Sustained release ocular implant for treating solid tumors
A slow-release implant, tumor technology, applied in the field of medicine, can solve the problems of unfavorable postoperative recurrence, difficult to control the maximum tolerated dose, and short release period of the sustained-release agent.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
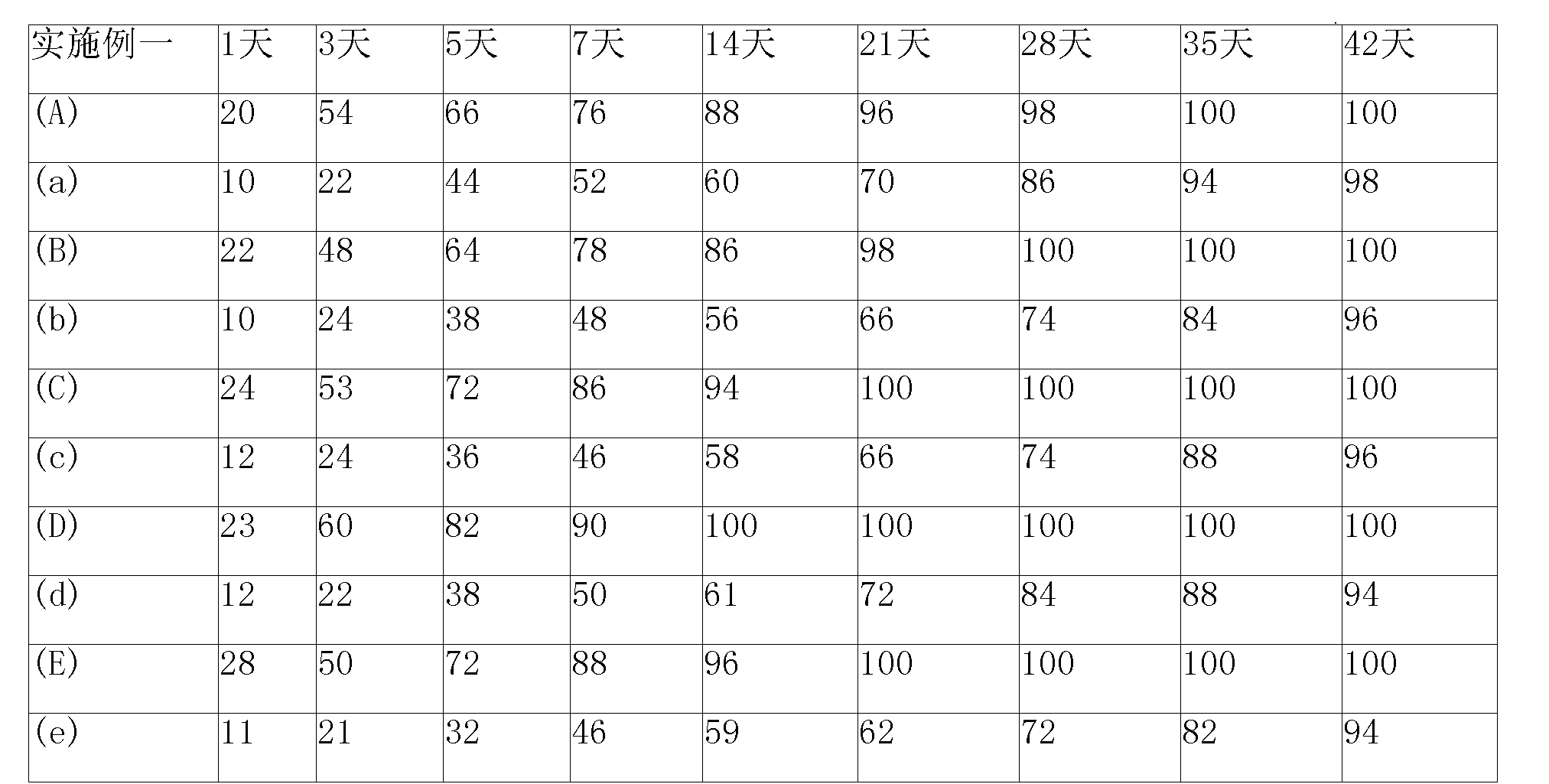
[0081] Put the weighed slow-release auxiliary material (molecular weight is 15000-25000 PLGA, 50:50) and slow-release modifier (mannitol) into different containers respectively, then add a certain amount of organic solvent to dissolve and mix well (to fully dissolve prevail) and then add different weights of nimustine, shake again and then vacuum dry to remove the organic solvent. The dried solid sustained-release implant was immediately shaped to obtain the following sustained-release implant:
[0082] (A) 1% Nimustine, containing 1mg Nimustine, 99mg PLGA
[0083] (a) 1% nimustine, containing 1mg nimustine, 95mgPLGA, 4mg mannitol;
[0084] (B) 5% nimustine, containing 5mg nimustine, 95mg PLGA;
[0085] (b) 5% nimustine, containing 5mg nimustine, 91mg PLGA, 4mg mannitol;
[0086] (C) 10% nimustine, containing 10mg nimustine, 90mg PLGA;
[0087] (c) 10% nimustine, containing 10mg nimustine, 80mg PLGA, 10mg mannitol;
[0088] (D) 15% Nimustine, containing 15mg Nimustine, 85...
Embodiment 2
[0093] 10 sustained-release implants in Example 1 were put into the dissolution apparatus respectively to measure the drug cumulative release amount (%) at different times, and it was found that in the sustained-release implants (A), (B), (C ), (D) and (E) five nimustine sustained-release implants that do not contain a sustained-release regulator (mannitol) have obvious burst release phenomena, and the burst release phenomenon mostly occurs on the 15th-20th day, Account for about 60-80% of the total drug content, while (a), (b), (c), (d) and (e) etc. contain sustained-release regulator (mannitol) nimustine sustained-release implants The sudden release phenomenon of the medicine is obviously not obvious, and the medicine is released slowly for 3-4 weeks.
Embodiment 3
[0095]Put the weighed sustained-release auxiliary material (PLGA with a molecular weight of 20000-30000, 75:25) and the sustained-release regulator (mannitol) into different containers respectively, then add a certain amount of organic solvent to dissolve and mix (to fully Dissolution prevails) and then add different weights of nimustine, shake again and then vacuum dry to remove the organic solvent. The dried solid sustained-release implant was immediately shaped to obtain the following sustained-release implant:
[0096] (A) 1% Nimustine, containing 1mg Nimustine, 99mg PLGA
[0097] (a) 1% nimustine, containing 1 mg nimustine, 95 mg PLGA and 4 mg sorbitol;
[0098] (B) 5% nimustine, containing 5mg nimustine, 95mg PLGA;
[0099] (b) 5% nimustine, containing 5 mg nimustine, 91 mg PLGA and 4 mg sorbitol;
[0100] (C) 10% nimustine, containing 10mg nimustine, 90mg PLGA;
[0101] (c) 10% nimustine, containing 10 mg nimustine, 80 mg PLGA and 10% sorbitol;
[0102] (D) 15% Nim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com