virus-like particles as immunopotentiators administered through the mucosa
InactiveUS7374766B1Adjuvant effectViral antigen ingredientsSnake antigen ingredientsWhole bodyPharmaceutical industry
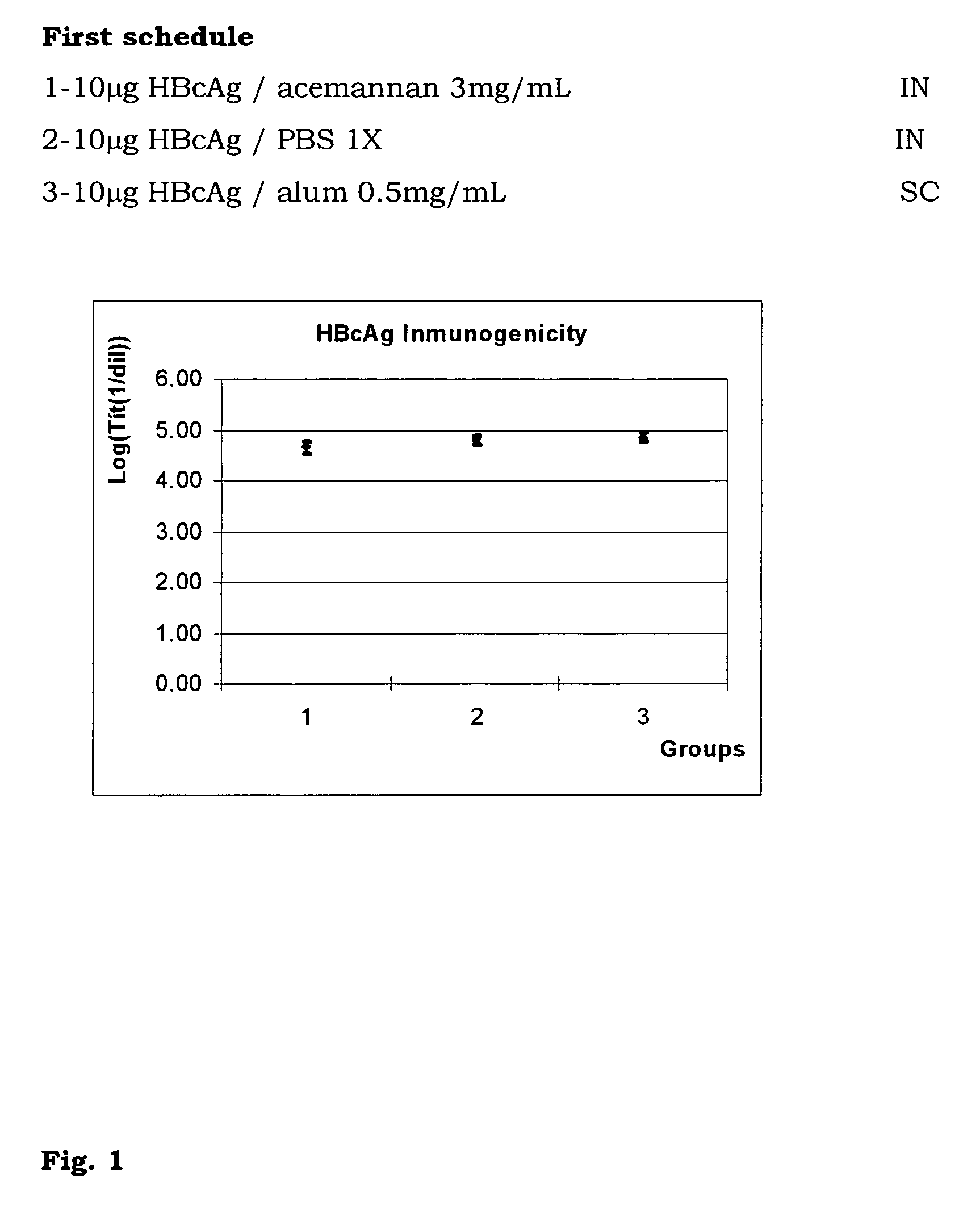
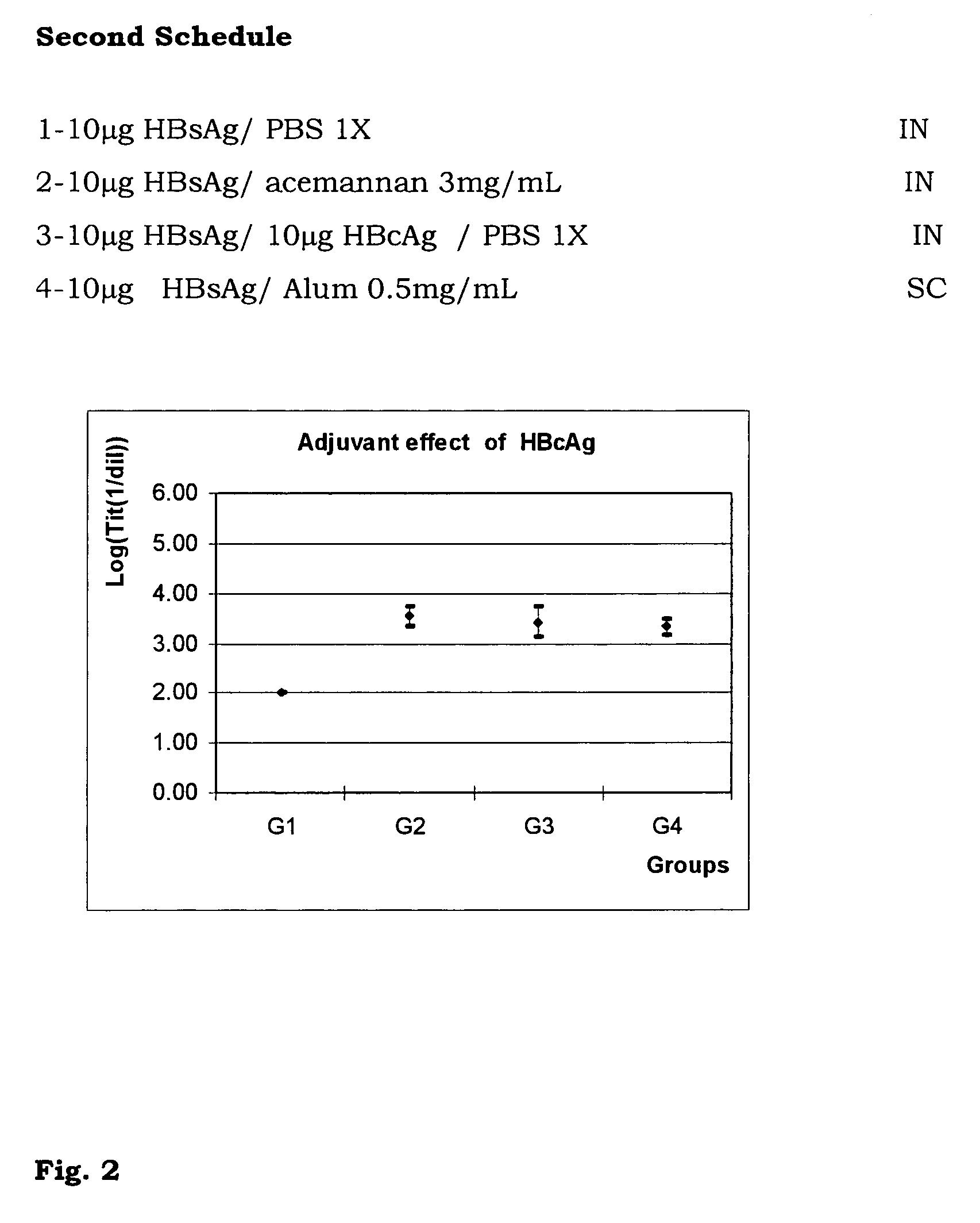
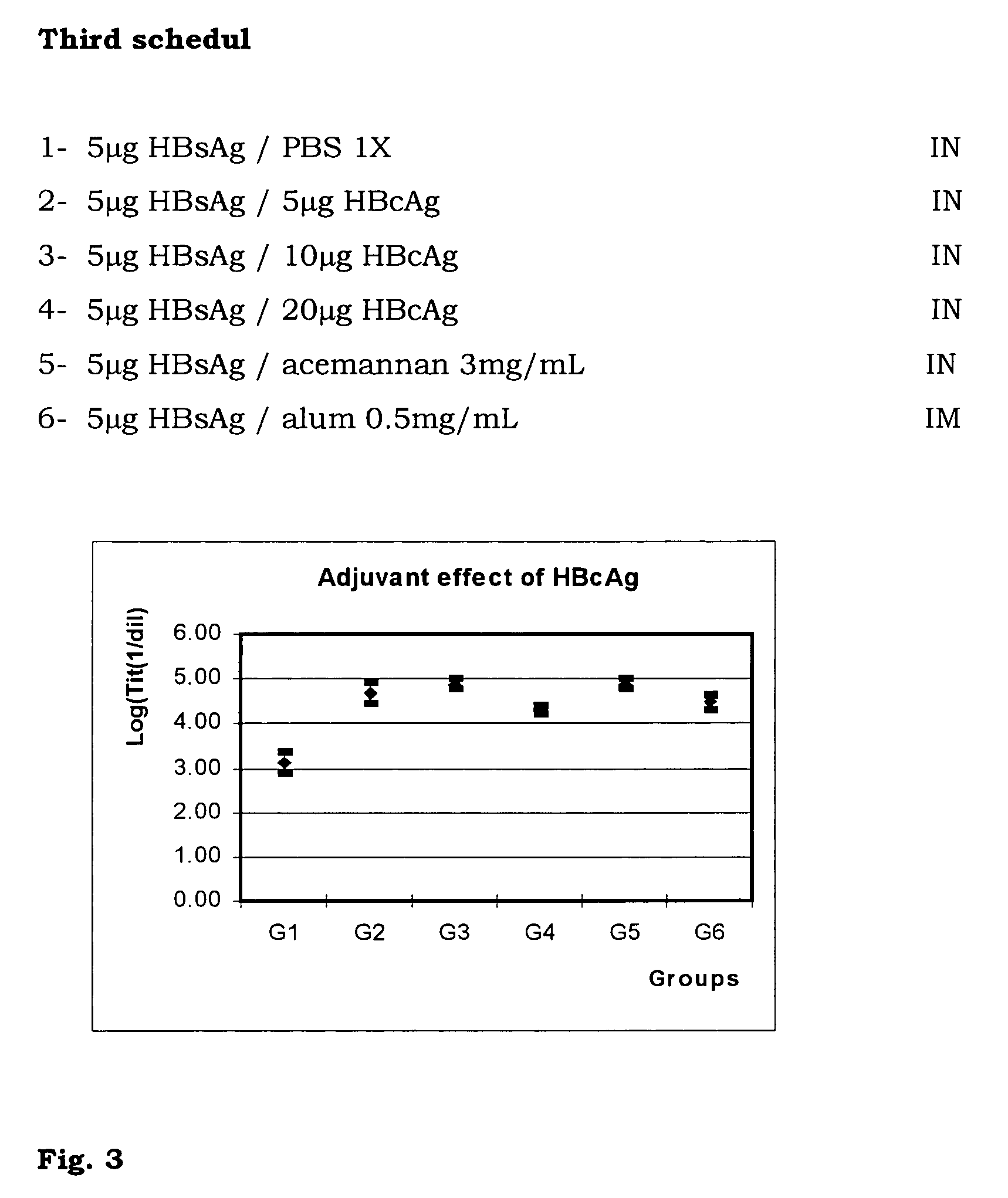
The present invention is related to the branch of medicine, particularly to the new formulations of vaccine antigens.The technical objective pursued with the present invention is, precisely, the development of formulations that are able to enhance the immune response to mucosally administered antigens, minimising the number of compounds in the formulation and generating strong mucosal and systemic responses through a synergic interaction between the antigens in the formulation.These formulations enable: a) to broaden the spectrum of the anti-hepatitis B immune response, containing as main compounds HBsAg and HBcAg, b) to enhance the response against HBsAg with a viral nucleocapsid c) to generate combined vaccines through the mucosal route with HBsAg as a central antigen. Stabilizers and preservatives can be introduced.The formulations of this invention can be applied in the pharmaceutical industry as human or veterinary vaccine formulations.
Owner:CENT DE ING GENETICA & BIOTECNOLOGIA
Anticancer composition loaded with anti-metabolism medicine fluorouracil and synergist thereof
InactiveCN101390829AOrganic active ingredientsPharmaceutical delivery mechanismAdditive ingredientSuspending Agents
Disclosed is an anticancer, which contains antimetabolics fluorouracil and the synergist thereof, and is a sustained-release injection. The anti-cancer combination is composed of sustained-release microspheres and solvent; wherein, the sustained-release microsphere contains effective anticancer ingredients and auxiliary material; the solvent is common solvent or special solvent which contains suspending agent. The viscosity of the suspending agent is 100cp-3000cp (20 DEG C-30 DEG C) and is selected from carboxymethyl cellulose; the antimetabolics is selected from methotrexate, fluorouracil, carmofur, tegafur, decitabine, capecitabine or gemcitabine; the synergist is tetrazine medicine or anticancer antibiotics; the auxiliary material is selected from p(LAEG-EOP), p(DAPG-EOP), or polyphosphate copolymer, or copolymer or mixture of polyphosphate, polylactic acid, polifeprosan, sebacic acid and PLGA; the anticancer combination also can be made into sustained-release implants which can be injected or implanted in tumor or the periphery of tumor with the effective medicine concentration maintained for more than 50 days; meanwhile, the systemic reaction of the medicine is obviously reduced; the treatment effects of the non-operative treatments such as chemotherapy or radiation treatment are selectively enhanced.
Owner:JINAN KANGQUAN PHARMA TECH
Anticancer composition containing tyrosine kinase restraining agent and taxane
InactiveCN101081209AOrganic active ingredientsPharmaceutical delivery mechanismWhole bodyMicrosphere
The slow released anticancer injection containing tyrosine kinase inhibitor and / or taxane consists of slow released microsphere and solvent. The slow released microsphere includes effective anticancer components of tyrosine kinase inhibitor and / or taxane and slow releasing supplementary material, and the solvent is common solvent or special solvent containing suspending agent. The suspending agent is carboxymethyl cellulose, etc. and has viscosity of 100-3000 cp at 20-30 deg.c. The slow releasing supplementary material is selected from p(LAEG-EOP), p(DAPG-EOP), p(BHET-EOP / TC), etc. The anticancer composition may be also prepared into slow released implant, and the released injection and slow released implant may be injected or set in tumor for slow releasing to maintain effective medicine concentration for over 40 days, and has obviously lowered systemic reaction on the medicine and capacity of enhancing the chemotherapeutic and radiotherapeutic effect.
Owner:JINAN KANGQUAN PHARMA TECH
Traditional Chinese medicine composition for treating atherosclerosis
The invention provides a traditional Chinese medicine composition for treating atherosclerosis. According to the invention, mulberry, sophora japonica, lotus stamen, dodder seed, aconite root, black sesame, altai anemone rhizome, intermediate peashrub flower, caulis spatholobi, trogopterus dung, ageratum, achyranthes and donkey-hide gelatin are used in the raw material medicine as monarch and adjuvant drugs for preventing and treating atherosclerosis, complex pulse and depletion, reinforcing liver and kidney, nourishing blood and brain, dispelling cold and relieving pain; auxiliary choerospondias fruit, ginseng flower, lily, gingko leaf, flos albiziae, radix linderae leaf, desertliving cistanche, longan pulp, salvia miltiorrhiza, curcuma root, fresh pears, oranges, lemons and royal jelly are used as auxiliary drugs to enhance effects of the monarch-and-adjuvant drugs, so as to tranquilize mind by nourishing the heart, replenish body vitality, restore physical strength and improve immunity; and combination of the drugs has better effects in preventing and treating symptoms of patients with atherosclerosis, reducing or inhibiting systemic reactions, protecting functions of various organ systems, nourishing the five internal organs and reinforcing yin and the essence.
Owner:朱延朋
Anticancer composition loaded with bortezomib and alkyl agent
InactiveCN101336926ADipeptide ingredientsBoron compound active ingredientsAdditive ingredientSuspending Agents
An anticancer composition comprising bortezomib and an alkylating agent thereof is in form of a sustained-released injection and comprises sustained-released microspheres and a solvent. The sustained-released microspheres comprise an anticancer effective ingredient and a sustained-released adjuvant, and the solvent is a common solvent or a special solvent containing a suspending agent. The suspending agent has a viscosity of 100-3,000cp (20-30 DEG C) and is selected from sodium carboxymethyl cellulose, etc.; the anticancer effective ingredient is bortezomib and an alkylating agent selected from melphalan, iphosphamide, 4-hydro-peroxy-cyclophosphamide and norcantharidin; and the sustained-released adjuvant is a copolymer selected from poly(lactic acid), PLGA, p(LAEG-EOP), p(DAPG-EOP), polifeprosan, poly(erucic acid dimmer-sebacic acid), difatty acid-sebacic acid copolymer, poly(fumaric acid-sebacic acid), etc. or a mixture thereof. The anticancer composition can also be made into a sustained-released implant, which can sustain effective drug concentration for more than 60 days by intratumoral or peritumoral injection or placement, and can distinctly reduce the systemic reaction of the drug and selectively enhance the curative effect of non-operative treatments such as chemotherapy and radiotherapy.
Owner:济南基福医药科技有限公司
Anticancer composition loaded with anti-metabolism medicine and synergist thereof
InactiveCN101390828AOrganic active ingredientsPharmaceutical delivery mechanismAdditive ingredientSuspending Agents
Disclosed is an anticancer combination which contains antimetabolics and the synergist thereof, and is a sustained-release injection. The anti-cancer combination is composed of sustained-release microspheres and solvent; wherein, the sustained-release microsphere contains effective anticancer ingredients and auxiliary material; the solvent is common solvent or special solvent which contains suspending agent. The viscosity of the suspending agent is 100cp-3000cp (20 DEG C-30 DEG C) and is selected from carboxymethyl cellulose; the antimetabolics is selected from methotrexate, fluorouracil, carmofur, tegafur, decitabine, capecitabine or gemcitabine; the synergist is tetrazine medicine or anticancer antibiotics; the auxiliary material is selected from p(LAEG-EOP), p(DAPG-EOP), or polyphosphate copolymer, or copolymer or mixture of polyphosphate, polylactic acid, polifeprosan, sebacic acid and PLGA; the anticancer combination also can be made into sustained-release implants which can be injected or implanted in tumor or the periphery of tumor with the effective medicine concentration maintained for more than 50 days; meanwhile, the systemic reaction of the medicine is obviously reduced; the treatment effects of the non-operative treatments such as chemotherapy or radiation treatment are selectively enhanced.
Owner:SHANDONG LANJIN PHARMA
Anticancer composition containing lomustine
The anticancer composition includes effective anticancer component selected from tyrosine kinase inhibitor and / or lomustine and slow releasing supplementary material, and may be prepared into slow released injection and slow released implant. The slow released injection includes also suspending agent and special solvent. The suspending agent has viscosity at 20-30 deg.c of 100-3000 cp and is selected from sodium carboxymethyl cellulose, etc. The slow releasing supplementary material is selected from p(LAEG-EOP), p(DAPG-EOP), p(BHET-EOP / TC), p(BHET-EOP / TC), p(BHDPT-EOP / TC), p(BHDPT-EOP / TC), p(CHDM-HOP), p(CHDM-EOP), etc. The released injection and slow released implant may be injected or set in tumor for slow releasing to maintain high medicine concentration for over 60 days to raise the treating effect and lower the systemic reaction on the medicine.
Owner:JINAN KANGQUAN PHARMA TECH
Anticancer composition
InactiveCN101088491APharmaceutical delivery mechanismPharmaceutical non-active ingredientsTreatment effectWhole body
The anticancer composition includes effective anticancer component selected from tyrosine kinase inhibitor and / or carmustine and slow releasing supplementary material, and may be prepared into slow released injection and slow released implant. The slow released injection includes also suspending agent and special solvent. The suspending agent has viscosity at 20-30 deg.c of 100-3000 cp and is selected from sodium carboxymethyl cellulose, etc. The slow releasing supplementary material is selected from p(LAEG-EOP), p(DAPG-EOP), p(BHET-EOP / TC), p(BHET-EOP / TC), p(BHDPT-EOP / TC), p( BHDPT-EOP / TC), p(CHDM-HOP), p(CHDM-EOP), etc. The released injection and slow released implant may be injected or set in tumor for slow releasing to maintain high medicine concentration for over 60 days to raise the treating effect and lower the systemic reaction on the medicine.
Owner:JINAN KANGQUAN PHARMA TECH
Temperature control sustained-release injection containing resist metabolism series medicine
InactiveCN101176706APharmaceutical delivery mechanismPharmaceutical non-active ingredientsPolyesterTreatment effect
The invention discloses a temperature control and sustained release injection containing anti-metabolism medicine, which comprises anticancer effective dose anti-metabolism medicine, amphiphilic block copolymer and certain amount of medicine releasing regulation agent; wherein, the amphiphilic block copolymer comprises polyethylene glycol and polyester; the mixture of the amphiphilic block copolymer and the solvent without organic solvent has the gelation property of the temperature sensitive, is the fluidity liquid in the environment below the body temperature, and can be automatically changed into the non-fluidity and biodegradation absorption water insoluble gel in the body of warm-blooded animal; the non-fluidity and biodegradation absorption water insoluble gel can release the anti-metabolism medicine slowly in the part of the tumor and maintain the effective medicine concentration of the effective medicine for a plurality of weeks or a plurality of months; the viscosity of the temperature control and sustained release injection is 10 cp to 3000 cp (5 DEG C to 30 DEG C), the temperature is 35 DEG C to 37 DEG C. The invention has the advantages of capability of injection inside or around the tumor or put inside the tumor cavity after the operation, obvious reduction of the systemic reaction of the medicine, and selective improvement of the therapeutic effect of the chemotherapy, the radiotherapy and other non-operation treatment method, and capability of curing the tumor in different stages.
Owner:SHANDONG LANJIN PHARMA +1
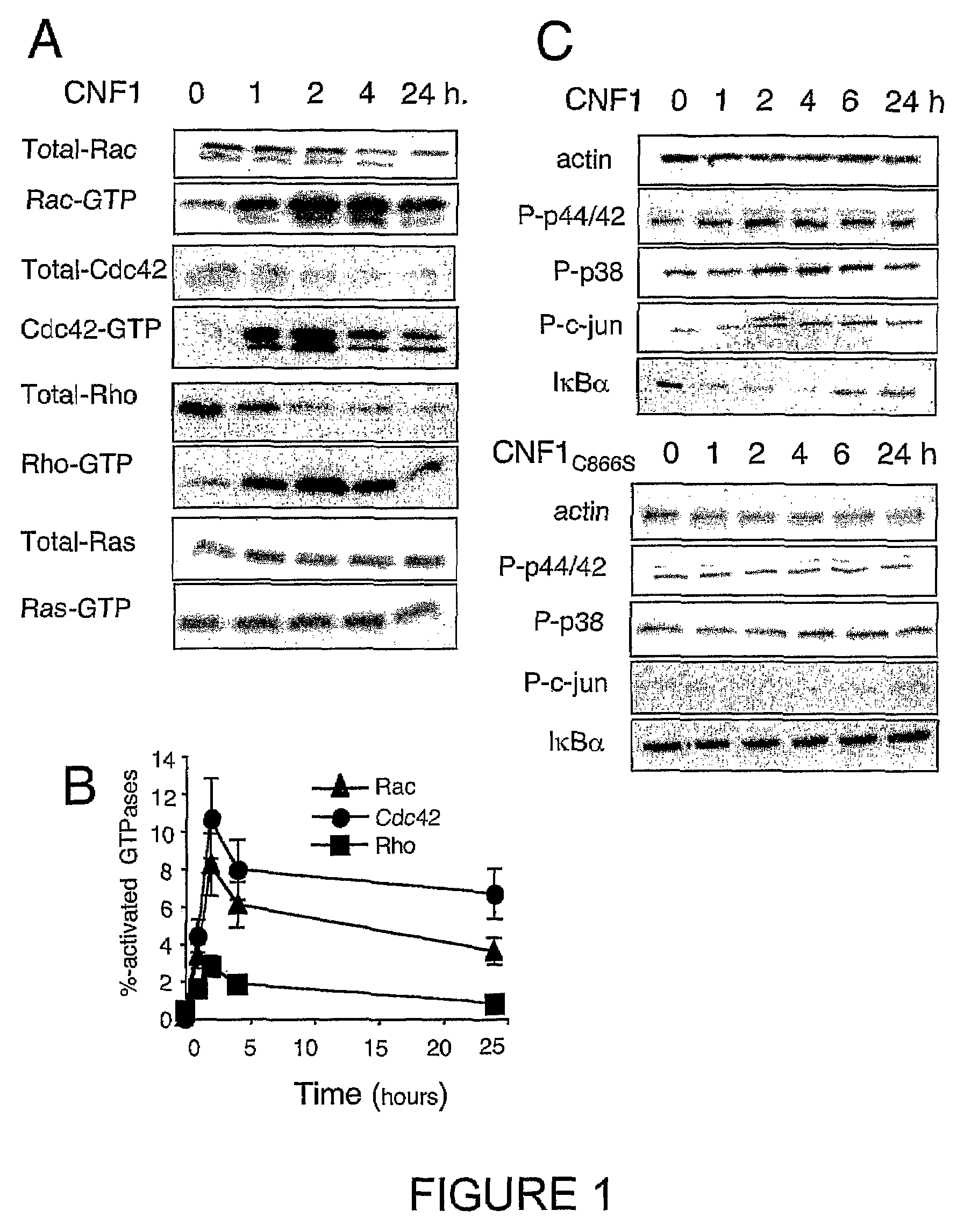
Vaccine composition comprising an immunoadjuvant compound consisting of a Rho GTPase family activator
InactiveUS7655240B2Bacterial antigen ingredientsPeptide/protein ingredientsGTPase ActivatorsChemical compound
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM)
Restrainer containing tyrosine kinase and anticancer hormone medicines composition
InactiveCN101138546APharmaceutical delivery mechanismPharmaceutical non-active ingredientsAdjuvantMicrosphere
An anti-cancer compound containing the PTK enzyme inhibitor (TKi) or the hormones medicine is a slow releasing injection, which consists of a slow-releasing micro sphere and a dissolvent. The slow-releasing micro sphere is selected from the effective component and the slow-releasing adjuvant of the TKi or the hormones medicine. The dissolvent comprises a common dissolvent and a special dissolvent containing the suspending agent. The viscosity of the dissolvent is 100cp-3000cp when the temperature is 20 DEG C to 30 DEG C. The dissolvent is selected from the sodium carboxymethycellulose. The adjuvant is selected from p(LAEG-EOP), p(DAPG-EOP), p(BHET-EOP / TC), p( BHET-EOP / TC), p(BHDPT-EOP / TC), p(BHDPT-EOP / TC), p(CHDM-HOP) or p(CHDM-EOP), which belong to the polyphosphoric acid ester copolymer or the copolymer of the polylactic acid, the Policeperson, the double fatty acid and the decanedioic acid, or the copolymer and the mixture of the poly-(druidic acid dimer-decanedioic acid) and the poly-(fumaric acid-decanedioic acid). The anti-cancer compound is also produced as a slow-releasing implantation preparation. The effective drug concentration can be maintained for over 40 days when the medicine is injected or arranged in the tumor or around the tumor. The medicine can greatly reduce the systemic reaction of the drug and selectively enhance the therapy effect of the non-surgical treatment such as the chemical therapy.
Owner:JINAN KANGQUAN PHARMA TECH
Application of human RTN4B protein in preparation of medicine for healing wound
This invention relates to the field of gene engineering and medicament, specifically relates to the application of RTN 4B in preparing wound healing drugs. Healing of wound is a very complicated process. Wound not only changes part of organism, but also initiate systemic reaction to different extent, meanwhile, wound part will bring pain to patients. The inventive RTN 4B can promote angiogenesis,enhance adhesion and migration of blood vessel cell; and can be used for preparing medicament for promoting healing of wound to shorten period of treatment and relieve pain.
Owner:FUDAN UNIV
Vaccine composition comprising an immunoadjuvant compound consisting of a rho gtpase family activator
InactiveUS20100266632A1Increase secretionAntibacterial agentsBacterial antigen ingredientsSystemic reactionImmunogenicity
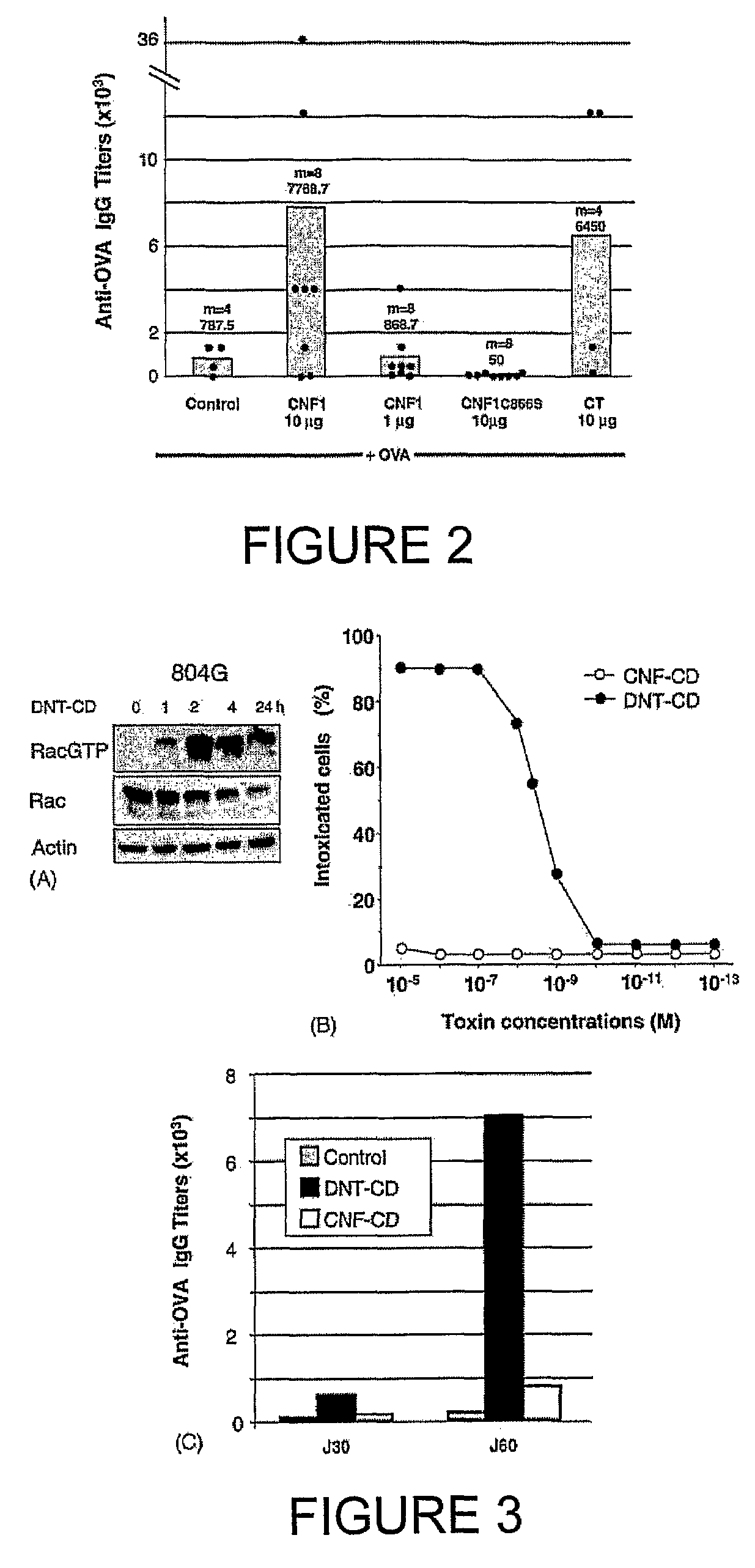
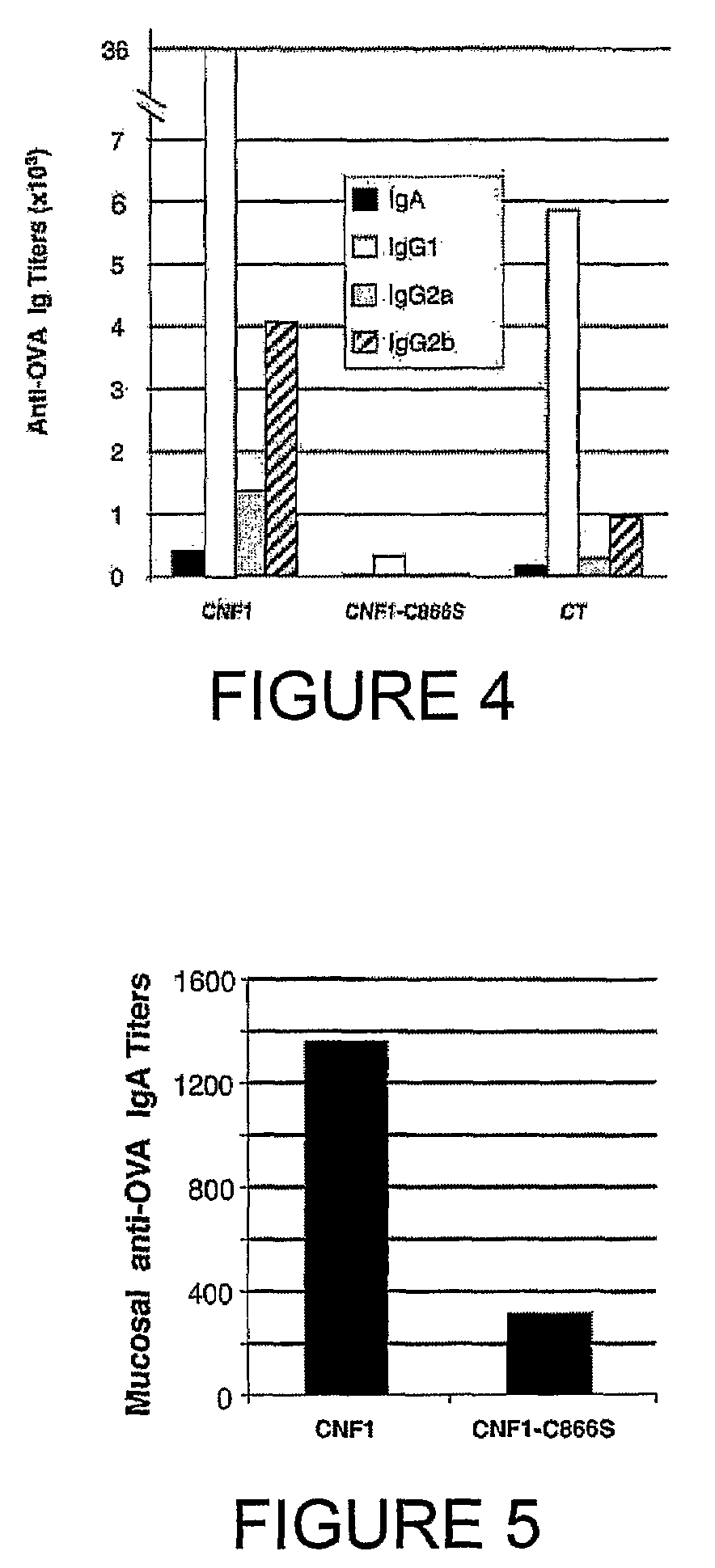
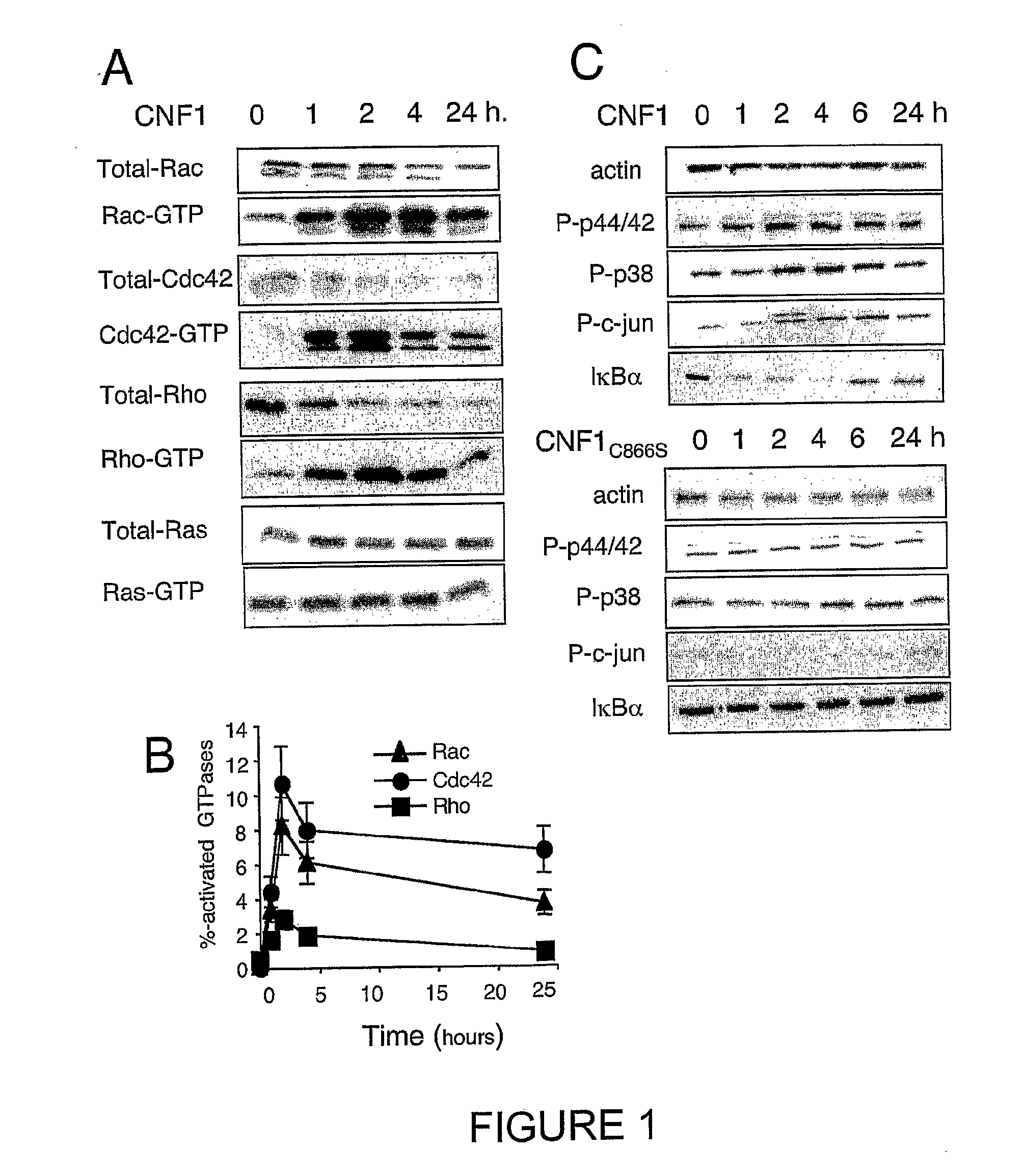
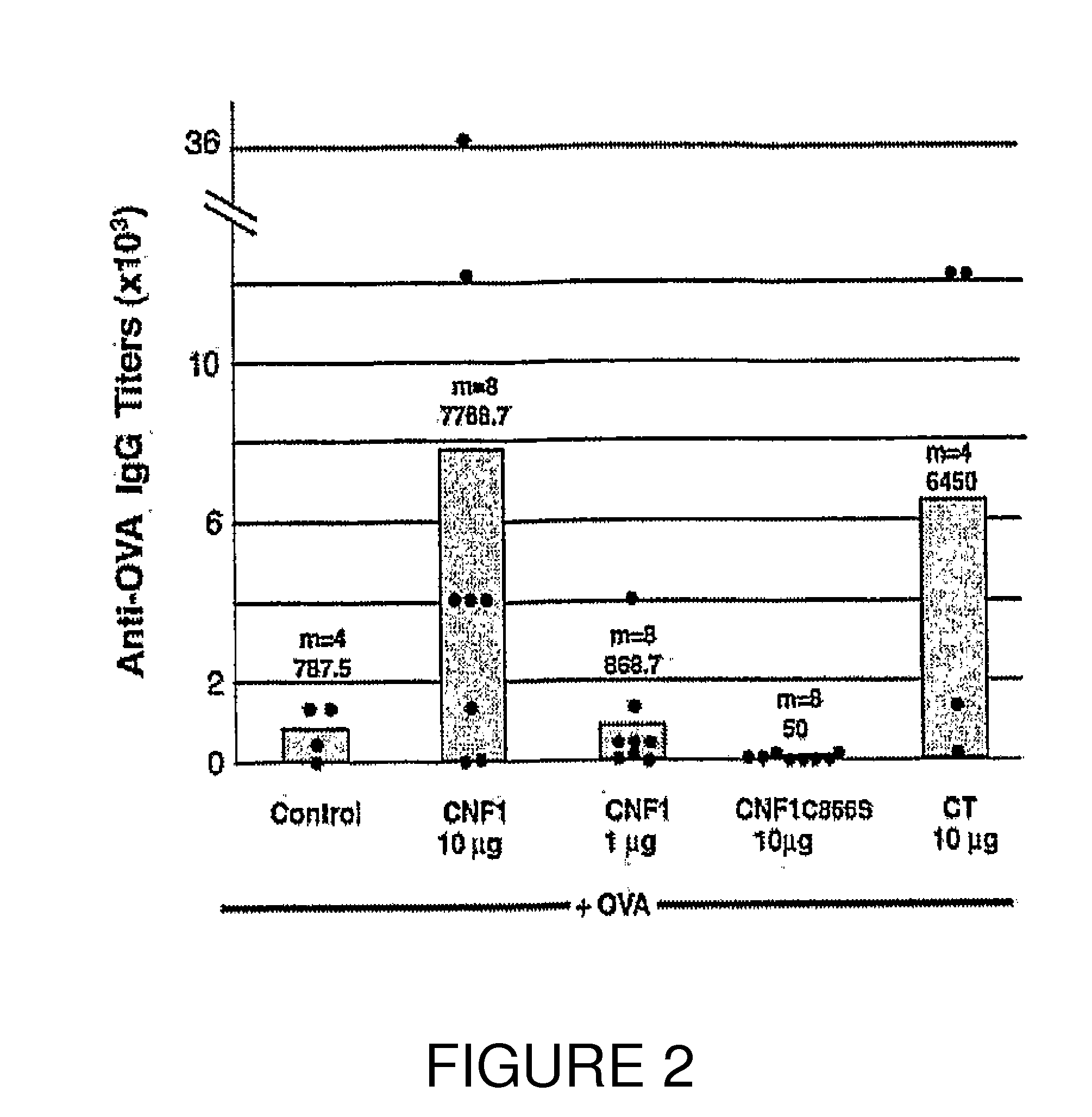
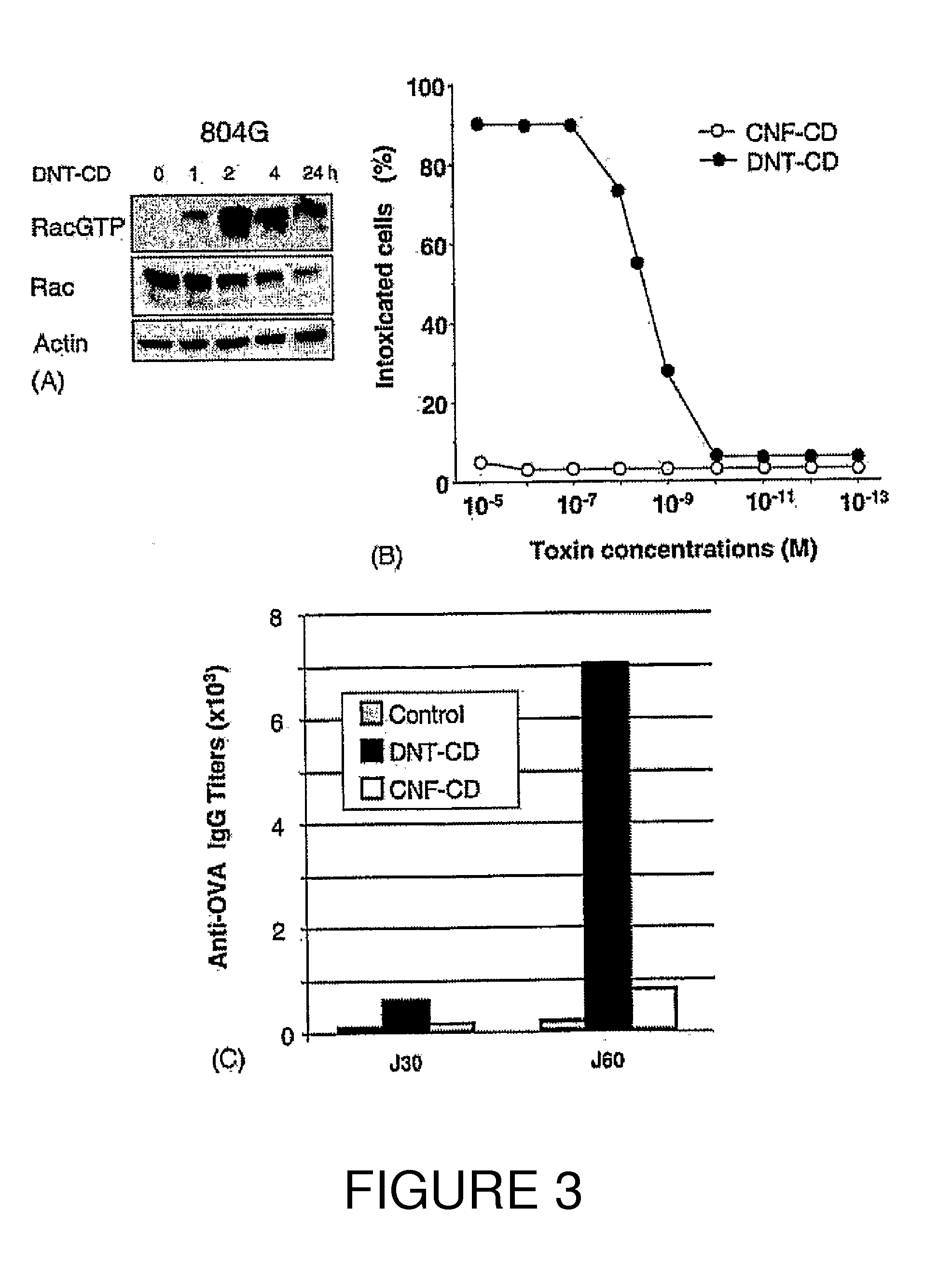
An immunogenic or vaccine composition comprising an immunoadjuvant compound consisting of a Rho GTPase activator. The Activators of Rho GTPases, namely the cytotoxic necrotizing factor 1 (CNF1), and DNT bear immunostimulatory properties towards the systemic response to orally administered ovalbumine.
Owner:LEMICHEZ EMMANUEL +5
Slow released compound anticancer prepn
InactiveCN1887266AEasy to operateGood repeatabilityOrganic active ingredientsSolution deliveryToxicantWhole body
The slow released compound anticancer injection consists of slow released microsphere and solvent. The slow released microsphere includes effective anticancer component and slow releasing supplementary material, and the solvent is special solvent containing suspending agent. The effective anticancer component is bendamustine and cell toxicant medicine, and the cell toxicant medicine is selected from taxane, alkaylating agent, topomerase inhibitor, etc. The slow releasing supplementary material is polylactic acid and its copolymer, polyethylene glycol-polylactic acid copolymer, EVAc, etc. The .suspending agent is carboxymethyl cellulose sodium, etc. and has viscosity of 100-3000 cp at 20-30 deg.c. The slow released microsphere may be also prepared into slow released implanting agent. The present invention has lowered systemic reaction and raised local concentration for 30-50 days, and may be used alone effectively and in enhancing the curative effect of chemotherapy, radiotherapy and other non-operative treatment.
Owner:JINAN SHUAIHUA PHARMA TECH
Slow-release injection containing tyrosine kinase restraining agent and platinum compound
InactiveCN101081208APharmaceutical delivery mechanismPharmaceutical non-active ingredientsTreatment effectMicrosphere
The slow released anticancer injection containing tyrosine kinase inhibitor and / or platinum compound consists of slow released microsphere and solvent. The slow released microsphere includes effective anticancer components of tyrosine kinase inhibitor and / or platinum compound and slow releasing supplementary material, and the solvent is common solvent or special solvent containing suspending agent. The suspending agent is carboxymethyl cellulose, etc. and has viscosity of 100-3000 cp at 20-30 deg.c. The slow releasing supplementary material is selected from p(LAEG-EOP), p(DAPG-EOP), p(BHET-EOP / TC), etc. The anticancer composition may be also prepared into slow released implant, and the released injection and slow released implant may be injected or set in tumor for slow releasing to maintain effective medicine concentration for over 50 days, and has obviously lowered systemic reaction on the medicine and capacity of enhancing the chemotherapeutic and radiotherapeutic effect.
Owner:JINAN KANGQUAN PHARMA TECH
Anti-cancer medicine composition carried with blood-vessel inhibiting agent and synergistic agent
The present invention relates to an anti-cancer medicine composition containing angiogenesis inhibitor and its synergist. It is formed from microsphere and solvent, in which the slow-release microsphere includes anti-cancer effective component and slow-release auxiliary material, and the solvent is general one or special one containing suspension adjuvant. The suspension adjuvant is selected from carboxymethylcellulose sodium, etc. and its viscosity is 100 cp-3000 cp (at 20 deg.C-30 deg.C). The slow-release auxiliary material is selected from polyphosphate copolymer of p(LAEG-EOP) and p(DAPG-EOP), etc. or polyphosphate and polylactic acid, difatty acid and sebacic acid copolymer, poly (erucidic acid dimmer-sebacic acid) or poly (fumaric acid-sebacic acid) copolymer or mixture. The anti-cancer effective component includes angiogenesis inhibitor and / or its synergist selected from anti-cancer antibiotic and / or tetrazine medicine. Said anti-cancer medicine composition also can be made into slow-release implantation.
Owner:JINAN KANGQUAN PHARMA TECH
Anticancer composition containing platinum compound and bortezomib
An anticancer composition comprising a platinum compound and bortezomib is in form of a sustained-released injection and comprises sustained-released microspheres and a solvent. The sustained-released microspheres comprise an anticancer effective ingredient and a sustained-released adjuvant, and the solvent is a common solvent or a special solvent containing a suspending agent. The suspending agent has a viscosity of 100-3,000cp (20-30 DEG C) and is selected from sodium carboxymethyl cellulose, etc.; the platinum compound is selected from cisplatin, carboplatin and oxaliplatin; and the sustained-released adjuvant is a copolymer or a blend selected from poly(phosphate ester)s such as p(LAEG-EOP) and p(DAPG-EOP), PLA, PLGA, polyphosphate ester-polylactic acid, polifeprosan, poly(erucic acid dimmer-sebacic acid) and poly(fumaric acid-sebacic acid). The anticancer composition can also be made into a sustained-released implant, which can sustain effective drug concentration for more than 40 days by intratumoral or peritumoral injection or placement, and can distinctly reduce the systemic reaction of the drug and selectively enhance the curative effect of non-operative treatments such as chemotherapy and radiotherapy.
Owner:济南基福医药科技有限公司
Traditional Chinese medicine preparation for radiotherapy post-operation conditioning
InactiveCN105582516ASignificant effectNo side effectsAntinoxious agentsUnknown materialsSystemic reactionOfficinalis
The invention belongs to the technical field of traditional Chinese medicine and particularly relates to a traditional Chinese medicine preparation for radiotherapy post-operation conditioning. The traditional Chinese medicine preparation is prepared from the following raw materials: rhizoma atractylodis macrocephalae, grewia biloba, rhizoma atractylodis, tangerine peel, turtle shell, orchidaceae dendrobium faulhaberianum, dendrobium densiflorum lindl ex wall., herminium monorchis, fructus corni, herba epimedii, radix morindae officinalis, radix aconiti carmichaeli, rhizoma zingiberis, cinnamon, bamboo shavings, rhizoma pinelliae, rhizoma corydalis and herba chelidonii. Through the conditioning of traditional Chinese medicines, the traditional Chinese medicine preparation provided by the invention has the functions of replenishing qi, nourishing yin and tonifying kidneys, is suitable for all general reactions after radiotherapy, contributes to the physical restoration of patients after radiotherapy, accelerates the discharge of vivotoxin, relieves the symptom of lump heat, increases the number of white cells and accelerates body recovery; and meanwhile, the traditional Chinese medicine preparation has a function of improving immunity, avoids tumor relapse to a greater degree and provides guarantee to the physical health of the patients.
Owner:QINGDAO CENT HOSPITAL
Temperature control gel rubber sustained-release injection containing resist metabolism series medicine
InactiveCN101176717APharmaceutical delivery mechanismPharmaceutical non-active ingredientsPolyesterWhole body
The invention discloses a temperature control and sustained release injection containing anti-metabolism medicine, which comprises anticancer effective dose anti-metabolism medicine, amphiphilic block copolymer and certain amount of medicine releasing regulation agent; wherein, the amphiphilic block copolymer comprises polyethylene glycol and polyester; the mixture of the amphiphilic block copolymer and the solvent without organic solvent has the gelation property of the temperature sensitive, is the fluidity liquid in the environment below the body temperature, can be automatically changed into the non-fluidity and biodegradation absorption water insoluble gel in the body of warm-blooded animal, the non-fluidity and biodegradation absorption water insoluble gel can release the anti-metabolism medicine slowly in the part of the tumor and maintain the effective medicine concentration of the effective medicine for a plurality of weeks or a plurality of months; the viscosity of the temperature control and sustained release injection is 10 cp to 3000 cp (5 DEG C to 30 DEG C), the temperature is 35 DEG C to 37 DEG C. The invention has the advantages of capability of injection inside or around the tumor or put inside the tumor cavity after the operation, obvious reduction of the systemic reaction of the medicine, and selective improvement of the therapeutic effect of the chemotherapy, the radiotherapy and other non-operation treatment method, and capability of curing the tumor in different stages.
Owner:SHANDONG LANJIN PHARMA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com