Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
133 results about "Absorbable polymers" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
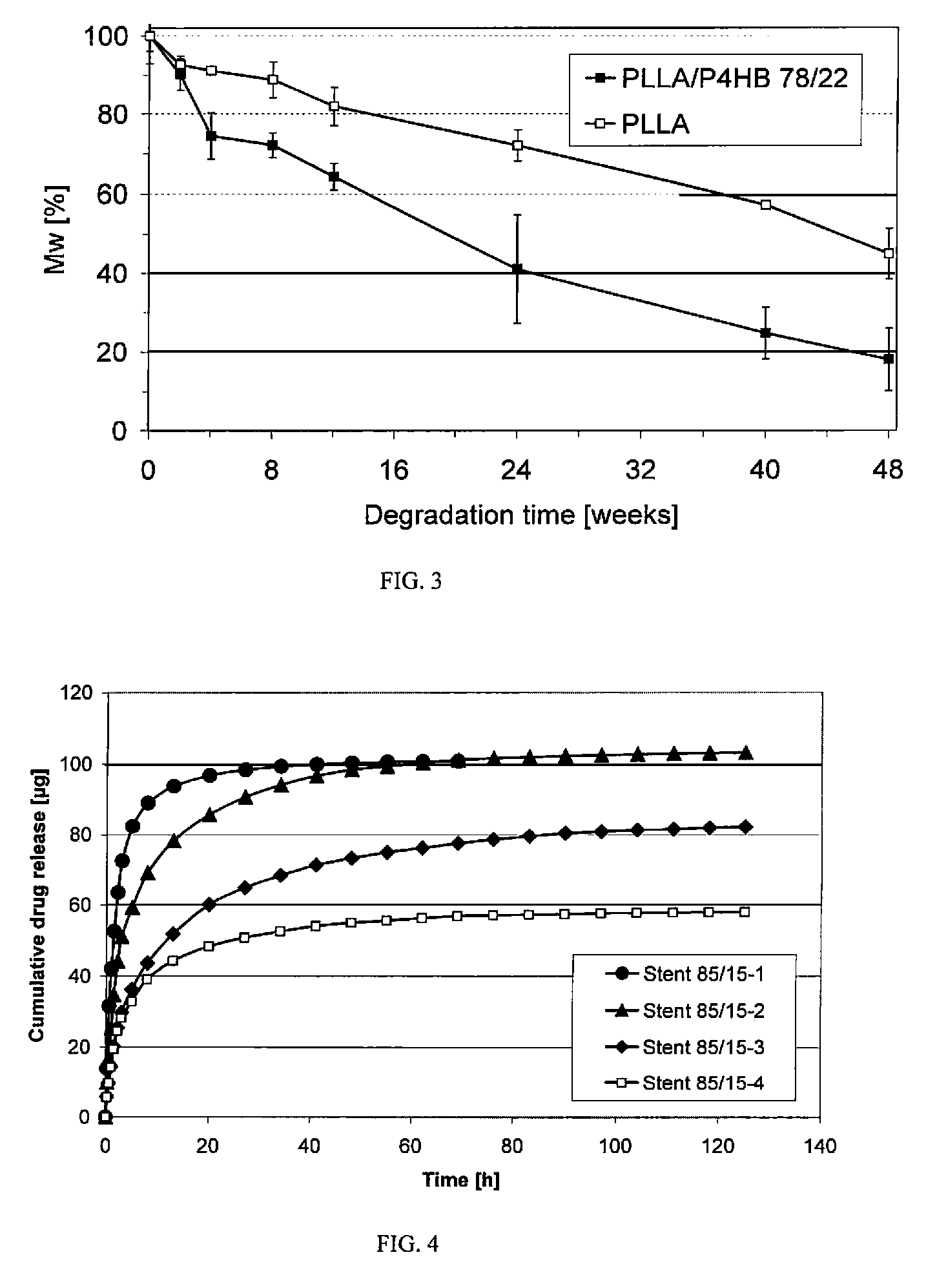
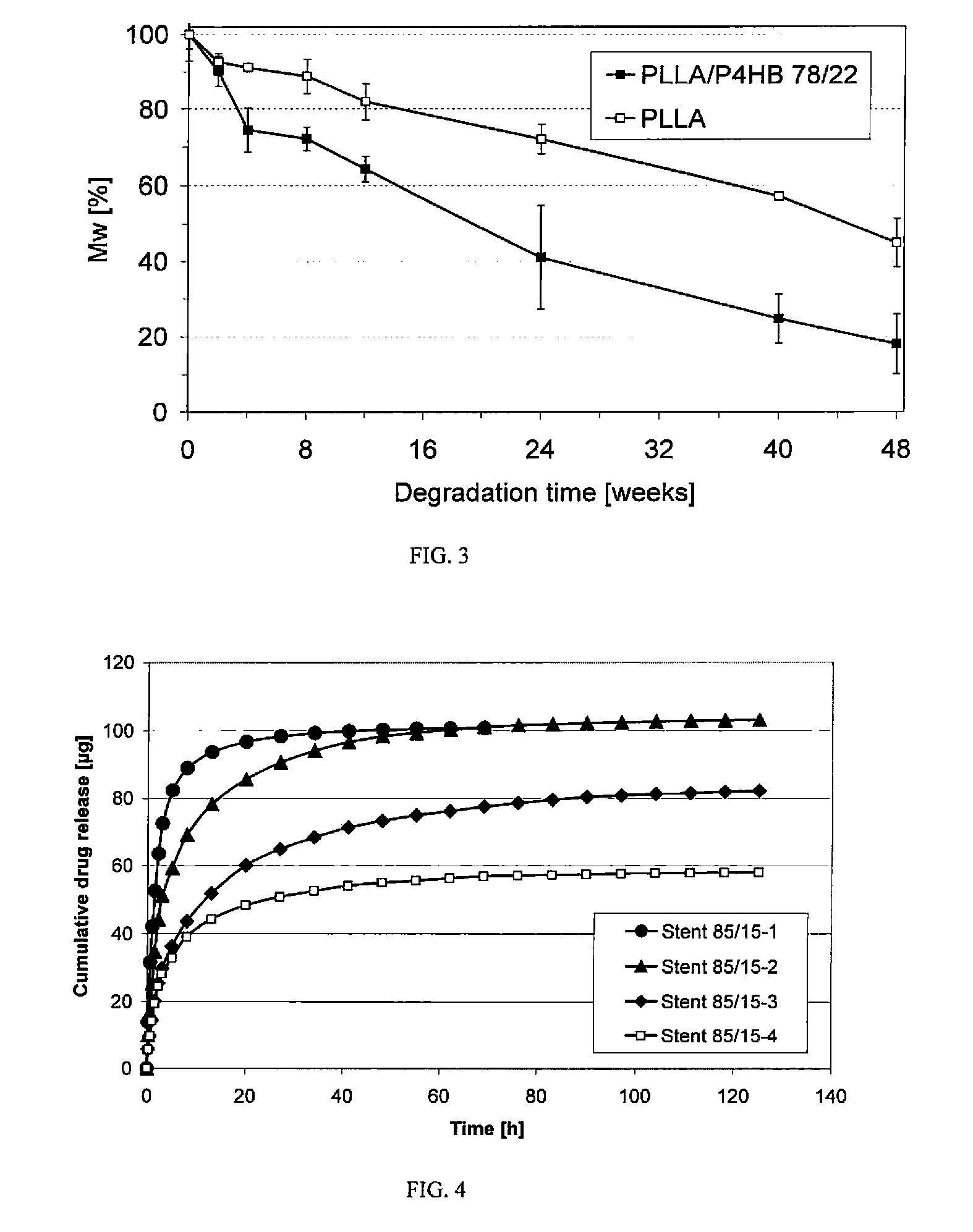
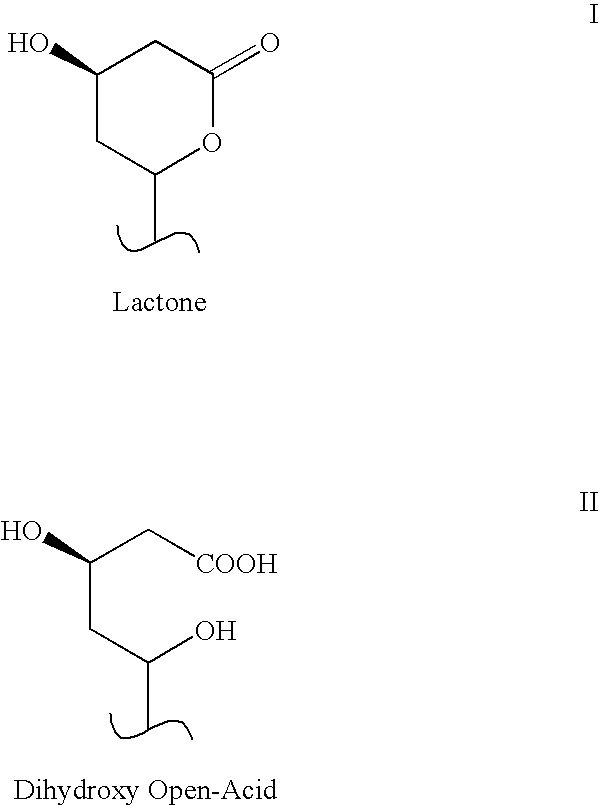
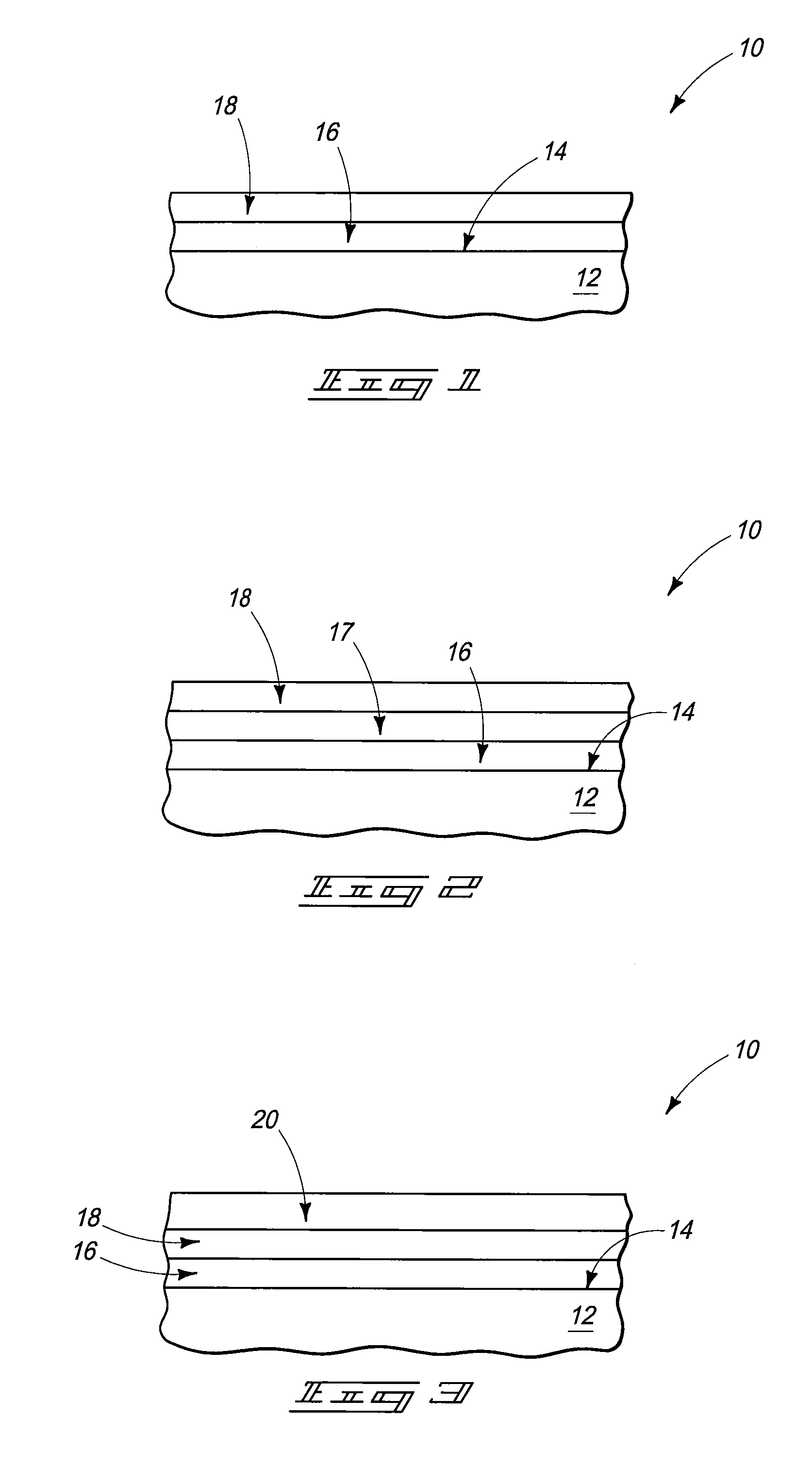
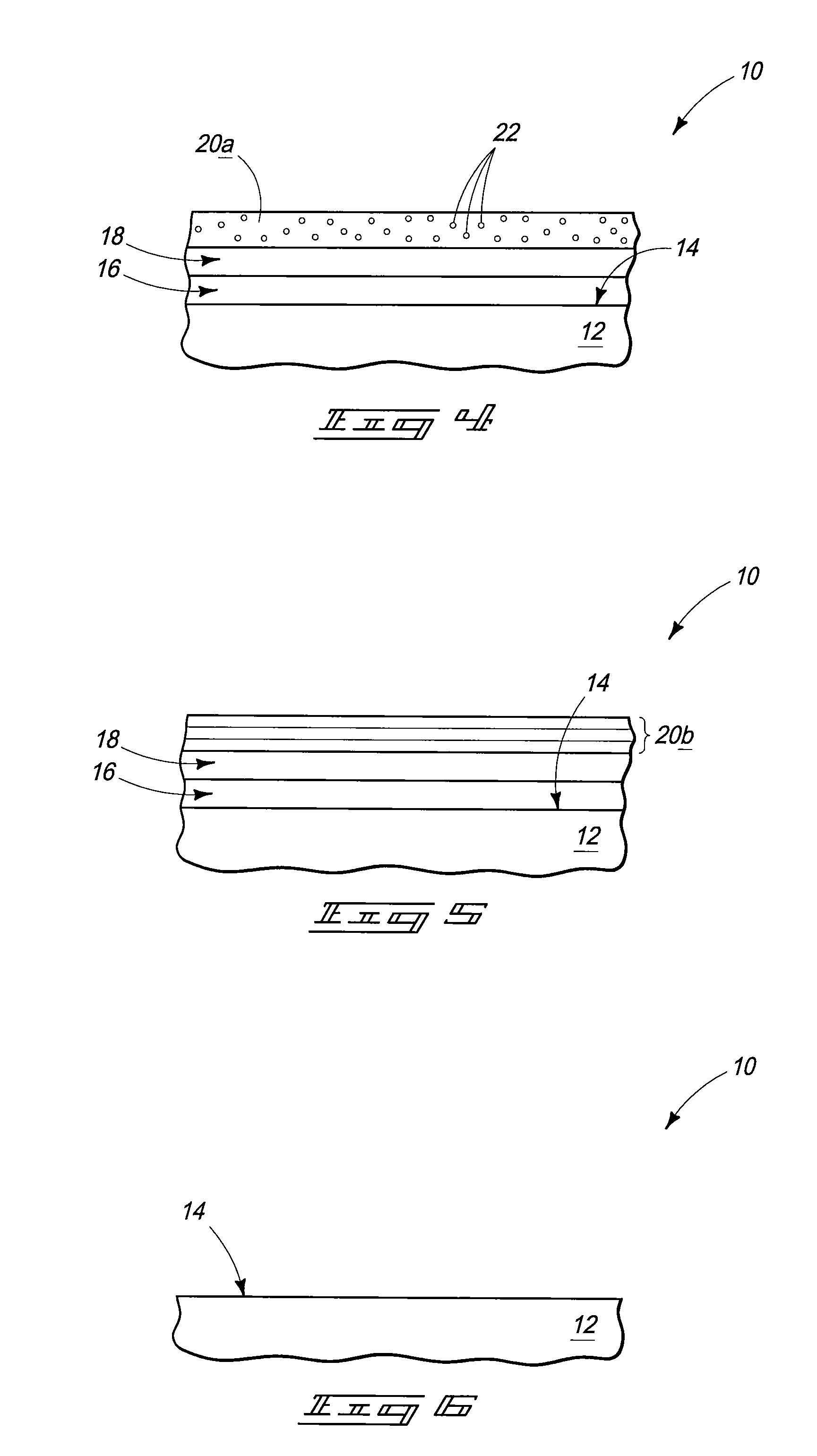
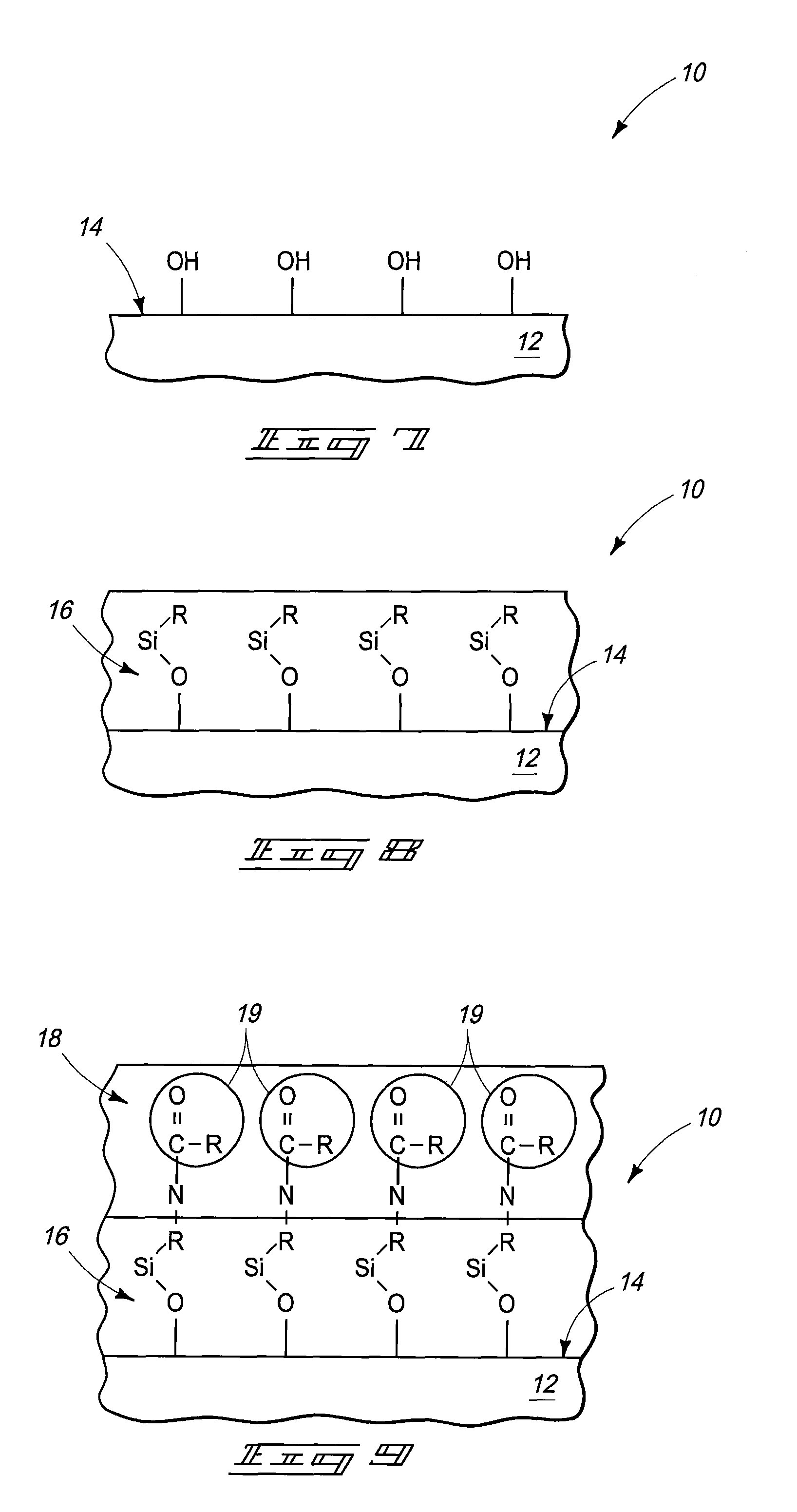
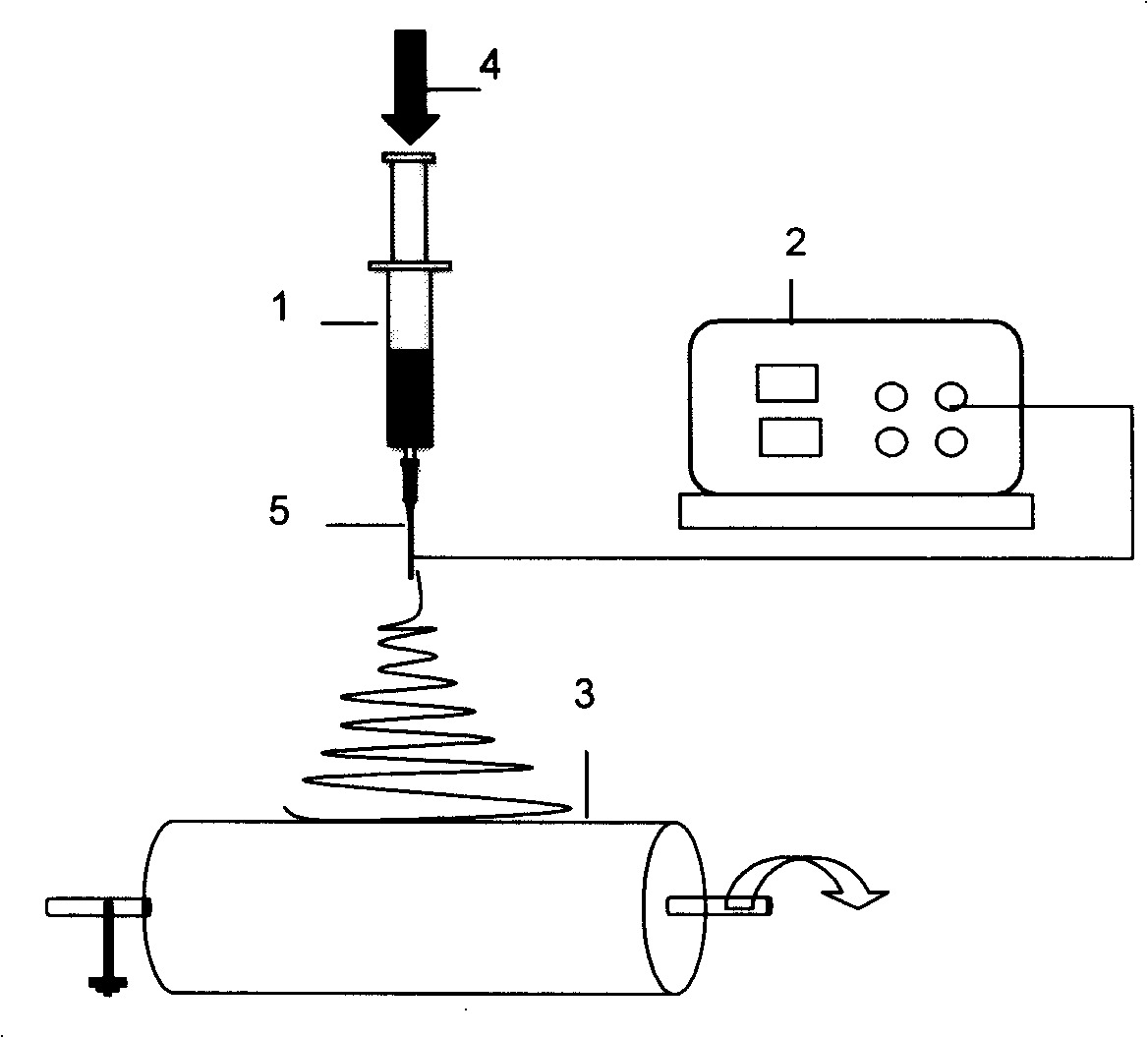
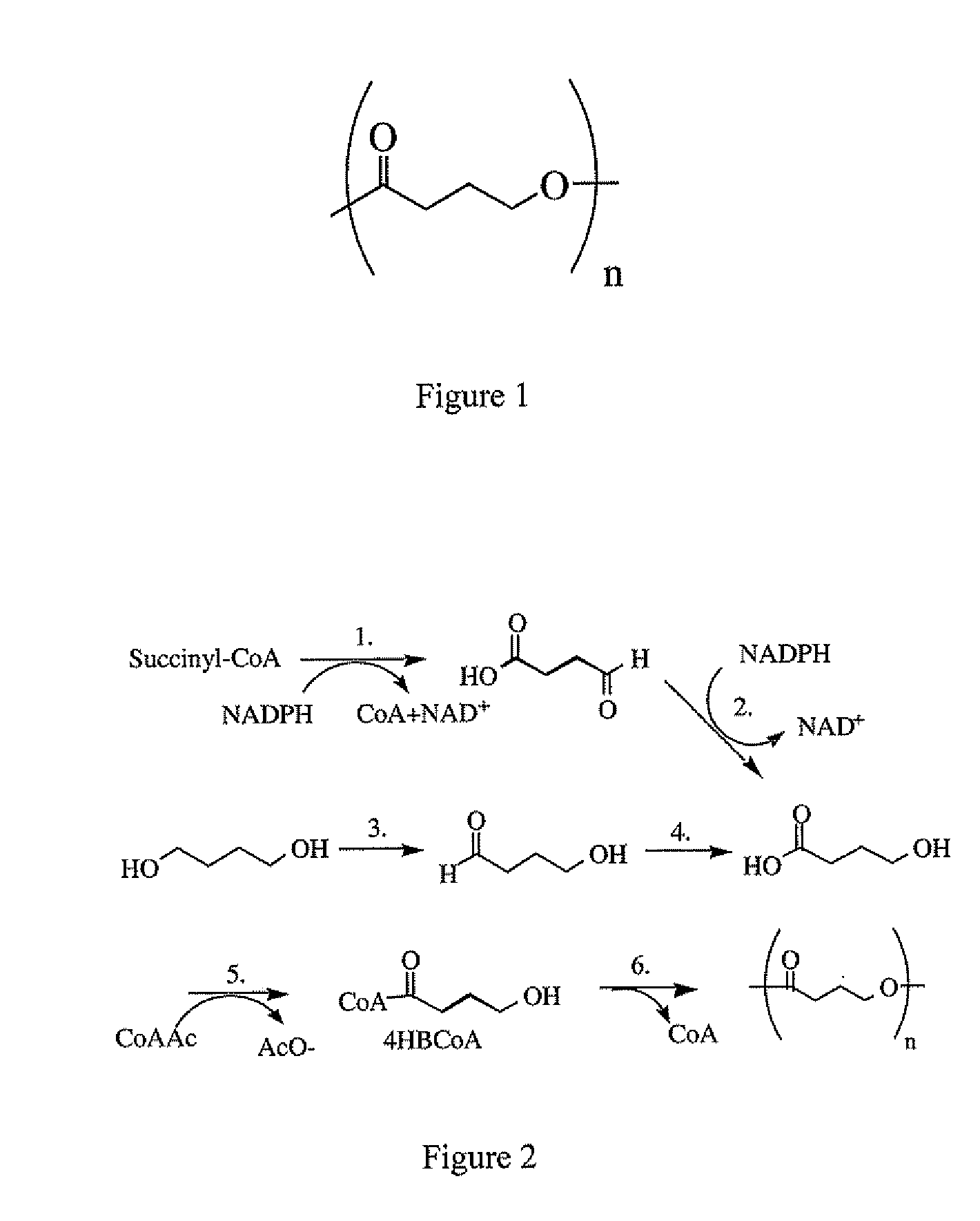
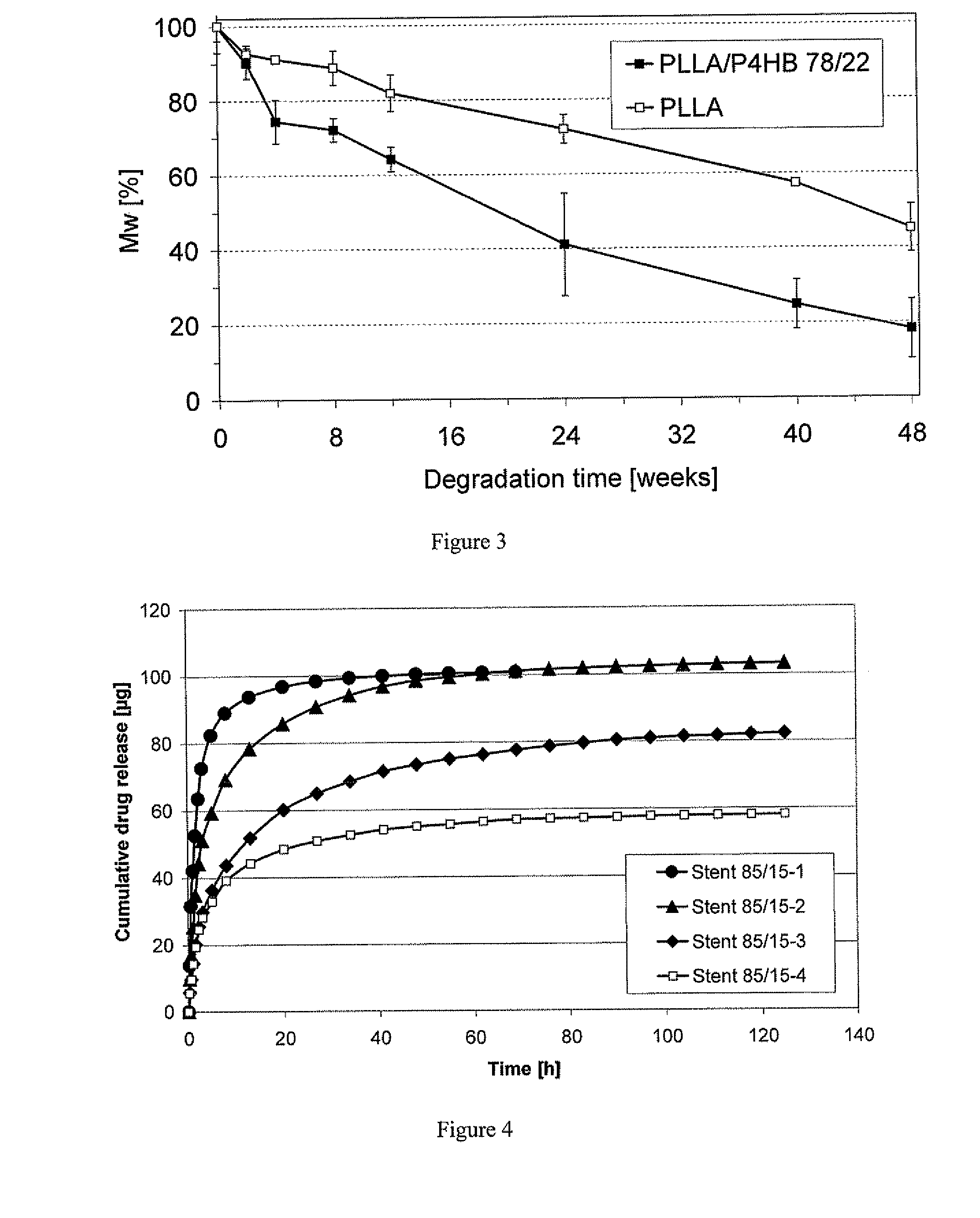
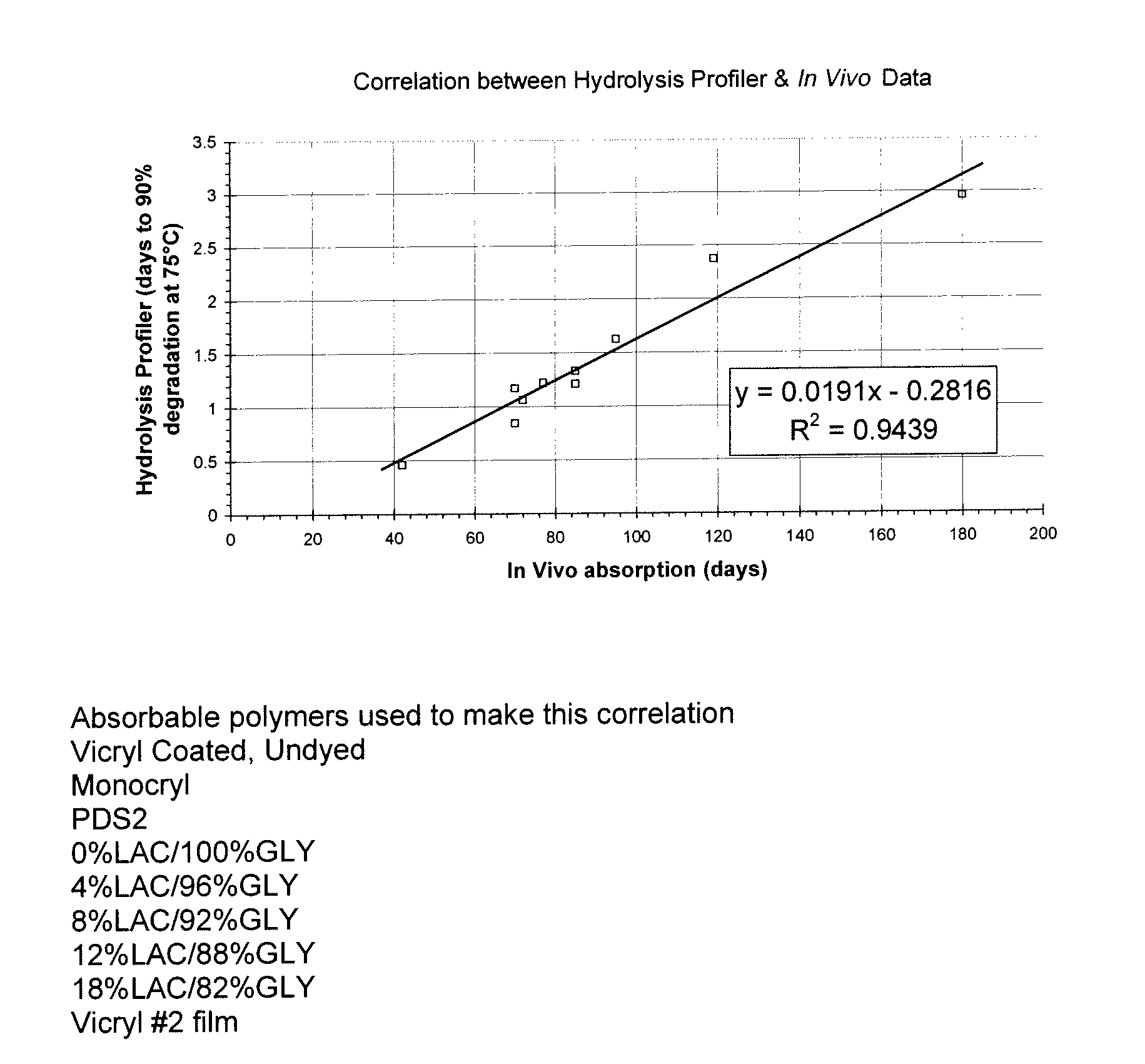
As the majority of absorbable polymers degrade by hydrolytic degradation, minimizing water content is of the utmost importance for enhancing device life and maintaining functionality. In general, absorbable polymers degrade by bulk hydrolysis of main-chain esters.
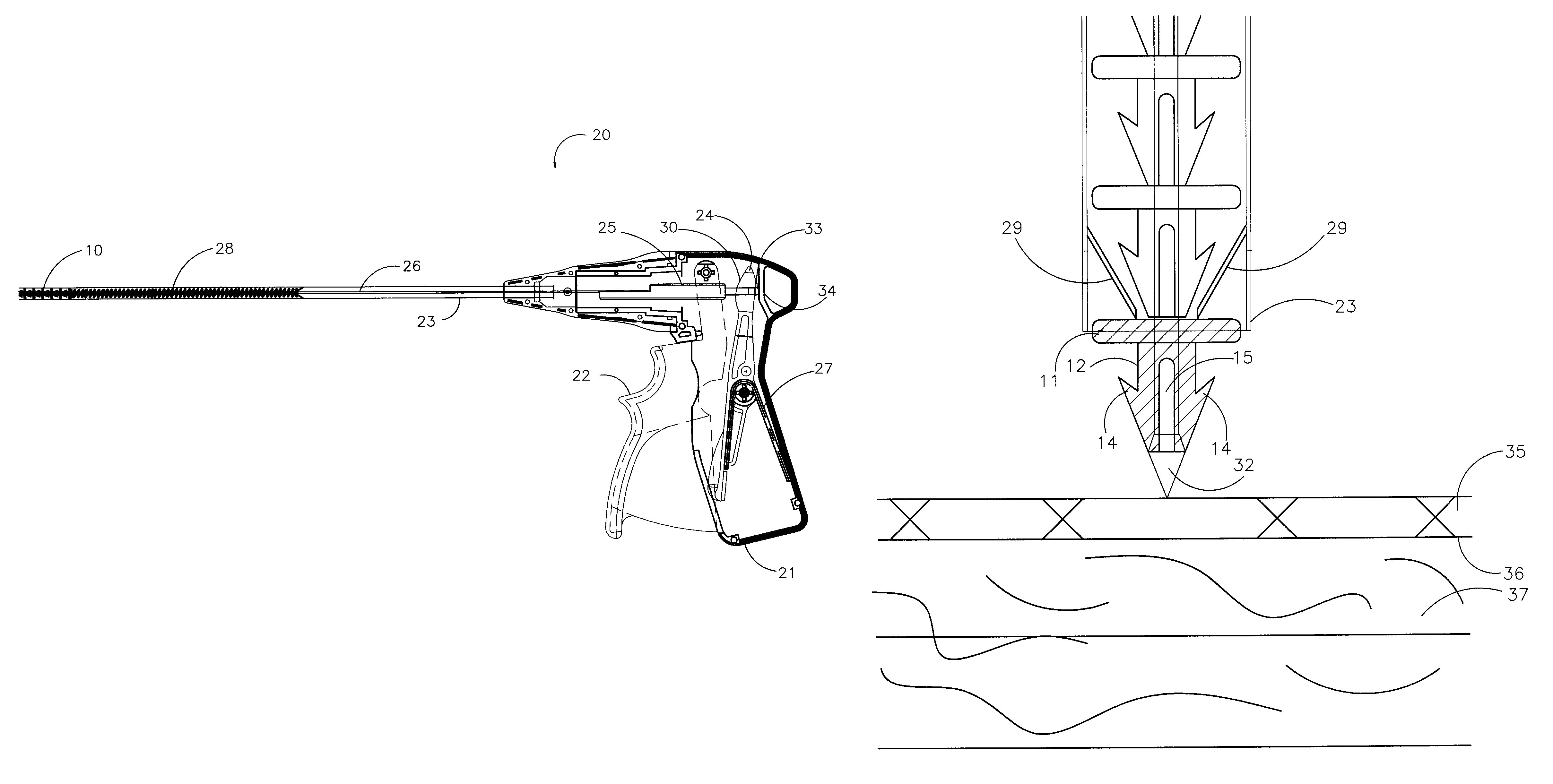
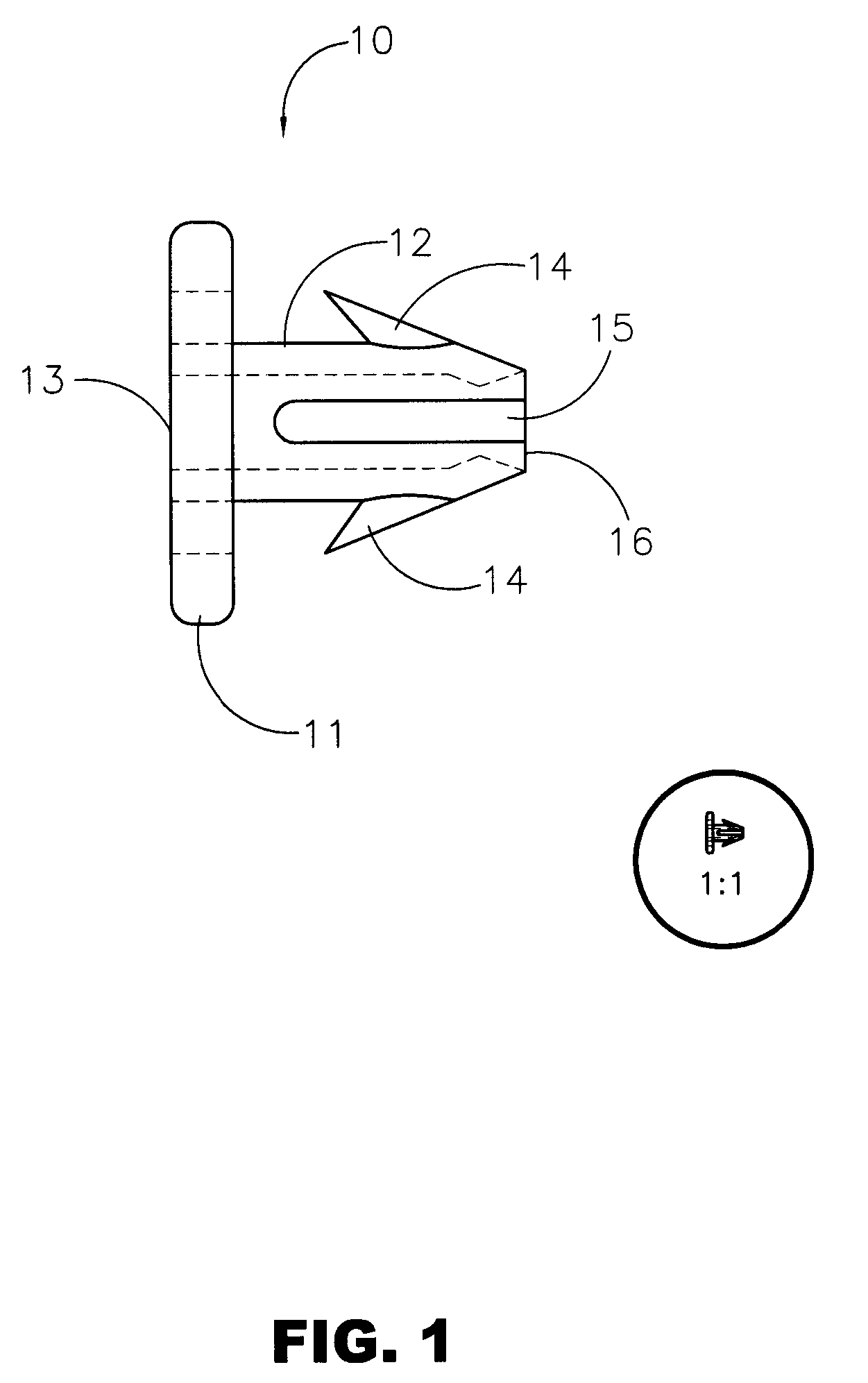
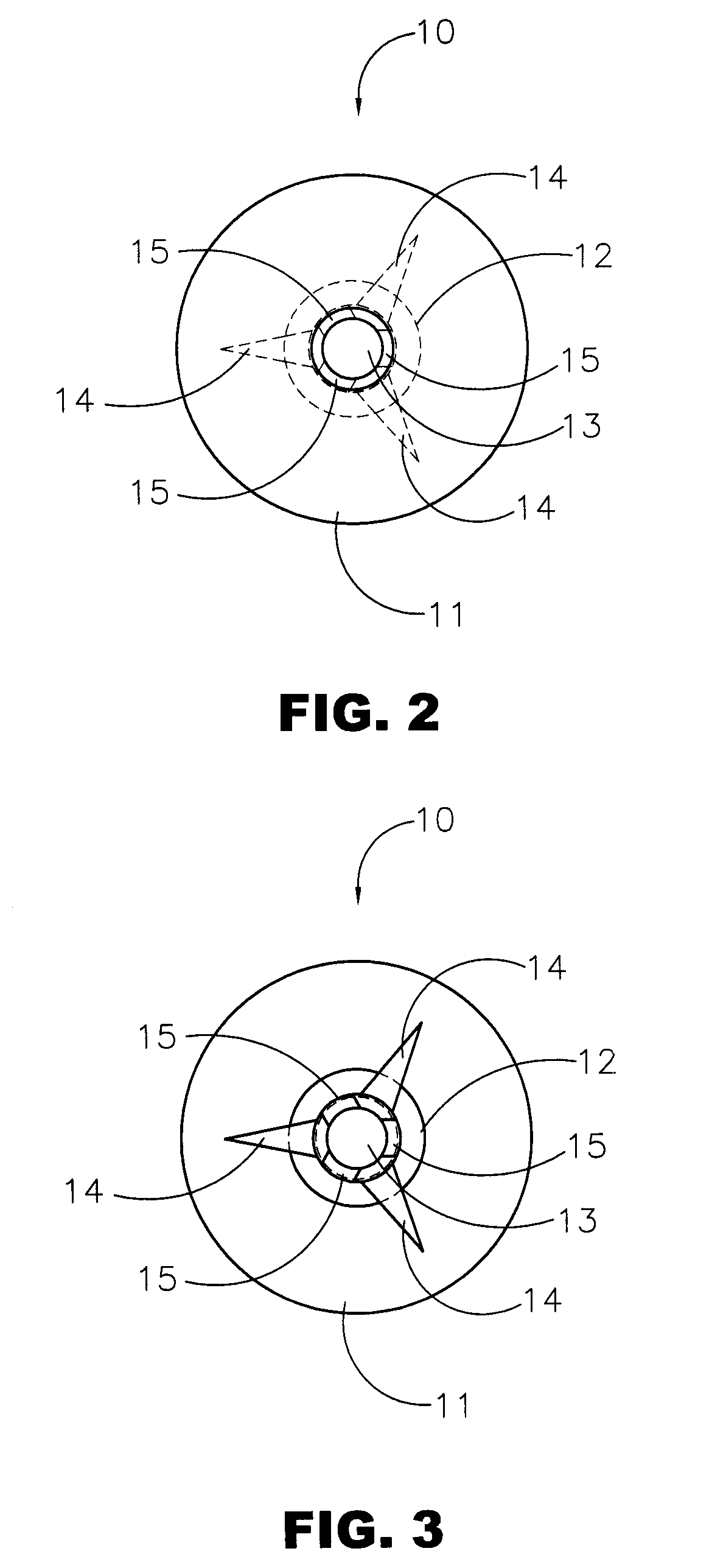
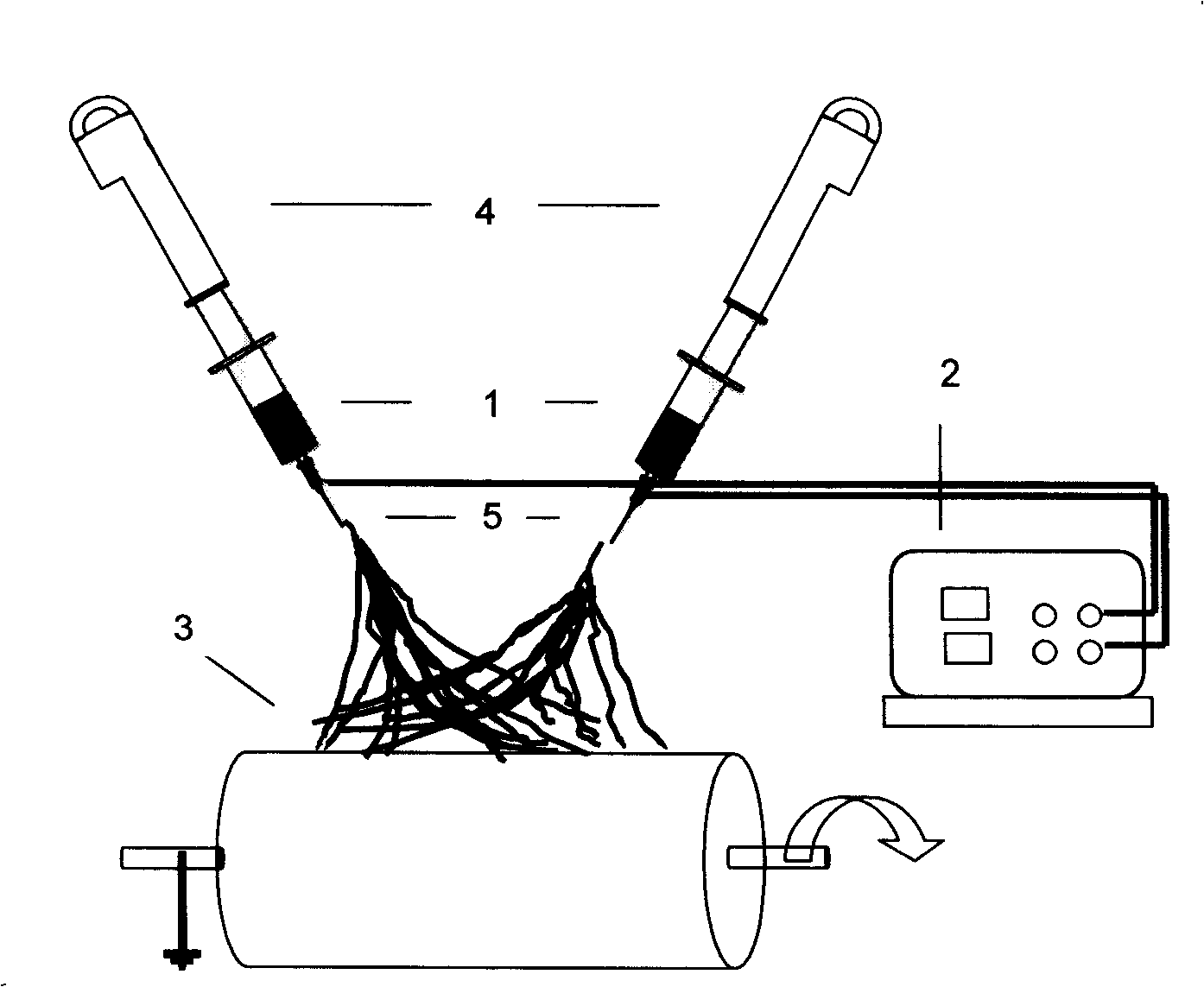
Surgery delivery device and mesh anchor
A delivery device for delivering a plurality of individual surgical anchors is disclosed. The delivery device includes a housing, a delivery tube, having a distal and a proximal end, an actuator, flexible anchor reaction members, a reciprocating anchor carrier, having a distal and a proximal end, the distal end terminating in a tissue penetrator. The device further includes at least one surgical anchor located in juxtaposition with the anchor carrier. Each of the surgical anchors has a penetration section and a head section. The surgical anchors are preferably made from an absorbable polymer. The actuator is connected to the anchor carrier and has at least two states. The first, or home state, is a position such that the surgical anchor is proximal the distal end of the tube. The second state is such that the penetrating section of the surgical anchor is exposed beyond the distal end of the delivery tube.
Owner:COVIDIEN LP
Biodegradable polymer coils for intraluminal implants
An endovascular cellular manipulation and inflammatory response are elicited from implantation in a vascular compartment or any intraluminal location of a separable coil comprised at least in part of at least one biocompatible and absorbable polymer or protein and growth factors. Typically a catheter associated with the separable coil is used to dispose the coil into a selected body lumen. The biocompatible and absorbable polymer or protein is thrombogenic. The coil further is comprised at least in part of a growth factor or more particularly a vascular endothelial growth factor, a basic fibroblast growth factor or other growth factors. The biocompatible and absorbable polymer is in the illustrated embodiment at least one polymer selected from the group consisting of polyglycolic acid, poly~glycolic acid poly-L-lactic acid copolymers, polycaprolactive, polyhydroxybutyrate / hydroxyvalerate copolymers, poly-L-lactide. Polydioxanone, polycarbonates, and polyanhydrides. The biocompatible and absorbable protein is at least one protein selected from the group consisting of collagen, fibrinogen, fibronectin, vitronectin, laminin, and gelatin. In one embodiment the coil is composed of the biocompatible and absorbable polymer or protein with a radio-opaque material is disposed thereon. Alternatively, the coil is composed of a radio-opaque material, and the biocompatible and absorbable polymer or protein is disposed thereon. This apparatus may be positioned within intracranial aneurysms or any aneurysm in the body as well as within other body cavities.
Owner:RGT UNIV OF CALIFORNIA
Bioabsorbable and biocompatible polyurethanes and polyamides for medical devices
ActiveUS20060188547A1Low toxicitySuture equipmentsOrganic active ingredientsAbsorbable polymersPolyester
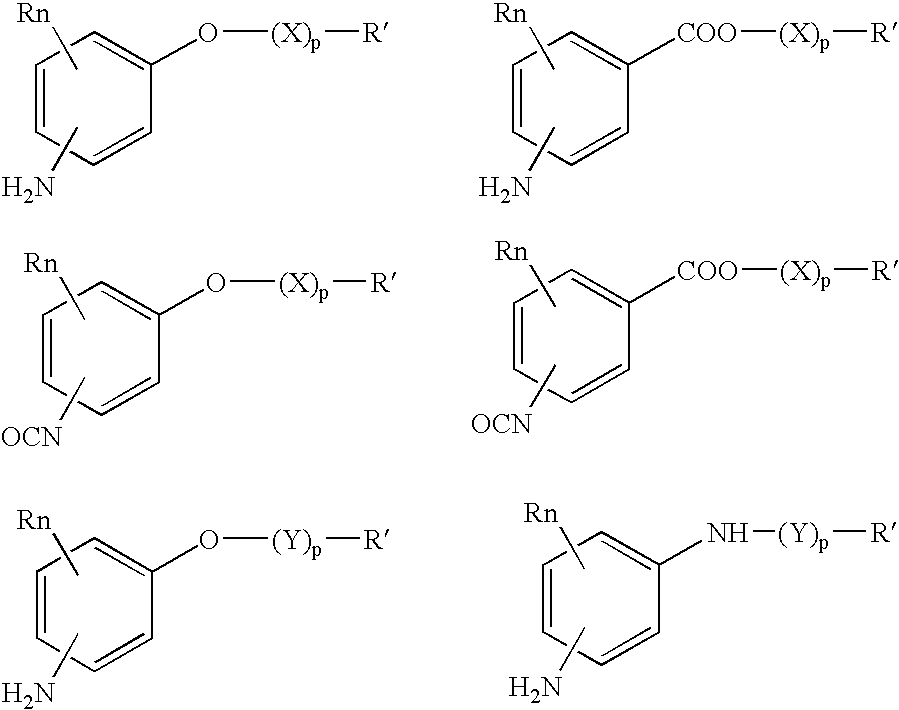
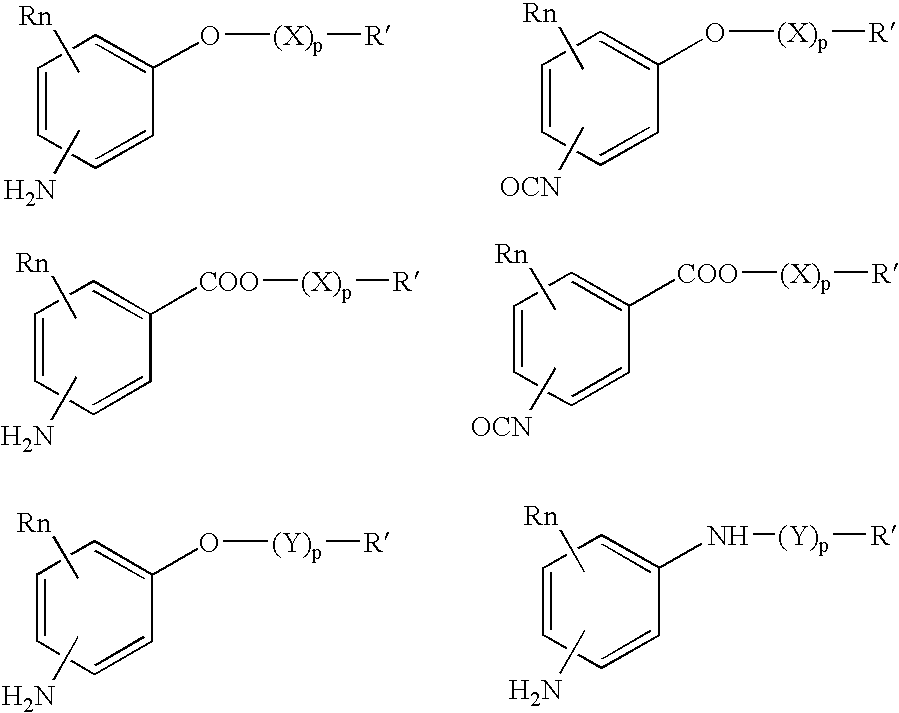
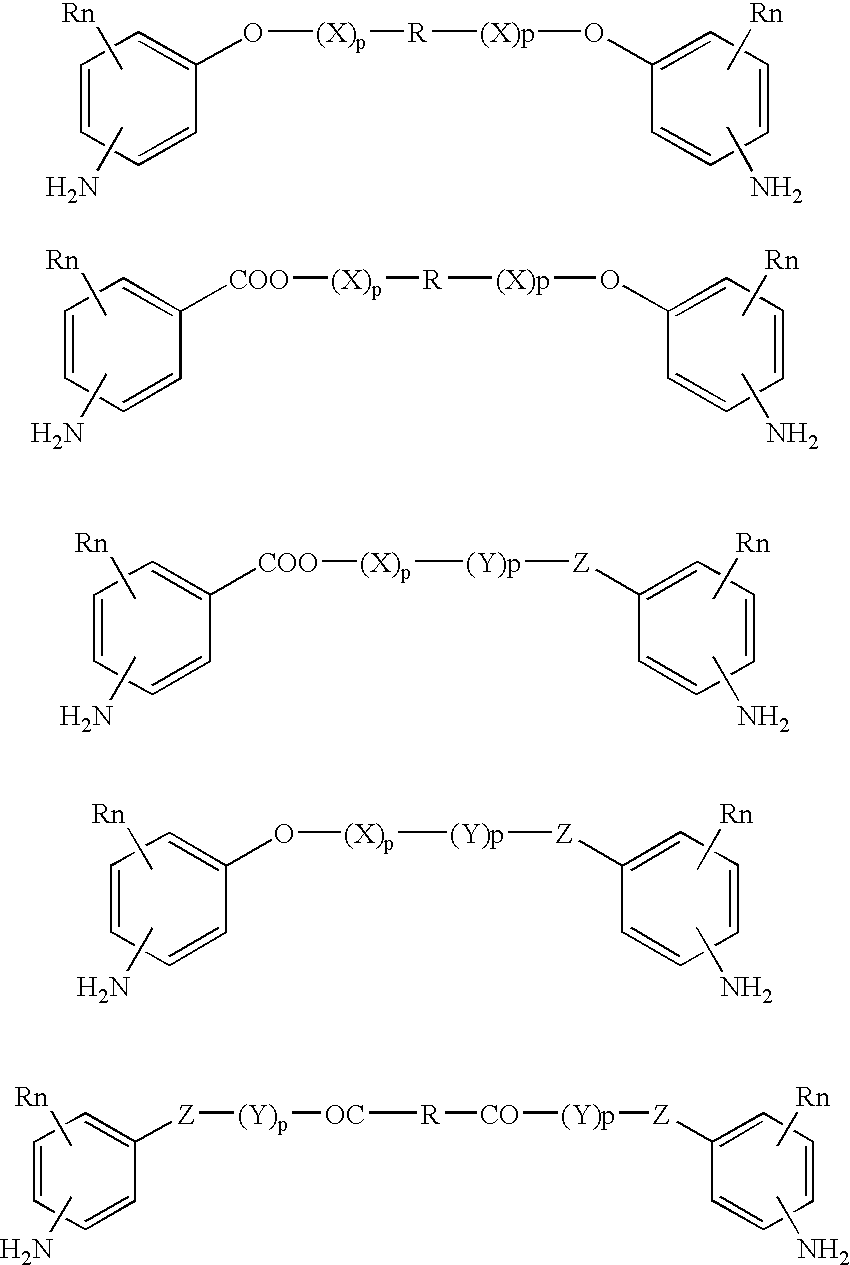
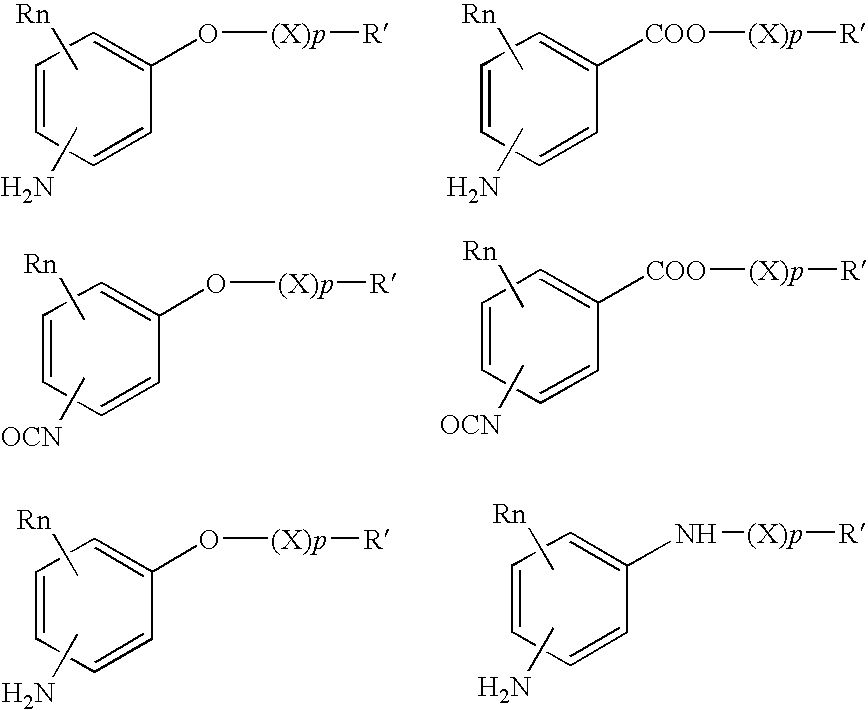
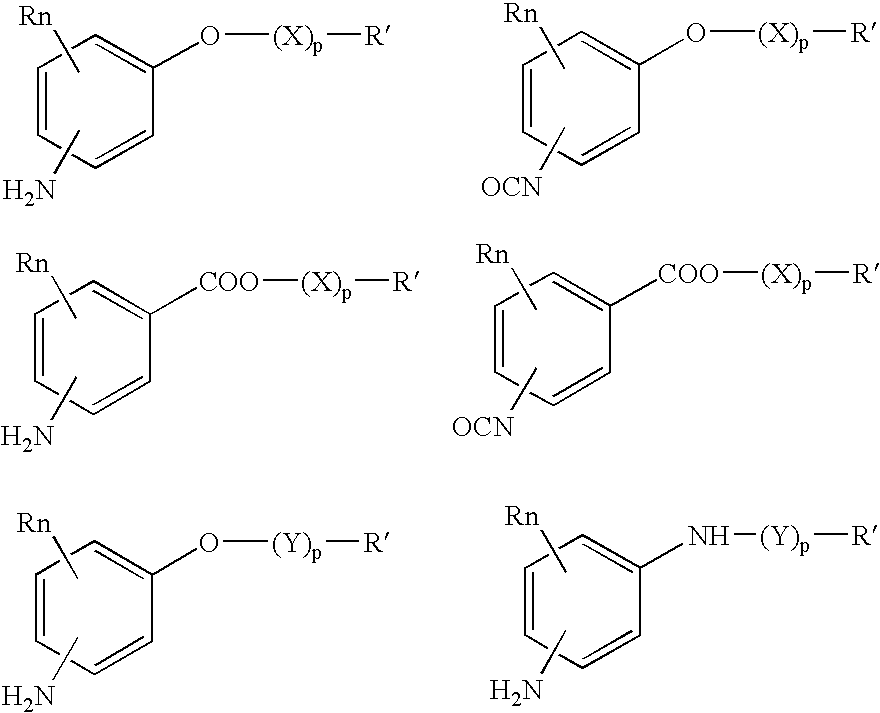
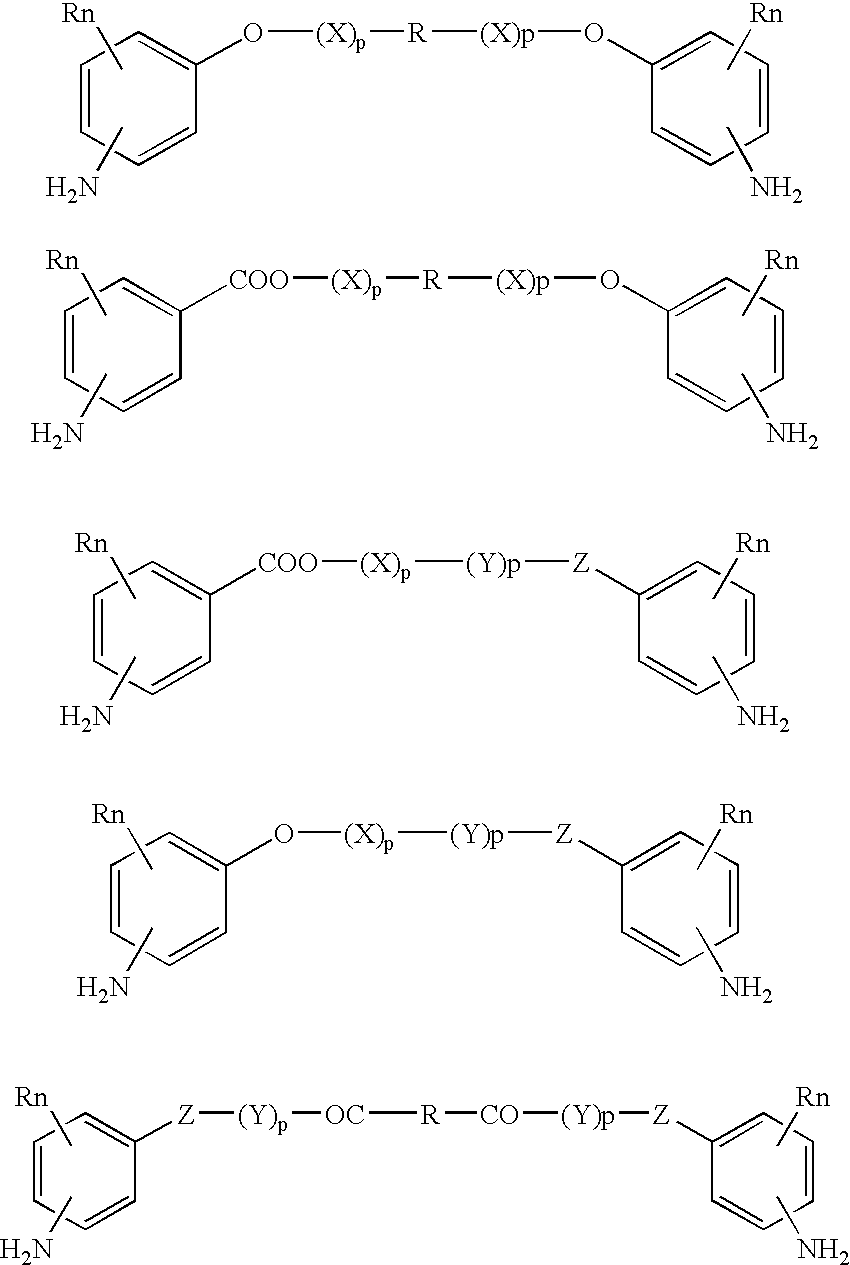
Absorbable polyurethanes, polyamides and polyester urethanes prepared from at least one compound selected from: or the diamines and diisocyanates thereof, wherein each X represents a member independently selected from —CH2COO— (glycolic acid moiety), —CH(CH3)COO— (lactic acid moiety), —CH2CH2OCH2COO— (dioxanone), —CH2CH2CH2CH2CH2COO— (caprolactone moiety), —(CH2)yCOO— where y is one of the numbers 2, 3, 4 or 6-24 inclusive, and —(CH2CH2O)z′CH2COO— where z′ is an integer between 2 and 24, inclusive; each Y represents a member independently selected from —COCH2O— (glycolic ester moiety), —COCH(CH3)O— (lactic ester moiety), —COCH2OCH2CH2O— (dioxanone ester), —COCH2CH2CH2CH2CH2O— (caprolactone ester), —CO(CH2)mO— where m is an integer between 2, 3, 4 or 6-24 inclusive, —COCH2O(CH2CH2O)n— where n is an integer between 2 and 24, inclusive; R′ is hydrogen, benzyl or an alkyl group, the alkyl group being either straight-chained or branched; p is an integer between 1 and 4, inclusive; and Rn represents one or more members selected from H, alkoxy, benzyloxy, aldehyde, halogen, carboxylic acid and —NO2, which is attached directly to an aromatic ring or attached through an aliphatic chain. Absorbable polymers prepared from these compounds are useful for drug delivery, tissue engineering, tissue adhesives, adhesion prevention and other implantable medical devices.
Owner:BEZWADA BIOMEDICAL LLC
Polymeric, degradable drug-eluting stents and coatings
ActiveUS7618448B2Improve performanceImprove propertiesSuture equipmentsPharmaceutical containersAbsorbable polymersEthylene Homopolymers
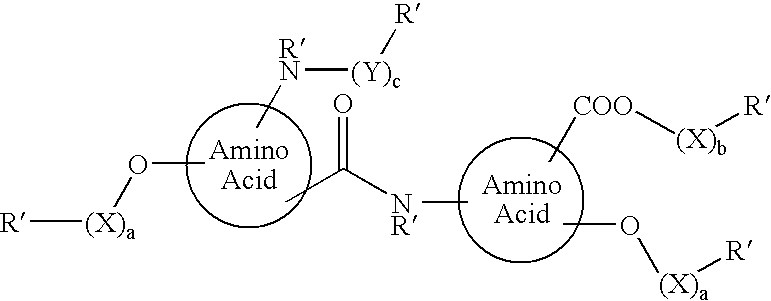
Absorbable stents and absorbable stent coatings have been developed with improved properties. These devices preferably comprise biocompatible copolymers or homopolymers of 4-hydroxybutyrate, and optionally poly-L-lactic acid and other absorbable polymers and additives. Compositions of these materials can be used to make absorbable stents that provide advantageous radial strengths, resistance to recoil and creep, can be plastically expanded on a balloon catheter, and can be deployed rapidly in vivo. Stent coatings derived from these materials provide biocompatible, uniform coatings that are ductile, and can be expanded without the coating cracking and / or delaminating and can be used as a coating matrix for drug incorporation.
Owner:TEPHA INC
Bioabsorbable and biocompatible polyurethanes and polyamides for medical devices
Absorbable polyurethanes, polyamides and polyester urethanes prepared from at least one compound selected from:or the diamines and diisocyanates thereof, wherein each X represents a member independently selected from —CH2COO— (glycolic acid moiety), —CH(CH3)COO— (lactic acid moiety), —CH2CH2OCH2COO— (dioxanone), —CH2CH2CH2CH2CH2COO— (caprolactone moiety), —(CH2)yCOO— where y is one of the numbers 2, 3, 4 or 6-24 inclusive, and —(CH2CH2O)z′CH2COO— where z′ is an integer between 2 and 24, inclusive; each Y represents a member independently selected from —COCH2O— (glycolic ester moiety), —COCH(CH3)O— (lactic ester moiety), —COCH2OCH2CH2O— (dioxanone ester), —COCH2CH2CH2CH2CH2O— (caprolactone ester), —CO(CH2)mO— where m is an integer between 2, 3, 4 or 6-24 inclusive, —COCH2O(CH2CH2O)n— where n is an integer between 2 and 24, inclusive; R′ is hydrogen, benzyl or an alkyl group, the alkyl group being either straight-chained or branched; p is an integer between 1 and 4, inclusive; and Rn represents one or more members selected from H, alkoxy, benzyloxy, aldehyde, halogen, carboxylic acid and —NO2, which is attached directly to an aromatic ring or attached through an aliphatic chain. Absorbable polymers prepared from these compounds are useful for drug delivery, tissue engineering, tissue adhesives, adhesion prevention and other implantable medical devices.
Owner:BEZWADA BIOMEDICAL LLC
Concentration gradient profiles for control of agent release rates from polymer matrices
The present invention generally encompasses the control of the release rate of agents from a polymeric matrix. This control over the release rate of agents provides for control over, inter alia, the therapeutic, prophylactic, diagnostic, and ameliorative effects that are realized by a patient in need of such treatment. In addition, the control of the release rate of agents also has an effect upon the mechanical integrity of the polymeric matrix, as well as a relationship to a subject's absorption rate of the absorbable polymers.
Owner:ABBOTT CARDIOVASCULAR
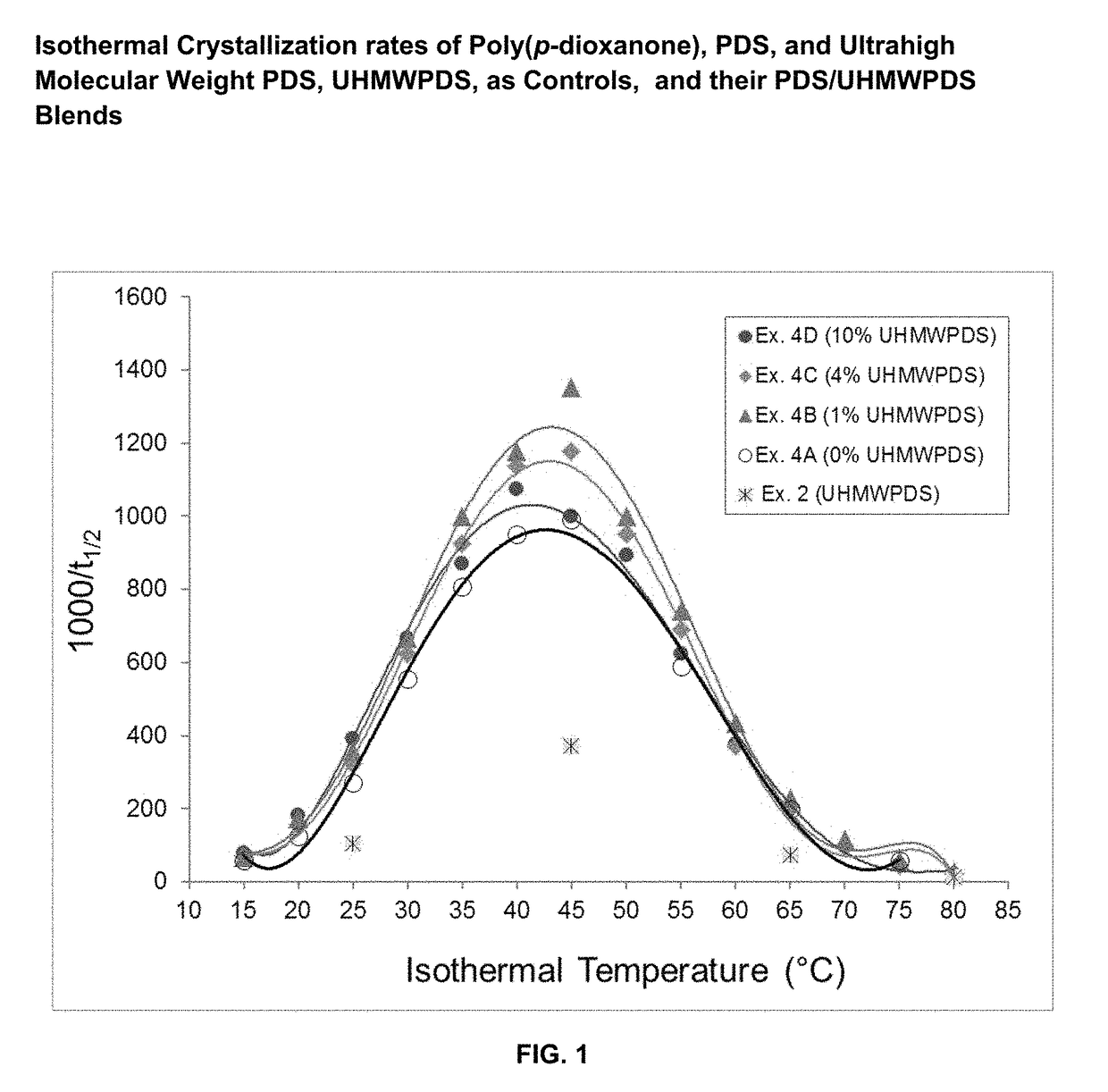
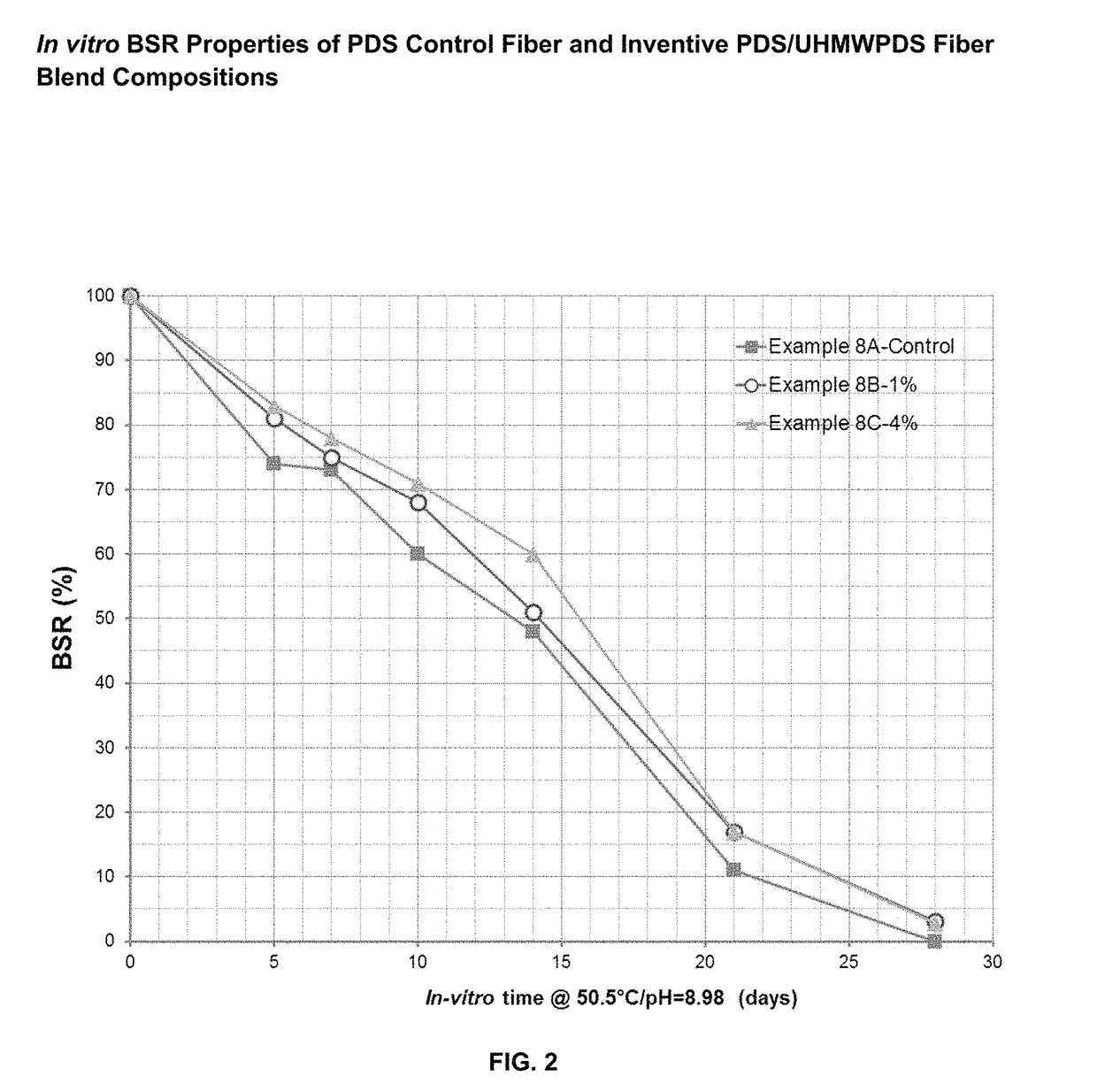
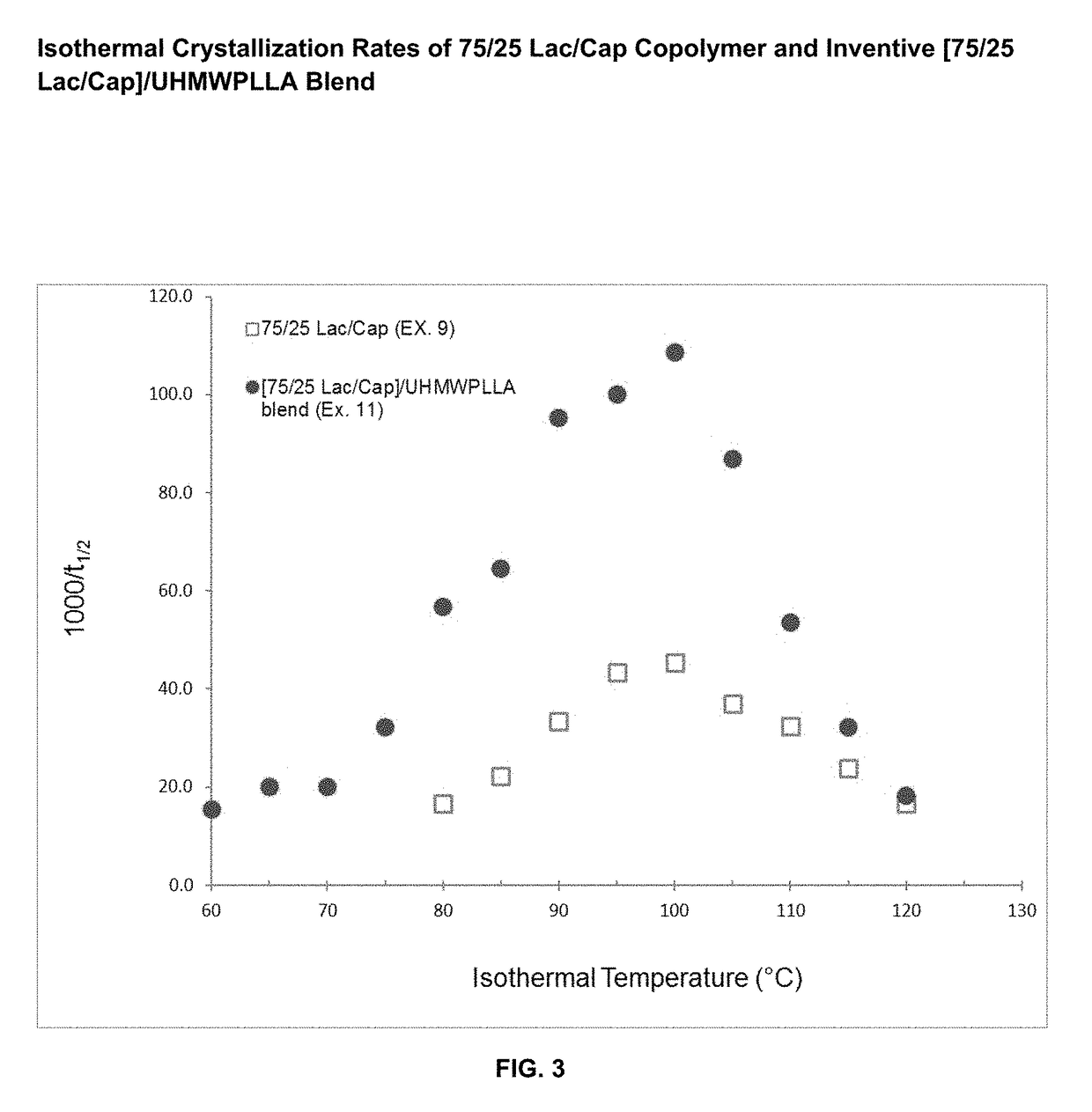
Absorbable polymer blend compositions having enhanced nucleation rates
ActiveUS9873790B1Increase the rate of crystallizationHigh crystallization kineticsSuture equipmentsPharmaceutical non-active ingredientsAbsorbable polymersFiber
Novel absorbable, semi-crystalline, polymer blend compositions are disclosed exhibiting enhanced crystallization and nucleation rates. Also disclosed are medical device constructs, such as fibers made from such blends. The blends have a first absorbable polymeric component having a first molecular weight distribution and a second absorbable polymeric component which has an ultrahigh molecular weight distribution. The first and second polymeric components may be the same polymer.
Owner:ETHICON INC
Hemostatic microfibrous constructs
Elastomeric fibrous constructs are formed from multicomponent, non-woven nano- / microfibers having a core and sheath configuration made primarily of an absorbable polymer and preferably a water-soluble one, respectively. Most preferably, the nano- / microfibers are produced by electrostatic spinning. Depending on clinical use of these constructs, suitable bioactive agents can be incorporated into the construct.
Owner:POLY MED
Polymeric, Degradable Drug-Eluting Stents and Coatings
ActiveUS20070185561A1Improve radial strengthRisk minimizationSuture equipmentsPharmaceutical containersAbsorbable polymersEthylene Homopolymers
Absorbable stents and absorbable stent coatings have been developed with improved properties. These devices preferably comprise biocompatible copolymers or homopolymers of 4-hydroxybutyrate, and optionally poly-L-lactic acid and other absorbable polymers and additives. Compositions of these materials can be used to make absorbable stents that provide advantageous radial strengths, resistance to recoil and creep, can be plastically expanded on a balloon catheter, and can be deployed rapidly in vivo. Stent coatings derived from these materials provide biocompatible, uniform coatings that are ductile, and can be expanded without the coating cracking and / or delaminating and can be used as a coating matrix for drug incorporation.
Owner:TEPHA INC
Drugs coated on a device to treat vulnerable plaque
A treatment for vulnerable plaque may include a device having a polymeric coating that contains a statin and / or one or more other drugs, where the statin and / or one or more other drugs may be locally released in a sustained fashion at the point of insertion of the device. The coating may coat a self-expanding or balloon expanding structure, such as a fibrous or thin-film structure, intended to reduce the occurrence or severity of restenosis. The coating may be applied to a gently expanding device that reduces vessel trauma by virtue of exerting a low force of expansion against the vessel wall. The coating may be an absorbable polymer on an implantable device such that the absorbable polymer coating degrades at a specified rate to reduce the risk of “late” thrombosis. An anti-thrombogenic agent may also be disposed on or in the absorbable polymer coating to eliminate residual polymer on the surface after the coating is absorbed.
Owner:PRESCIENT MEDICAL
Medical Implants and Methods of Making Medical Implants
A medical implant device having a substrate with an oxidized surface and a silane derivative coating covalently bonded to the oxidized surface. A bioactive agent is covalently bonded to the silane derivative coating. An implantable stent device including a stent core having an oxidized surface with a layer of silane derivative covalently bonded thereto. A spacer layer comprising polyethylene glycol (PEG) is covalently bonded to the layer of silane derivative and a protein is covalently bonded to the PEG. A method of making a medical implant device including providing a substrate having a surface, oxidizing the surface and reacting with derivitized silane to form a silane coating covalently bonded to the surface. A bioactive agent is then covalently bonded to the silane coating. In particular instances, an additional coating of bio-absorbable polymer and / or pharmaceutical agent is deposited over the bioactive agent.
Owner:BATTELLE MEMORIAL INST
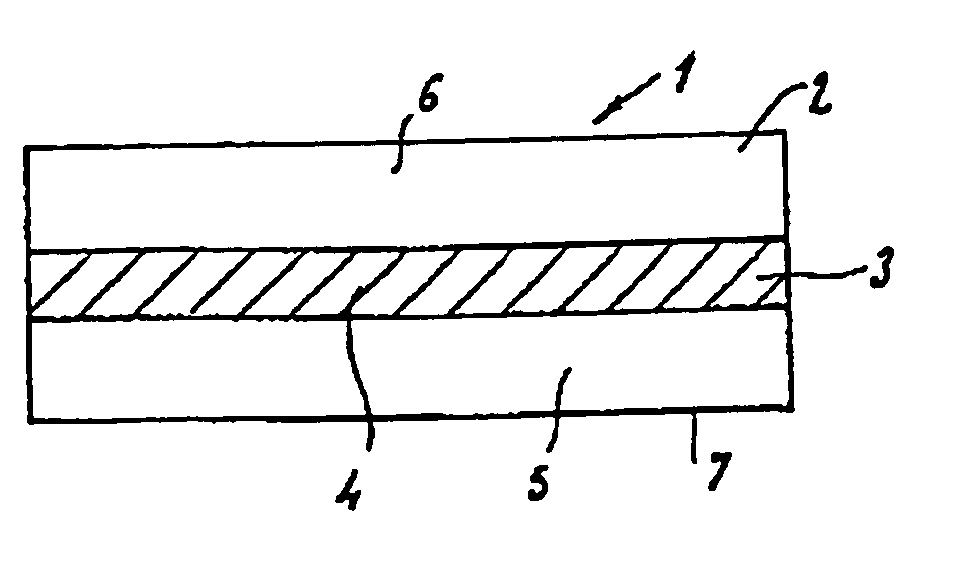
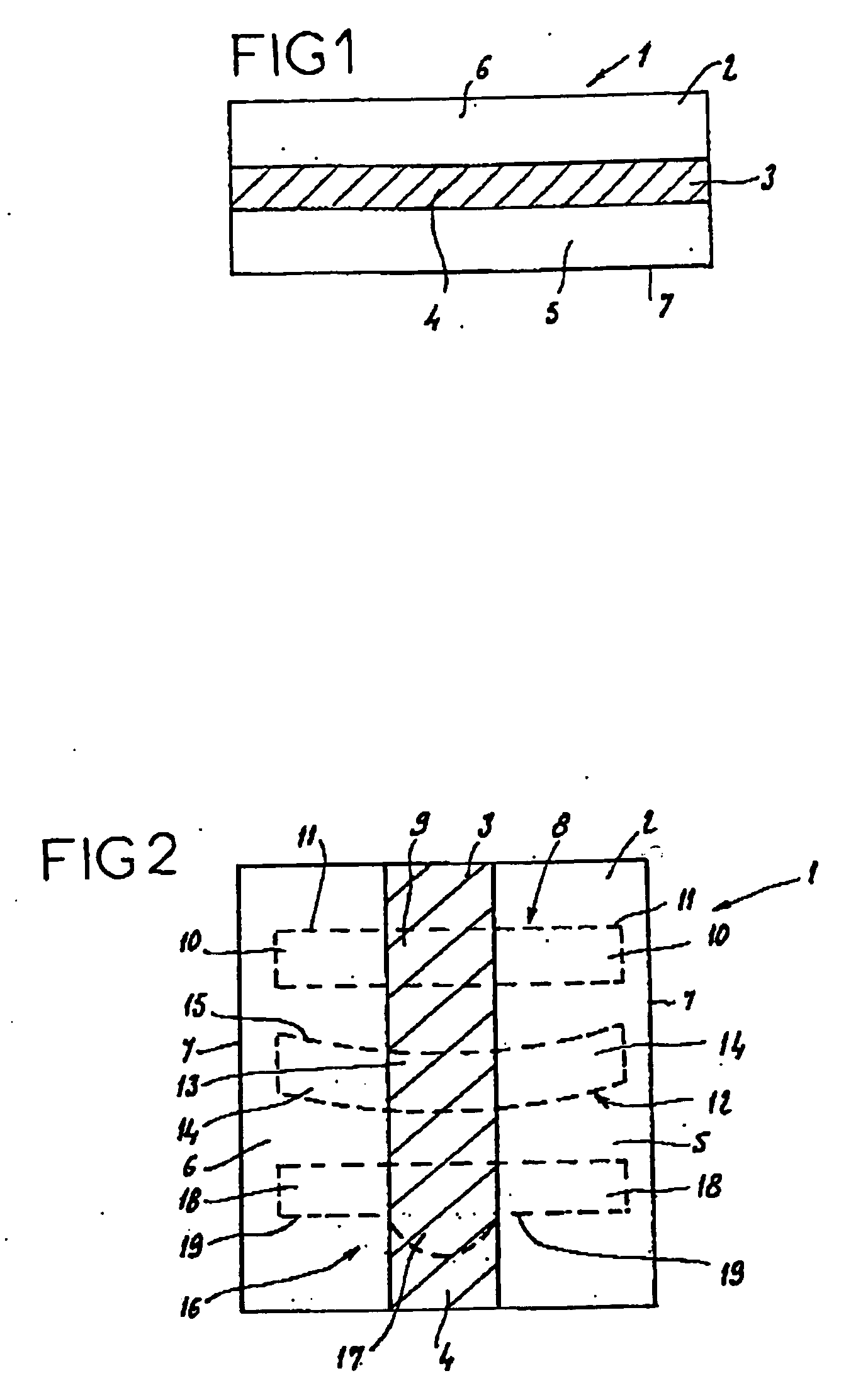
Absorbable fiber reinforced multilayer film material and its prepn
The present invention relates to one kind of biomedical material. The three-layered material includes one middle layer is cloth, net or non-woven fabric of absorbable polymer fiber, and upper and lowr porous layers of absorbable polymer material. The absorbable polymer fiber contains chitin, chitosan, their derivative or other absorbable polymer material, and the absorbable fiber reinforced multilayer film material may contains additive. The absorbable fiber reinforced multilayer film material is prepared through weaving cloth or with polymer fiber, painting polymer solution to the upper and lower sides of the cloth or net, and post-treatment. It has high strength and is suitable for use in preventing tissue adhesion.
Owner:SOUTHEAST UNIV
Medical implants and methods of making medical implants
A medical implant device having a substrate with an oxidized surface and a silane derivative coating covalently bonded to the oxidized surface. A bioactive agent is covalently bonded to the silane derivative coating. An implantable stent device including a stent core having an oxidized surface with a layer of silane derivative covalently bonded thereto. A spacer layer comprising polyethylene glycol (PEG) is covalently bonded to the layer of silane derivative and a protein is covalently bonded to the PEG. A method of making a medical implant device including providing a substrate having a surface, oxidizing the surface and reacting with derivitized silane to form a silane coating covalently bonded to the surface. A bioactive agent is then covalently bonded to the silane coating. In particular instances, an additional coating of bio-absorbable polymer and / or pharmaceutical agent is deposited over the bioactive agent.
Owner:BATTELLE MEMORIAL INST
Biodegradable and absorbable polymer superfine fibre film with radioactive nuclide marker and preparation and use thereof
InactiveCN101301496AImprove effectivenessRadioactiveSurgeryRadioactive preparation carriersFiberAbsorbable polymers
The invention relates to a bio-degradable and bio-absorbable macromolecule superfine fiber film with a radioactive nuclide mark and a method for making the same as well as medical use. The invention comprises the bio-degradable and bio-absorbable macromolecule superfine fiber film material or a composite superfine fiber film material with physically embedding radioactive nuclide mark, or the bio-degradable and bio-absorbable macromolecule superfine fiber film material or the composite superfine fiber film material with chelate radioactive nuclide mark having a bi-functional group coupling agent with surface chemical modification. The film material is a non-woven material consisting of fibers with diameters ranging from scores of nanometers to thousands of nanometers which is made through an electrostatic spinning process, and the radioactive nuclide is compounded inside the fibers or on the surfaces of the fibers by means of physics and chemic. The fiber film material with the radioactive nuclide mark is covered at a tumour part or a lesion tissue part after resection, thereby effectively killing the residual tumour cells, and playing roles in stanching, healing wound and preventing adhesion and so on.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Medical materials sterilized by radiaton and their ways in use
InactiveUS20020197296A1Efficient mixingImprove mechanical propertiesSuture equipmentsImpression capsAbsorbable polymersBiological body
Present invention provides medical material sterilized by radioisotope, comprising polymer composite using in living body, containing multifunctional triazine compounds at weight ratio range of 0.01 to 20 weight percent to the polymer. The present invention shows the fabrication of polymer composite having good heat and radiation resistance, by preventing heat molding record and irradiation on sterilized processes from deteriorating molecular weight caused on heat and radiation decomposition of the polymer. It is possible that the polymer composite is applied for the medical field of decomposable and bio-absorbable polymers and even bio-nonabsorbent polymers such as suture of operation or bonding agent for broken bone as a result. Furthermore, it is possible that the polymer composite is applied for not only medical material but also food wrapping material of industrial use.
Owner:BERTELSMANN MUSIC GROUP
Hard tissue repairing material and its prepn
InactiveCN1403167APromote bone healingPromote absorptionSurgeryProsthesisAbsorbable polymersPolyester
The present invention relates to surgical material. The material includes biological absorbable polymer 10-85 wt%, un-sintered calcium phosphate salt 5-80 wt%, and demineralized bone 1-70 wt%. The biologically absorbable polymer includes polyester, chitosan, hondroitin sulfate, collagen, alginatel, etc.; and the calcium phosphate salt is un-sintered hydroxyapatite or tricalcium phosphate of granularity less than 100 microns. The hard tissue repairing material is prepared through mixing biologically absorbable polymer, un-sintered calcium phosphate salt and demineralized bone dispersed in solvent and freeze drying to obtain the hard tissue repairing material. The composition has the function of promoting bone healing and excellent biocompatibility.
Owner:SOUTHEAST UNIV
Inorganic-organic hybrid micro-/nanofibers
InactiveUS20070112115A1Material nanotechnologyMonocomponent polyetheresters artificial filamentElectrospinningMelt blowing
Inorganic-organic hybrid micro- / nanofibers having a cross-sectional area not exceeding 140μ2 include micro- / nanoparticulate inorganic components dispersed in an organic, absorbable or non-absorbable polymeric matrix at a weight concentration of at least about 4 percent by weight and are produced by electrostatic spinning or melt-blowing.
Owner:POLY MED
Intermediate composite part for forming reinforcement prosthesis
The present invention relates to an intermediate composite part (1) designed for forming a composite reinforcement prosthesis and comprising: i) a porous textile support (2) which includes an arrangement of threads each composed of at least one filament of nonabsorbable polymer material, said textile support defining the free edge or edges (7) of the part, and ii) a hydrophilic absorbable material (3) covering said textile support, at least on one side of the latter, extending across the surface of what is called a protected zone (4) and creating, on each side of the latter, two unprotected zones (5, 6) of the textile support which are limited by said free edges and are devoid of any of said absorbable material. It also relates to a process for preparing such a part.
Owner:SOFRADIM PROD SAS
Polymeric, degradable drug-eluting stents and coatings
ActiveUS20090012604A1Improve radial strengthRisk minimizationSurgeryCoatingsAbsorbable polymersEthylene Homopolymers
Absorbable stents and absorbable stent coatings have been developed with improved properties. These devices preferably comprise biocompatible copolymers or homopolymers of 4-hydroxybutyrate, and optionally poly-L-lactic acid and other absorbable polymers and additives. Compositions of these materials can be used to make absorbable stents that provide advantageous radial strengths, resistance to recoil and creep, can be plastically expanded on a balloon catheter, and can be deployed rapidly in vivo. Stent coatings derived from these materials provide biocompatible, uniform coatings that are ductile, and can be expanded without the coating cracking and / or delaminating and can be used as a coating matrix for drug incorporation.
Owner:TEPHA INC
Absorbable active tissue matter for repairing hard tissue and its prepn
The present invention relates to surgical material. The material includes biologically absorbable polymer 10-90 wt%, un-sintered calcium phosphate salt 5-80 wt%, and bone growth factor less than 10 wt%. The biologically absorbable polymer includes polyester, chitosan, chondroitin sulfate, collagen, alginate, etc.; and the calcium phosphate salt is un-sintered hydroxyapatite or tricalcium phosphate of granularity less than 100 microns. The hard tissue repairing material is prepared through mixing biologically absorbably polymer, un-sintered calcium phosphate salt and bone growth factor dispersed in solvent, molding, freeze drying to eliminate solvent and obtain the composition. The composition has the function of promoting bond healing and excellent biocompatibility.
Owner:SOUTHEAST UNIV
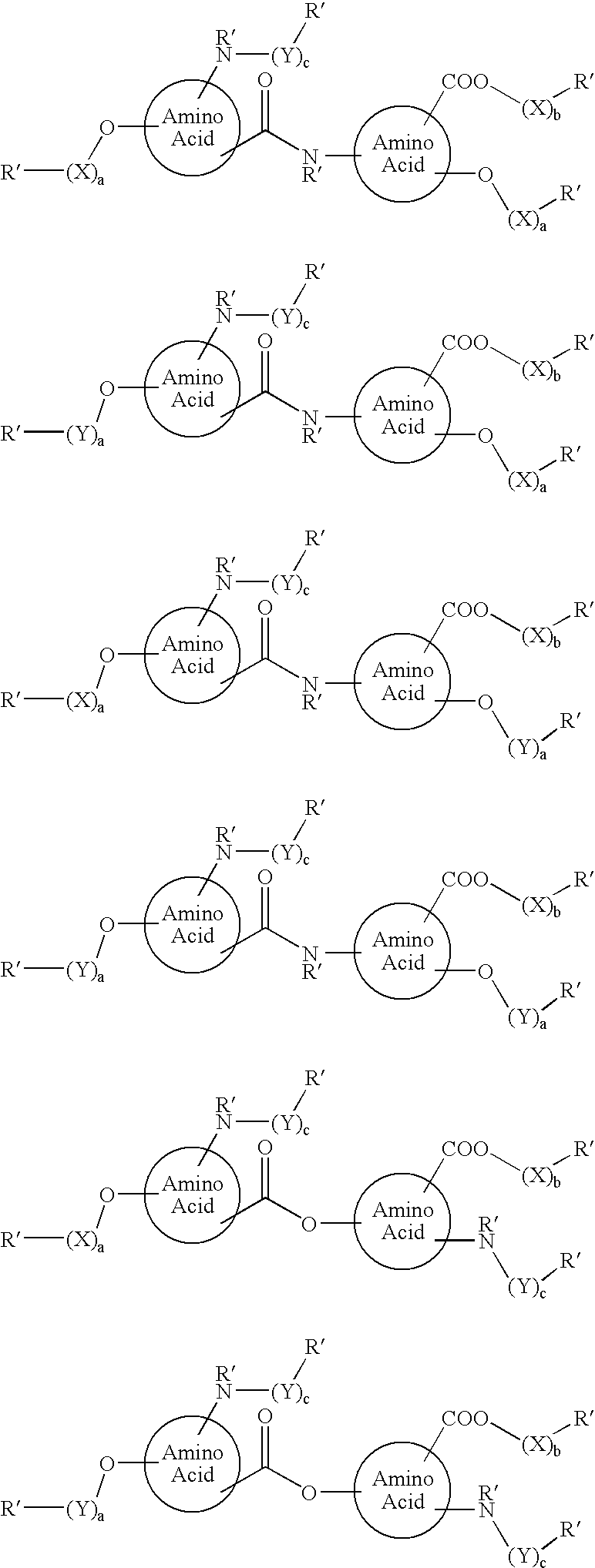
Functionalized diphenolics and absorbable polymers therefrom
InactiveUS20070135355A1Useful applicationBiocidePeptide/protein ingredientsAbsorbable polymersTime range
The present invention relates to dephenolic compounds, an example of which is shown below, which are functionalized, and polymers formed from the same. Polymers formed from the functionalized diphenolics are expected to have controllable degradation profiles, enabling them to release an active component over a desired time range. The polymers are also expected to be useful in a variety of medical applications.
Owner:BEZWADA BIOMEDICAL LLC
Medical materials sterilized by radiation and their ways in use
InactiveUS6897245B2Good heat and radiation resistanceSuture equipmentsImpression capsAbsorbable polymersDecomposition
Present invention provides medical material sterilized by radioisotope, comprising polymer composite using in living body, containing multifunctional triazine compounds at weight ratio range of 0.01 to 20 weight percent to the polymer.The present invention shows the fabrication of polymer composite having good heat and radiation resistance, by preventing heat molding record and irradiation on sterilized processes from deteriorating molecular weight caused on heat and radiation decomposition of the polymer. It is possible that the polymer composite is applied for the medical field of decomposable and bio-absorbable polymers and even bio-nonabsorbent polymers such as suture of operation or bonding agent for broken bone as a result. Furthermore, it is possible that the polymer composite is applied for not only medical material but also food wrapping material of industrial use.
Owner:BMG INC
Active composition for repairing hard tissue and its prepn
InactiveCN1403165APromote bone healingPromote absorptionSurgeryProsthesisSurgical materialAbsorbable polymers
The present invention relates to surgical material. The material includes biological absorbable polymer 10-90 wt%, un-sintered calcium phosphate salt 9-80 wt% and active matter less than 50 wt%. The biologically absorbable polymer includes polyester, chitosan, chondroitin sulfate, collagen, alginate, etc.; and the calcium phosphate salt is un-sintered hydroxyapatite or tricalcium phosphate of granularity less than 100 microns. The hard tissue repairing material is prepared through mixing biologically absorbable polymer, un-sintered calcium phosphate salt, etc. dispersed in solvent, freeze drying to eliminate solvent and obtain porous material and mixing with active matter. The composition has the function of promoting bone healing and excellent biocompatibility.
Owner:SOUTHEAST UNIV
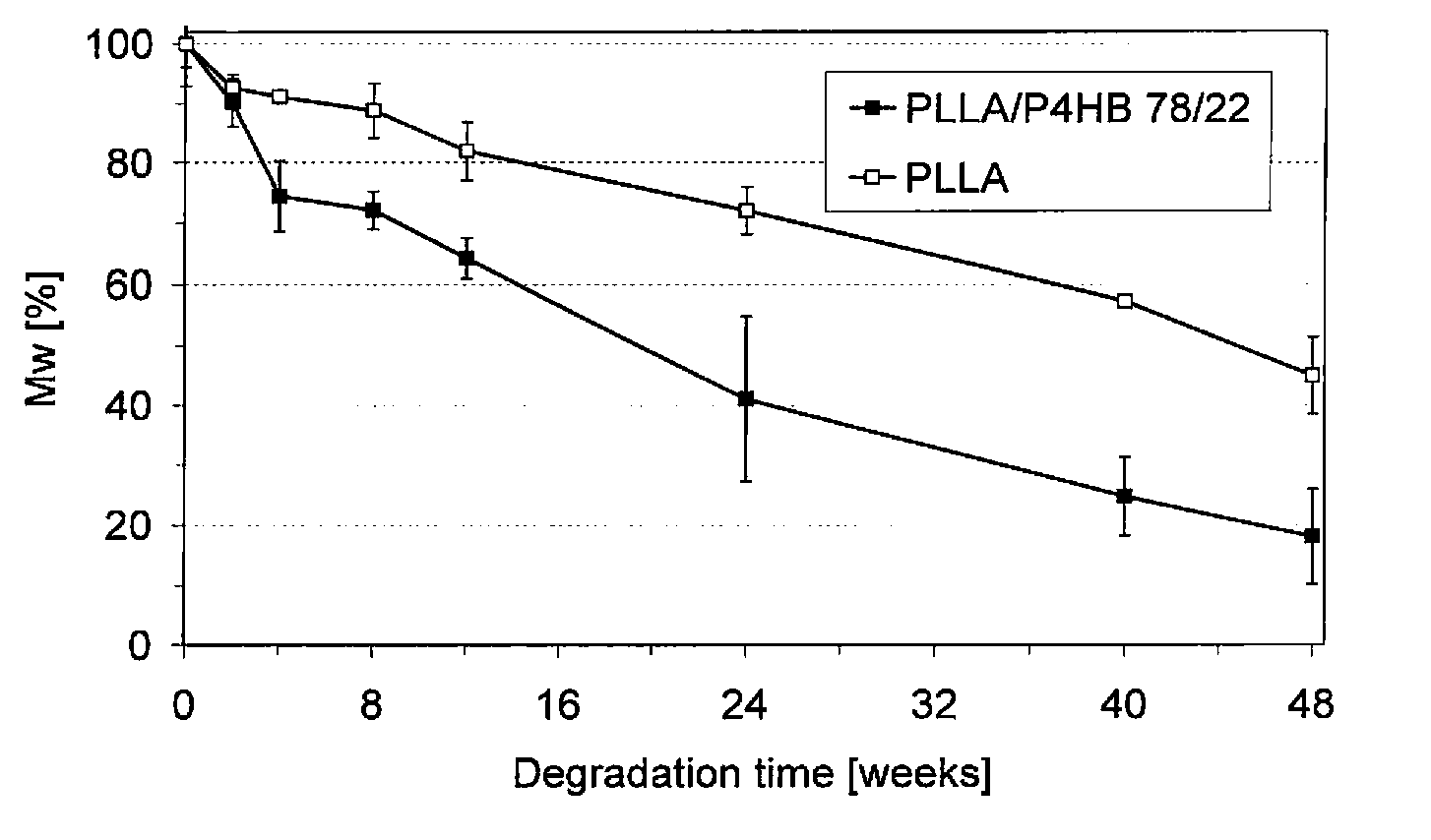
Mechanically Strong Absorbable Polymeric Blend Compositions of Precisely Controllable Absorption Rates, Processing Methods, And Products Therefrom
ActiveUS20130315963A1Improve mechanical propertiesHigh breaking strengthSuture equipmentsBiocideBreaking strengthAbsorbable polymers
Novel absorbable polymer blends are disclosed. The blends are useful for manufacturing medical devices having engineered degradation and breaking strength retention in vivo. The blends consist of a first absorbable polymeric component and a second absorbable polymeric component. The weight average molecular weight of the first polymeric component is higher than the weight average molecular weight of the second polymeric component. At least at least one of said components is at least partially end-capped by a carboxylic acid group. Further aspects are medical devices made therefrom.
Owner:CILAG GMBH INT
Absorbable polymer formulations
A co-polyester which includes the reaction product of a polycondensation polyester and epsilon-caprolactone, wherein the polycondensation polyester comprises the reaction product of diglycolic acid and / or a derivative thereof and a diol. The co-polyester is injectable and absorbable into animal, such as human, tissue and can be used for facial cosmetic or reconstructive surgery of soft tissue. Another embodiment is directed to a method for preventing adhesion using a co-polyester comprising the reaction product of a polycondensation polyester and epsilon-caprolactone, wherein the polycondensation polyester comprises the reaction product of diglycolic acid and / or a derivative thereof and a diol, and the co-polyester comprises about 40 to 50% by weight of the polycondensation polyester based on the total weight of the co-polyester.
Owner:ETHICON INC
Concentration Gradient Profiles For Control of Agent Release Rates From Polymer Matrices
The present invention generally encompasses methods of coating which control of the release rate of agents from a polymeric matrix. This control over the release rate of agents provides for control over, inter alia, the therapeutic, prophylactic, diagnostic, and ameliorative effects that are realized by a patient in need of such treatment. In addition, the control of the release rate of agents also has an effect upon the mechanical integrity of the polymeric matrix, as well as a relationship to a subject's absorption rate of the absorbable polymers.
Owner:ABBOTT CARDIOVASCULAR
Prestress-reinforced light high-strength controllable-degradation medical composite material and preparation method thereof
InactiveCN103330959AMake full use of the strengthening effectReduce dosageSurgeryAbsorbable polymersProtection layer
The invention relates to a prestress-reinforced light high-strength controllable-degradation medical composite material and a preparation method thereof. The medical composite material is characterized in that a magnesium alloy wire subjected to prestress processing is taken as a reinforced phase to improve strength and stiffness of the composite material, an absorbable high polymer material is taken as a matrix, and meanwhile, the early-stage degradation velocity of the composite material can be further regulated and controlled by regulating the thickness of a shell protection layer formed by the high polymer material of the matrix. According to the composite material, bars or plates are manufactured by utilizing methods of thermal die pressing, extrusion or drawing or the like, and various degradable high-toughness bone repairing and fixing instruments such as bone nails and bone plates can be obtained by carrying out subsequent machining; and compared with the conventional absorbable polymer bone surgery instruments, the composite material has the advantages that the mechanical fixing effect is relatively good, and furthermore, the problems that the degradation speed of the absorbable magnesium alloy bone surgery instruments is difficult to control and the hydrogen release quantity during degradation is large and the like can be overcome.
Owner:SOUTHEAST UNIV
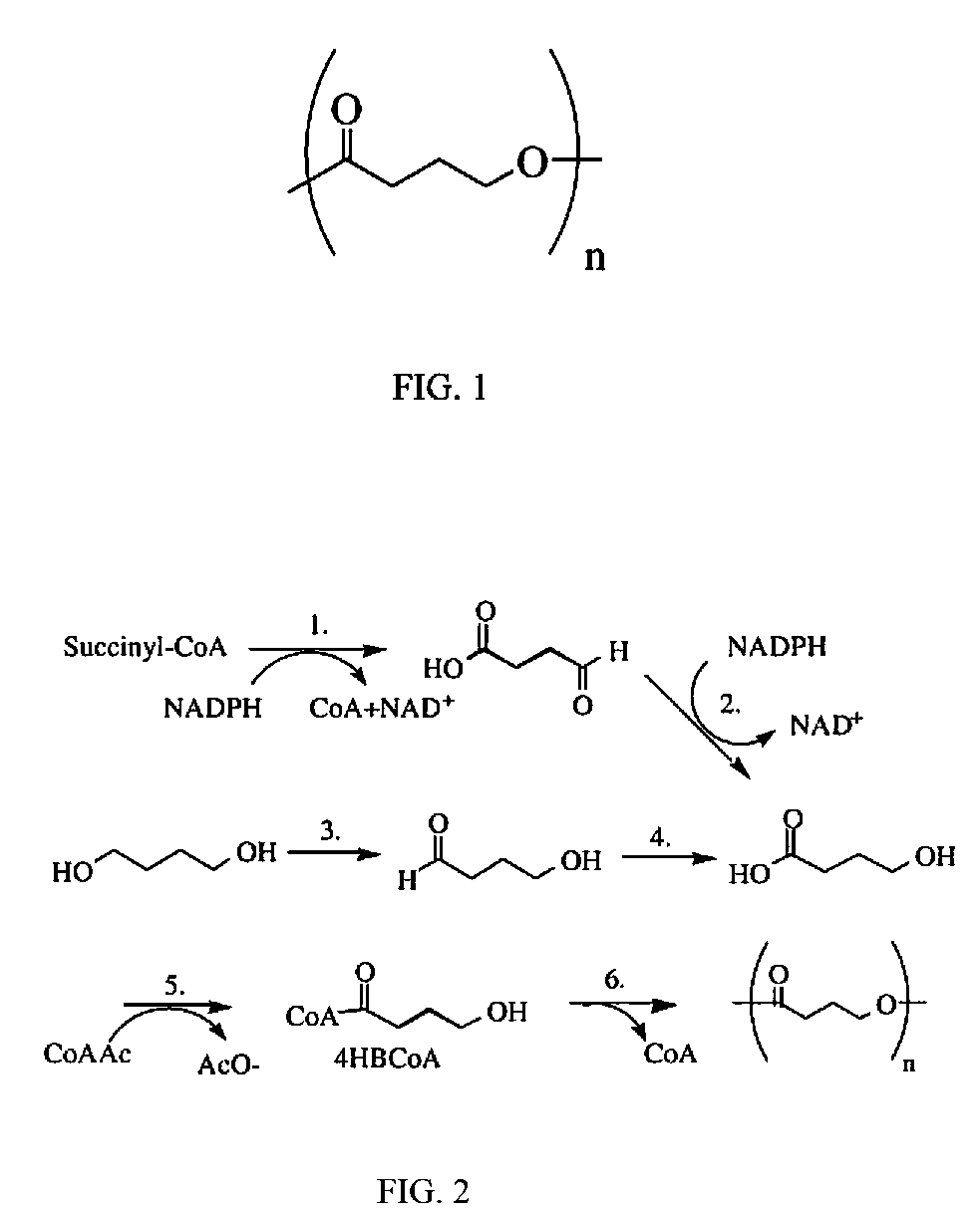
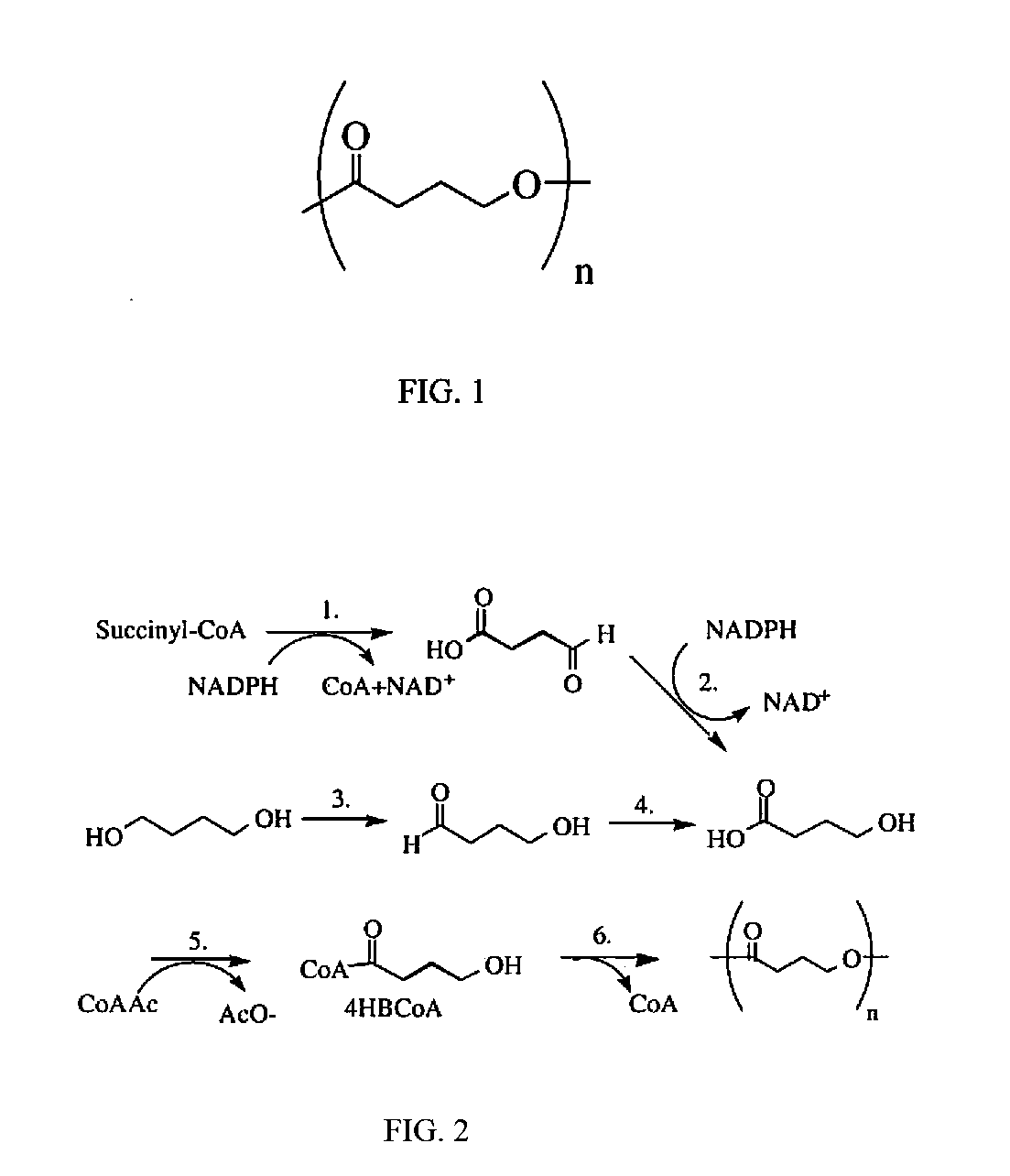
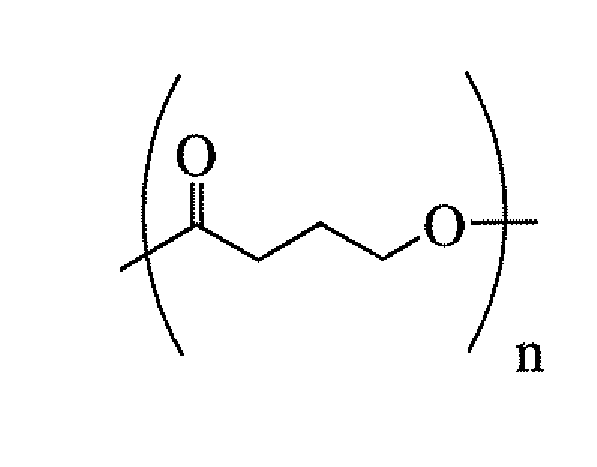
Medical devices containing dry spun non-wovens of poly-4-hydroxybutyrate and copolymers
ActiveUS20120150285A1Improve toughnessImprove cohesionSurgeryCatheterAbsorbable polymersPolymer science
Continuous processing methods for making absorbable polymeric dry spun non-wovens with one or more of the following properties: high burst strength, fine fibers of average diameter from 0.01 μm to 50 μm, and thickness from 10 μm to 10 mm, have been developed. Improved fiber cohesion is made possible by controlling the tackiness of the fibers of the non-woven during web collection. The polymer is preferably a polyhydroxyalkanoate, more preferably, a 4-hydroxybutyrate polymer or copolymer. A non-woven of poly-4-hydroxybutyrate is most preferred. The non-wovens have fine fibers with average diameters ranging from 0.01 μm to 50 μm, and are derived by dry spun processing, during which a solution of polymer(s) is injected into a stream of high velocity air with a pressure of 1 to 500 psi for solvent stripping and polymer strand attenuation. The non-wovens can be used for a variety of purposes including fabrication of medical devices.
Owner:TEPHA INC
Absorbable microparticles
This invention pertains to a sustained release complex of one or more peptides, one or more proteins or a combination thereof immobilized on an absorbable polymer microparticle optionally having an absorbable polymer coating. The microparticle complex of this invention comprises a peptide(s) and / or protein(s) which have at least one amino group and / or at least one carboxyl group per molecule and a solid absorbable polyester microparticle having surface and subsurface carboxylic group or amino groups in sufficient amounts to bind the peptide(s) and / or protein(s) so that the immobilized peptide(s) or protein(s) represent 0.1% to 30% of the total mass of the microparticle complex. The microparticle complex with immobilized peptide(s) and / or protein(s) are optionally further encased individually or in groups with an absorbable polymer to control, further, the release of the immobilized peptide(s) and / or protein(s). To control the release of the immobilized peptide(s) and / or protein(s) even further, the encased microparticles can be incorporated into a composition with an absorbable gel-forming liquid that transforms to a flexible gel or semi-solid upon contacting water in the biologic environment.
Owner:POLY MED
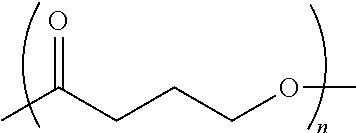
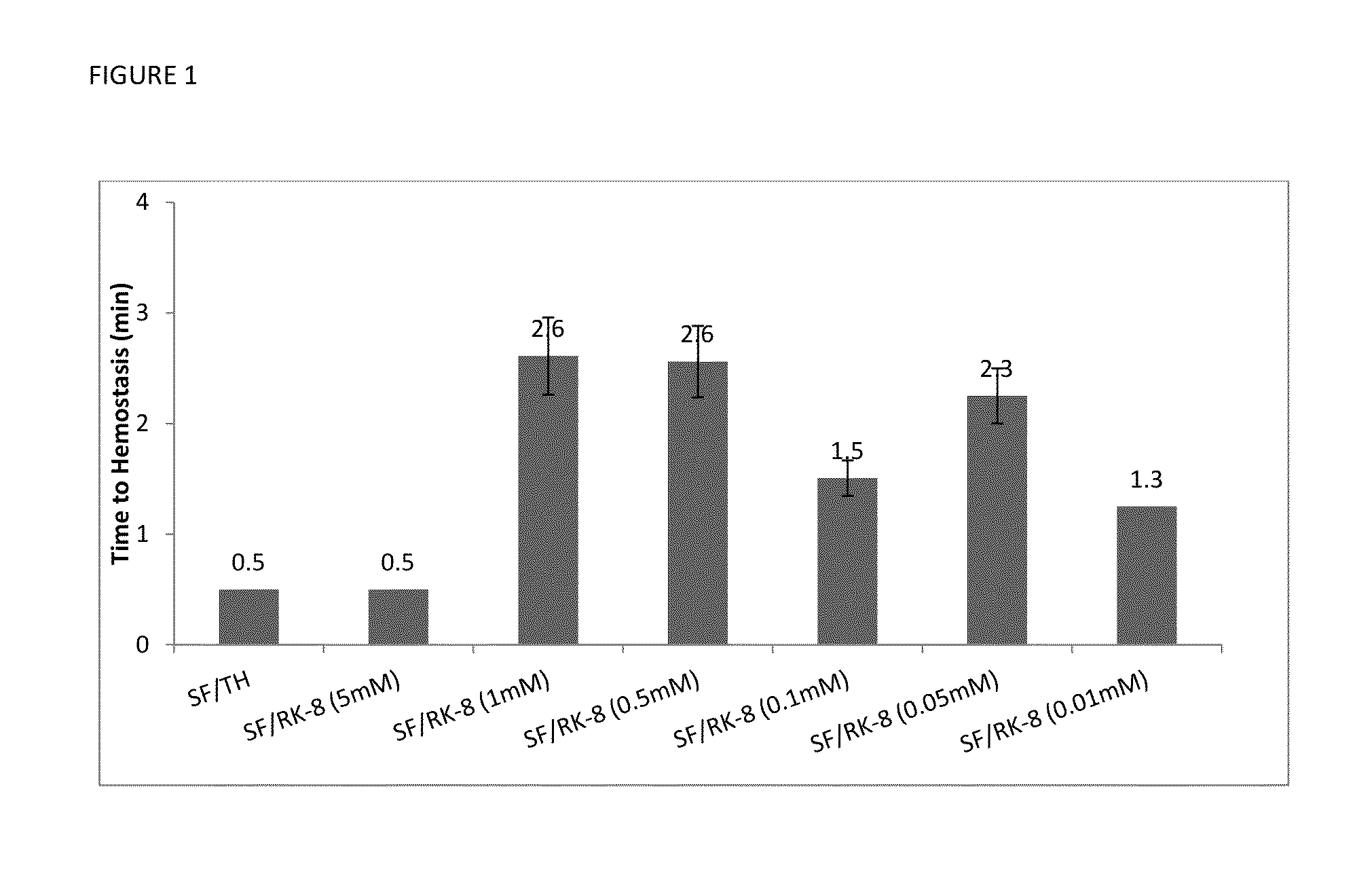
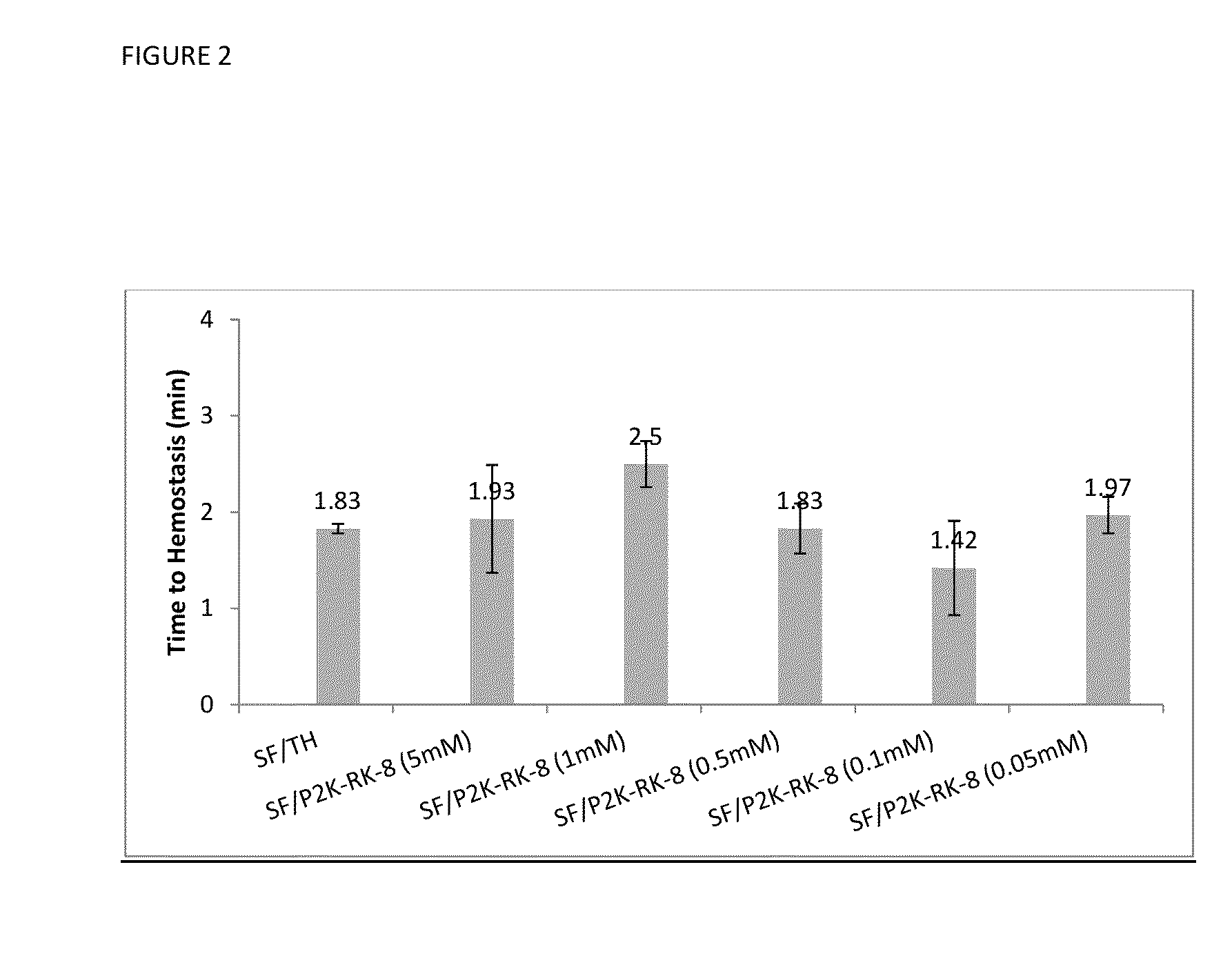
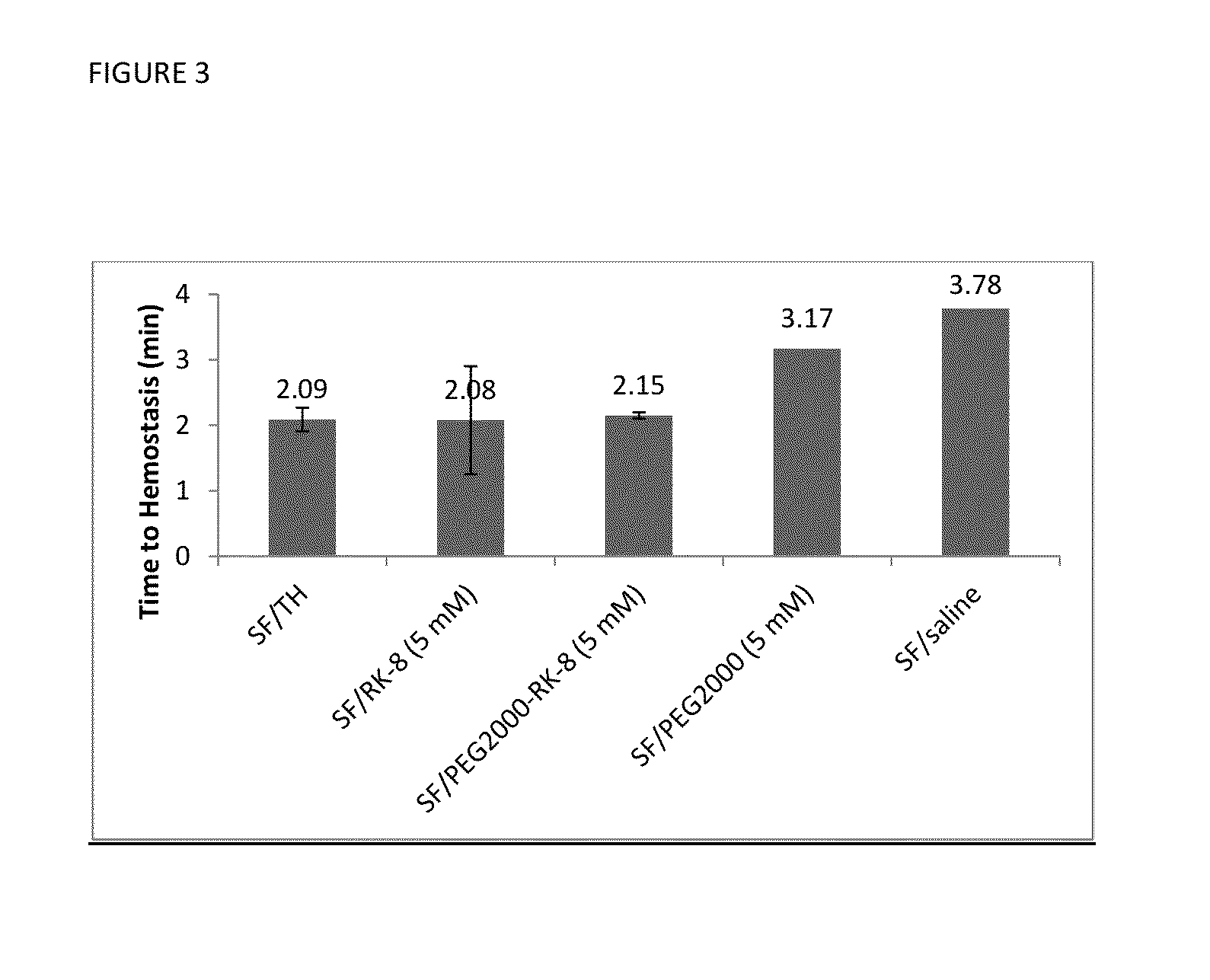
Procoagulant Peptides and Their Derivatives and Uses Therefor
ActiveUS20130004478A1Improve solubilityCost effectiveFibrinogenSurgical adhesivesAbsorbable polymersGenetically engineered
The present invention is directed to a hemostatic or tissue sealing material having (a) a peptide having a sequence SEQ ID NO: 1 or an amino acid analog sequence thereof, and (b) a scaffold for said peptide or amino acid analogue sequence. The scaffold is preferably hemostatic, such as a natural or genetically engineered absorbable polymer, a synthetic absorbable polymer, or combinations thereof. The natural or genetically engineered absorbable polymers can be selected from the group consisting of a protein, a polysaccharide, or combinations thereof.
Owner:ETHICON INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com