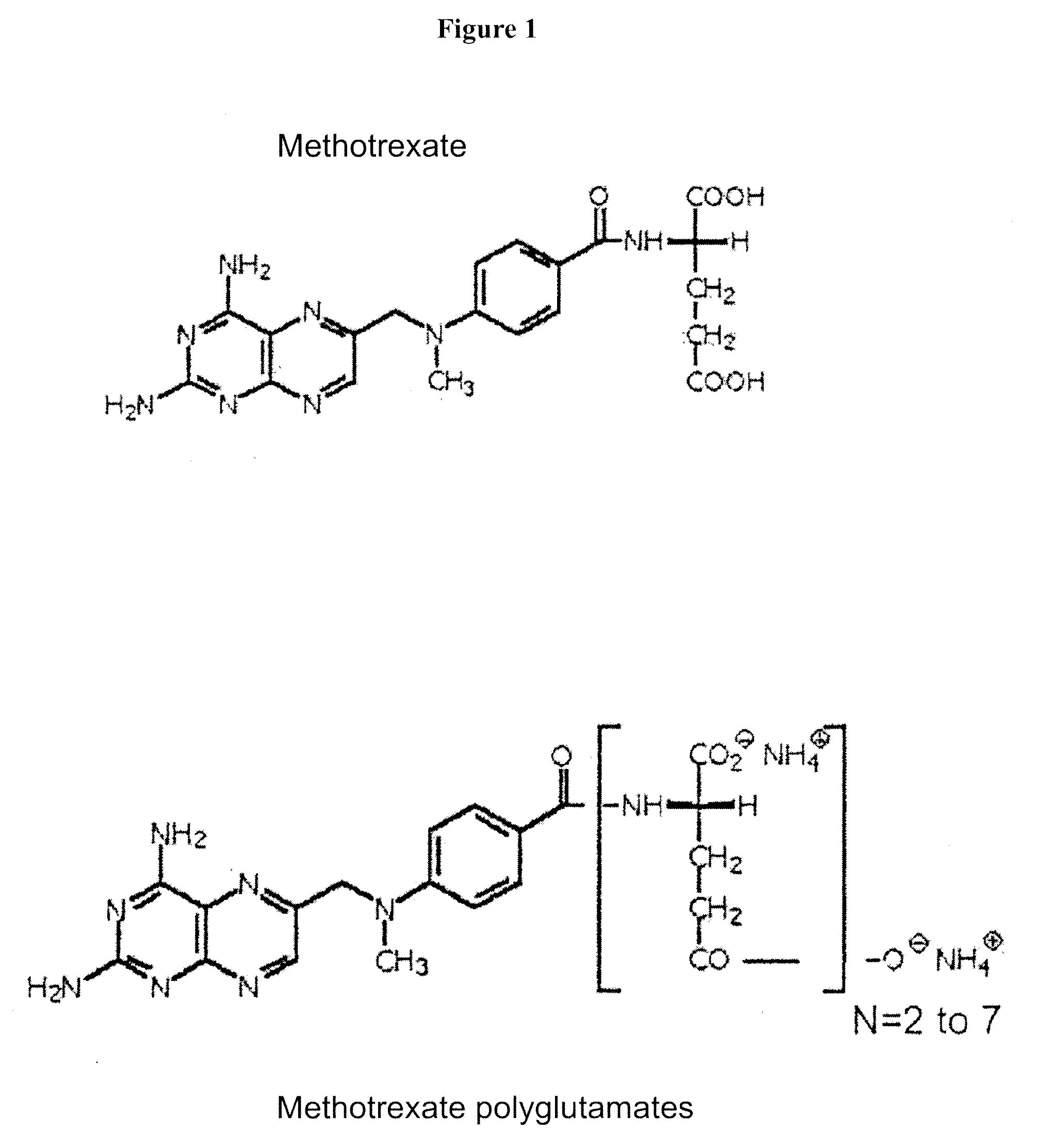
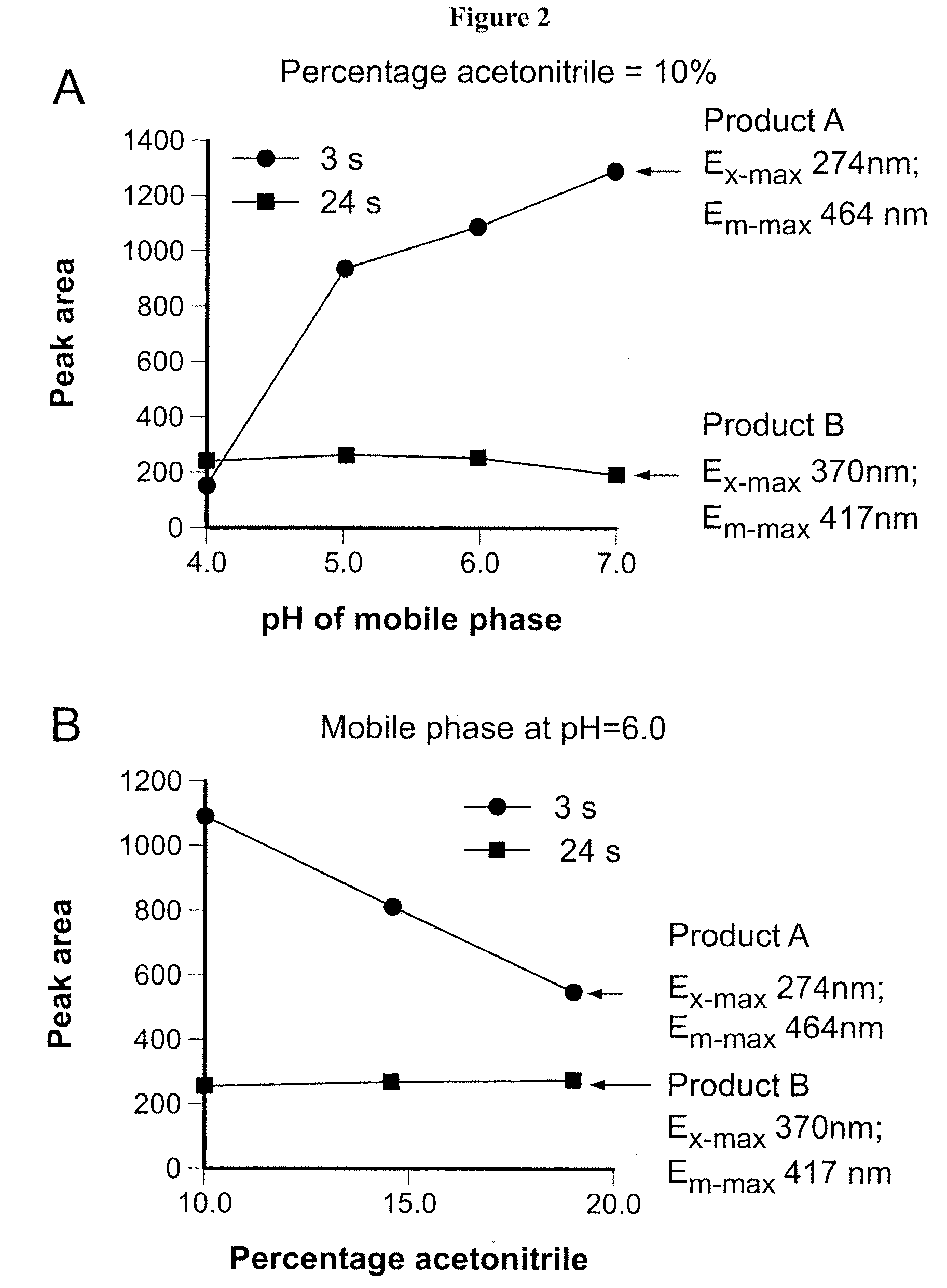
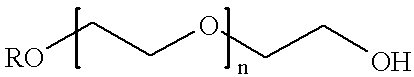
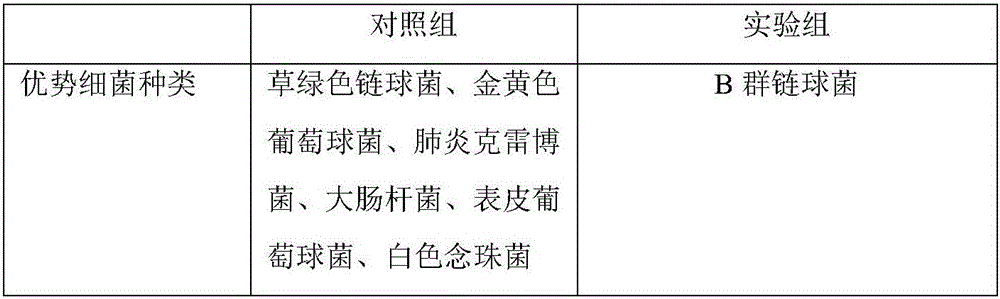
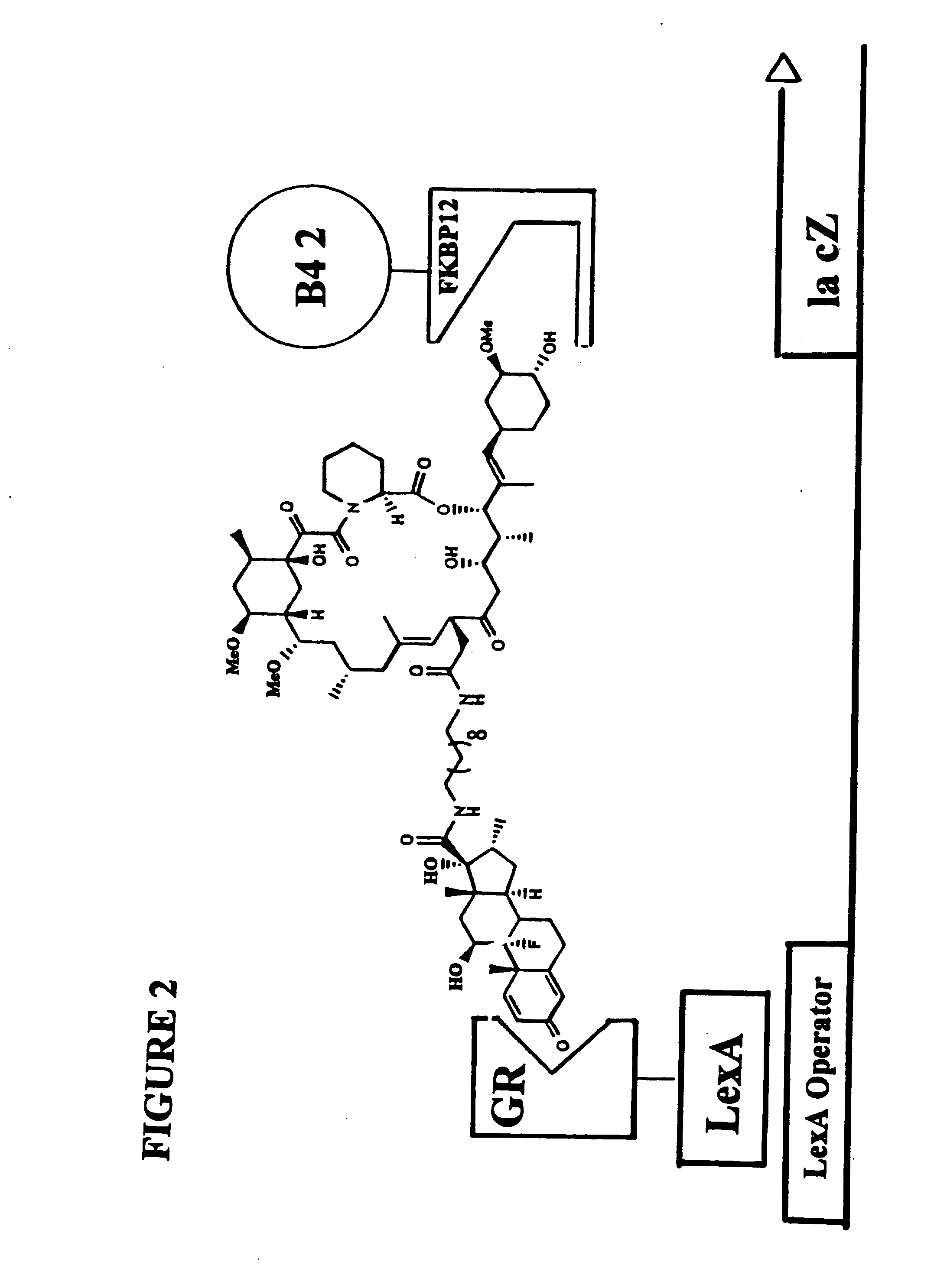
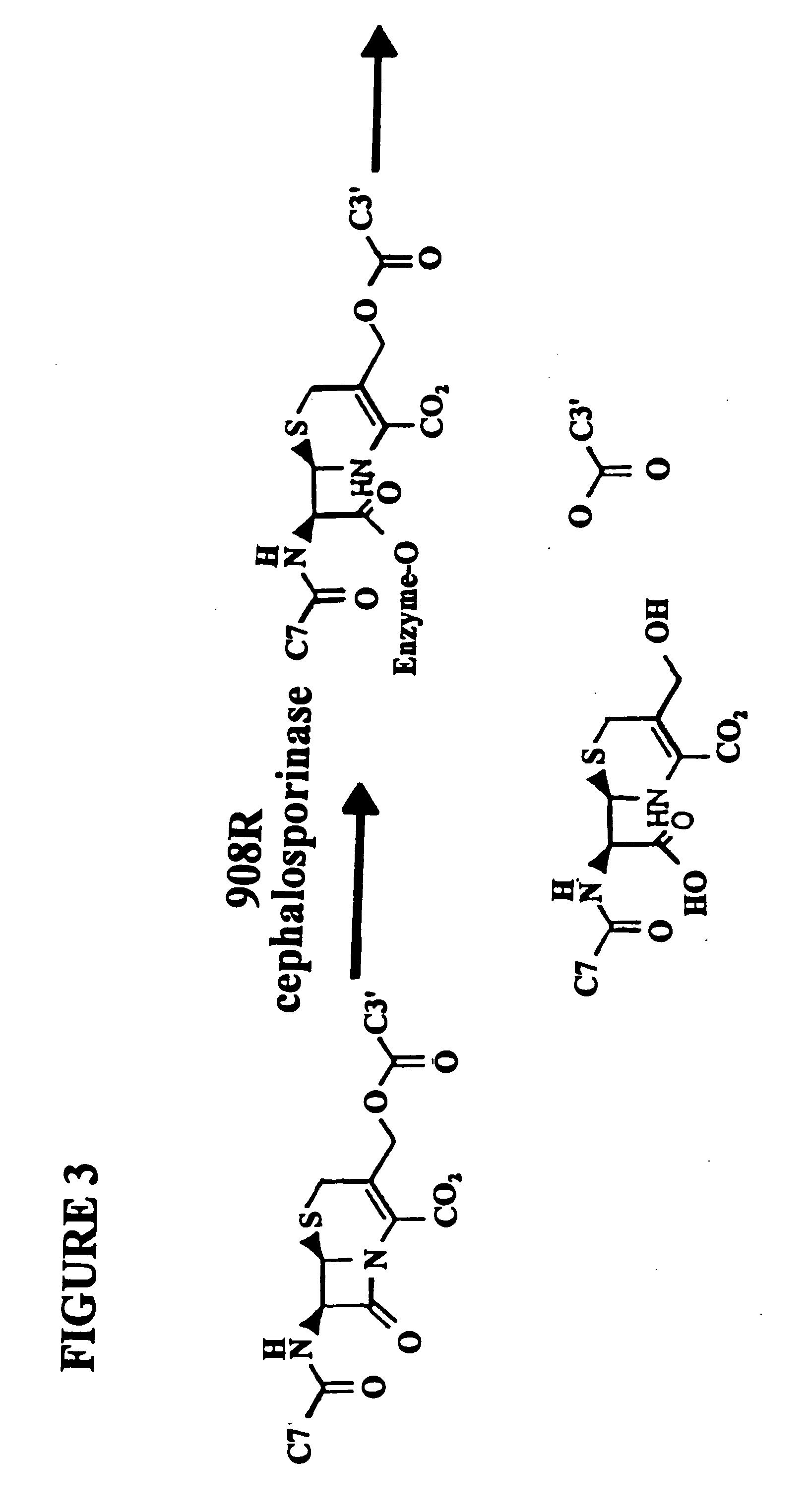
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
84 results about "Amethopterin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cancer treatment drug
Antibacterial and antitumor orthopaedic implantation material and preparation method thereof
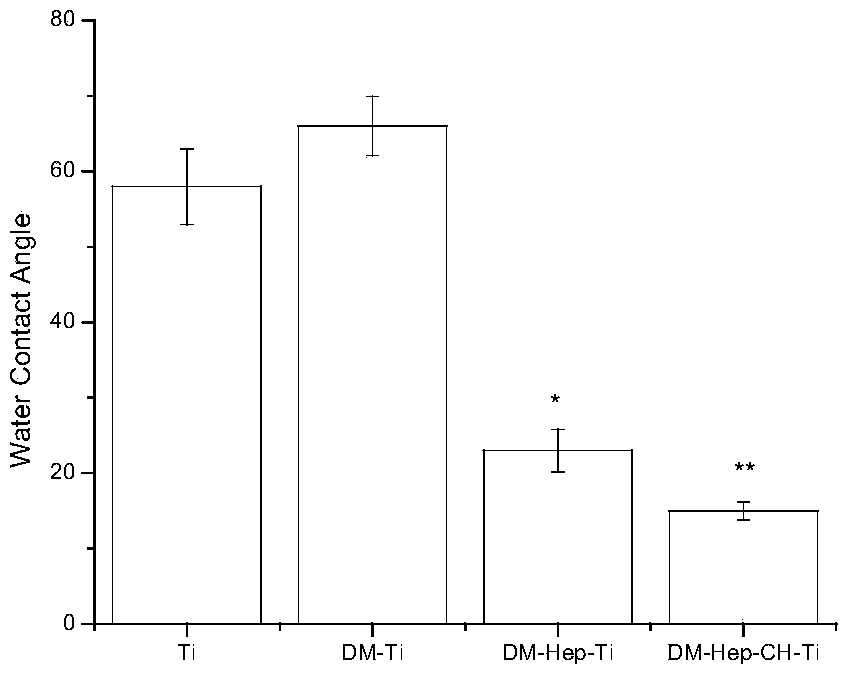
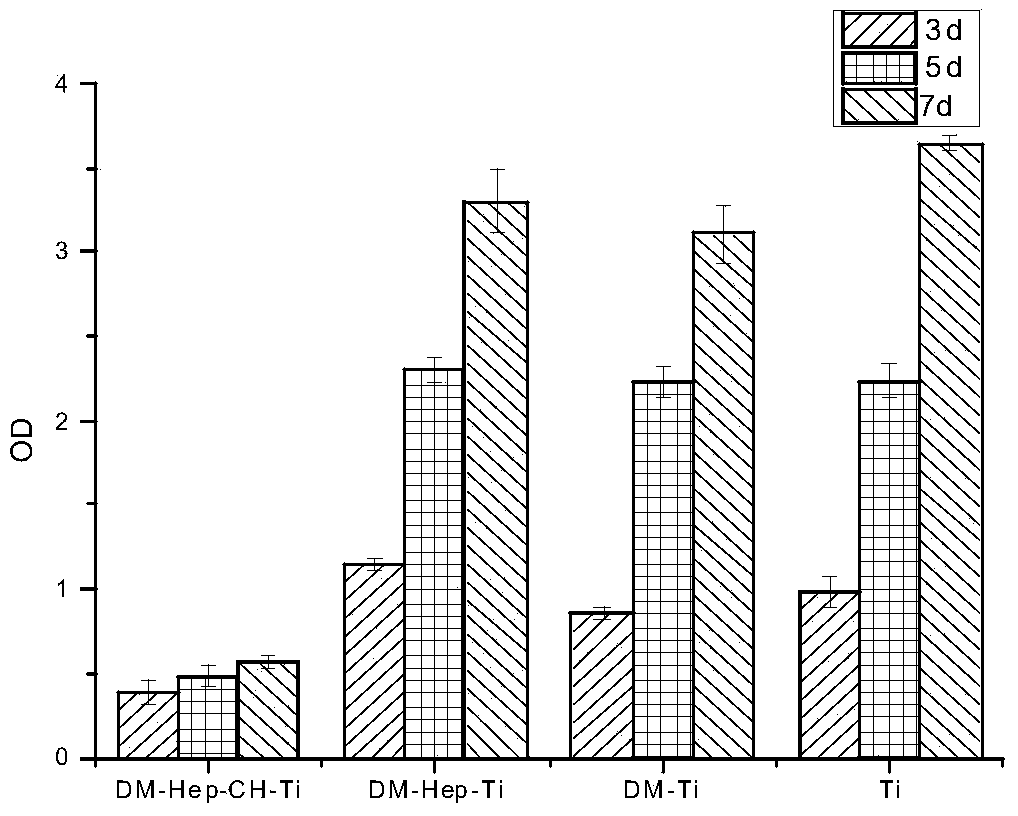
The invention discloses an antibacterial and antitumor orthopaedic implantation material and a preparation method thereof. The implantation material is a medical pure titanium sheet with the surface modified by chitosan, methotrexate, heparin sodium, polylysine and dopamine. According to the material, dopamine serves as a bridge to allow polylysine and a heparin sodium particle to be bonded on the surface of titanium; heparin, as a good anticoagulant, can improve biocompatibility between the material and blood, and is bonded with chitosan and methotrexate with antibacterial and antitumor effects through an electrostatic effect. Physiological-biochemical characteristics of drugs are utilized ingeniously to form an orderly and uniform antibacterial and antitumor coating with high biocompatibility on the surface of the titanium, and the prepared implantation material has good antibacterial and antitumor activity, and can effectively prevent bacterial adhesion and reproduction, as well as recurrence and transfer of a cancer.
Owner:GUANGZHOU GENERAL HOSPITAL OF GUANGZHOU MILITARY COMMAND
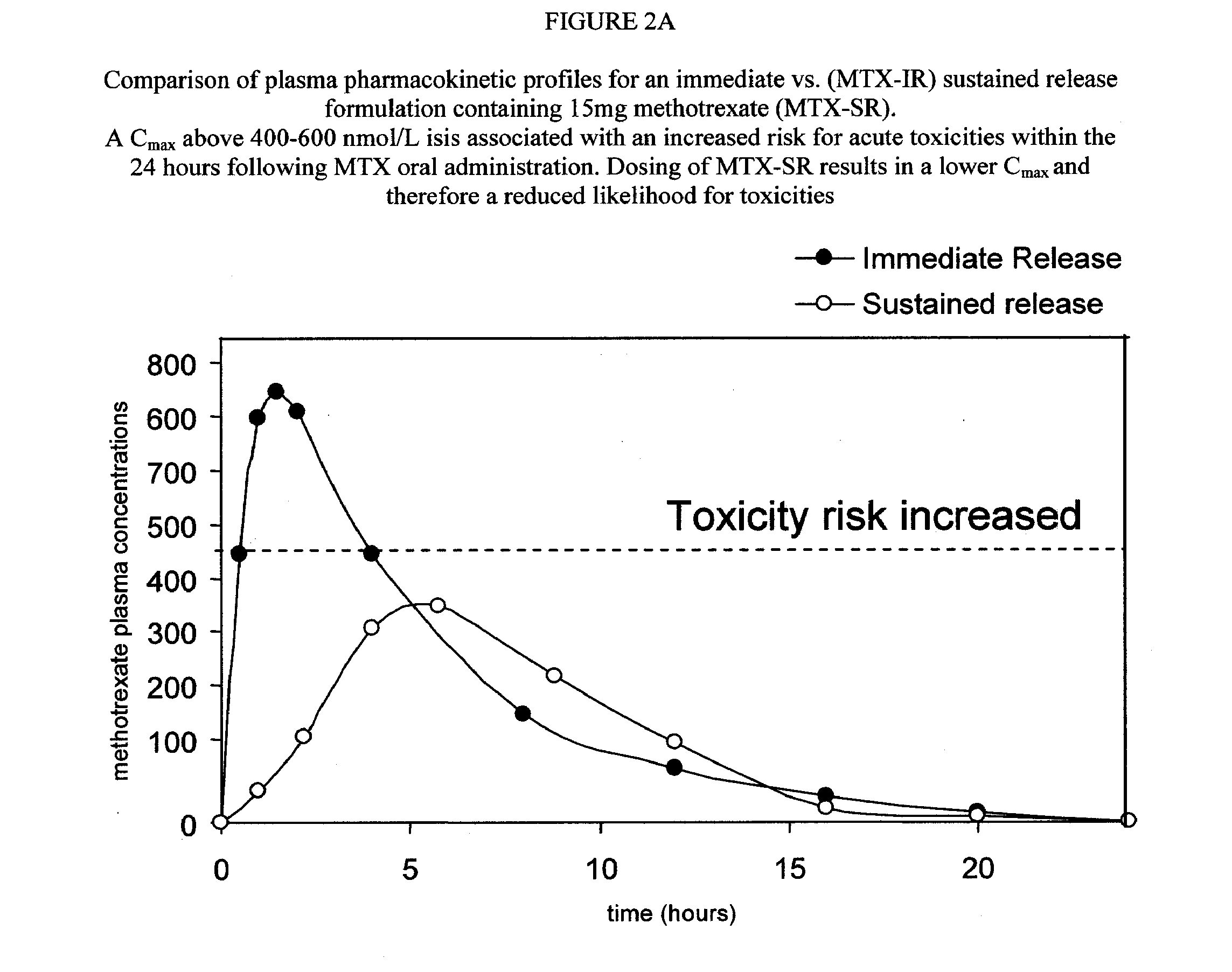
Sustained release methotrexate formulations and methods of use thereof
InactiveUS20080268045A1Reduce morbidityGood curative effectBiocideSkeletal disorderDiseaseOral medication
Described herein are methods of treating a disease by treatment with oral sustained release methotrexate alone or in combination with folates. In some embodiments, these approaches improve the pharmacotherapeutic performance of methotrexate therapy.Described herein are novel pharmaceutical compositions for oral administration. Also described herein are novel pharmaceutical compositions for the controlled, sustained delivery of one or more drugs to the stomach or upper gastrointestinal tract. Further described are novel pharmaceutical compositions with increased gastrointestinal residence time. More particularly, novel pharmaceutical compositions which can simultaneously, float in gastric fluid, adhere to the mucosal surfaces of the gastrointestinal tract, swell to a size which delays passage through the pylorus, are described herein. In some embodiments, the pharmaceutical compositions comprise methotrexate. In some embodiments, the pharmaceutical compositions comprise methotrexate and a folate compound. Also described herein are methods for treating or preventing diseases, by administration of the pharmaceutical compositions described herein.
Owner:CYPRESS BIOSCI
Use of regularly scheduled high dose intravenous methotrexate therapy, with interim administration of immunomodulatory agents, to treat multiple sclerosis and other diseases of the central nervous system
InactiveUS6903100B2Halted deteriorationImprove scoreBiocidePeptide/protein ingredientsCytotoxicityHigh doses
The present invention is directed to the treatment of multiple sclerosis by periodically administering a high dose of methotrexate at a level sufficiently high to cross the blood brain barrier. The methotrexate administration is accompanied by leucovorin rescue of the periphery. The high dose methotrexate is preferably administered at 1 to 4 month intervals. The periodic high dose methotrexate treatment may be used in conjunction with interim treatments using a therapeutic agent that is effective in treating MS, but does not cross the BBB in cytotoxic amounts. It is contemplated that the method of the present invention may be employed to treat other non-infectious, non-neoplastic inflammatory conditions of the CNS.
Owner:MIDAMERICA NEUROSCI RES FOUND
Bone repairing material with calcium and phosphor base and its preparation method
InactiveCN1569256AIncreased flexural (bending) strengthGood treatment effectProsthesisFiberBone formation
The invention relates to a biological tissue material and its preparation, in particular to a calcium phosphor salt base bone repairing material and its preparation. Said material provided by the invention contains calcium phosphor salt and chitin / chitosan fiber, which existes in form of fiber thread or fiber bunch. It can also be combined with 0.1-10% chitin derivants like chitosan, phosphorylate chitin, phosphorylate chitosan, carboxylation sulation chitosan and or 0.01-0.1% collagen, bone formation protein amethopterin or adriacin. Cell experiments and animal experiments all indicate that the material has favorable biological compatibility and induction activity for osteogenesis. It can raise averagely 1 to 10 times of flexural resistant intensity of calcium phosphor base bone repairing material. The invention is a reliable in vivo degradable material for tissue repairing and treatment.
Owner:TSINGHUA UNIV
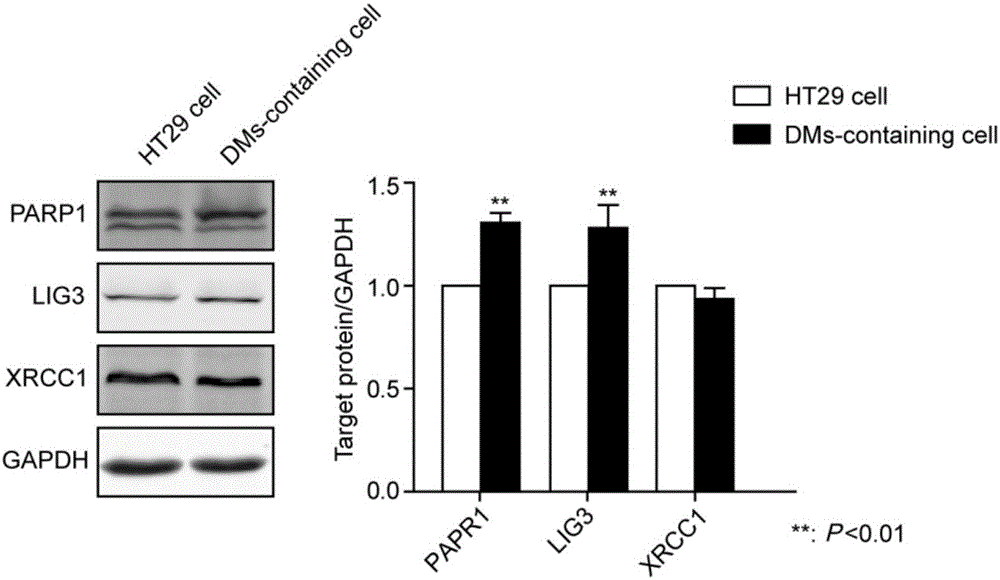
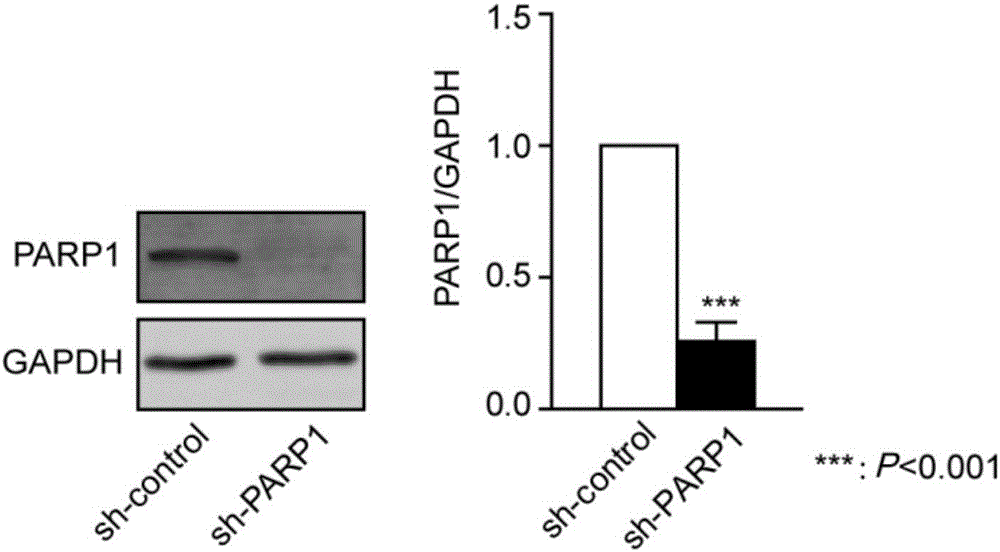
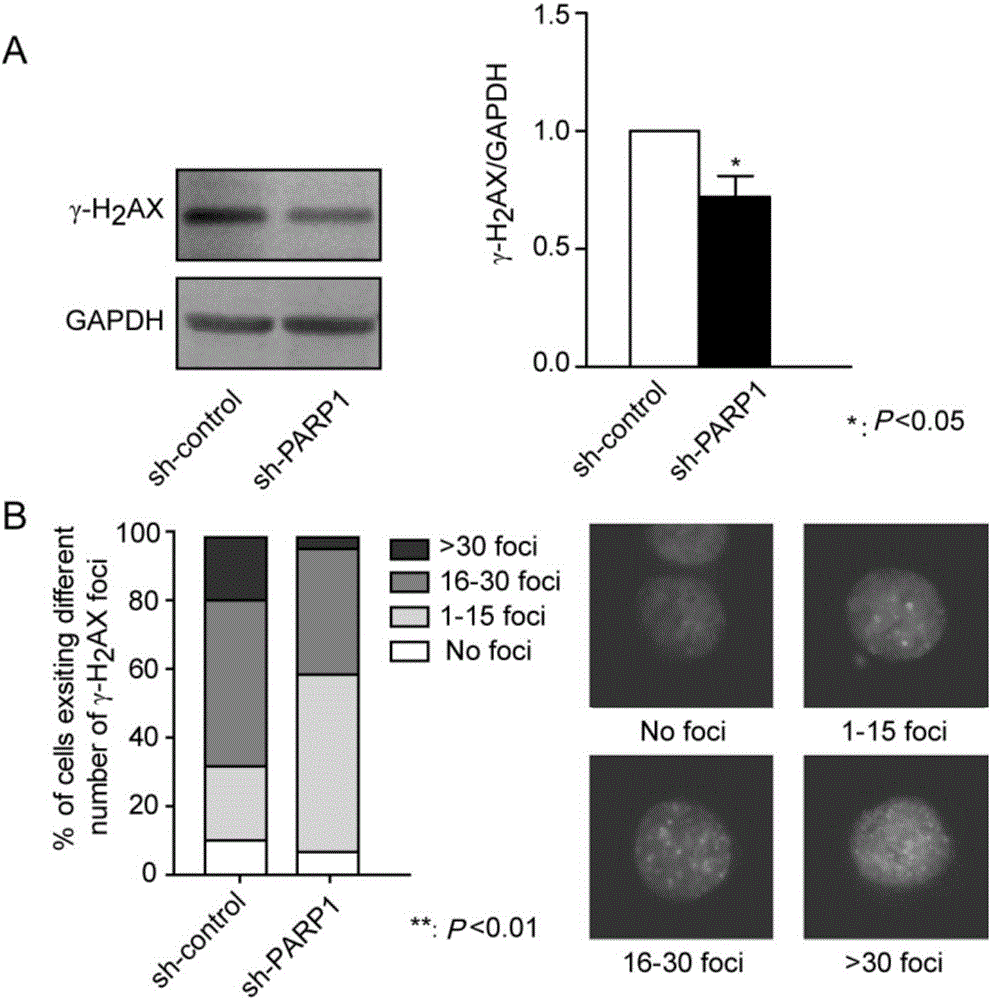
Application of PARP1 inhibitor in preparation of medicine for reversing drug resistance of tumor cells to amethopterin
ActiveCN106492217AOrganic active ingredientsAntineoplastic agentsIndividualized treatmentTumor therapy
The invention discloses application of a PARP1 inhibitor in the preparation of a medicine for reversing drug resistance of tumor cells to amethopterin and belongs to the technical field of tumour biotherapy. It is found by the inventor through researches that as for malignant cells with MTX resistance and DHFR gene high-amplification, by specifically inhibiting the key protein RARP1 of A-NHEJ pathway, DHFR gene copy number can be reduce and DMs in cells can be decreased so as to reverse drug resistance of tumors and enhance efficiency of tumor therapy. Therefore, on the basis of the above researches, the invention brings forward the application of the PARP1 inhibitor in the preparation of a medicine for reversing the drug resistance of tumor cells to MTX by reducing DHFR gene copy number. The invention provides a new targeting therapeutic scheme for the MTX as the main therapeutic drug and for the biotherapy of malignant tumors which are easy to resist drugs and also provides a scientific basis for effectively fighting against MTX drug resistance. In addition, the invention is also of great positive significance for deeply understanding the nature of chemotherapy resistance and finding resistance target for individualized treatment.
Owner:HARBIN MEDICAL UNIVERSITY
Hyaluronic Acid-Methotrexate Conjugate
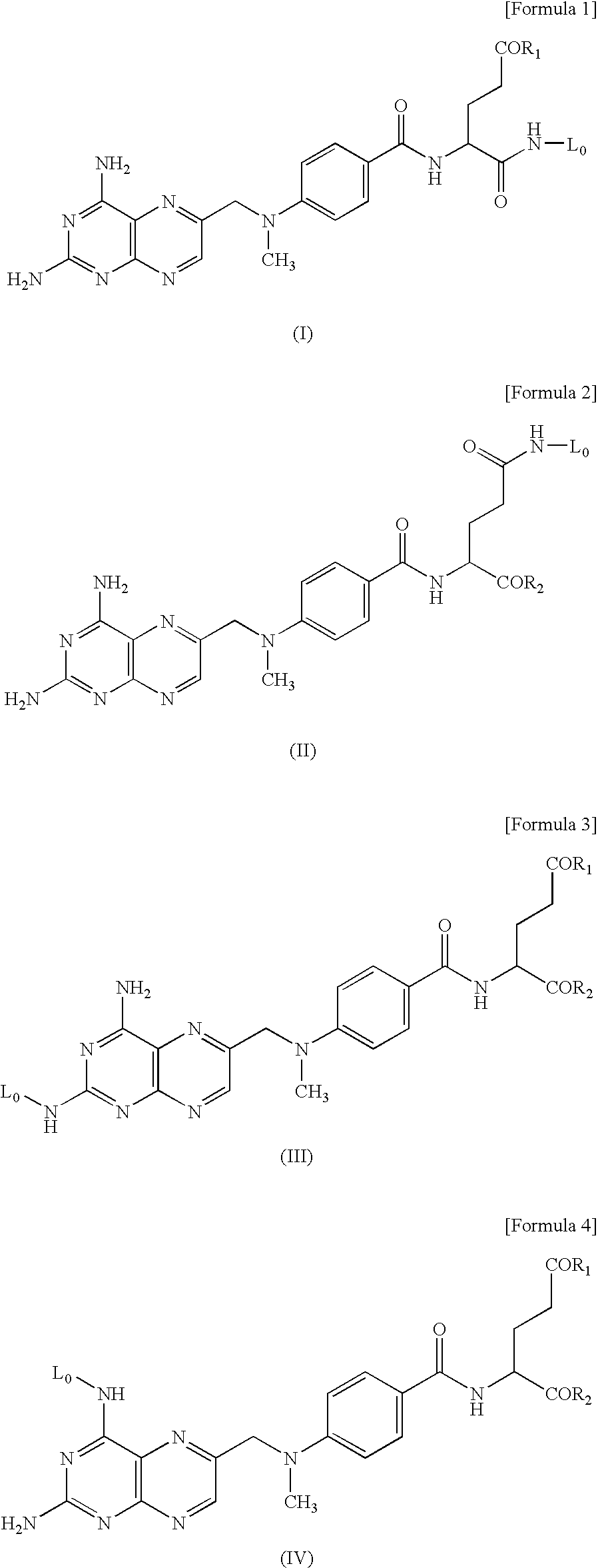
An object of the present invention is to provide a hyaluronic acid-methotrexate conjugate useful as a therapeutic drug for joint disorders. There is provided a hyaluronic acid-methotrexate conjugate useful for the treatment of joint disorders, wherein methotrexate is conjugated with a hydroxy group of hyaluronic acid through a linker containing a peptide chain consisting of 1 to 8 amino acids, and the linker is conjugated with the hyaluronic acid through a carbamate group.
Owner:DENKA CO LTD
Preparation method of hyaluronic acid-alendronate sodium- methotrexate nanometer granules
PendingCN110237266AImprove stabilityGood dispersionPowder deliveryOrganic active ingredientsTumor targetSide effect
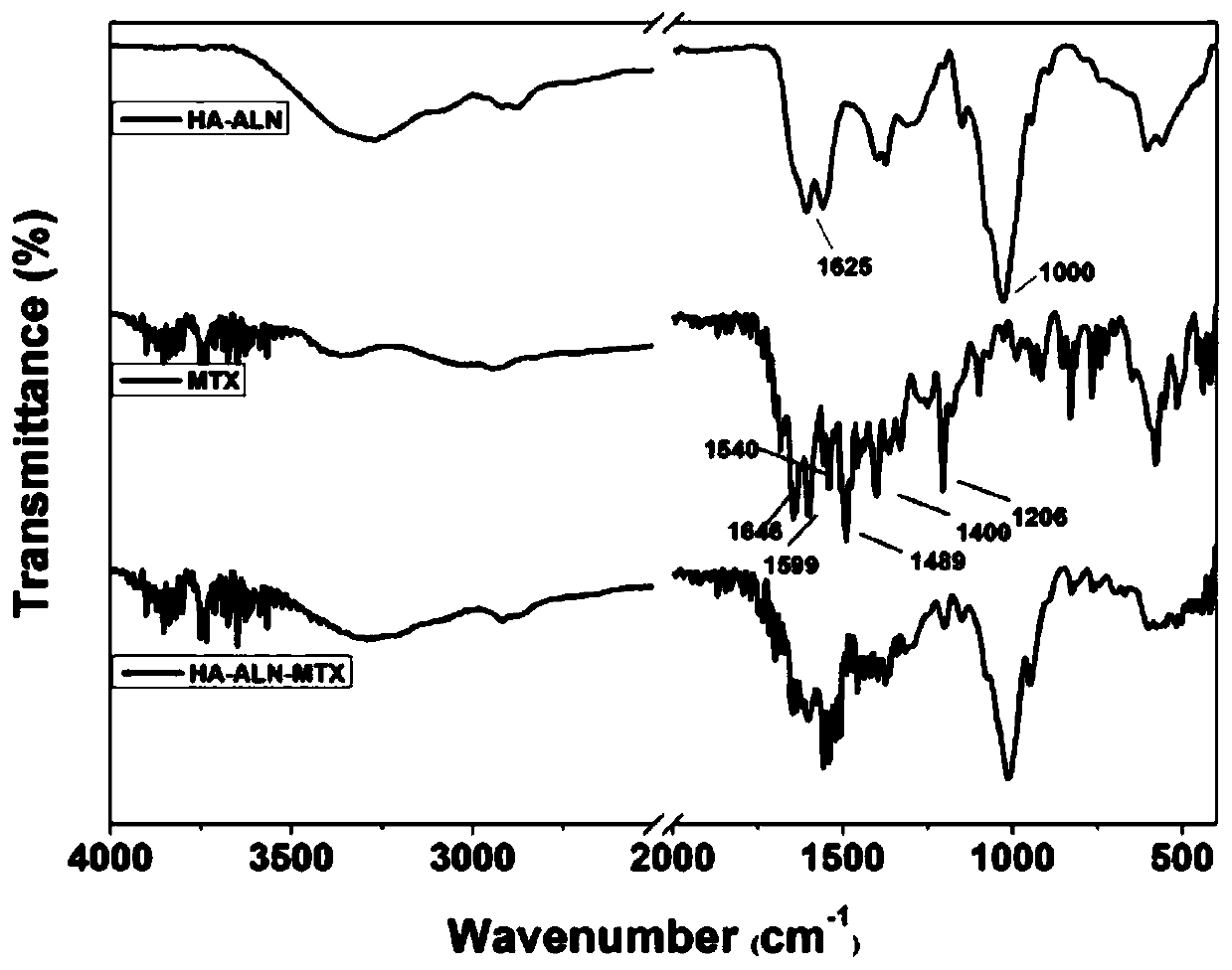
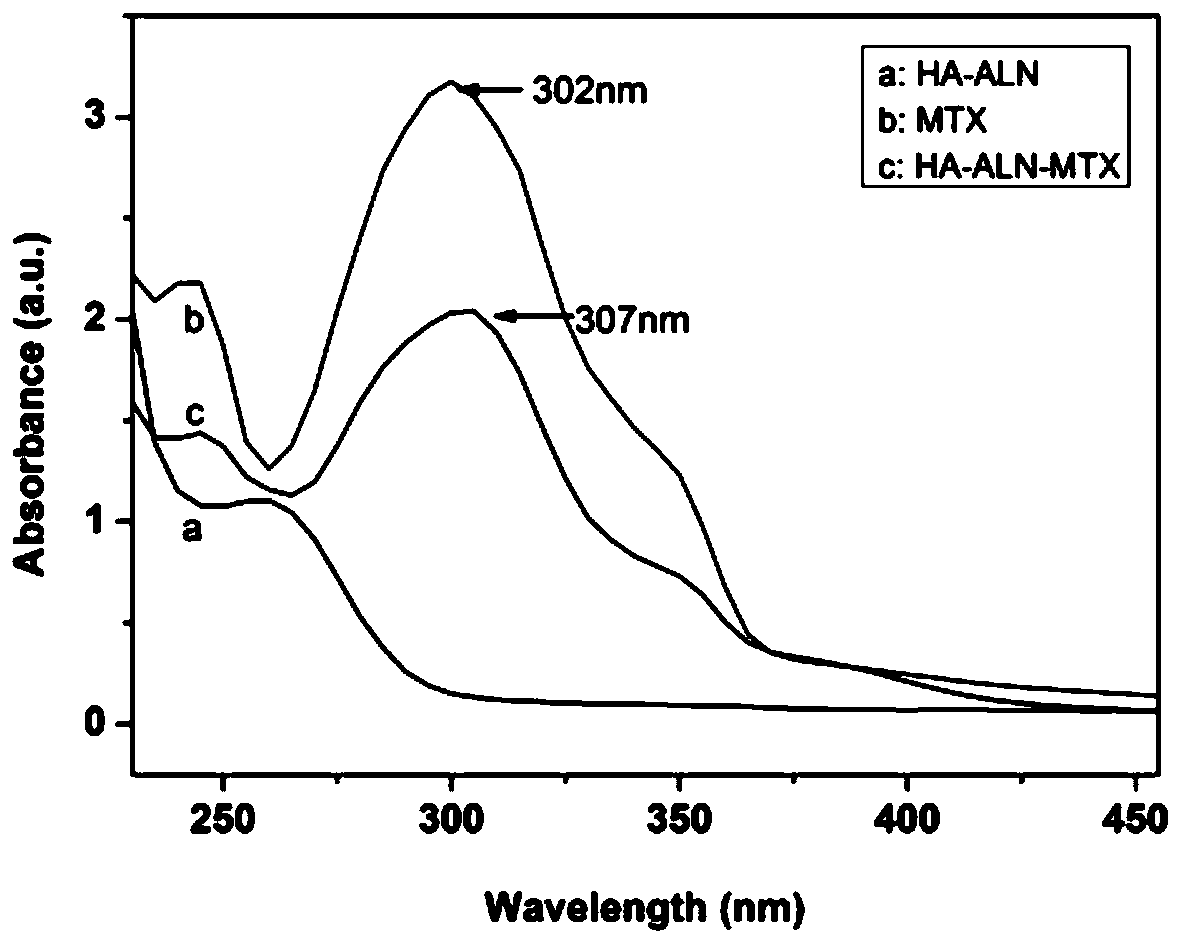
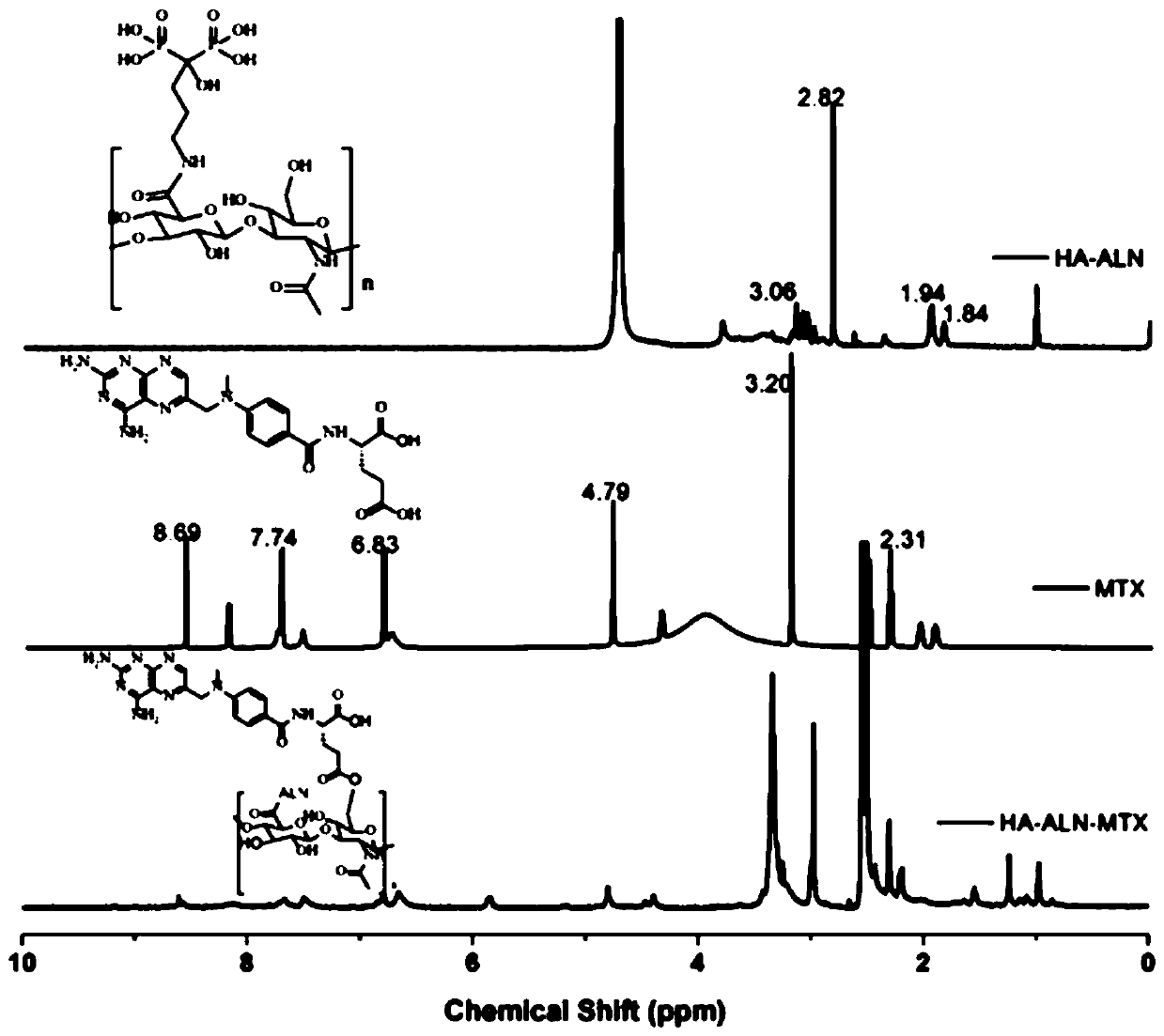
The invention discloses a preparation method of hyaluronic acid-alendronate sodium-methotrexate nanometer granules. The method comprises the steps of adding hyaluronic acid to an aqueous solution of EDC and NHS, after carboxyl activation, adding alendronate sodium, continuing a stirring reaction, and performing dialysis to obtain an HA-ALN coupling substance; then enabling the HA-ALN coupling substance to dissolve in DMF, adding DMSO, performing diluting to prepare an HA-ALN coupling substance solution, enabling methotrexate to dissolve in dimethylsulfoxide, and adding EDC and DMAP, to prepare a methotrexate solution; and finally, mixing the HA-ALN coupling substance solution with the methotrexate solution, and performing a normal-temperature reaction to prepare the hyaluronic acid-alendronate sodium-methotrexate nanometer granules. The prepared HA-ALN-MTX coupling substance is high in stability and favorable in dispersibility, can be targeted to cancer cells to effectively kill cancer cells, and besides, is low in toxic and side effect, and has application prospect in tumor targeted therapy.
Owner:YANGZHOU UNIV
Marker genes for screening of drug-induced toxicity in human cells and screening method using the same
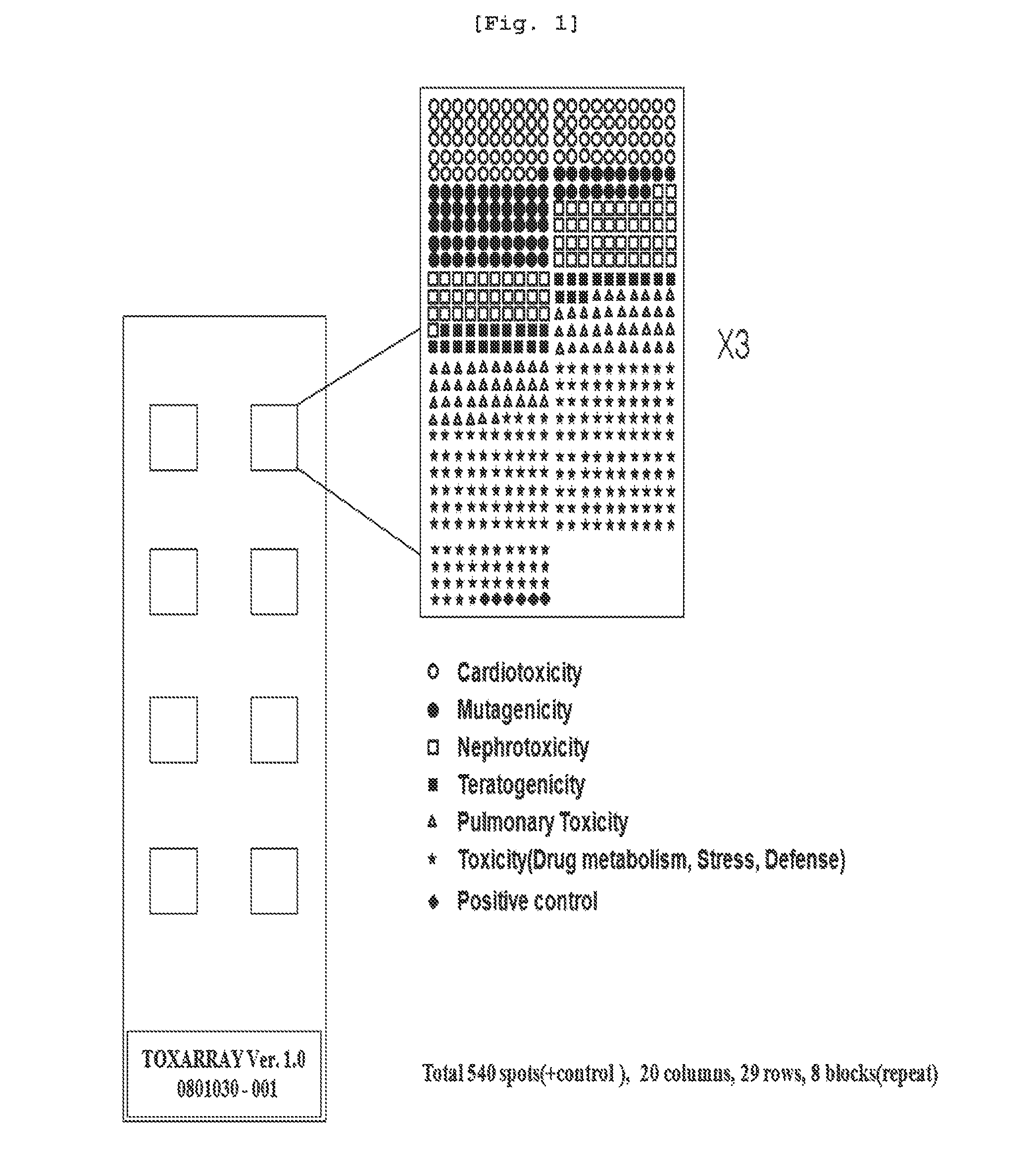
InactiveUS20110118127A1Microbiological testing/measurementLibrary screeningDrug induced toxicityCardiotoxicity
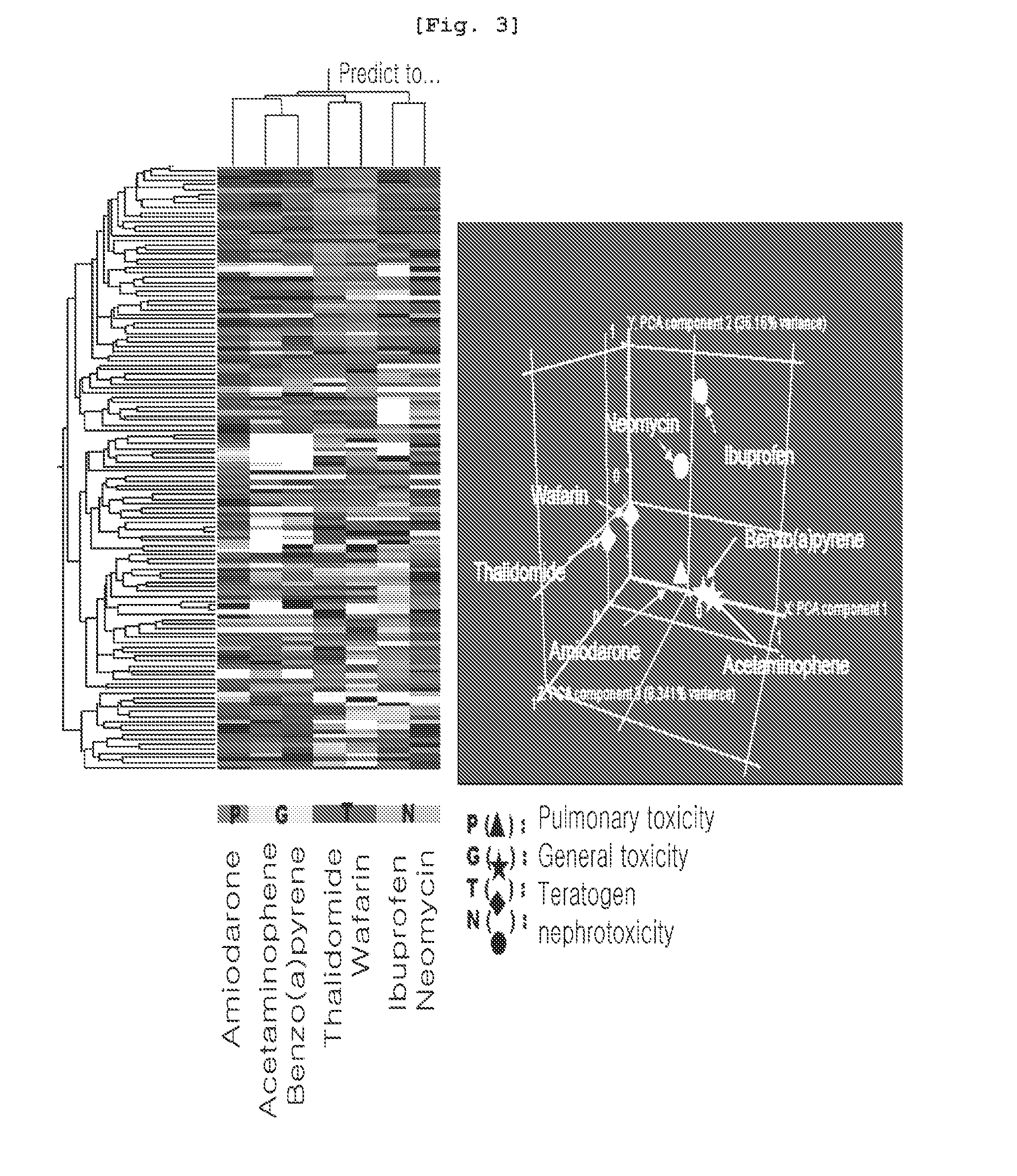
The present invention relates to a marker gene for screening a drug inducing toxicity in human and a screening method using the same. More precisely, the invention relates to a microarray on which marker genes up-or down-regulated specifically by 16 drugs inducing pulmonary toxicity, teratogenicity, nephrotoxicity, cardiotoxicity or mutation (Methotrexate, Nitrofurantoin, Amiodarone, Carbamazepine, Valproic acid, Thalidomide, Cisplatin, Gentamycin, Amphotericine, Furylfuramide, N-nitroso-N-methylurea, methylmethanesulfonate, 4-nitroquinoline-N-oxide, 2-nitrofluorene, Doxorubicin and Daunorubicin) are integrated, a kit comprising the said microarray, and a screening method of a drug inducing toxicity in human using the same. The DNA microarray containing the marker gene of the present invention facilitates the construction of Toxtarget Array for screening a drug inducing toxicity in human using drug-specific genes, suggesting that this chip can be effectively used for monitoring drugs or chemicals carrying toxicity to human or determining risks thereof and also it can be used as a tool for examining mechanisms of toxicity / side effects caused by the drugs.
Owner:KOREA INST OF SCI & TECH
Coprecipitation method for preparing methotrexate/ layered double hydroxides nanocomposite material
InactiveCN102631685ASimple operation processLow operating costOrganic active ingredientsPharmaceutical non-active ingredientsCentrifugationPolyethylene glycol
The invention discloses a coprecipitation method for preparing a methotrexate / layered double hydroxides (MTX / LDHs) nanocomposite material, and belongs to the technical field of materials and pharmaceutical preparations. The production method comprises the following steps that: polyethylene glycol (PEG) / water is used as a solvent, and is reacted in an environment of 5 to 20 wt% of alkali solution, and the resultant product is separated by centrifugation and then washed by centrifugation, and finally, the MTX / LDHs nanocomposite material is prepared by peptization and drying. The method has theadvantages that the MTX / LDHs nancomposite material with good monodispersity can be synthesized by adding one solvent, the operation is simple, the synthetic effect is good, and no pollution to a synthetic product exists.
Owner:江苏顺德缘文化科技股份有限公司
Use of ulinastatin in preparation of drugs for treating rheumatoid arthritis and pharmaceutical composition thereof
InactiveCN101954072ALittle side effectsPeptide/protein ingredientsAntipyreticSide effectCurative effect
The invention relates to an application of ulinastatin in the preparation of drugs for treating rheumatoid arthritis. The related drugs are derived from natural proteins which are extracted from human urine, have stable and reliable quality and few adverse reactions and can overcome the shortcomings of more side effects and the like in the traditional drugs for treating the rheumatoid arthritis. The efficacy comparison studies of rheumatoid arthritis animal models prove that the efficacy of the ulinastatin is better than an amethopterin group, and the side effects are few.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
Methods of quantifying methotrexate metabolites
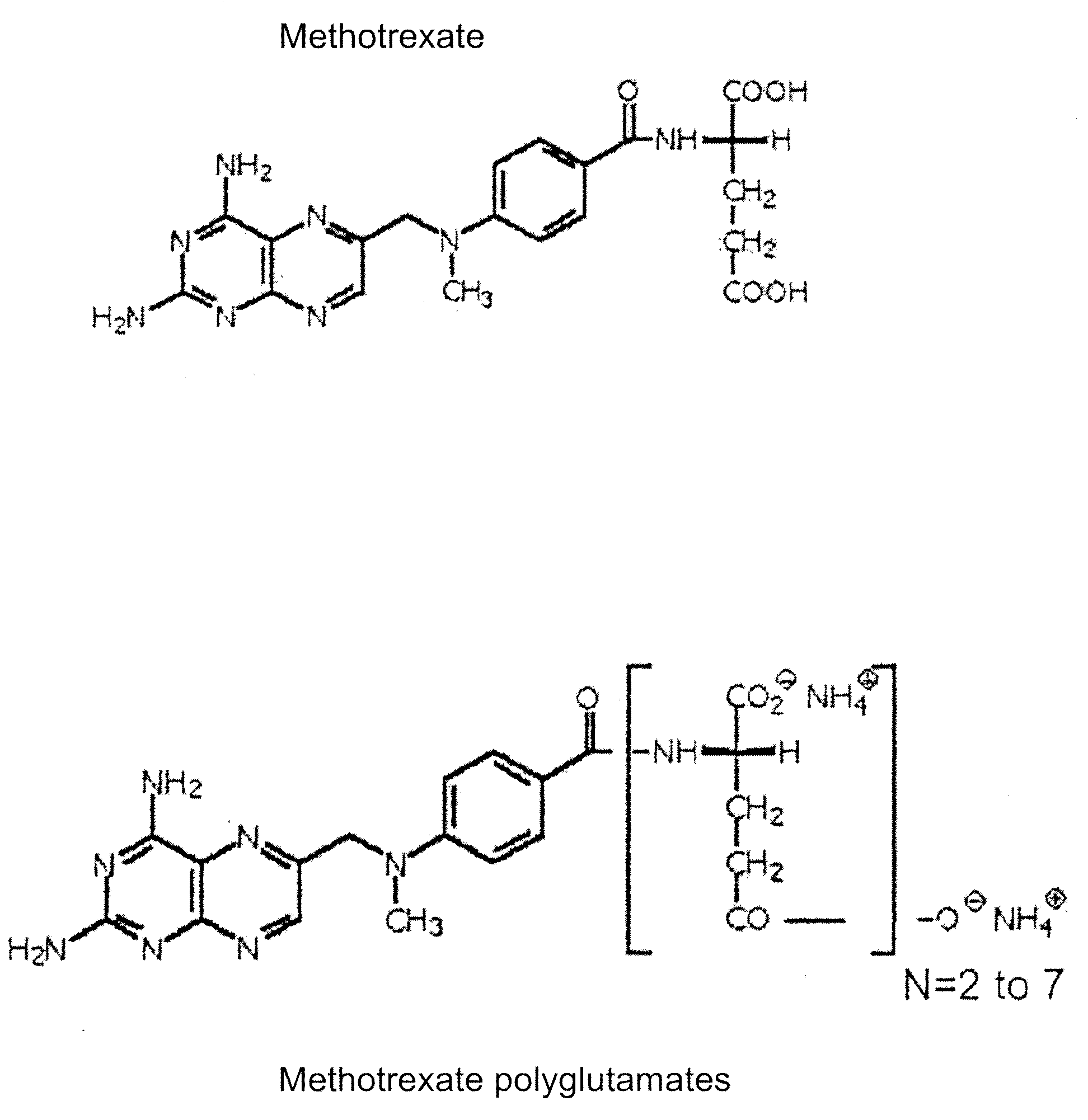
InactiveUS20100159493A1Efficiently converting methotrexate polyglutamatesEfficient conversionMicrobiological testing/measurementMammal material medical ingredientsCell extractionMetabolite
The present invention provides a method for efficiently converting methotrexate polyglutamates to methotrexate in a cellular extract by contacting the cellular extract with gamma glutamyl hydrolase under conditions suitable for efficient conversion of methotrexate polyglutamates to methotrexate.
Owner:CYPRESS BIOSCI +1
Antineoplastic conjugates of transferin, albumin and polyethylene glycol
Conjugates of transferrin, albumin and polyethylence glycol consisting of native or thiolated transferrin or albumin or of polyethylene glycol (MW between approximately 5,000 and 20,0000) with at least one HS-, HO- or H2N group and cytostatic compounds derived through maleinimide or N-hydroxysuccinimide ester compounds, such as doxorubicin, daunorubicin, epirubicin, idarubicin, mitoxandrone, chloroambucil, melphalan, 5-fluorouracyl, 5'-desoxy-5-fluorouridine, thioguanine, methotrexate, paclitaxel, docetaxel, topotecan, 9-aminocamptothecin, etoposide, teniposide, mitopodoside, vinblastine, vincristine, vindesine, vinorelbine or a compound of general formula A, B, C or D, where n=0-6, X=-NH2, -OH, -COOH, -O-CO-R-COR*, -NH-CO-R-COR*, where R is an aliphatic carbon chain with 1-6 carbon atoms or a substituted or unsubstituted phenylene group and R*H, phenyl, alkyl with 1-6 carbon atoms.
Owner:KRATZ FELIX
Culture medium for selectively separating and culturing group B streptococci and preparation method thereof
InactiveCN106434843APromoting logarithmic growthShorten the formation timeMicrobiological testing/measurementMicroorganism based processesColistin SulfateUltimate tensile strength
The invention discloses a culture medium for selectively separating and culturing group B streptococci and a preparation method thereof. Culture supernatant fluid of group B streptococci at the logarithmic phase and an antibiotic combination are added to a group B streptococcus selective developing basic culture medium. The culture supernatant fluid contains quorum sensing substances capable of accelerating the growth of bacteria and facilitating the speed of color development reaction and color development intensity, thereby greatly shortening the detection time; and the antibiotic combination comprises amethopterin, colistin sulfate and metronidazole, which can inhibit the growth of mixed bacterium, except the group B streptococci, thereby achieving a purpose of selectively separating and culturing the group B streptococci. When the culture medium is used for detecting the group B streptococci, the characteristics of high specificity and sensitivity, convenience in observation and the like are provided, and the culture medium can be effectively applied to rapid detection to clinic samples.
Owner:JIANGNAN UNIV
Preparation method of medicine sustained-release system with pH and near-infrared light dual response
InactiveCN111214655ATo achieve the purpose of controlled releaseGood light to heatOrganic active ingredientsEnergy modified materialsCarboxyl radicalControl release
The invention relates to a preparation method of a medicine sustained-release system with pH and near-infrared light dual response. The preparation method comprises the following steps of preparing graphene oxide, preparing graphene oxide / aminated mesoporous silica, preparing methotrexate / graphene oxide / aminated mesoporous silica, and preparing a methotrexate / graphene oxide / mesoporous silica / sodium alginate medicine sustained-release system. The preparation method provided by the invention has the beneficial effects that amino groups on the surface of aminated mesoporous silica and carboxyl onsodium alginate take amidation reaction, so that amido bonds can be generated to coat the surface of the mesoporous silica with the sodium alginate, and a sealing and blocking effect is achieved on medicine methotrexate in mesopores. The amido bonds have certain pH sensitivity, and the graphene oxide has good light-to-heat conversion characteristics, so that the methotrexate / graphene oxide / mesoporous silica / sodium alginate medicine sustained-release system with pH and near-infrared light dual response is obtained. The methotrexate / graphene oxide / mesoporous silica / sodium alginate medicine sustained-release system can achieve the goal of controlled release of medicine through dual stimulation of near-infrared light and pH.
Owner:CHANGZHOU UNIV
Yttrium-based metal-organic framework material and application thereof
ActiveCN112280054AGood biocompatibilityImprove water stabilityPowder deliveryPharmaceutical non-active ingredientsBenzoic acidMetal-organic framework
The invention discloses a yttrium-based metal organic framework material and application thereof. The chemical formula of the yttrium-based metal organic framework material (Y-MOF) is Y<4> (M)< 2>. (DMF)<3.5>. (H<2>O), and in the chemical formula, M is 2, 4, 6-tri (4-carboxyl phenyl) 1, 3, 5-triazine, 2, 4, 6-tri (4-pyridine) 1, 3, 5-triazine or 1, 3, 5-tribenzoic acid pyridine. The yttrium-basedmetal organic framework material (Y-MOF) prepared by the invention is of a porous structure, has better biocompatibility and water stability, can be used as a carrier material for simultaneously loading metal ions (Mn < 2 + >, Fe < 2 + > and the like) and drug molecules (methotrexate, busulfan, adriamycin and the like), and has the effects of magnetic resonance imaging, chemodynamic therapy and chemical drug therapy; the yttrium-based metal organic framework material (Y-MOF) is prepared by adopting a solvothermal method, so that the method is simple, convenient and safe to operate, the raw materials are easy to obtain, and the conditions are mild.
Owner:ZHEJIANG SCI-TECH UNIV
Methotrexate metal coordination polymer, and preparation method and application thereof
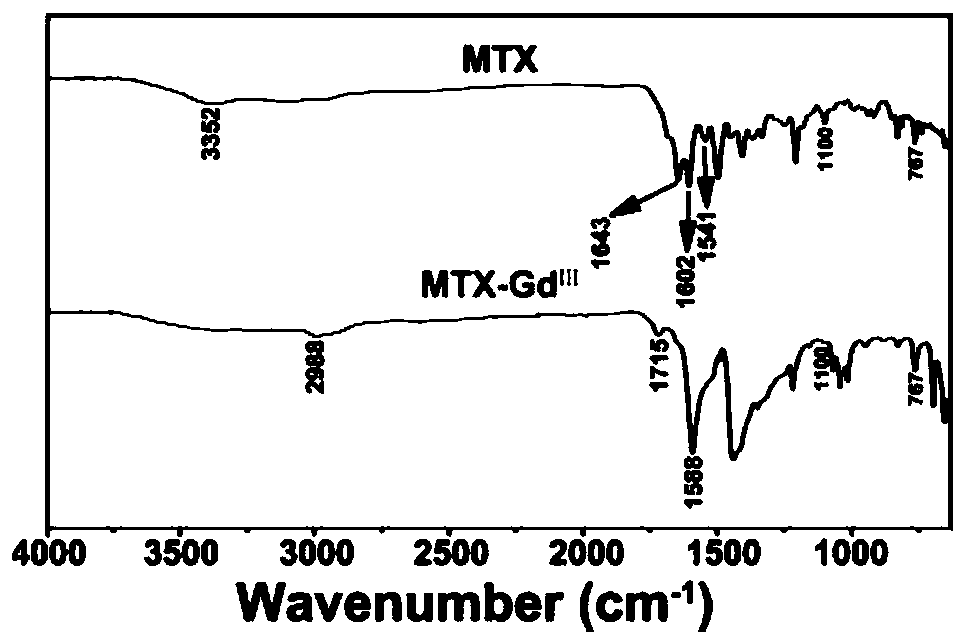
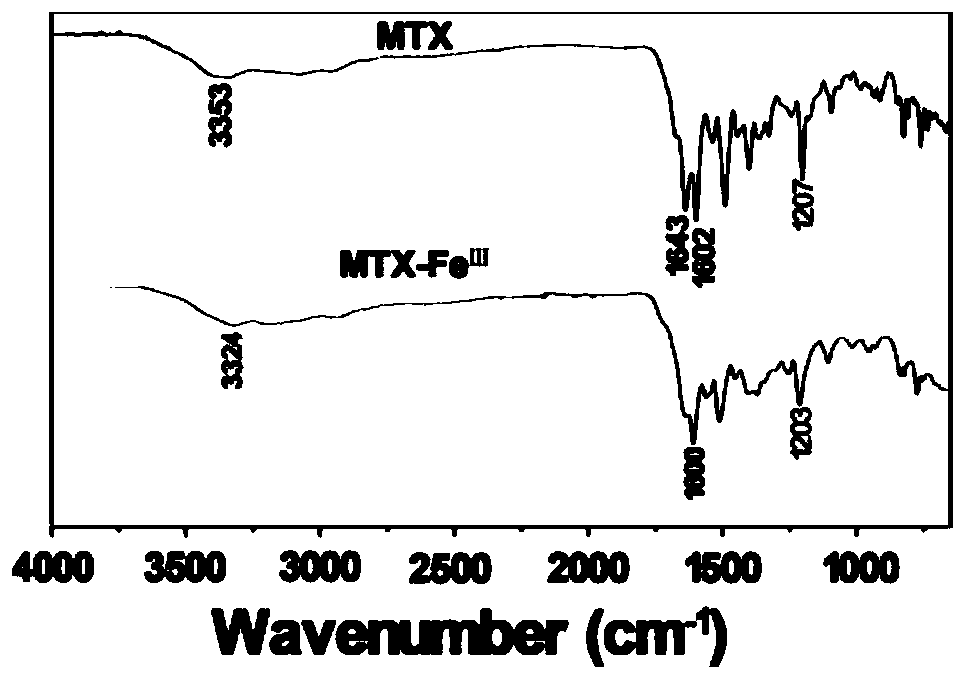
ActiveCN110862546AEffective treatmentAccurate and efficient diagnosis and treatmentSynthetic polymeric active ingredientsImmunological disordersDiseaseStructural formula
The invention discloses a methotrexate metal coordination polymer, and a preparation method and application thereof. The structural formula of the methotrexate metal coordination polymer is as described in the description, wherein R is a functional metal ion. According to the invention, GdIII and FeIII capable of being imaged by magnetic resonance imaging (MRI) are introduced into a structure of MTX to obtain a coordination polymer of MTX-GdIII and MTX-FeIII, and the polymer can be efficiently delivered to tumor cells with overexpressed folate receptors under active targeting mediation (the MTX and the folate receptor have extremely strong affinity), so that the administration dosage is reduced, and accurate and efficient diagnosis and treatment of tumor focus are realized. An MTX-ZnII coordination polymer is obtained by introducing ZnII with an immune promotion effect into the structure of the MTX, and the polymer is expected to be used for efficiently treating tumors and immune diseases.
Owner:XIAMEN UNIV
Hyaluronic acid-methotrexate conjugate
Owner:DENKA CO LTD
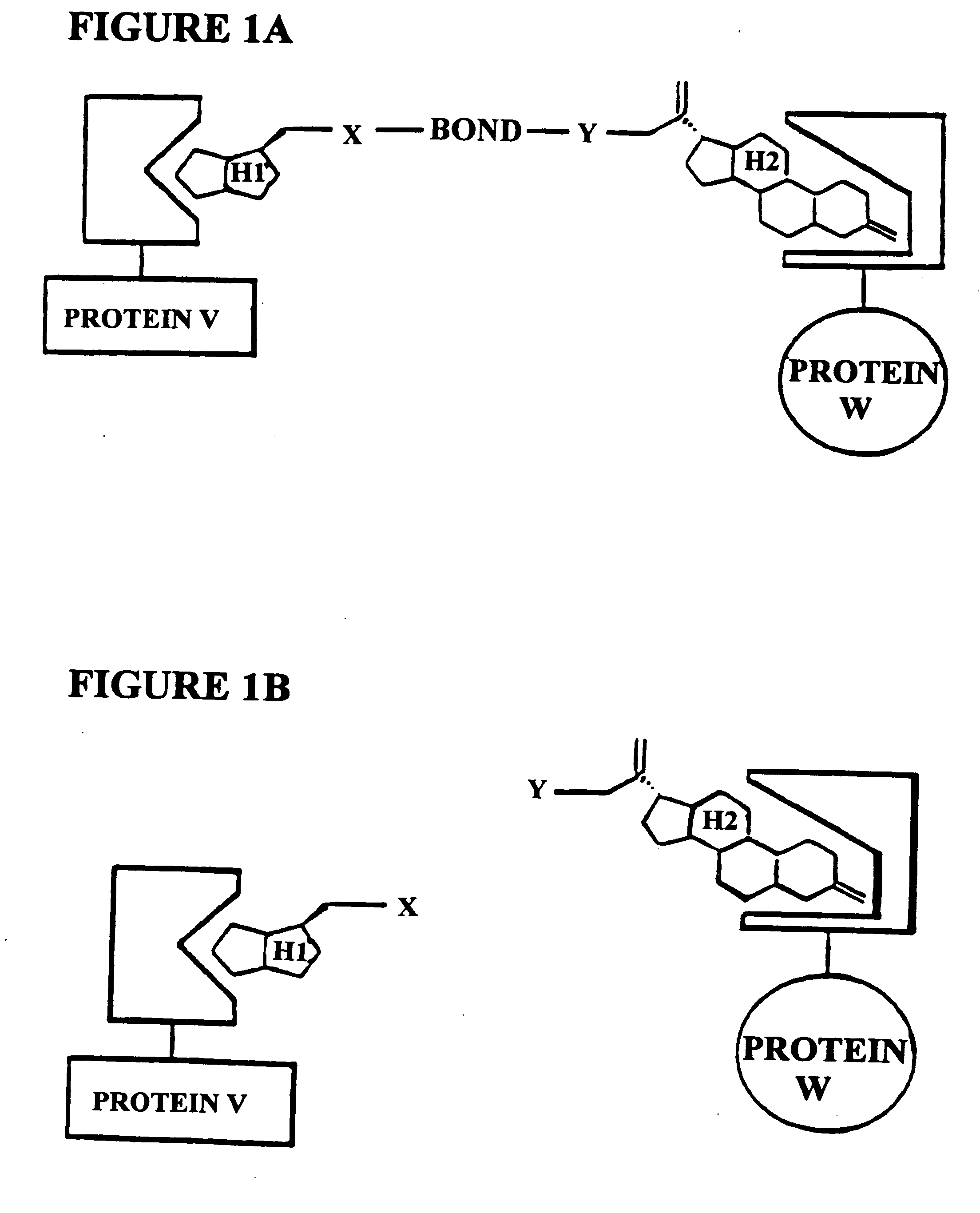
Methods and assays for screening protein targets
A method for identifying a protein as being able to bind a ligand comprising, providing a molecule composed of a methotrexate moiety that is covalently bonded to the ligand; introducing the molecule into a cell which a) expresses a first fusion protein comprising a dihydrofolate reductase capable of binding methotrexate, expresses b) a second fusion protein comprising the protein, wherein one of the first and second fusion proteins also comprises a transcription activator domain and the other comprises a DNA-binding domain, and c) has a reporter gene wherein expression of the reporter gene is conditioned on the proximity of the first fusion protein to the second fusion protein; permitting the molecule to bind to the first fusion protein and to the second fusion protein so as to activate the expression of the reporter gene; and selecting the cell if it expresses the reporter gene.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Sustained release anticancer agent carrying angiogenesis inhibitor and cytotoxic drug
Disclosed is an anticancer slow release injection carrying both anti-angiogenesis agent and cytotoxic drugs, which comprises slow release micro-balloons and dissolvent, wherein the slow release microballoons comprise anti-cancer active constituents and slow release auxiliary materials, the dissolvent being specific dissolvent containing suspension adjuvant. The anticancer active constituents being the combination of anti-angiogenesis agent selected from Marimastat, SU5416, SU6688, Fumagillin or TNP-470, with cytotoxic drugs selected from Eptaplatin, Nedaplatin, Melphalan, 4-hydroperoxycyclophosphamide, hydroxyl radical Paclitaxel, 10-desacetyltaxuyunnanin, 7-epi-taxol, Vinorelbine, Tamoxifen, Amethopterin, Adriamycin, pidorubicin, actinomycin D, Tallimustine, Atrimustine, Semustine or Ranimustine, the slow release auxiliary materials are selected from di-aliphatic acid and sebacylic acid copolymer, ethylene-vinylacetate copolymer or lactic acid polymer, the viscosity of the suspension adjuvant is 100-3000cp and is selected from sodium carboxymethylcellulose.
Owner:JINAN KANGQUAN PHARMA TECH
ELISA (Enzyme-linked Immunosorbent Assay) kit for detecting methotrexate and application thereof
InactiveCN107219357ASmall standard deviationChromatography mass spectrometry is easyMaterial analysisSerum igeElisa kit
The invention provides an ELISA (Enzyme-linked Immunosorbent Assay) kit for detecting methotrexate and application thereof. The kit is prepared from the following reagents: a methotrexate coated 96-pore microporous plate, a methotrexate monoclonal antibody, an antibody diluting solution, an HRP (Hypothalamic Regulatory Peptide)-labeled goat anti-mouse secondary antibody, a methotrexate standard product, a washing liquid concentrated solution, a sample diluting solution, a TMB (Tetramethylbenzidine) color-developing solution and a stopping solution. The stable, rapid and simple methotrexate detection kit is obtained through improving the combination of all reagents and improvement on parameters of the reagents; when the ELISA kit is applied, the standard deviation value of the kit is relatively small. The ELISA kit can be used for detecting the plasma drug concentration of patients who utilize the methotrexate with a large dosage and detecting the plasma drug concentration of patients who utilize the methotrexate with a small dosage.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Synthesis process of methotrexate
PendingCN112851676AIncreased recrystallization yieldLow toxicityOrganic chemistryMedicinal chemistryPharmacology
The invention relates to a synthesis process of methotrexate, which comprises the following steps: synthesizing a methotrexate crude product, preparing the synthesized methotrexate crude product into methotrexate disodium salt, adjusting the pH value to 5-6, and refining into a methotrexate pure product, the reaction synthesis steps are less, the raw materials are easy to purchase, the cost is low, and the experimental operation is simple; the methotrexate disodium salt is recrystallized and separated out by acetone, the yield is improved, and the purity of the compound after detection reaches 99% or above.
Owner:贵州中森医药有限公司
Bone repairing material with calcium and phosphor base and its preparation method
InactiveCN1298395CIncreased flexural (bending) strengthGood treatment effectProsthesisFiberBone formation
The invention relates to a biological tissue material and its preparation, in particular to a calcium phosphor salt base bone repairing material and its preparation. Said material provided by the invention contains calcium phosphor salt and chitin / chitosan fiber, which existes in form of fiber thread or fiber bunch. It can also be combined with 0.1-10% chitin derivants like chitosan, phosphorylate chitin, phosphorylate chitosan, carboxylation sulation chitosan and or 0.01-0.1% collagen, bone formation protein amethopterin or adriacin. Cell experiments and animal experiments all indicate that the material has favorable biological compatibility and induction activity for osteogenesis. It can raise averagely 1 to 10 times of flexural resistant intensity of calcium phosphor base bone repairing material. The invention is a reliable in vivo degradable material for tissue repairing and treatment.
Owner:TSINGHUA UNIV
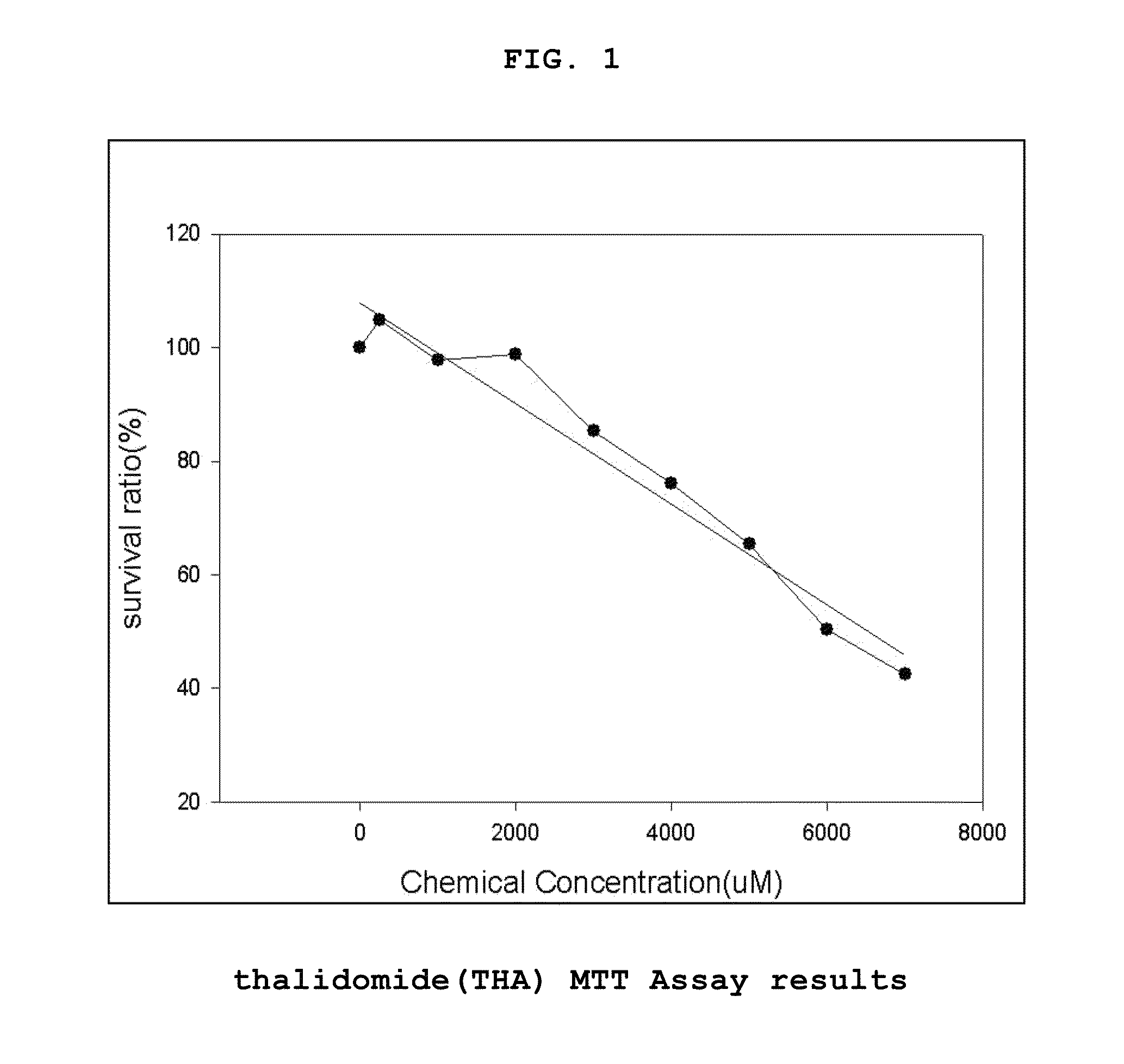
Genes based on thalidomide, valproic acid and methotrexate treatment for screening of drug inducing teratogenicity and screening method using thereof
ActiveUS8445207B2Microbiological testing/measurementLibrary screeningDNA Microarray ChipTeratogenic risk
The present invention relates to a screening method using the genes related to teratogenicity, more precisely the genes up- or down-regulated by a drug inducing teratogenicity such as thalidomide, valproic acid, and methotrexate and a method for screening of thalidomide, valproic acid and methotrexate using the genes. The genes of the present invention is based on reactive genes selected by DNA microarray chip, so that it is very effective in risk assessment and monitoring drugs or chemicals having high risk of teratogenicity and at the same time it can be used as a tool to examine mechanism of teratogenicity.
Owner:EAN HIGHTECH CO LTD
In vivo screen using chemical inducers of dimerization
InactiveUS20100261167A1Compounds screening/testingMicrobiological testing/measurementBinding domainIn vivo
A method for identifying a molecule that binds a known target in a cell from a pool of candidate molecules, comprising: (a) covalently bonding each molecule in the pool of candidate molecules to a methotrexate moiety to form a screening molecule; (b) introducing the screening molecule into a cell which expresses a first fusion-protein comprising a binding domain capable of binding methotrexate, a second fusion protein comprising the known target, and a reporter gene wherein expression of the reporter gene is conditioned on the proximity of the first fusion protein to the second fusion protein; (c) permitting the screening molecule to bind to the first fusion protein and to the second fusion protein so as to activate the expression of the reporter gene; (d) selecting which cell expresses the reporter gene; and (e) identifying the small molecule that binds the known target.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
cRGD-quaternized chitosan oligosaccharide modified ES2 peptide-methotrexate conjugate as well as a preparation method and application thereof
PendingCN113499447AImprove stabilityImprove biological activityAntibacterial agentsOrganic active ingredientsTumor targetTumor targeting
The invention relates to a cRGD-quaternized chitosan oligosaccharide modified ES2 peptide-methotrexate conjugate as well as a preparation method and application thereof, and belongs to the technical field of biological medicines. The CREM peptide conjugate can be prepared by controlling the conditions such as the supply quantity of the cRGD, the ES2 peptide and the MTX, the pH of a reaction system, the reaction time and the like. Compared with the ES2 peptide, the cRGD-quaternized chitosan oligosaccharide modified and methotrexate combined conjugate of the ES2 peptide keeps the anti-angiogenesis and anti-tumor activity of the ES2 peptide, integrates the tumor targeting property of cRGD and the anti-tumor activity of methotrexate and the like, has the characteristics of higher stability, hydrophilicity, targeting property and the like; therefore, the oligosaccharide has better use effect and application value.
Owner:SHANDONG UNIV
Solid tumor resisting release agent including nimustine and the intensifier
InactiveCN101040843AEasy injectionIncreased sensitivitySolution deliveryPharmaceutical non-active ingredientsTherapeutic effectPolymer
A nimustine slow release injection for treating solid cancer comprises slow release finding and nimustine, or the combination of nimustine and relative booster (pidorubicin, osaliplatinum, adriablastina, amethopterin or the like). The viscidity of slow-release injection is 10-650cp (20-30Deg. C). The anti-cancer effective component can be made into slow release plant agent. The slow release finding substantially comprises macromolecule polymer with biological soluble degradable and absorb property, which can slow release the anti-cancer effective component to cancer part in the degradation process, significantly reduce general reaction and hold effective drug density on cancer. The anti-cancer compound can be arranged part of cancer to reduce the general toxicity reaction, selectively improve the drug density locally, and strengthen the effect of non-surgery treatments as chemotherapy, and radiation therapy or the like. The solid cancer comprises glioma, lung carcinoma, intestinal cancer, breast cancer or the like.
Owner:JINAN KANGQUAN PHARMA TECH
Detection kit for methotrexate metabolic markers and detection method and application of detection kit
PendingCN113528629AShorten the timeImprove throughputMicrobiological testing/measurementDNA/RNA fragmentationDrug utilisationSLCO1B1
The invention discloses a detection kit of methotrexate metabolism markers, and a detection method and application of the detection kit. The kit is used for detecting the gene polymorphism of the methotrexate metabolism markers ABCB1 (C3435T), MTHFR (C677T) and SLCO1B1 (c.1865+4846T>C). According to the invention, multiple RPA amplification and optimized pyrosequencing technologies are combined to detect the methotrexate drug dosage and the gene polymorphism related to adverse reaction prediction, the kit can be used for simultaneously detecting ABCB1 (C3435T), MTHFR (C677T) and SLCO1B1 (c.1865+4846T>C) gene polymorphism methotrexate drugs, and suggestions from the gene perspective are provided for clinical personalized medication.
Owner:湖南菲思特精准医疗科技有限公司
Ion chromatography-carbon nanotube-modified electrode electrochemical detection analysis system
InactiveCN102735769ASmall particle sizeLarge particle sizeComponent separationMaterial electrochemical variablesElectrochemical detectorIon chromatography
The invention relates to an application system of chemical apparatus analysis, and especially relates to an ion chromatography-carbon nanotube-modified electrode electrochemical detection analysis system used for simultaneously detecting folic acid and methotrexate. The invention relates to an ion chromatography-carbon nanotube-modified electrode electrochemical detection analysis system. According to the invention, the carbon nanotubes are positively charged through covalent modification, and are absorbed on the surface of bare glass carbon electrode, such that novel carbon nanotube membrane-modified electrode is formed. The modified electrode is adopted as a working electrode, such that methotrexate and folic acid in anion states can be easily absorbed. Compared to bare glass carbon electrode, electrocatalytic oxidation of methotrexate and folic acid are greatly promoted. According to the invention, the modified electrode is applied in combination with ion chromatography in an ion chromatography electrochemical detector, such that methotrexate and folic acid rapid separation and detection are realized. Therefore, a problem that separation cannot be realized by using merely electroanalysis, and defects of low sensitivity, high interference and complicated operation of other detection methods, are overcome. The system is suitable for detections of low-concentration methotrexate and folic acid in complex substrate biological samples.
Owner:ZHEJIANG UNIV
Artificially synthesized cationic peptide and applications thereof in preparing anti-tumor drug
ActiveCN103992394ARelatively small molecular massImprove anti-tumor activityPeptide/protein ingredientsDepsipeptidesSide effectApoptosis
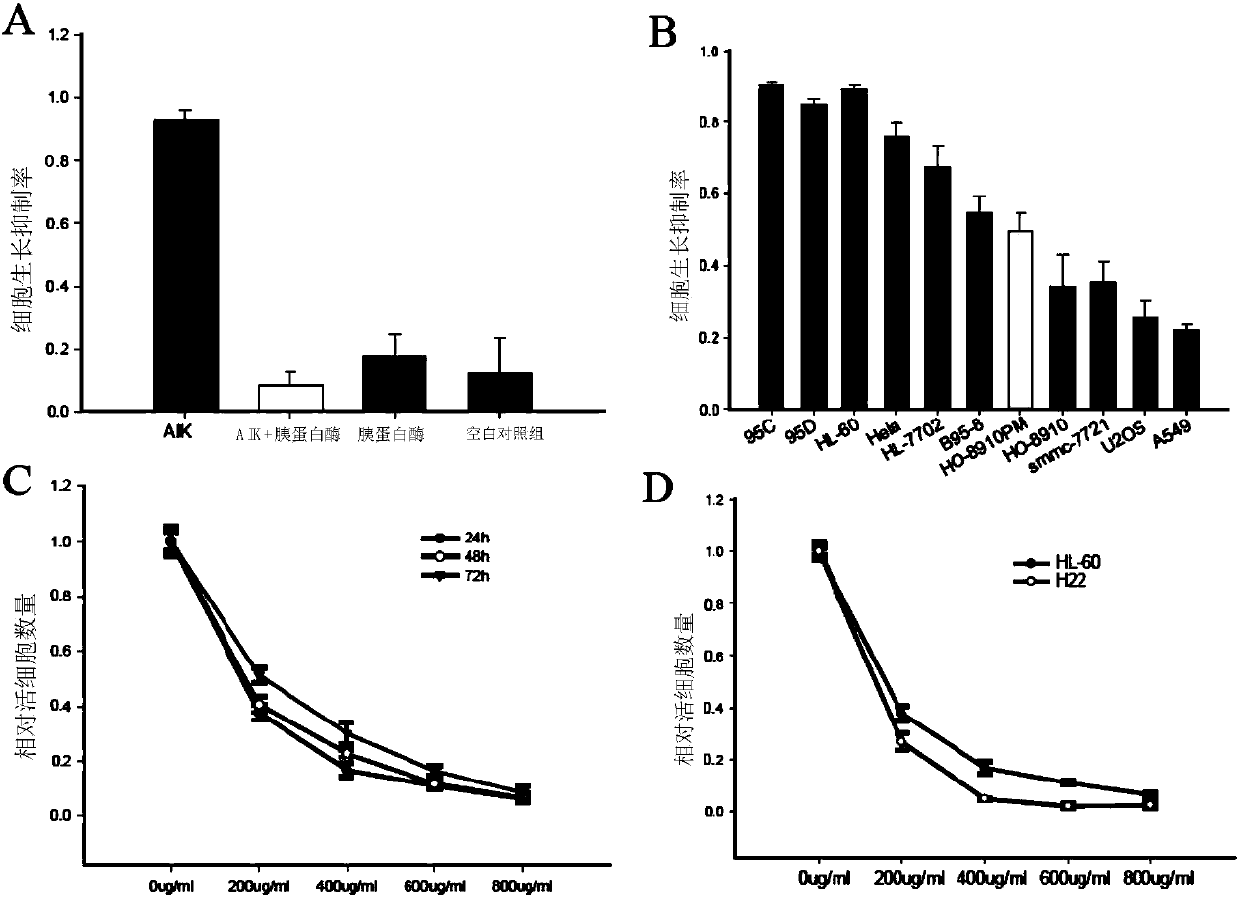
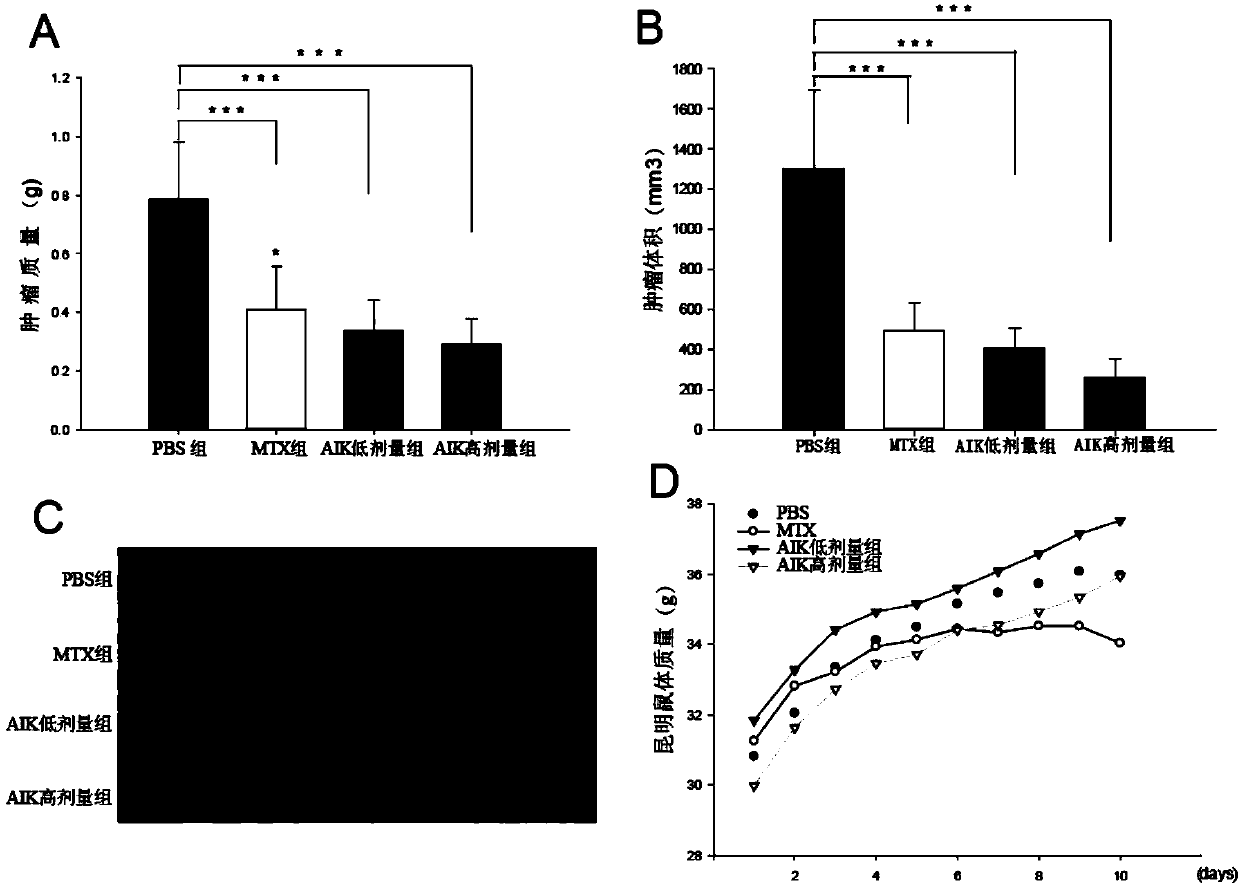
The invention discloses an artificially synthesized cationic peptide (named as AIK) and applications thereof in preparing an anti-tumor drug. The amino acid sequence of the cationic peptide is shown as SEQ ID NO.1. A research shows that the AIK has an obvious inhibition effect on various solid tumor cells, especially leukemic cells, cells of pulmonary giant cell cancer and hepatoma ascites cells, the anti-tumor activity is possibly combined an anionic acceptor on the surface of tumor cells so as to induce the tumor cells to be subjected to apoptosis and necrosis, and the anti-tumor activity is higher than that of the anti-tumor peptide molecules reported by other researchers. More importantly, the artificially synthesized 25-peptide drug molecules have higher specificity on the tumor cells. After the anti-tumor drug is locally administrated by subcutaneous tumor-bearing mice, the subcutaneous growth of liver cancer cells H22 of the tumor-bearing mice can be obviously inhibited and toxic and side effects are lower than chemotherapeutic drugs amethopterin. The artificially synthesized cationic peptide can provide theoretical basis and experimental materials for further researching and developing polypeptide anti-tumor new drugs and small-size targeted anti-tumor new drugs.
Owner:HARBIN MEDICAL UNIVERSITY
Preparation method of methotrexate compound
ActiveCN113461689AHigh purityEasy to separateOrganic chemistryPhysical/chemical process catalystsChlorobenzenePtru catalyst
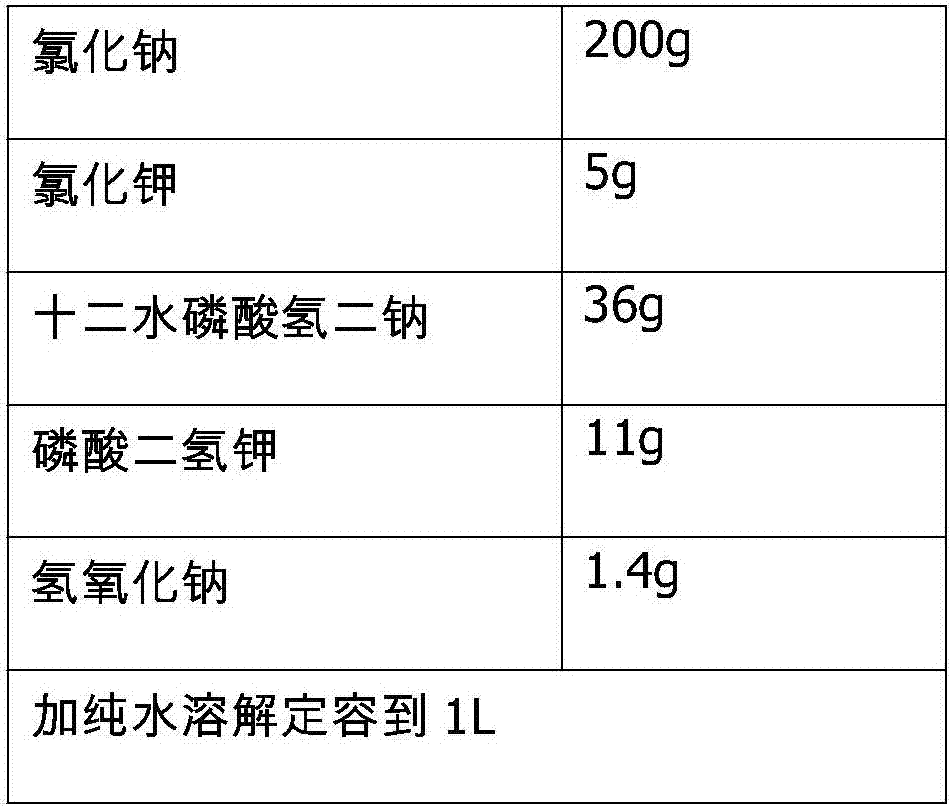
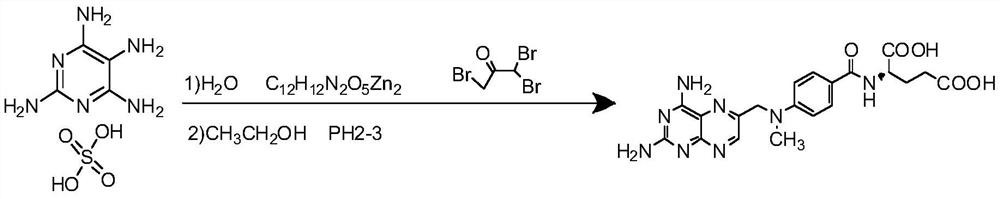
The invention belongs to the field of methotrexate synthesis, and particularly relates to a preparation method of a methotrexate compound, which comprises the following steps: under the action of a high-activity copper catalyst, synthesizing p-methylamino benzoyl L-glutamic acid zinc salt from chlorobenzoyl L-glutamic acid, methylamine and zinc chloride, and then synthesizing methotrexate from the p-methylamino benzoyl L-glutamic acid zinc salt, tetraaminopyrimidine sulfate and tribromoacetone, and the preparation method of the chlorobenzoyl L-glutamic acid is limited. According to the method, the problem of low purity of methotrexate is solved, efficient catalysis of the high-activity copper-based catalyst is utilized, and a fixed bed reaction system is matched, so that the catalytic efficiency is ensured, rapid separation of the catalyst is realized, and the stability of the product is effectively improved.
Owner:ZHEJIANG ZHEBEI PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com