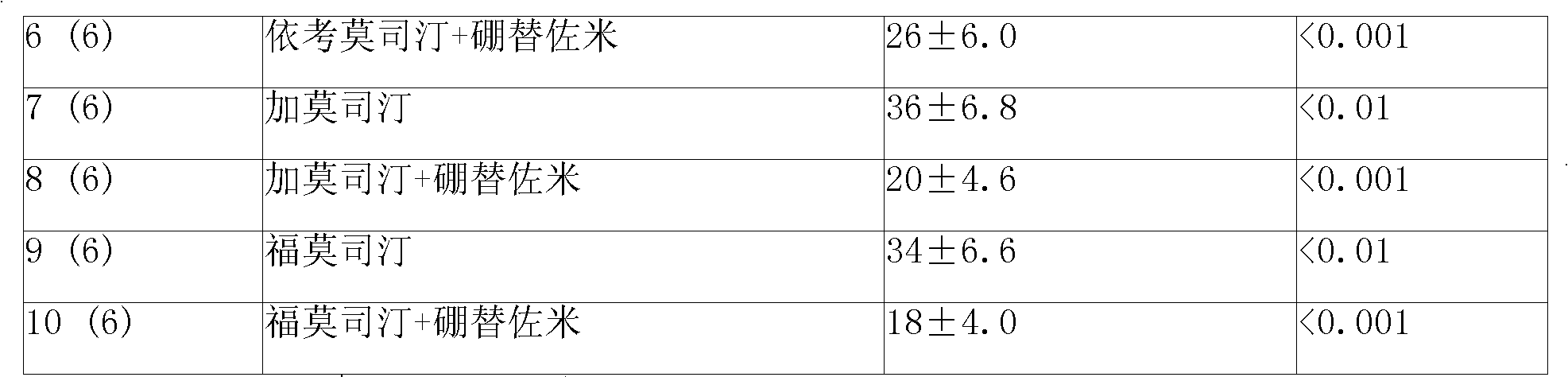Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
41 results about "Nimustine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nimustine (INN) is a nitrosourea alkylating agent.
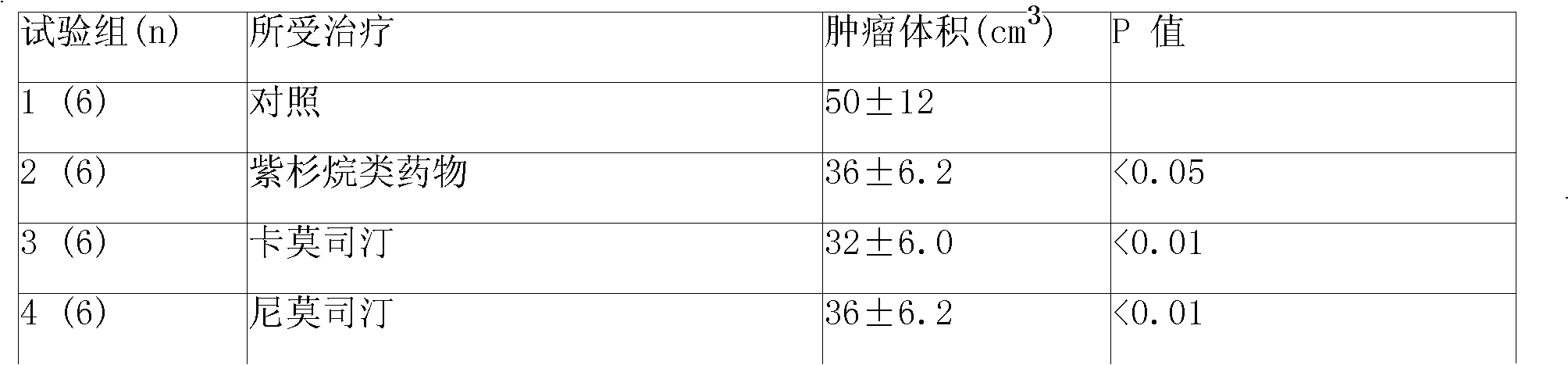
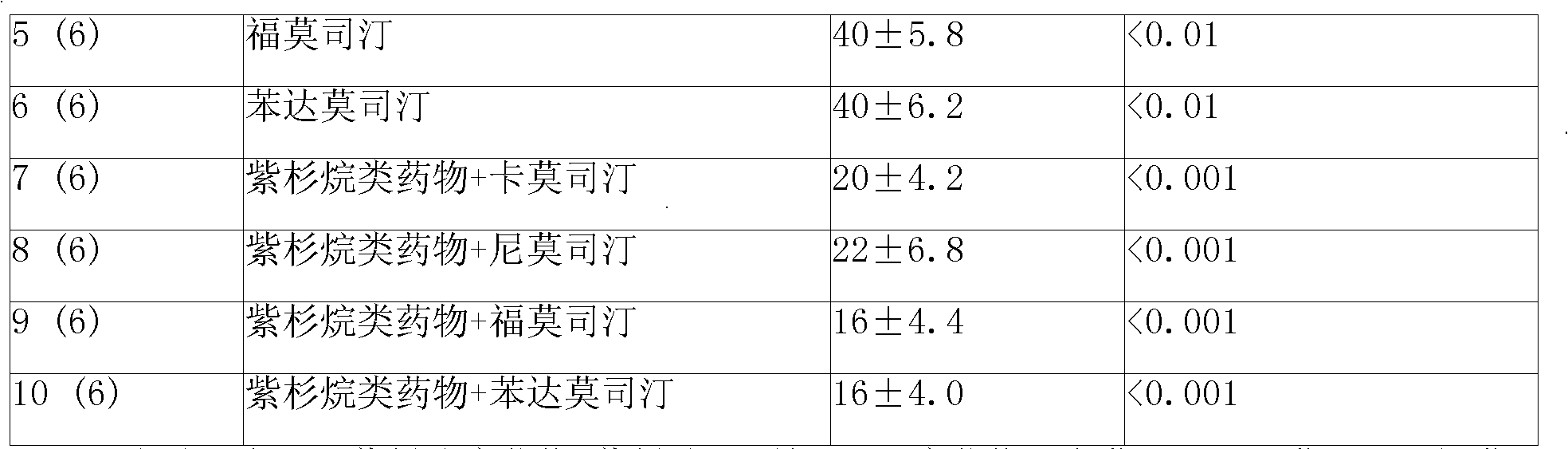
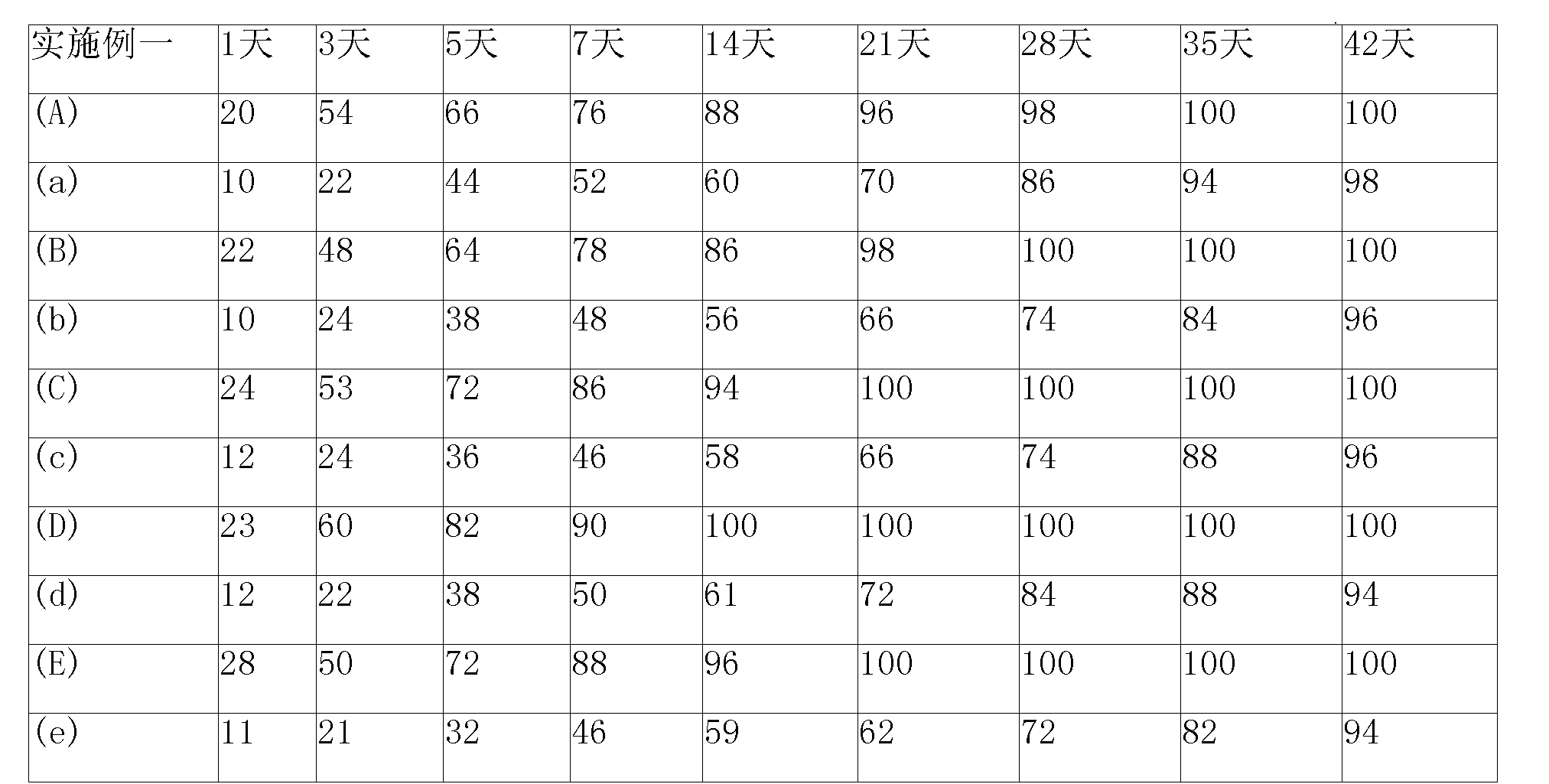
Anticancer sustained-release gel injection containing stines medicine
The invention relates to an anticancer sustained release gel injection with stine drugs, comprising sustained release microspheres with stine drugs, a amphiphilic block copolymer, solvent and releasing moderator; wherein, the mixture of the amphiphilic block copolymer and the solvent possesses sensitive gelation property; after in vivo injection, the injection can be transformed into nonflowing, biodegradable gel insoluble in water; the insoluble gel can release the contained drugs in local tumor for weeks, even months. Intra-tumor injection or local injection can be used to treat different tumors and unresectable tumors, control late complications and the postoperative residual tumor cell recurrent, reinforce the effect of radiotherapy and chemotherapy and the effect of radiotherapy particles; nimustine and carmustine and other stines; the amphiphilic block copolymer is a PLGA-PEG-PLGA copolymer with the molecular weight of PEG 1200-1600 accounting for 20% of the amphiphilic block copolymer weight; in the poly lactide coglycolide copolymer, the molar ratio of glycolide and lactide is 6:1.
Owner:济南基福医药科技有限公司
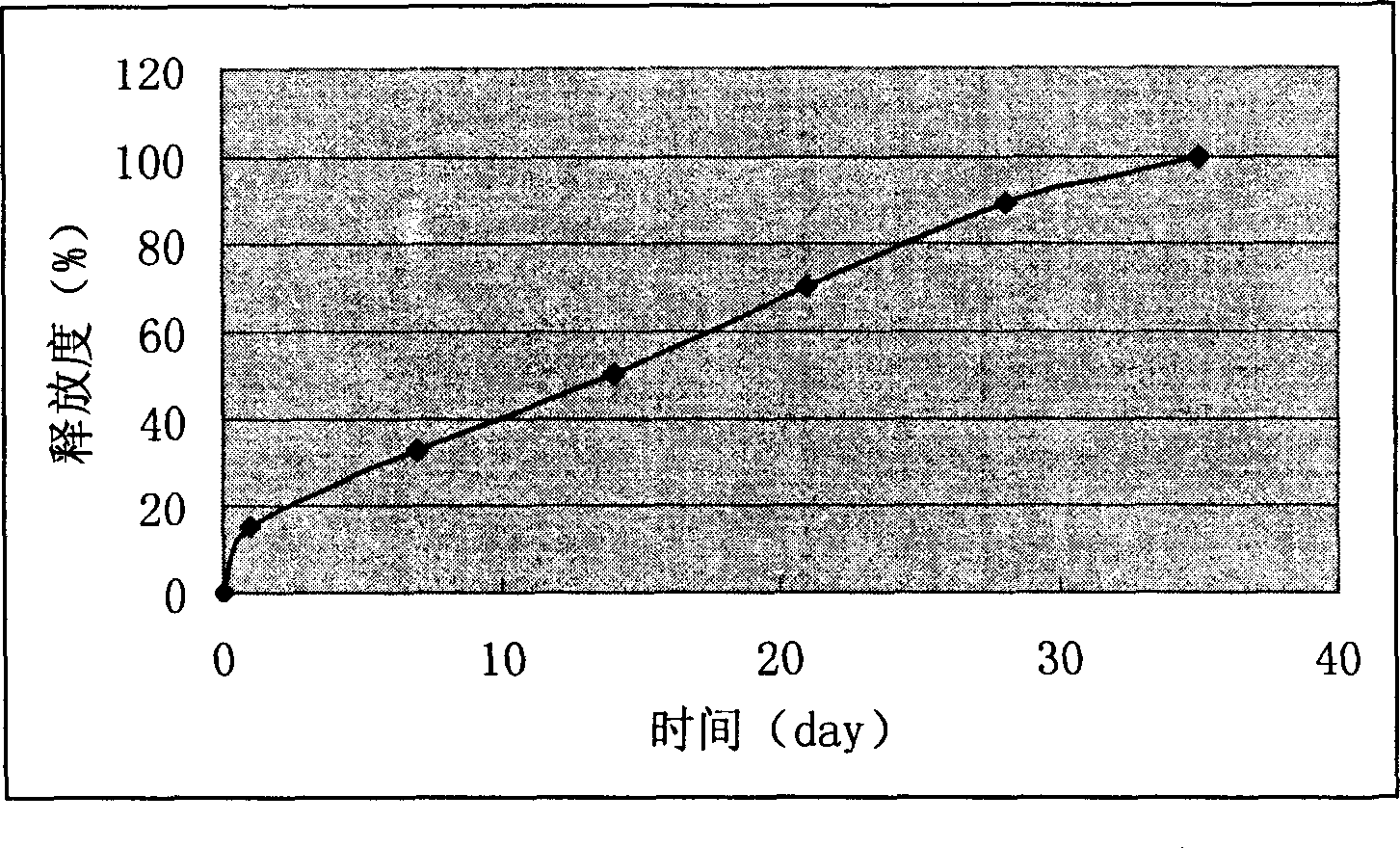
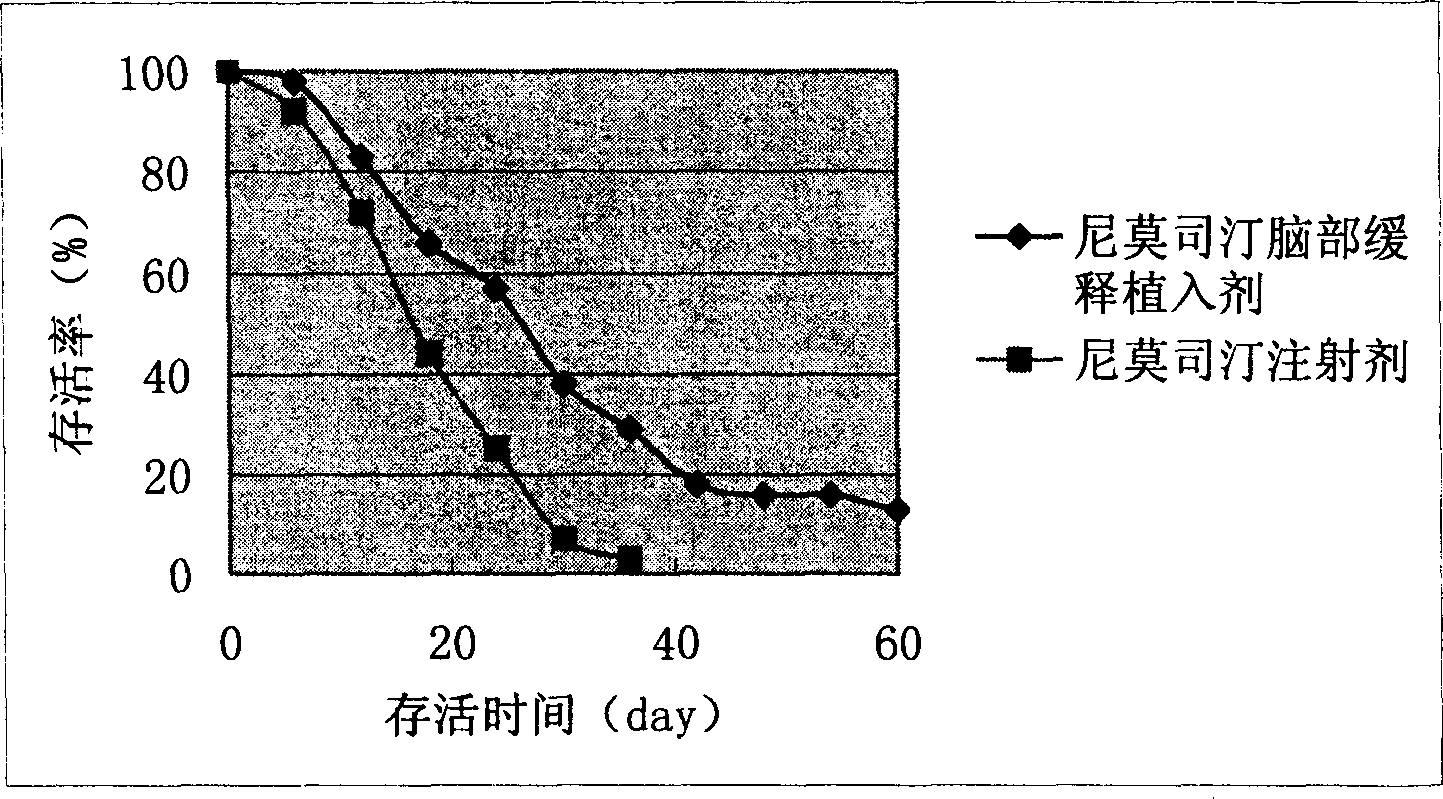
Nimustine brain slow release implantation agent and its preparation method
InactiveCN1686134AInhibit gliomaOrganic active ingredientsPharmaceutical delivery mechanismAcetic acidMicrosphere
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Fluorouracil containing anti-cancer sustained-release injection
InactiveCN101234084AEasy to operateGood repeatabilityOrganic active ingredientsPharmaceutical delivery mechanismPolyethylene glycolSuspending Agents
The invention relates to anticancer sustained release injection which comprises sustained release microspheres and menstruum, wherein, the sustained release microspheres comprise anticancer active components and sustained release auxiliary material; the menstruum is special menstruum that contains suspending agent. The anticancer active components are fotemustine, nimustine, carmustine or combination of bendamustine and mitozolomide, docetaxel, etoposide, teniposide, vinblastine, anastrozole, tamoxifen, fluorouracil or mitomycin C; the sustained release auxiliary material is polylactic acid and polylactic acid copolymer, polyethylene glycol and polylactic acid copolymer of polyethylene glycol, terminal carboxyl group polylactic acid copolymer, EVAc, fatty acid and decanedioic acid copolymer, etc.; viscosity of the suspending agent is 100cp-3,000cp (at 25 DEG C-30 DEG C), and the suspending agent is selected from sodium carboxymethylcellulose, etc. The sustained release microspheres can also be made into sustained release implant; the injection or implant is injected or placed in or around tumor so as to reduce general reaction of the drug and selectively improve and keep local concentration for about 30-50 days. The anticancer sustained release injection can be used solely and can also promote anti-tumor effects of non-operative treatments, such as chemotherapy and / or radiotherapy, etc.
Owner:JINAN SHUAIHUA PHARMA TECH
Anticancer sustained-released gel injection and preparation method thereof
InactiveCN101336891APharmaceutical delivery mechanismPharmaceutical non-active ingredientsTumor vesselSolvent
The invention relates to an anticancer slow-release gel injection and a preparation method thereof. The anticancer slow-release gel injection contains a taxane-like drug such as paclitaxel, docetaxel, hydroxypaclitaxel, epi-paclitaxel or deacetylpaclitaxel, a stine-like drug, an amphiphilic block polymer and a solvent. The composition exhibits the property of temperature-sensitive gelation, is a flowable liquid at the room temperature and turns into a stagnant and biodegradable water-insoluble gel in vivo in warm-blooded animals. The stine-like drug is selected from atrimustine, ambnmustine, nimustine, bendamustine, carmustine, elmustine, galamustine, fotemustine, estramustine, hesmustine, neptamustine, lomustine, semustine, ranimustine, semustine, tauromustine, tallimustine and spiromustine. The anticancer slow-release gel injection can release the drug slowly and locally around the tumor and retain the effective blood concentration for a plurality of weeks to a plurality of months, can not only kill tumor cells but also effectively inhibit tumor vessels, can reduce the general drug toxicity and can enhance the curative effect of chemotherapy particularly radioactive seed implantation.
Owner:济南基福医药科技有限公司
Sustained-release injection containing nitrosourea drugs
The invention provides a sustained-release injection containing nitrosourea drug (galamustine), which contains sustained-release microspheres and solvents. The sustained-release microspheres each comprise an anticancer-active component selected from nitrosourea drugs (such as nimustine and carmustine) and / or topoisomerase inhibitors, and a sustained-release agent. The solvents are common solvents or special solvents containing suspending agent. The viscosity of the suspending agent ranges from 100cp to 3000cp (at a temperature ranging from 20 DEG C to 30 DEG C). The suspending agent is selected from sodium carboxymethylcellulose and the like. The sustained-release agent is selected from p(LAEG-EOP) or p(DAPG-EOP) or other polyphosphate ester copolymers, or copolymer or blend of polyphosphate ester and PLA, polifeprosan, PLGA or poly(erucidic acid dipolymer-sebacic acid). The topoisomerase inhibitor is selected from camptothecin, hydroxycamptothecine, topotecan, lartotecan, irinotecan, etoposide or teniposide. The anticancer composition is also available in the dosage form of sustained-release implant, can retain the effective drug concentration for more than 60 days after intratumoral or local injection or implantation, can obviously reduce the systemic reaction to the drug, and can selectively enhance the curative effect of non-operative treatments such as radiotherapy and chemotherapy.
Owner:JINAN SHUAIHUA PHARMA TECH
Anticancer slow release agent of nimustine and its progression agent
InactiveCN1919172AEasy injectionIncreased sensitivityOrganic active ingredientsSolution deliveryTreatment effectWhole body
Disclosed is slow release anticancer agent carrying both Nimustine and its synergistic agents, which comprises slow release auxiliary materials and active anticancer constituents, wherein the active anticancer constituents include Nimustine and its synergistic agents (such as Docetaxel, hydroxycamptothecin or 4-hydroperoxycyclophosphamide). The viscosity of the slow release injection is 10-650cp (at 20-30 deg C), and the active anticancer constituents can also be made into slow release implanting agent. The slow release subsidiary materials mainly comprise bio-compactable and degradable macromolecular polymers, when locally dispensed on the tumor, the composition not only can lower down the whole body toxicity reaction of the anti-cancer medicament, but also can selectively increase the tumor local medicinal concentration, the treatment effect of the non-operative treatment methods such as chemotherapy, medicament and radiation can also be improved. The solid tumors include glioma, osteosarcoma, lymphoma, lung carcinoma, intestinal cancer and breast cancer.
Owner:JINAN KANGQUAN PHARMA TECH
Medicinal composition of nimustine and its progression agent
InactiveCN1919171AEasy injectionIncreased sensitivityOrganic active ingredientsSolution deliveryPolymerDrug concentration
Disclosed is a pharmaceutical composition of slow release injection carrying both Nimustine and its synergistic agent, which comprises slow release auxiliary materials and active anticancer constituents, wherein the active anticancer constituents include Nimustine and its synergistic agents (such as Triptorelin, Anastrozole or Decitabine). The viscosity of the slow release injection is 10-650cp (at 20-30 deg C), and the active anticancer constituents can also be made into slow release implanting agent. The slow release subsidiary materials mainly comprise bio-compactable and degradable macromolecular polymers, when locally dispensed on the tumor, the composition not only can lower down the whole body toxicity reaction of the anti-cancer medicament, but also can selectively increase the tumor local medicinal concentration, the treatment effect of the non-operative treatment methods such as chemotherapy, medicament and radiation can also be improved. The solid tumors include hepatic carcinoma, lung carcinoma, breast cancer, cancer of pancreas, intestinal cancer, renal carcinoma, and gastric carcinoma.
Owner:JINAN KANGQUAN PHARMA TECH
Novel anticancer sustained-release agent
InactiveCN101269008AEasy to operateGood repeatabilitySolution deliveryPharmaceutical non-active ingredientsPolyethylene glycolVinorelbine
A new anticarcinogenic sustained-release injection consists of sustained-release microballoon sphere and menstruum; wherein, the sustained-release microballoon sphere comprises anticarcinogenic active ingredient and sustained-release accessory material; the menstruum is special menstruum containing suspending agent. The anticarcinogenic active ingredient is the combination of cytotoxic drugs of fotemustine, bendamustine, tallimustine, carmustinum, nimustine, lomustine, semustine or ranimustine and adriamycin, epirubicin, melphalan, 4H peroxide cyclophosphamide, actinomycin D, vinorelbine or nolvadex, etc.; the sustained-release accessory material is selected from polylactic acid and the multipolymer or mixture thereof, mono-methyl polyethyleneglycol, polyethyleneglycol or polylactic acid multipolymer, EVAc, the multipolymer of fatty acid and decanedioic acid, etc.; the viscosity of the suspending agent is 100cp-3000cp (at the temperature of 25 DEG C-30 DEG C), the suspending agent is selected from sodium carboxymethyl cellulose, etc. The sustained-release microballoon sphere also can be made into sustained-release implant which can be injected and placed in tumor or around tumor and can release medicine at the local parts of the tumor for 30 to 40 days; therefore, the anticarcinogenic sustained-release injection can be separately united together with non-operativetreatment such as chemo-treatment and / or radiation treatment, etc. for application.
Owner:JINAN SHUAIHUA PHARMA TECH
Nimustine sustained-release implantation agent for curing entity tumour
InactiveCN101176712AIncreased sensitivityHigh clinical application valueOrganic active ingredientsPharmaceutical delivery mechanismWhole bodyPotassium
The invention relates to a slow-release implant agent of nimustine, which is characterized in comprising amounts of nimustine, slow-release excipients and releasing regulatory agents in the slow-release implant agent. The excipients comprise macromolecules which are biologically compatible and degradable, mainly PLA and PLGA. The releasing regulatory agents are selected from one or more items from mannitol, sorbitol, xylitol, oligosaccharide, chitin, potassium salt, sodium salt, hyaluronic acid, collagen, chondroitin, gelatin and albumin. The slow-release implant agent slowly releases nimustine on the local tumor in the process of degradation and absorption, so the argent can evidently reduce the systemic toxicity and simultaneously apply to the effective drug concentration control on the local tumor. Therefore, the argent can be applied separately or combined with the non-surgical treatments such as chemotherapy drug and radiotherapy, which can also be widely used for tumor treatment of different phases, not only selectively improving the drug concentration on local tumor but also reinforcing the therapeutic effect of non-surgical treatments such as chemotherapy drug and radiotherapy.
Owner:JINAN SHUAIHUA PHARMA TECH
Slow release injection containing platinum compound and alkylating agent
InactiveCN101011345APharmaceutical delivery mechanismPharmaceutical non-active ingredientsCarboplatinAdjuvant
Disclosed is a slow release injection containing platinum-group compounds and / or alkylating agents, which comprises slow release microspheres and dissolvent, the slow release microspheres include platinum-group compounds selected from Tegafur, Capecittabine, Pemetrexed, Carboplatin or Gemcitabine, and / or alkylating agent anticancer active constituents and slow release auxiliary materials, the dissolvent being conventional dissolvent or specific dissolvent containing suspension adjuvant. The viscosity of the suspension adjuvant is 100-3000cp (at 20-30 deg C), and is selected from sodium carboxymethylcellulose, the slow release auxiliary materials are selected from polyphosphate ester copolymers such as p(LAEG-EOP), p(DAPG-EOP), copolymer or blend of polyphosphate ester with PLA, Polifeprosan, poly(dodecanedioic acid-tetradecanedioic acid) or poly(fumaric acid-sebacylic acid). The alkylating agent is selected from Carmustine, Nimustine, Fotemustine, Lomustine or bendamustine. The anticancer composition can also be prepared into slow release implanting agent, for injection or placement in or around tumor with a period of effective concentration maintenance over 60 days, as well as the treatment effect of appreciably lowering general reaction of the drugs, and improving the treatment effect of the non-operative treatment methods such as chemotherapy.
Owner:JINAN SHUAIHUA PHARMA TECH
Docetaxel-containing anti-cancer sustained-release injection
InactiveCN101234085AEasy to operateGood repeatabilityOrganic active ingredientsPharmaceutical delivery mechanismPolyethylene glycolSuspending Agents
The invention relates to anticancer sustained release injection which comprises sustained release microspheres and menstruum, wherein, the sustained release microspheres comprise anticancer active components and sustained release auxiliary material; the menstruum is special menstruum that contains suspending agent. The anticancer active components are fotemustine, nimustine, carmustine or combination of bendamustine and mitozolomide, docetaxel, etoposide, teniposide, vinblastine, anastrozole, tamoxifen, fluorouracil or mitomycin C; the sustained release auxiliary material is polylactic acid and polylactic acid copolymer, polyethylene glycol and polylactic acid copolymer of polyethylene glycol, terminal carboxyl group polylactic acid copolymer, EVAc, fatty acid and decanedioic acid copolymer, etc.; viscosity of the suspending agent is 100cp-3,000cp (at 25 DEG C-30 DEG C), and the suspending agent is selected from sodium carboxymethylcellulose, etc. The sustained release microspheres can also be made into sustained release implant; the injection or implant is injected or placed in or around tumor so as to reduce general reaction of the drug and selectively improve and keep local concentration for about 30-50 days. The anticancer sustained release injection can be used solely and can also promote anti-tumor effects of non-operative treatments, such as chemotherapy and / or radiotherapy, etc.
Owner:JINAN SHUAIHUA PHARMA TECH
Solid tumor resisting release agent including nimustine and the intensifier
InactiveCN101040843AEasy injectionIncreased sensitivitySolution deliveryPharmaceutical non-active ingredientsTherapeutic effectPolymer
A nimustine slow release injection for treating solid cancer comprises slow release finding and nimustine, or the combination of nimustine and relative booster (pidorubicin, osaliplatinum, adriablastina, amethopterin or the like). The viscidity of slow-release injection is 10-650cp (20-30Deg. C). The anti-cancer effective component can be made into slow release plant agent. The slow release finding substantially comprises macromolecule polymer with biological soluble degradable and absorb property, which can slow release the anti-cancer effective component to cancer part in the degradation process, significantly reduce general reaction and hold effective drug density on cancer. The anti-cancer compound can be arranged part of cancer to reduce the general toxicity reaction, selectively improve the drug density locally, and strengthen the effect of non-surgery treatments as chemotherapy, and radiation therapy or the like. The solid cancer comprises glioma, lung carcinoma, intestinal cancer, breast cancer or the like.
Owner:JINAN KANGQUAN PHARMA TECH
Anti-cancer composition loading both mtrosourea medicament and synergist
InactiveCN101011346APharmaceutical delivery mechanismPharmaceutical non-active ingredientsMitozolomideTreatment effect
Disclosed is a slow release injection agent of anticancer composition containing nitrosourea drugs and synergistic agent, which comprises slow release microspheres and dissolvent, wherein the slow release microspheres comprise anti-cancer active constituents and slow release auxiliary materials, the dissolvent being conventional dissolvent or specific dissolvent containing suspension adjuvant. The viscosity of the suspension adjuvant is 100-3000cp (at 20-30 deg C), and is selected from sodium carboxymethylcellulose, the nitrosourea drugs are selected from Carmustine, Nimustine or Fotemustine, the synergistic agent can be selected from tetrazine drugs such as Mitozolomide or temozolomide and / or anticancer antibiotics such as Adriamycin, Aclarubicin, Epirubicin, mitomycin or pidorubicin, the slow release auxiliary materials are selected from polyphosphate ester copolymers such as p(LAEG-EOP), p(DAPG-EOP), copolymer or blend of polyphosphate ester with polylactic acid, Polifeprosan, sebacylic acid and PLGA. The anticancer composition can also be prepared into slow release implanting agent, for injection or placement in or around tumor with a period of effective concentration maintenance over 60 days, as well as the treatment effect of appreciably lowering general reaction of the drugs, and improving the treatment effect of the non-operative treatment methods such as chemotherapy.
Owner:JINAN SHUAIHUA PHARMA TECH
Slow release injection containing anti-metabolism medicament and alkylating agent
InactiveCN101011343APharmaceutical delivery mechanismPharmaceutical non-active ingredientsAdjuvantMicrosphere
Disclosed is a slow release injection containing antimetabolites and / or alkylating agents, which comprises slow release microspheres and dissolvent, the slow release microspheres include antimetabolites selected from Tegafur, Capecittabine, Pemetrexed, Carmofur or Gemcitabine, and / or alkylating agent anticancer active constituents and slow release auxiliary materials, the dissolvent being conventional dissolvent or specific dissolvent containing suspension adjuvant. The viscosity of the suspension adjuvant is 100-3000cp (at 20-30 deg C), and is selected from sodium carboxymethylcellulose, the slow release auxiliary materials are selected from polyphosphate ester copolymers such as p(LAEG-EOP), p(DAPG-EOP), copolymer or blend of polyphosphate ester with PLA, Polifeprosan, poly(dodecanedioic acid-tetradecanedioic acid) or poly(fumaric acid-sebacylic acid). The alkylating agent is selected from Carmustine, Nimustine, Fotemustine, Lomustine or bendamustine. The anticancer composition can also be prepared into slow release implanting agent, for injection or placement in or around tumor with a period of effective concentration maintenance over 50 days, as well as the treatment effect of appreciably lowering general reaction of the drugs, and improving the treatment effect of the non-operative treatment methods such as chemotherapy.
Owner:JINAN KANGQUAN PHARMA TECH
Anticancer sustained-release gel injection and preparation method thereof
InactiveCN101283974APharmaceutical delivery mechanismPharmaceutical non-active ingredientsTherapeutic effectSolvent
A sustained-release gel injection comprises angiogenesis inhibitor and / or carmustine or nimustine, amphiphilic block copolymer and solvent, wherein the angiogenesis inhibitor is selected from SU5416, SU6668, alemtuzumab, axitinib, ibritumomab, bevacizumab, sutent, dasatinib, tarceva, vandetanib, gefitinib, lapatinib, lapatinib, sunitinib, sorafenib, endostatain, engiostatin, tositumomab, tipifarnib, sirolimus, cetuximab, ematinib, lenalidomide and thalidomide. The mixture of amphiphilic block copolymer and solvent has temperature sensitive gelatinization characteristic, is fluid liquid at room temperature, and automatically becomes non-flowing biodegradable water insoluble gel in warm-blooded animals. The preparation can locally slowly release at tumor foci and maintain effective drug concentration for several weeks to several months; and has effects on killing tumor cells, effectively inhibiting tumor angiogenesis, reducing systemic toxicity of drug, and enhancing the treatment effect of chemotherapy and radiotherapy (particularly radioactive particles).
Owner:济南基福医药科技有限公司
Anticancer sustained release injection containing tumor drug resistance reversal agent and cytotoxic drug
Disclosed is an anticancer slow release injection containing tumor drug resistance reversal agents and cytotoxic drugs, which comprises slow release micro-balloons and dissolvent, wherein the slow release microballoons comprise anti-cancer active constituents and slow release auxiliary materials, the dissolvent being specific dissolvent containing suspension adjuvant. The anticancer active constituents include nelarabine, Tipifarnib, Ronafarnib, Valspodar, and / or selected from cytotoxic drugs including Cyclophosphamide, Carmustine, Nimustine, camptothecine, Paclitaxel, cisplatin, Carboplatin, Vincristine, O6-benzylguanine, or O6-benzylfolacin. The slow release auxiliary materials are selected from Polifeprosan, di-aliphatic acid and sebacylic acid copolymer, polylactic acid copolymer and EVAc. The viscosity of the suspension adjuvant is 100-3000cp (at 25-30 deg C) and can be sodium carboxymethylcellulose.
Owner:JINAN KANGQUAN PHARMA TECH
Novel anticancer injection
InactiveCN101269009AEasy to operateGood repeatabilityOrganic active ingredientsSolution deliveryMicrospherePolyethylene glycol
A new anticarcinogenic sustained-release injection consists of sustained-release microballoon sphere and menstruum; wherein, the sustained-release microballoon sphere comprises anticarcinogenic active ingredient and sustained-release accessory material; the menstruum is special menstruum containing suspending agent. The anticarcinogenic active ingredient is the combination of cytotoxic drugs of fotemustine, bendamustine, tallimustine, carmustinum, nimustine, lomustine, semustine or ranimustine and adriamycin, epirubicin, melphalan, 4H peroxide cyclophosphamide, actinomycin D, vinorelbine or nolvadex, etc.; the sustained-release accessory material is selected from polylactic acid and the multipolymer or mixture thereof, mono-methyl polyethyleneglycol, polyethyleneglycol or polylactic acid multipolymer, EVAc, the multipolymer of fatty acid and decanedioic acid, etc.; the viscosity of the suspending agent is 100cp-3000cp (at the temperature of 25 DEG C-30 DEG C), the suspending agent is selected from sodium carboxymethyl cellulose, etc. The sustained-release microballoon sphere also can be made into sustained-release implant which can be injected and placed in tumor or around tumor and can release medicine at the local parts of the tumor for 30 to 40 days; therefore, the anticarcinogenic sustained-release injection can be separately united together with non-operativetreatment such as chemo-treatment and / or radiation treatment, etc. for application.
Owner:JINAN SHUAIHUA PHARMA TECH
Anticancer composition containing nimustine
An anti-cancer composition comprises anti-cancer effective ingredient selected from tyrosine kinase inhibitor and / or nimustine and sustained release adjuvant, and can be made into sustained release injection and sustained release implant agent. Sustained release injection also comprises special dissolvent containing suspending agent. The Suspending agent has a viscocity of 100cp-3000cp (at 20deg.C-30deg.C) and is selected from sodium carboxymethylcellulose, and so on. the sustained release adjuvant is selected from p(LAEG-EOP), p(DAPG-EOP), p(BHET-EOP / TC), p(BHET-EOP / TC), p(BHDPT-EOP / TC), p( BHDPT-EOP / TC), p(CHDM-HOP) or p(CHDM-EOP).Slow releasing injection and implantable agent can keep high medicinal concentration in tumour part through slow releasing for over 60 days after being injected or implanted in or around tumour. The anticancer composition may also be prepared into sustained-release implant. It can reduce systemic toxic reaction of anticancer drugs, and also selectively improve the therapeutic effect of non-operative therapy such as chemotherapy.
Owner:JINAN KANGQUAN PHARMA TECH
Medical composition loaded with nimustine and synergist thereof
InactiveCN101416941BEasy to operateGood repeatabilityOrganic active ingredientsSolution deliveryAdditive ingredientTherapeutic effect
The invention relates to a drug combination, namely, a slow-release injection, carrying nimustine and a synergistic agent thereof at the same time, which consists of slow-release accessories and active anti-cancer ingredients. The active anti-cancer ingredients comprise the nimustine and the synergistic agent thereof (vinorelbine, melphalan or mitomycin C) respectively. The viscosity of the slow-release injection is 10cp to 650cp (at the temperature of 20 DEG C to 30 DEG C). The active anti-cancer ingredients can also be prepared into a slow-release implant. The slow-release accessories are mainly biological soluble and degradable macromolecular polymerizers that can be absorbed. During the process of degradation and adsorption, the macromolecular polymerizers can slowly release the active anti-cancer ingredients to parts of tumors, thus obviously reducing the toxic reaction of the whole body as well as maintaining the concentration of effective drugs at the parts of the tumors. Putting the anti-cancer drug combination at the parts of the tumors can not only reduce the toxic reaction of the whole body active against cancer, but also selectively raise the drug concentration of the parts of the tumors and enhance the curative effect of nonspecific treatment, such as chemotherapy drugs and radiotherapy treatment, and the like. Solid tumors include glioma, osteoma sarcomatosum, lymphoma, lung cancer, intestinal cancer, oophoroma, and the like.
Owner:SHANDONG LANJIN PHARMA
Anticancer slow release preparation of nimustine and its progression agent
InactiveCN1919179AEasy injectionIncreased sensitivityOrganic active ingredientsPeptide/protein ingredientsTreatment effectWhole body
Disclosed is slow release anticancer agent carrying both Nimustine and its synergistic agents, which comprises slow release auxiliary materials and active anticancer constituents, wherein the active anticancer constituents include Nimustine and its synergistic agents (such as Vinblastine, Goserelin or Carmofur). The viscosity of the slow release injection is 10-650cp (at 20-30 deg C), and the active anticancer constituents can also be made into slow release implanting agent. The slow release subsidiary materials mainly comprise bio-compactable and degradable macromolecular polymers, when locally dispensed on the tumor, the composition not only can lower down the whole body toxicity reaction of the anti-cancer medicament, but also can selectively increase the tumor local medicinal concentration, the treatment effect of the non-operative treatment methods such as chemotherapy, medicament and radiation can also be improved. The solid tumors include glioma, osteosarcoma, lymphoma, lung carcinoma, intestinal cancer, and breast cancer.
Owner:JINAN KANGQUAN PHARMA TECH
Medical composition loaded with nimustine and synergist thereof
InactiveCN101416941AEasy injectionIncreased sensitivityOrganic active ingredientsSolution deliveryAdditive ingredientVinorelbine
The invention relates to a drug combination, namely, a slow-release injection, carrying nimustine and a synergistic agent thereof at the same time, which consists of slow-release accessories and active anti-cancer ingredients. The active anti-cancer ingredients comprise the nimustine and the synergistic agent thereof (vinorelbine, melphalan or mitomycin C) respectively. The viscosity of the slow-release injection is 10cp to 650cp (at the temperature of 20 DEG C to 30 DEG C). The active anti-cancer ingredients can also be prepared into a slow-release implant. The slow-release accessories are mainly biological soluble and degradable macromolecular polymerizers that can be absorbed. During the process of degradation and adsorption, the macromolecular polymerizers can slowly release the active anti-cancer ingredients to parts of tumors, thus obviously reducing the toxic reaction of the whole body as well as maintaining the concentration of effective drugs at the parts of the tumors. Putting the anti-cancer drug combination at the parts of the tumors can not only reduce the toxic reaction of the whole body active against cancer, but also selectively raise the drug concentration of the parts of the tumors and enhance the curative effect of nonspecific treatment, such as chemotherapy drugs and radiotherapy treatment, and the like. Solid tumors include glioma, osteoma sarcomatosum, lymphoma, lung cancer, intestinal cancer, oophoroma, and the like.
Owner:SHANDONG LANJIN PHARMA
Delayed-release injection containing nitrous carbamide type medicine and topographic enzyme inhibitor
A slow-release injection or implant containing the nitrosourea-type medicines (nimustine, carmustine, etc) and topostine as the active anticancer components is composed of the slow-release microballs containing said active anticancer components and the slow-release auxiliary chosen from p(LAEG-EOP), p(DAPG-EOP), etc, and the solvent which may be ordinary solvent or a special solvent containing suspending aid.
Owner:JINAN SHUAIHUA PHARMA TECH
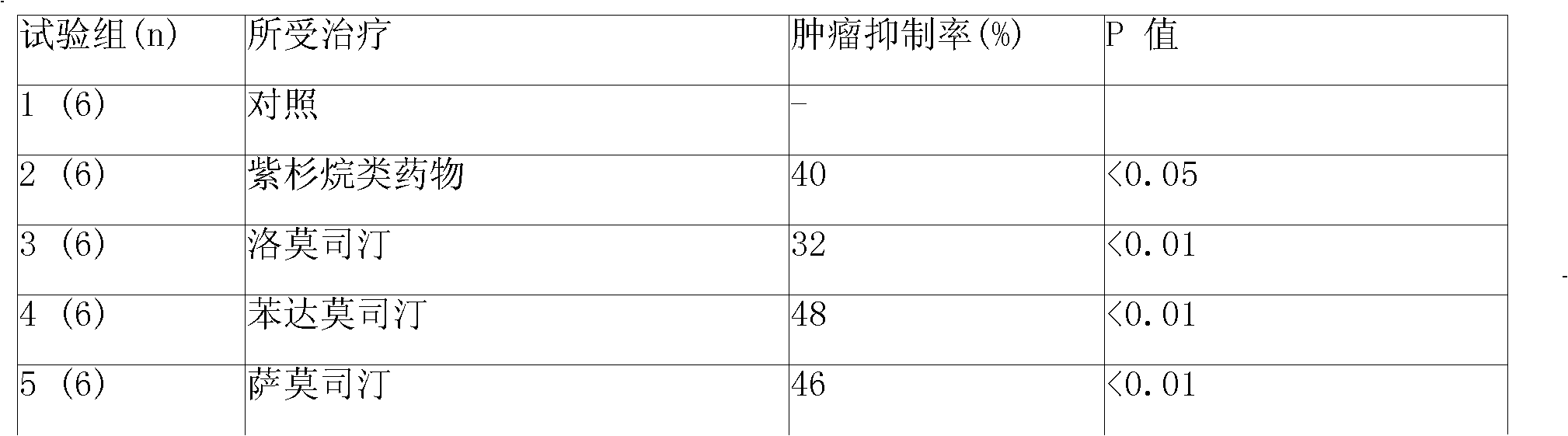
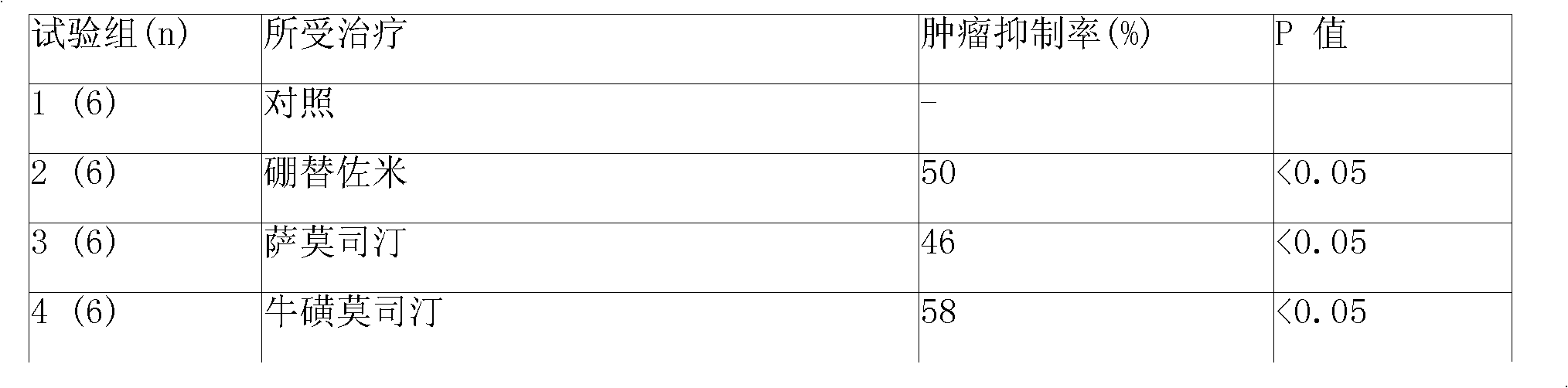
Anticancer composition containing nitrosourea medicament and bortezomib
InactiveCN101301469ABoron compound active ingredientsPharmaceutical non-active ingredientsTreatment effectAdditive ingredient
The invention provides a sustained-release injection which is an anti-tumor composite containing nitrosoureas drug and bortezomib. The sustained-release injection comprises a sustained-release microsphere and a solution medium, wherein the sustained-release microsphere comprises effective anti-tumor ingredient and sustained-release excipient, the solution medium is a common solution medium or a special solution medium containing a suspending drug. The suspending drug has the viscosity between 100cp and 3000cp (under twenty centi degrees to thirty centi degrees) and is selected from sodium carboxymethylcellulose and others; the nitrosoureas drug is selected from nimustine,bendamustine,carmustine,galamustine,fotemustine,ranimustine,samustine or lomustine; the sustained-release excipient is selected from polyphosphonate copolymer like p(LAEG-EOP)or p(DAPG-EOP), PLA, PLGA, polyphosphonate with PLA, polifeprosan, the polymer or blending polymer of ( erucic acid dipolymer-sebacic acid) or poly(boletic acid-sebacic acid); the anti-tumor composite can also be prepared to be a sustained-release implant which can maintain the effective concentration of drug over forty days for intratumor or tumor circumference injection or placement, can also reduce general reaction obviously and enhance the treatment effect of non-operative therapeutics such as chemotherapy and radiotherapy.
Owner:济南基福医药科技有限公司
Novel anticancer sustained-release injection
InactiveCN101427996AEasy to operateGood repeatabilityOrganic active ingredientsSolution deliveryChemical treatmentPolyethylene glycol
The invention relates to novel sustained-release injection for cancer therapy. The sustained-release injection comprises sustained-release microspheres and dissolvent, wherein the sustained-release microspheres comprise active ingredients for cancer therapy and sustained-release auxiliary materials, and the dissolvent is special dissolvent containing suspending agent. The active ingredients for cancer therapy are combination of fotemustine, bendamustine, tallimustine, carmustine, nimustine, lomustine, semustine or ranimustine with cytotoxic drug of adriacin, epidoxorubicin, melphalan, 4H- cyclophosphamide peroxide, actinomycin D, vinorelbine or tamoxifen; the sustained-release auxiliary materials comprise polylactic acid and the copolymer or mixture thereof, monomethyl polyethylene glycol, polyethylene glycol / polylactic copolymer, EVAc, fatty acid and decanedioic copolymer; the viscocity of the suspending agent is 100cp to 3000cp (at 25 to 30 DEG C), and the suspending agent is selected from sodium carboxymethyl cellulose; the sustained-release microspheres can also be produced into sustained-release implant, and by injecting or positioning the sustained-release agent in tumor or tumor margin, drug release in local part of tumor can be performed for approximately 30 to 40 days; therefore, the sustained-release injection can be applied in combination with non-operative treatment such as chemical treatment and / or radiation treatment.
Owner:JINAN SHUAIHUA PHARMA TECH
Anticancer slow release agent of nimustine and its progression agent
InactiveCN1919176AEasy injectionIncreased sensitivitySolution deliveryPharmaceutical non-active ingredientsCarboplatinWhole body
Disclosed is a slow release injection for treating solid tumors which comprises slow release auxiliary materials and active anticancer constituents, wherein the active anticancer constituents include Nimustine or the combination of Nimustine and its synergists (such as adriamycin, Carboplatin or Amethopterin). The viscosity of the slow release injection is 10-650cp (at 20-30 deg C), and the active anticancer constituents can also be made into slow release implanting agent. The slow release subsidiary materials mainly comprise bio-compactable and degradable macromolecular polymers, when locally dispensed on the tumor, the composition not only can lower down the whole body toxicity reaction of the anti-cancer medicament, but also can selectively increase the tumor local medicinal concentration, the treatment effect of the non-operative treatment methods such as chemotherapy, medicament and radiation can also be improved. The solid tumors include glioma, osteosarcoma, lymphoma, lung carcinoma, intestinal cancer, and breast cancer.
Owner:JINAN KANGQUAN PHARMA TECH
Anticancer slow release agent of nimustine and its progression agent
InactiveCN1919180AEasy injectionIncreased sensitivityOrganic active ingredientsSolution deliveryWhole bodyTherapeutic effect
Disclosed is a slow release injection for treating solid tumors which comprises slow release auxiliary materials and active anticancer constituents, wherein the active anticancer constituents include Nimustine or the combination of Nimustine and its synergists (such as Paclitaxe, camptothecine or cytophosphanel). The viscosity of the slow release injection is 10-650cp (at 20-30 deg C), and the active anticancer constituents can also be made into slow release implanting agent. The slow release subsidiary materials mainly comprise bio-compactable and degradable macromolecular polymers, when locally dispensed on the tumor, the composition not only can lower down the whole body toxicity reaction of the anti-cancer medicament, but also can selectively increase the tumor local medicinal concentration, the treatment effect of the non-operative treatment methods such as chemotherapy, medicament and radiation can also be improved. The solid tumors include glioma, osteosarcoma, lymphoma, lung carcinoma, intestinal cancer, and breast cancer.
Owner:JINAN KANGQUAN PHARMA TECH
Nimustine slow release injection for treating noumenal tumour
InactiveCN1919178AEasy injectionIncreased sensitivitySolution deliveryPharmaceutical non-active ingredientsEsophagus CancersLung cancer
Disclosed is a Nimustine slow release injection for treating solid tumors, which comprises effective anticancer dose of Nimustine or the combination of Nimustine and its synergists (such as 5-FU, cisplatin or 06-benzyl guanine). The solid tumors include osteosarcoma, lymphoma, gastric carcinoma, cancer of pancreas, lung carcinoma, bladder carcinoma, hepatic carcinoma, breast cancer, esophagus cancer, intestinal cancer, thyroid carcinoma, and glioma. The viscosity of the slow release injection is 10-650cp (at 20-30 deg C), and the active anticancer constituents can also be made into slow release implanting agent. The slow release subsidiary materials mainly comprise bio-compactable and degradable macromolecular polymers, when locally dispensed on the tumor, the composition not only can lower down the whole body toxicity reaction of Nimustine, but also can selectively increase the tumor local medicinal concentration, the treatment effect of the non-operative treatment methods such as chemotherapy, medicament and radiation can also be improved.
Owner:JINAN KANGQUAN PHARMA TECH
Slow release injection containing mtrosourea medicament and topoisomerase inhibitor
InactiveCN101011349APharmaceutical delivery mechanismPharmaceutical non-active ingredientsAdjuvantTreatment effect
Disclosed is a slow release injection containing nitrosourea drugs and / or topoisomerase depressant, which comprises slow release microspheres and dissolvent, the slow release microspheres include nitrosourea drugs selected from Nimustine, Carmustine, and / or topoisomerase depressant anticancer active constituents and slow release auxiliary materials, the dissolvent being conventional dissolvent or specific dissolvent containing suspension adjuvant. The viscosity of the suspension adjuvant is 100-3000cp (at 20-30 deg C), and is selected from sodium carboxymethylcellulose, the slow release auxiliary materials are selected from polyphosphate ester copolymers such as p(LAEG-EOP), p(DAPG-EOP), copolymer or blend of polyphosphate ester with PLA, Polifeprosan, PLGA and poly(dodecanedioic acid-tetradecanedioic acid), the topoisomerase depressant is selected from camptothecine, monohydric camptothecine, Topotecan, Lartotecan, Irinotecan, Etoposide or Teniposide, the anticancer composition can also be prepared into slow release implanting agent for injection or placement in or around tumor with a period of effective concentration maintenance over 60 days, as well as the treatment effect of appreciably lowering general reaction of the drugs, and improving the treatment effect of the non-operative treatment methods such as chemotherapy.
Owner:JINAN SHUAIHUA PHARMA TECH
Anti-cancer composition loading both mtrosourea medicament and alkaloids medicament
InactiveCN101011348APharmaceutical delivery mechanismPharmaceutical non-active ingredientsAdjuvantTreatment effect
Disclosed is a slow release injection agent of anticancer composition containing nitrosourea drugs and alkaloid drugs, which comprises slow release microspheres and dissolvent, wherein the slow release microspheres comprise anti-cancer active constituents and slow release auxiliary materials, the dissolvent being conventional dissolvent or specific dissolvent containing suspension adjuvant. The viscosity of the suspension adjuvant is 100-3000cp (at 20-30 deg C), and is selected from sodium carboxymethylcellulose, the nitrosourea drugs are selected from Carmustine, Nimustine, Fotemustine, Lomustine or Bendamustine, the alkaloid drugs include Vincristine, Vinblastine, Vinorelbine, Vindesine and Vinrosidine, the slow release auxiliary materials are selected from polyphosphate ester copolymers such as p(LAEG-EOP), p(DAPG-EOP), copolymer or blend of polyphosphate ester with polylactic acid, Polifeprosan, sebacylic acid and PLGA. The anticancer composition can also be prepared into slow release implanting agent, for injection or placement in or around tumor with a period of effective concentration maintenance over 60 days, as well as the treatment effect of appreciably lowering general reaction of the drugs, and improving the treatment effect of the non-operative treatment methods such as chemotherapy.
Owner:JINAN SHUAIHUA PHARMA TECH
Sustained release ocular implant for treating solid tumors
InactiveCN101229119AOrganic active ingredientsPharmaceutical delivery mechanismTherapeutic effectExcipient
A sustained release implant for treating solid cancer is characterized in that the sustained release implant comprises nimustine, sustained release excipients and a release regulator with an effective amount for anti-cancer. The sustained release excipients essentially include macromolecule polymer which has a biotic capacitability and can be degraded and absorbed, mainly relating to PLA, and PLGA; the release regulator is selected from one or combination of mannitol, sorbitol, xylitol, oligosaccharide, chitin, potassium salt, sodium salt, hyaluronic acid, collagen, chondroitin, gelatin and albumin. The sustained release implant can slowly release carmustine in partial knub during the process of degrading and absorbing; thereby being able to maintain effective medicine concentration on partial knub simultaneously when remarkably reducing the general toxicity reaction; consequently, the invention can be widely applied for the treatment of the solid knub in various stages by single or cooperated with non operative treatments like chemotherapy medicines and radiotherapy, which can not only selectively enhance medicine concentration on partial knub , but also enhance the curing effects of non operative treatments like medicine chemotherapy and radiotherapy.
Owner:JINAN SHUAIHUA PHARMA TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com