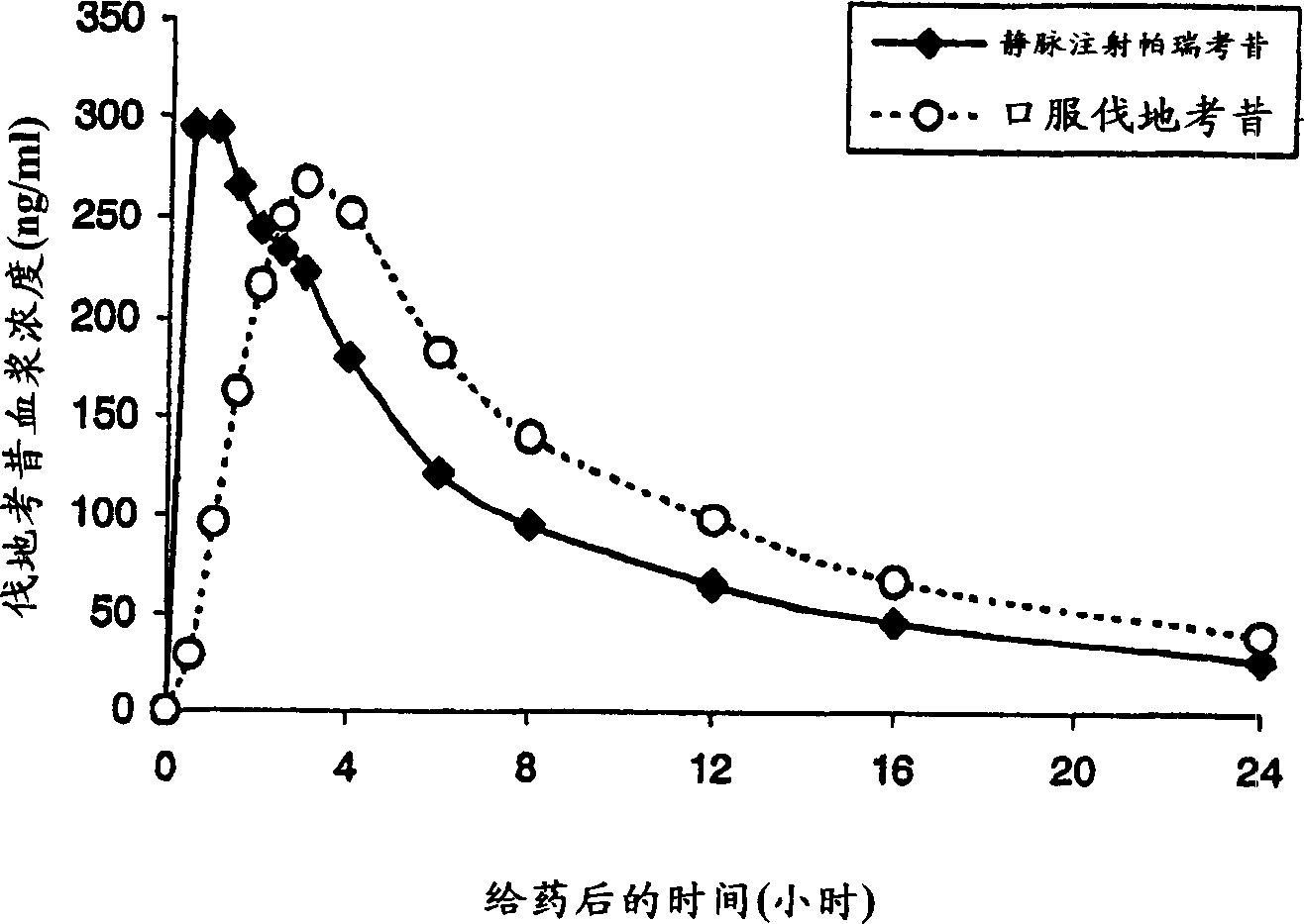
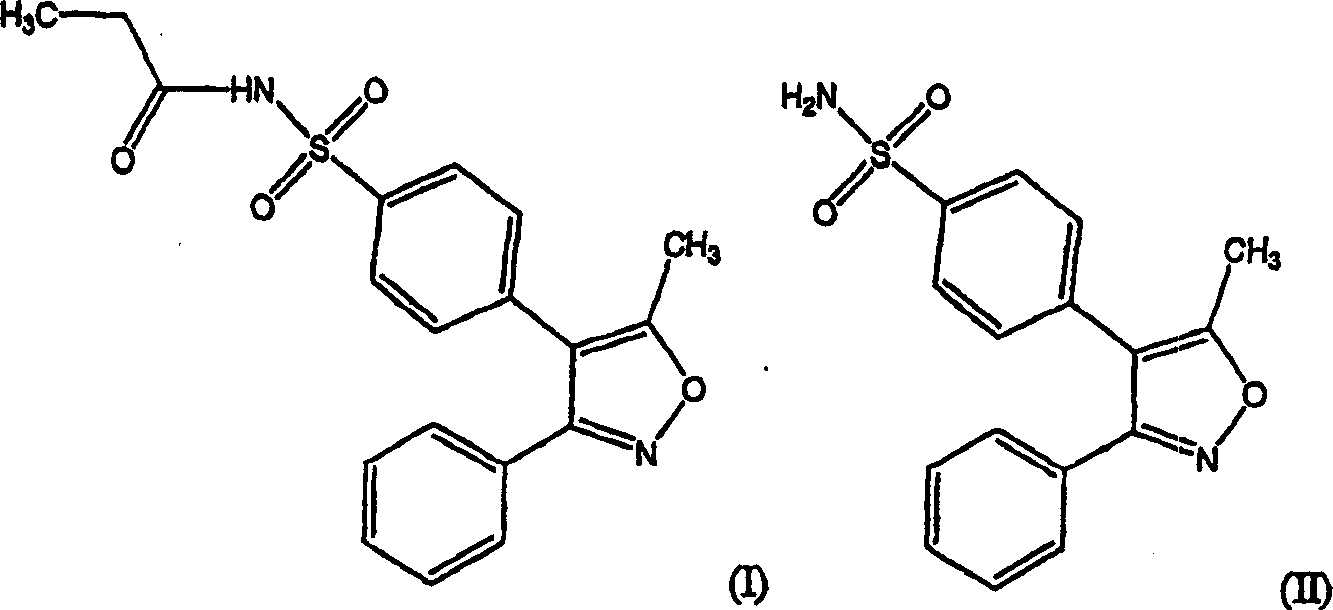
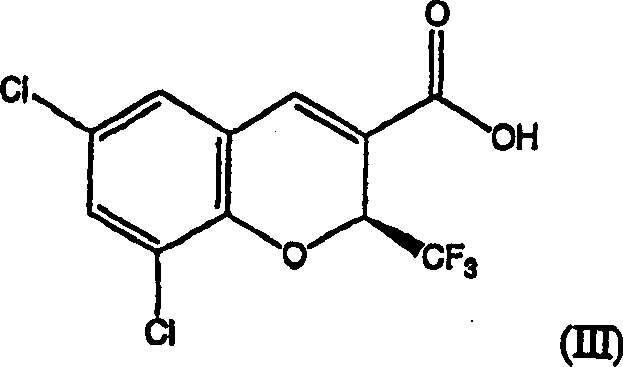
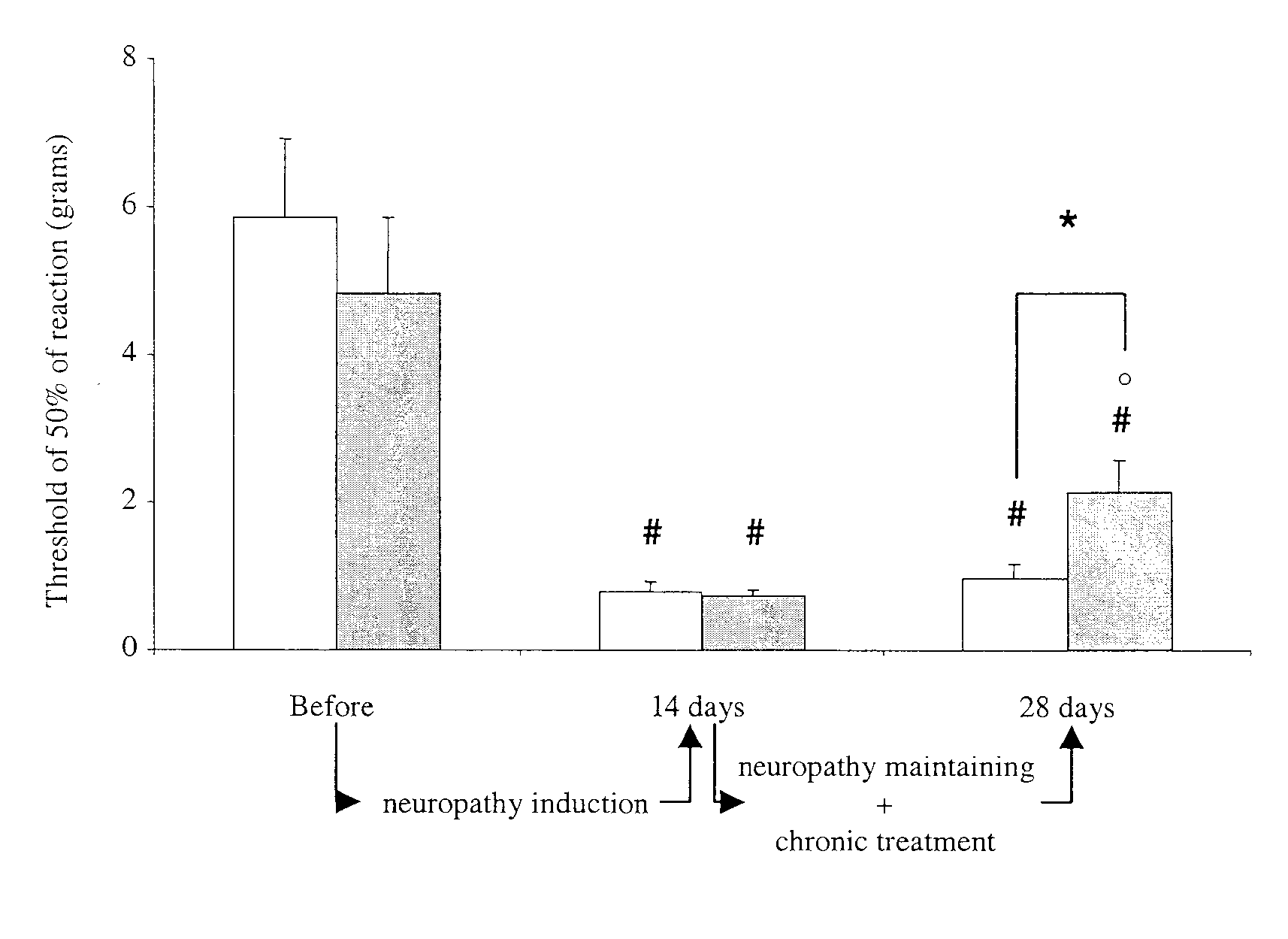
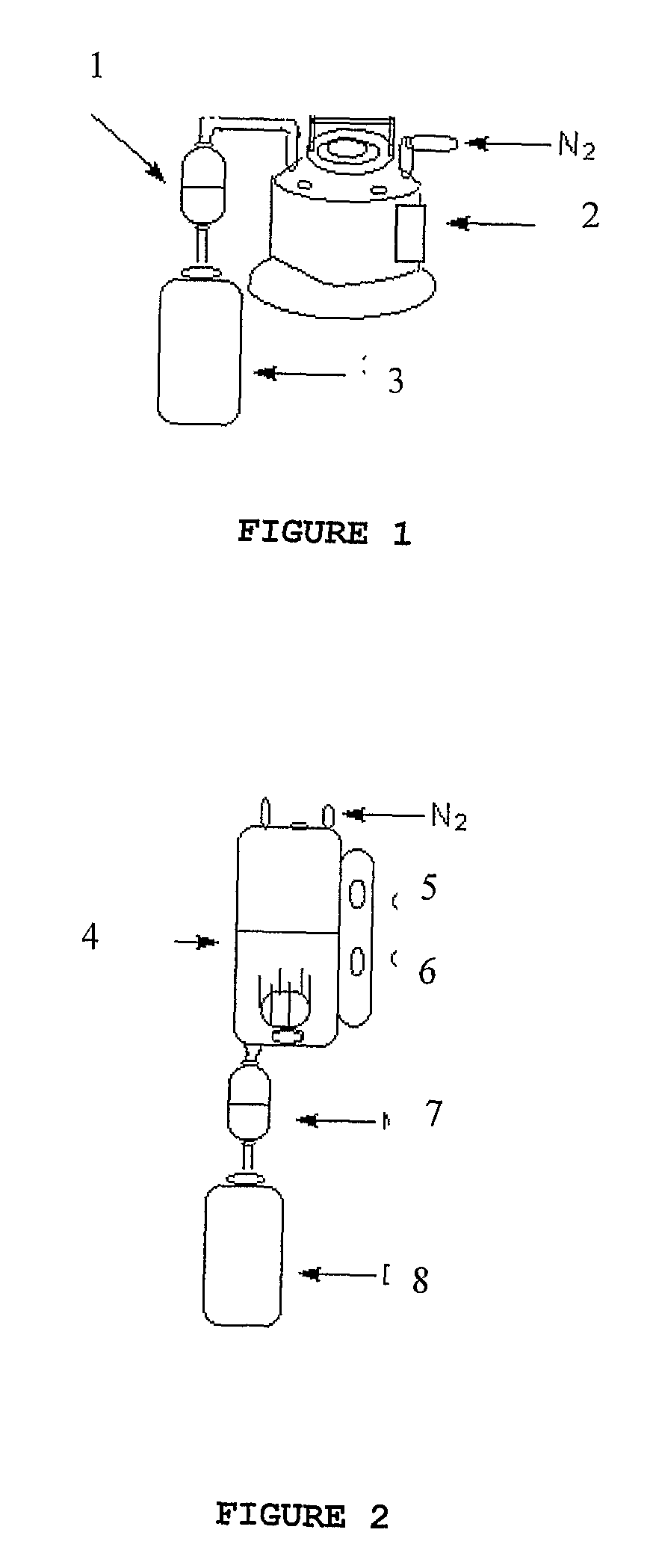
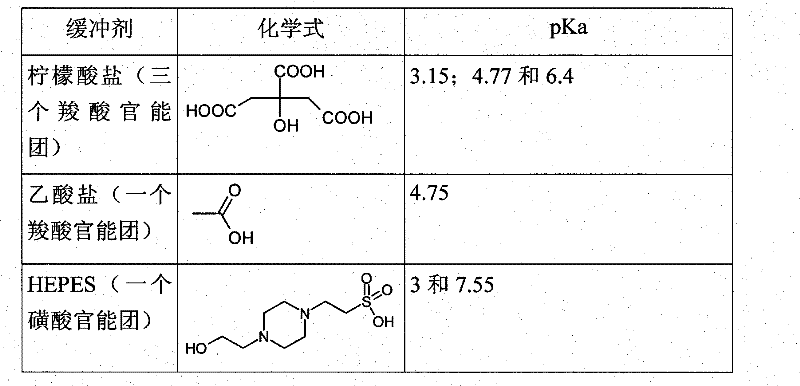
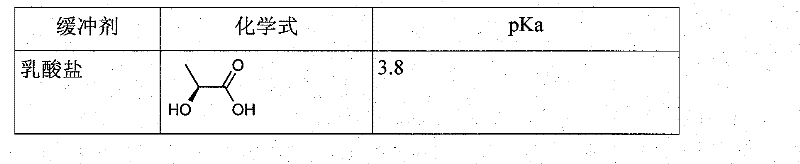
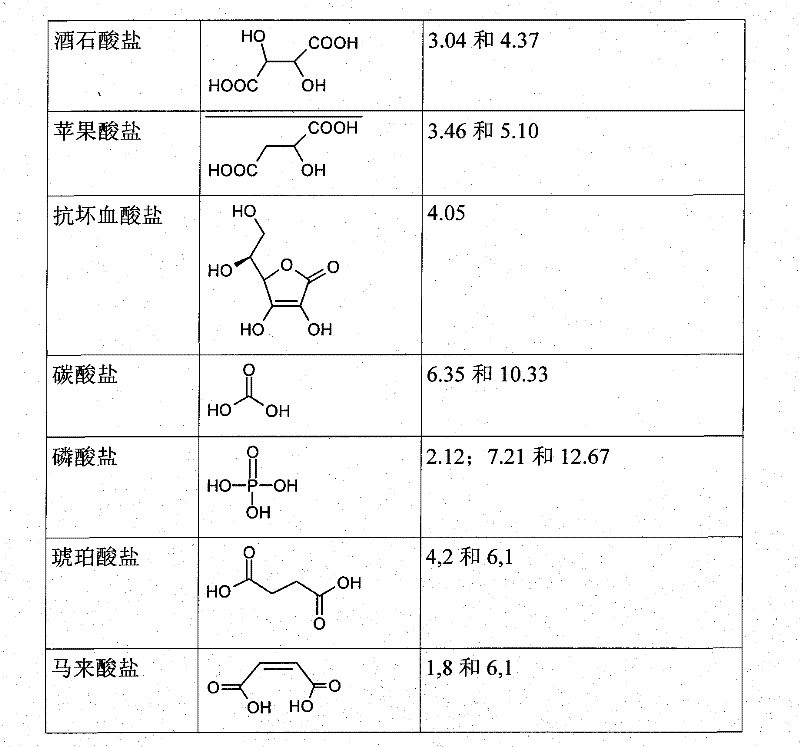
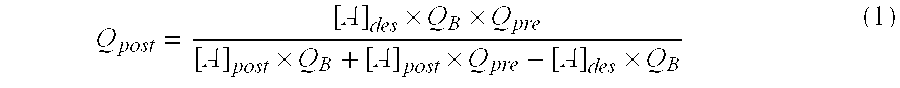Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
70 results about "Injectable Solution" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Banamine Injectable Solution Indications. Horse: BANAMINE (flunixin meglumine injection) is recommended for the alleviation of inflammation and pain associated with musculoskeletal disorders in the horse. It is also recommended for the alleviation of visceral pain associated with colic in the horse.
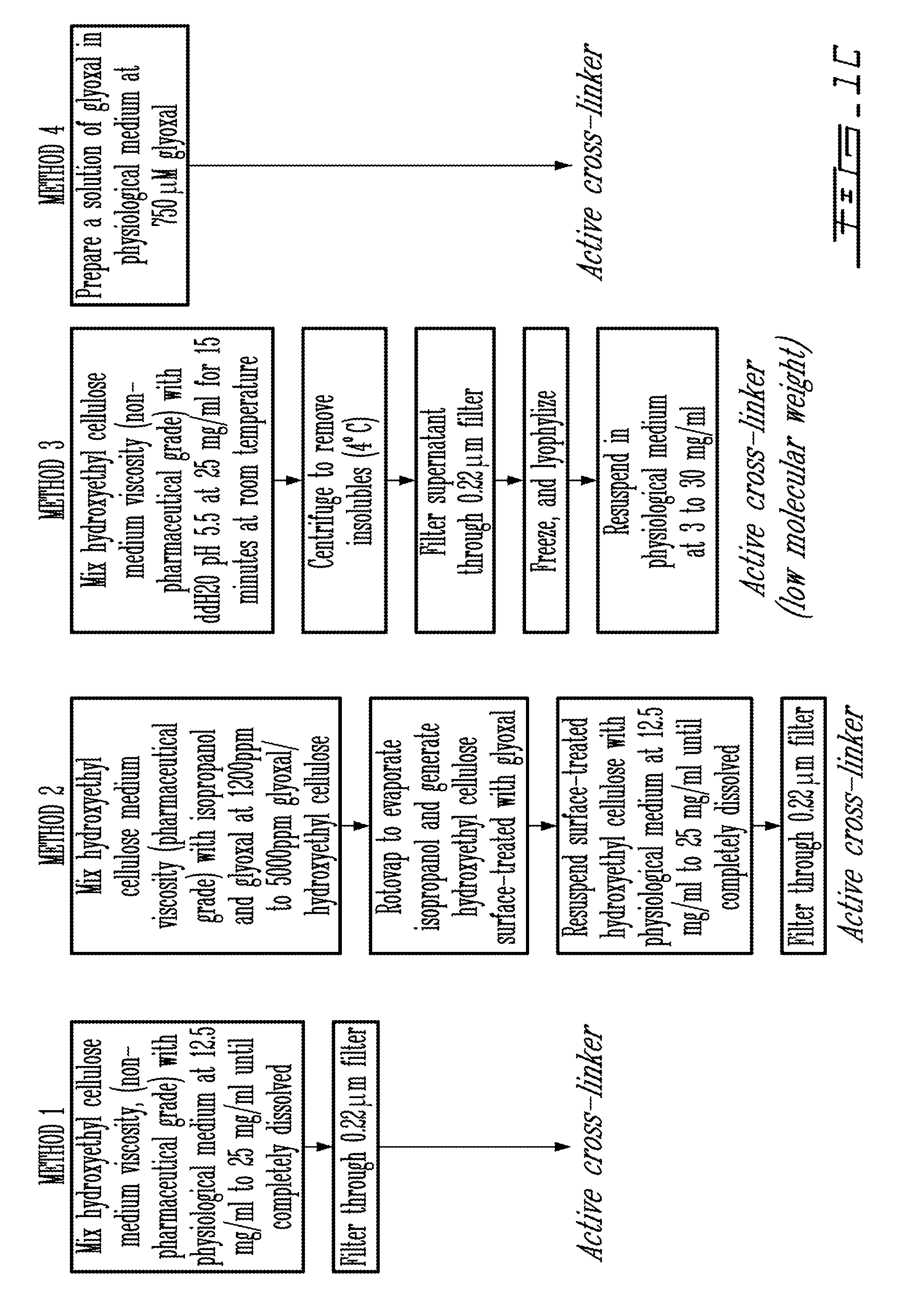
Injectable cross-linked polymeric preparations and uses thereof
InactiveUS20050003010A1Promote regenerationFunction increaseOrganic active ingredientsPowder deliveryCross-linkDamages tissue
A composition for promoting repair of damaged tissues, being a cross-linked alginate solution, which can be maintained in liquid form indefinitely (under constant conditions) and only gels in vivo. This cross-linked alginate solution is an ideal material to be used for tissue repair. Injection of said material into cardiac tissue post-myocardial infarct induced tissue regeneration. The invention provides such injectable solution, as well as compositions and method of preparation thereof. The invention also provides various methods and uses of the cross-linked alginate solution, for cardiac tissue regeneration, induction of neo-vascularization, enhancing SDF-1 expression and guiding stem cell chemotaxis, among others. A kit for tissue repair is also provided.
Owner:BEN GURION UNIVERSITY OF THE NEGEV
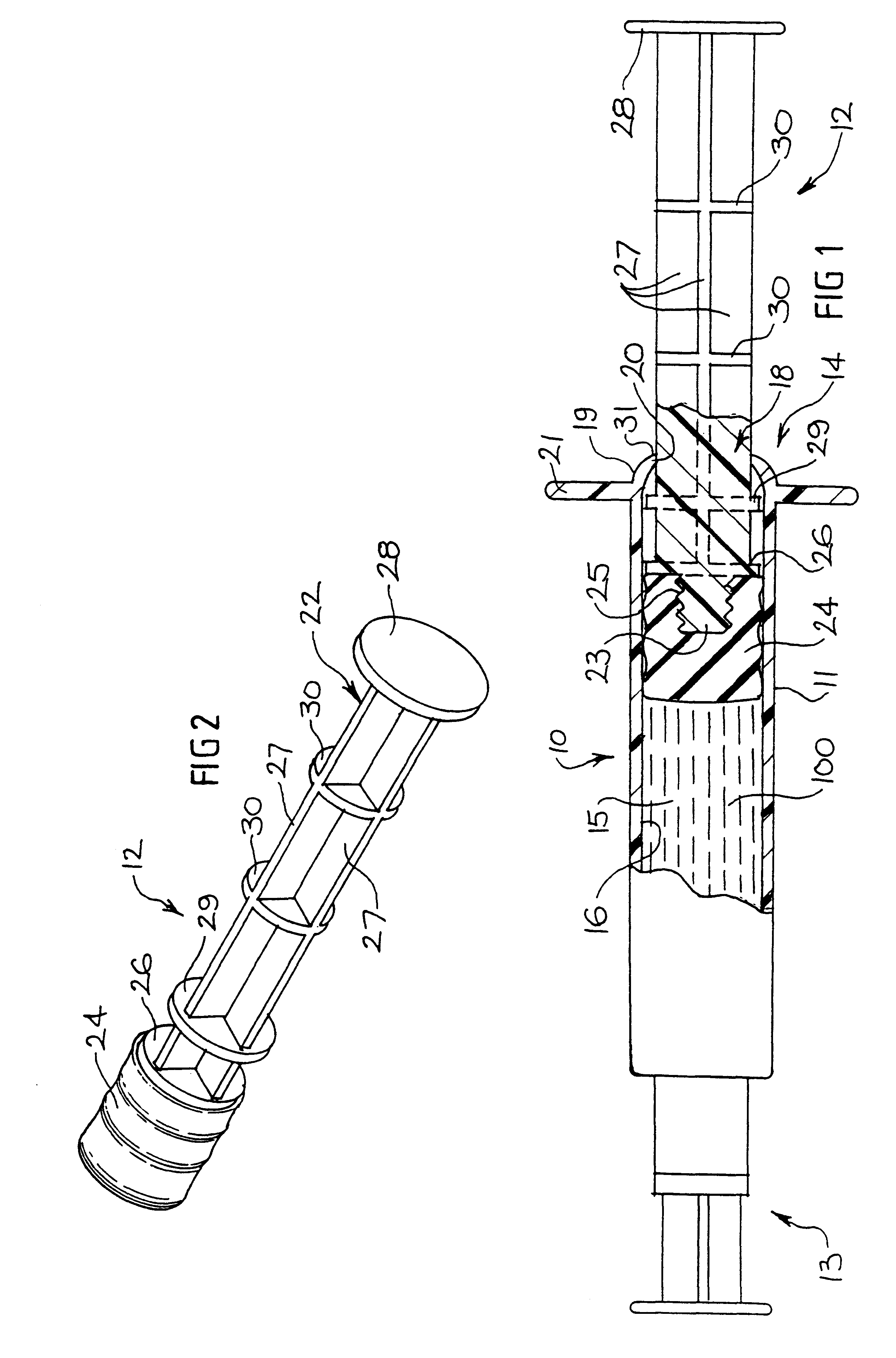
Tamper evident syringe design
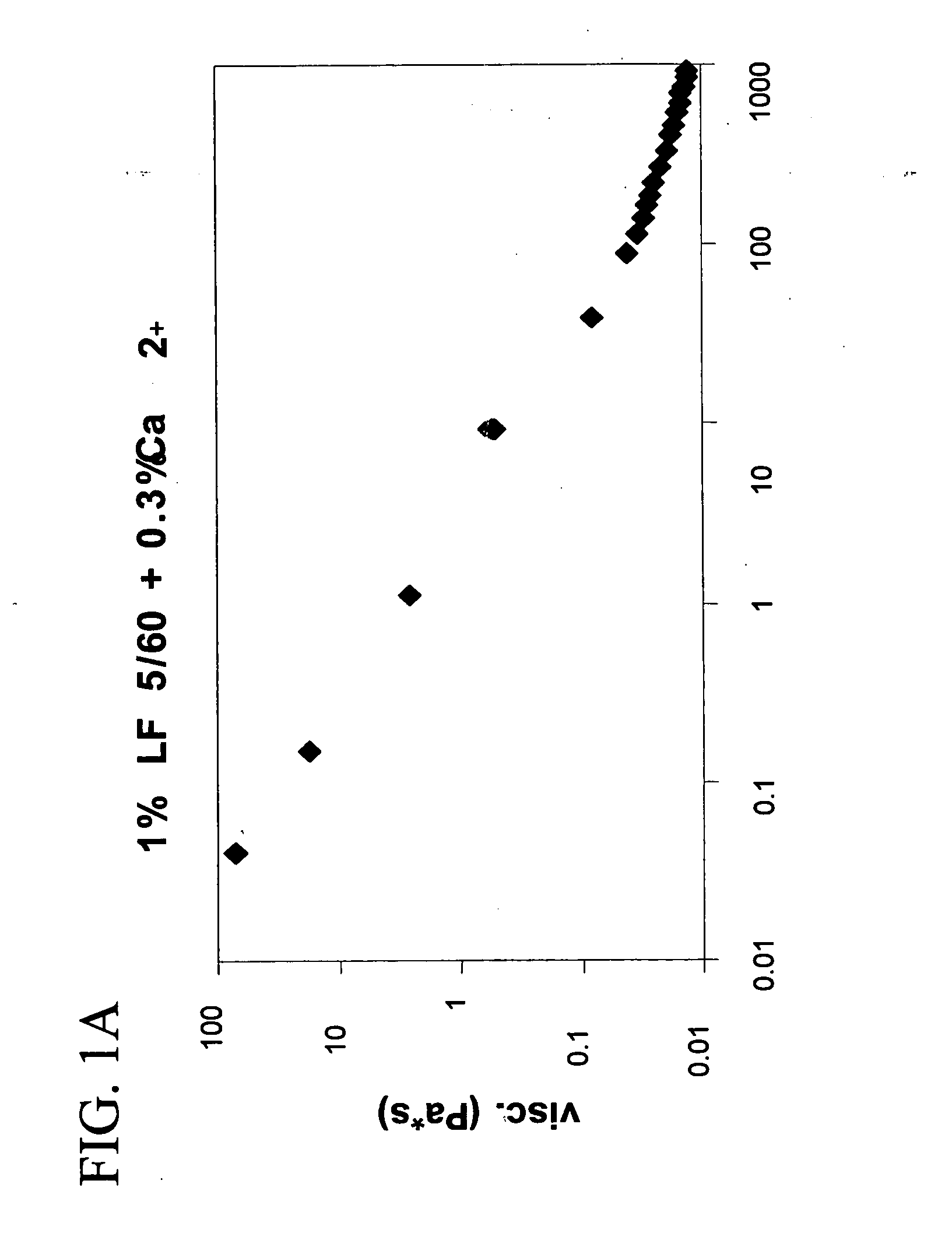
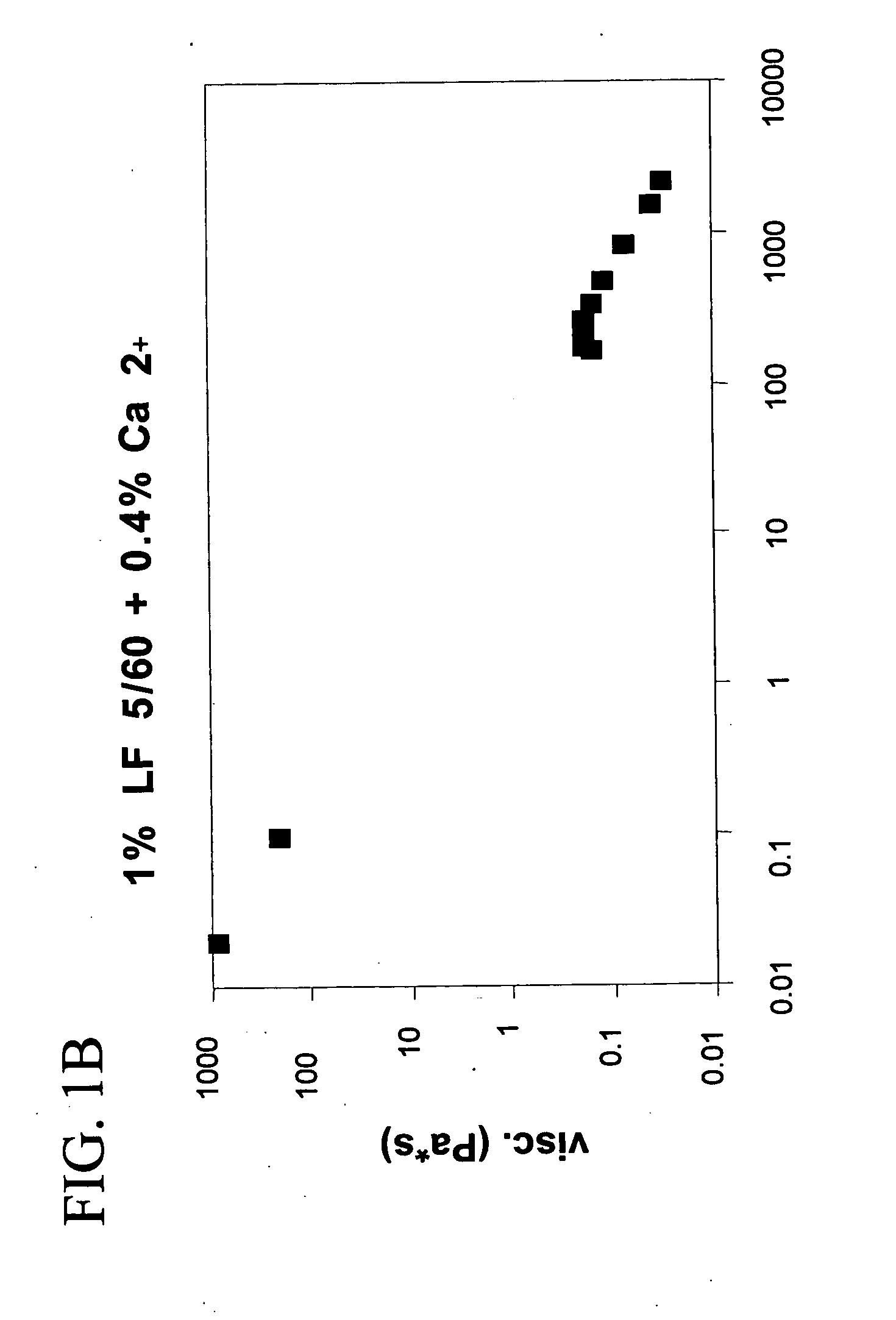
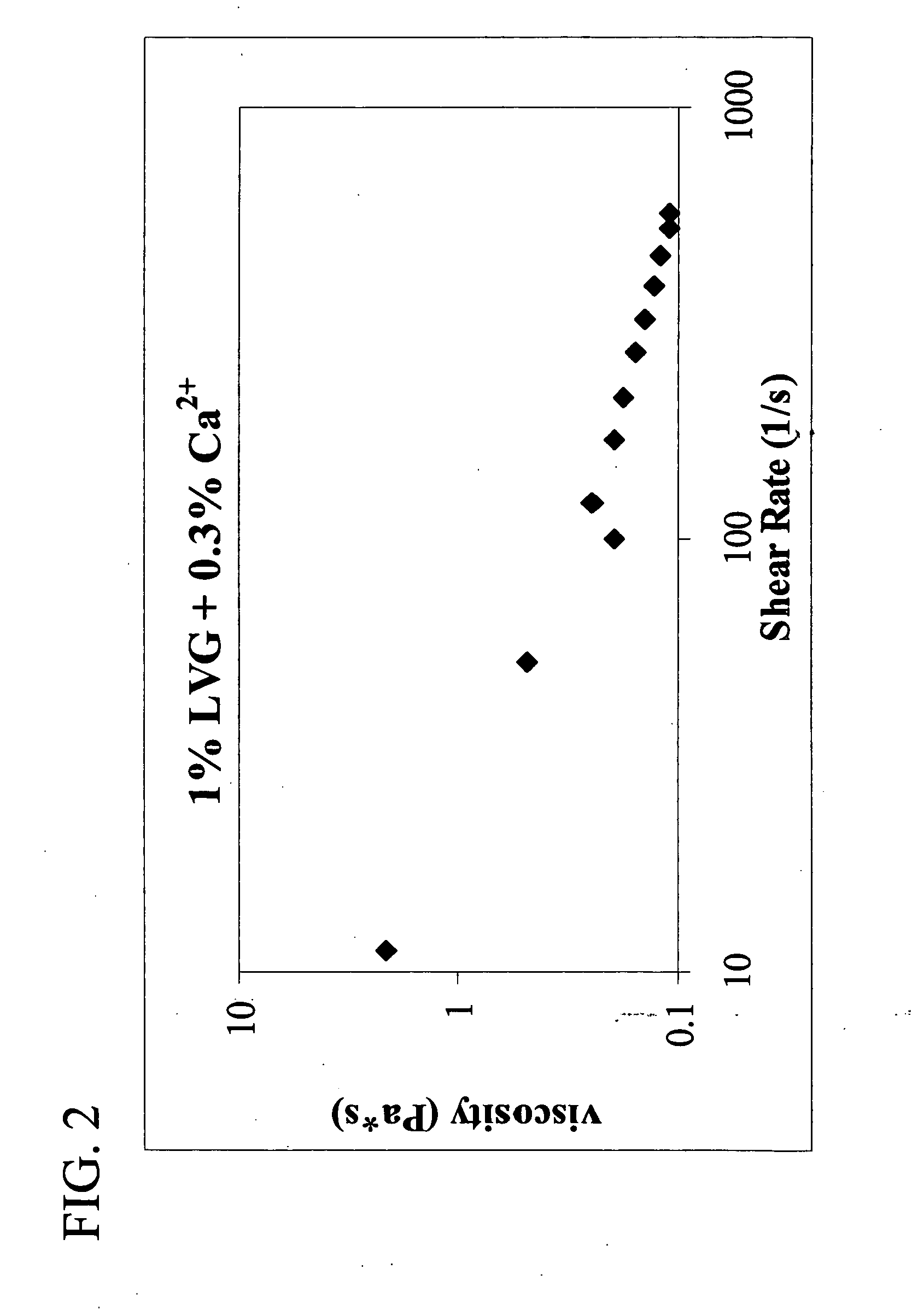
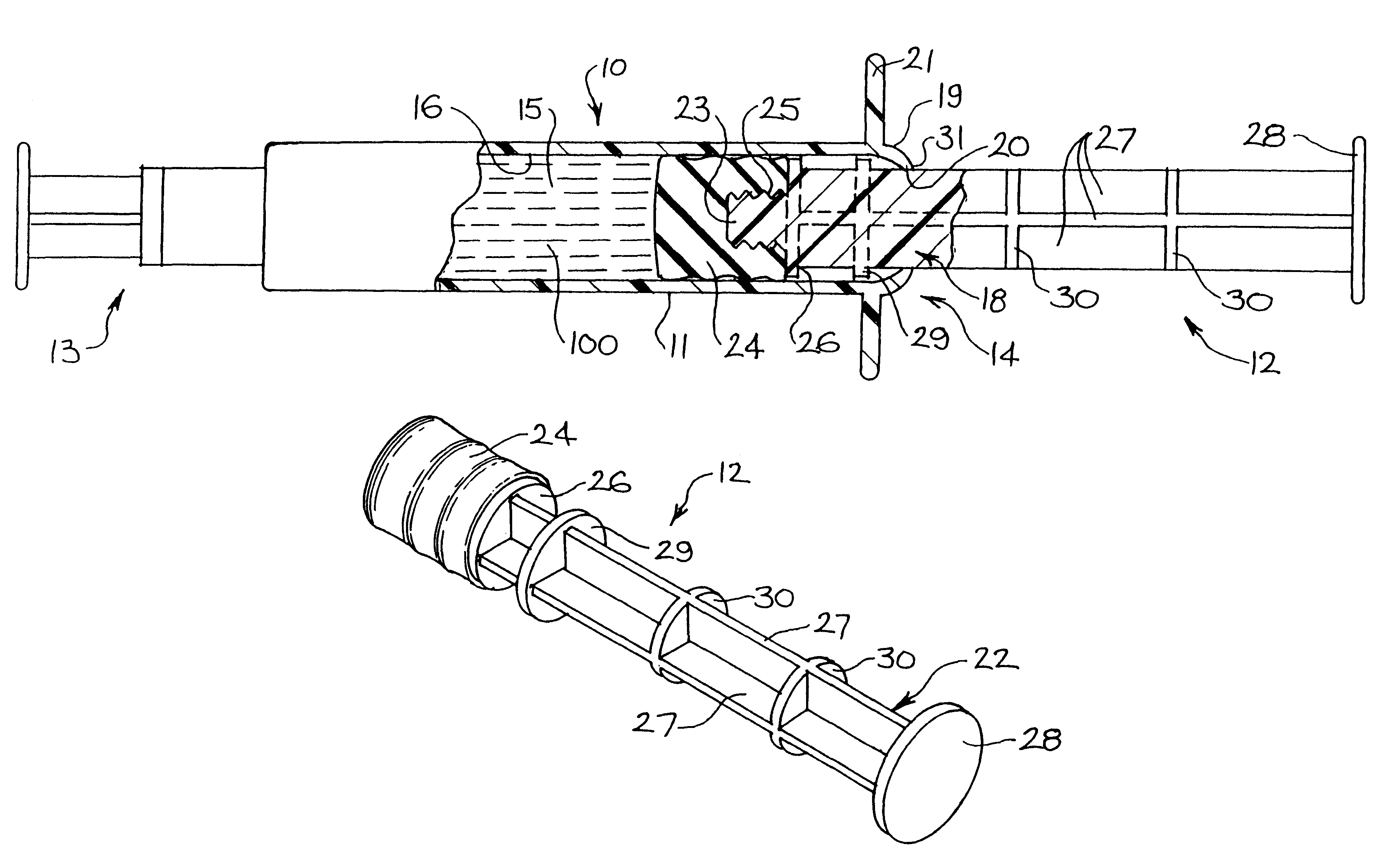
A plastic prefilled syringe (10) including a syringe body (11) and a plunger assembly (12), the syringe body (11) having opposed first (13) and second (14) ends and an inner wall (16) defining a cylindrical chamber (15) which contains an injectable solution (100), the first end (13) of the syringe body (11) being sealed by a closure and the second end (14) incorporating an opening (18), the plunger assembly (12) including a plunger shaft (22) extending through said opening (18) and a stopper (24) secured at an end of said shaft (22) within said chamber, the plunger assembly (12) being movable within the chamber with the stopper (24) being operable to seal the opening (18) wherein the plunger assembly (12) includes barrier means (26 and 29) on said shaft (22) the barrier means (26 and 29) being adapted, in conjunction with a part of the syringe body (16 and 19), to inhibit access to the injectable solution (100) through the opening (18).
Owner:ASTRA PHARMA PTY LTD
Storage stable perfusion solution for dihydropteridinones
Owner:BOEHRINGER INGELHEIM INT GMBH
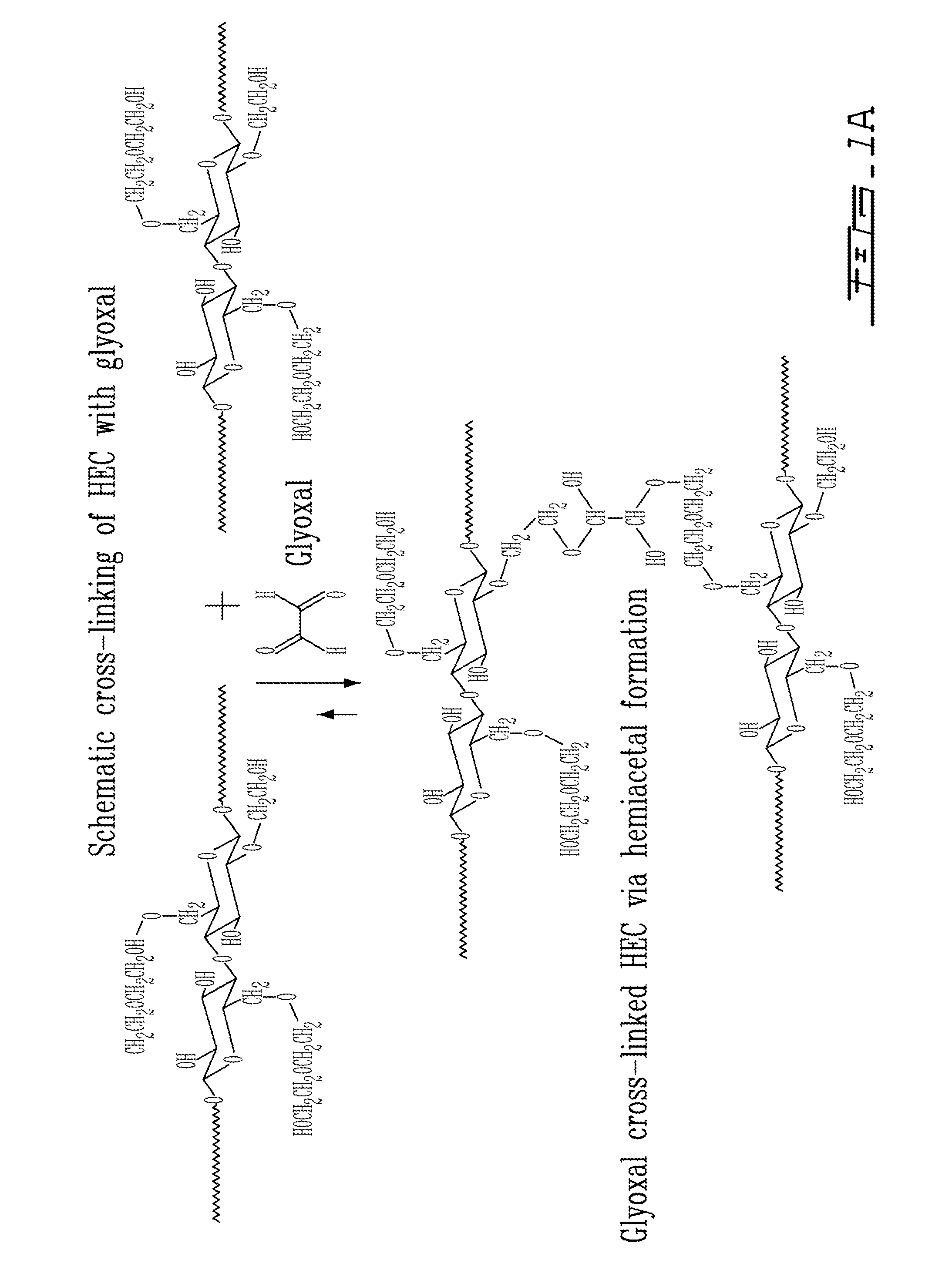
Composition for cytocompatible, injectable, self-gelling polysaccharide solutions for encapsulating and delivering live cells or biologically active factors
InactiveUS20090202430A1Control releaseMaintain their viabilityOrganic active ingredientsBiocideCross-linkInjection site
The present invention provides compositions and methods for tissue repair using a cytocompatible self-gelling cross-linked hydrogel. The composition comprises a biocompatible mixture of chitosan, bifunctional dialdehyde, and hydroxylated polymer, which can be used to immobilize or encapsulate viable cells, or bioactive substances. The method includes the process of mixing bioactive substances, live cells, and / or extracellular matrix components with a cross-linking solution comprising a bifunctional aldehyde-treated hydroxylated polymer such as hydroxyethyl cellulose. The cross-linking solution is then mixed homogenously with a neutral isotonic chitosan solution. The chitosan becomes cross-linked by the bifunctional aldehyde, while the cells are protected from potentially nocive effects of the aldehyde cross-linker by the hydroxylated polymer. The injectable solution retains cell viability and bioactivity, and immobilizes cells at the site of injection or delivery. Depending on the particular application, mixtures of chitosan and bifunctional dialdehyde may be employed. The injectable solution also liberates bioactive substances with controlled release kinetics from the site of injection.
Owner:PIRAMAL HEALTHCARE CANADA
Paclitaxel-based antitumor formulation
InactiveUS20070020337A1Simplifies plantFinal yieldPowder deliveryBiocideNanoparticle ProductionAlbumin solution
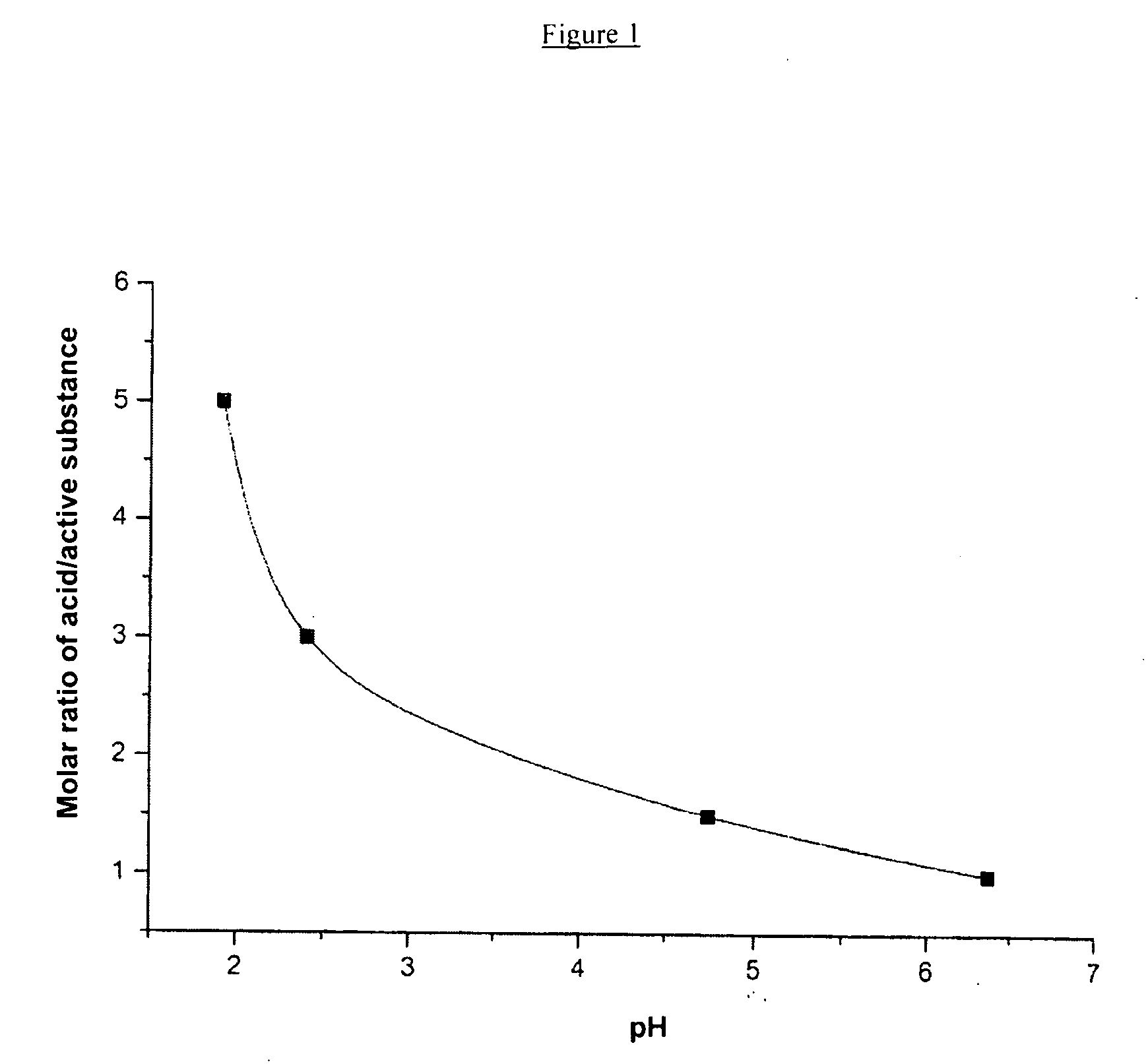
Antitumor formulation based on nanoparticles of paclitaxel and human serum albumin as obtained by the addition of a biocompatible acid to an aqueous albumin solution before this is mixed with paclitaxel during the nanoparticle production process, the injectable solutions of this formulation having a pH between 5.4 and 5.8 and having stability and inalterability with time.
Owner:ABRAXIS BIOSCI LLC
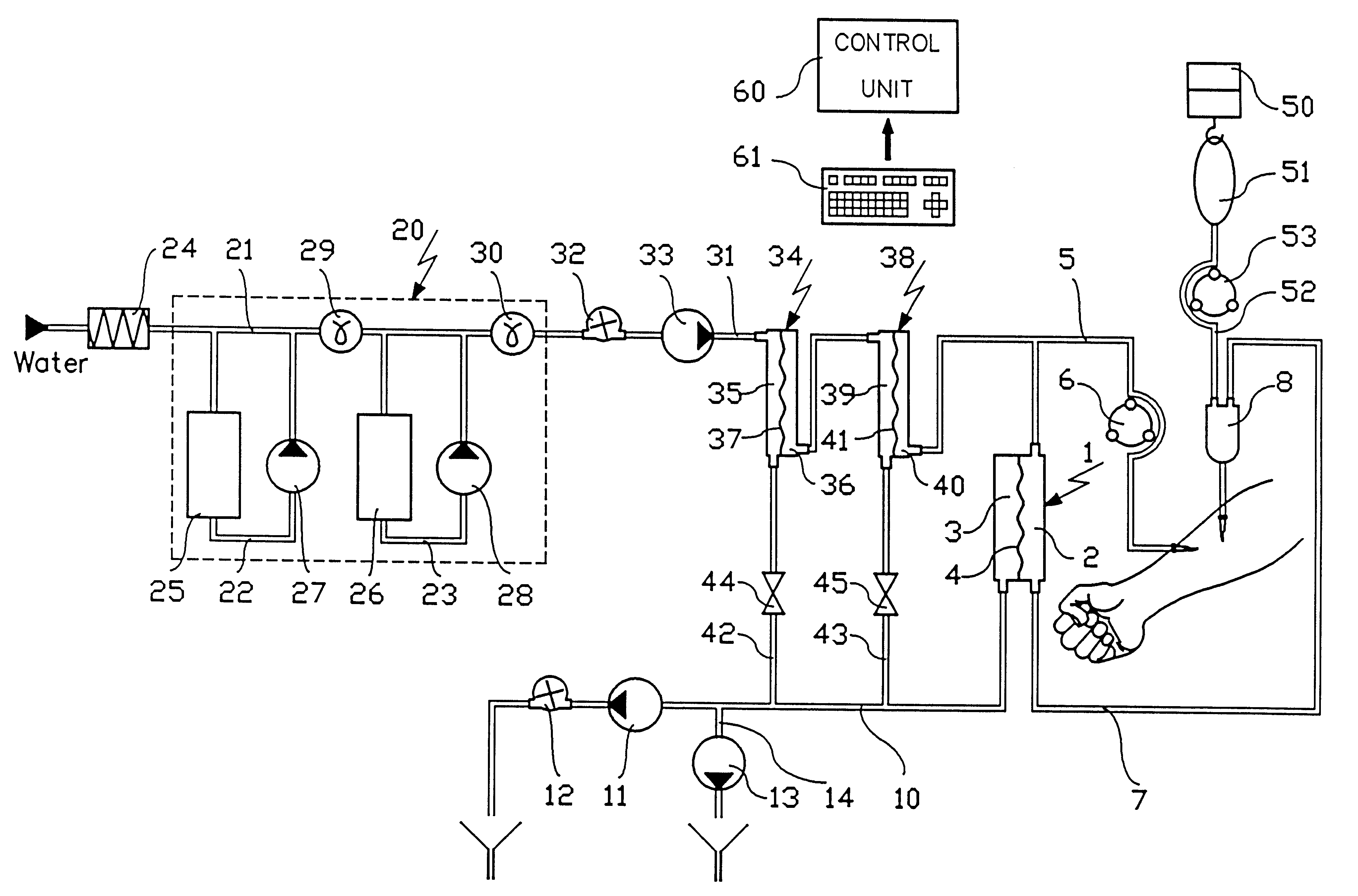
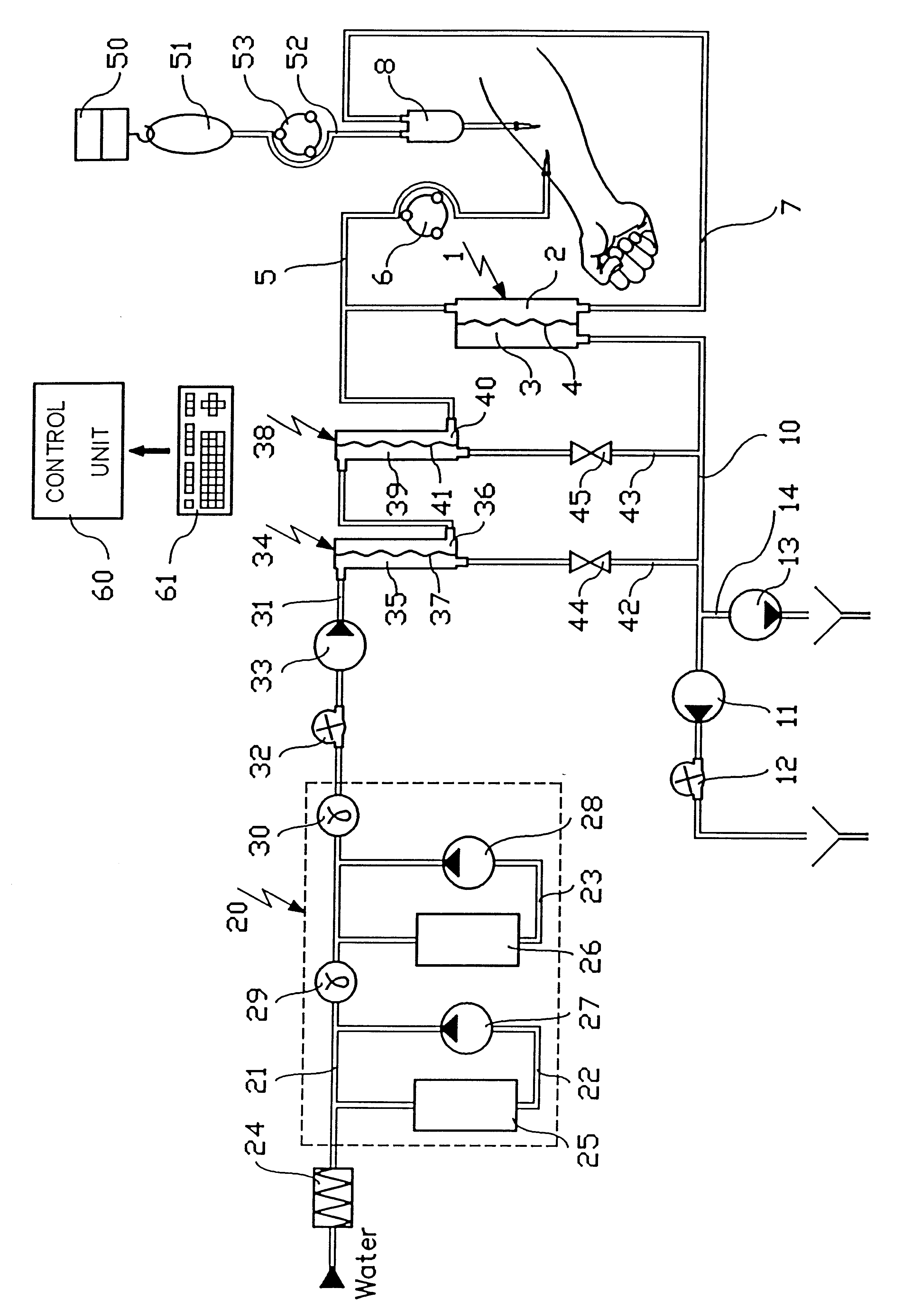
Haemofiltration machine for independently controlling the concentration of at least two ionic substances in a patient's internal medium
InactiveUS6635026B1Mitigate such drawbackReduce riskSemi-permeable membranesSolvent extractionInjectable SolutionMachine design
A haemofiltration machine designed to work with a haemofilter comprises two compartments separated by a semipermeable membrane, one compartment having an inlet connected to a withdrawing tube for taking blood from the patient and an outlet connected to a blood return tube, the other having an outlet connected to a tube for removing used liquid. The machine itself comprises a solution generator for preparing a first injectable solution from at least one concentrated solution; a circulation pump for injecting the first solution at a flow rate of Qpre into the withdrawing tube; an infusion pump for injecting a patient with a second solution containing a ionic substance [A] with a fixed concentration [A]post different from its concentration [A]pre in the first solution; and a control unit for determining an infusion flow rate Q(post) for the second solution such that the concentration of the ionic substance [A] inside the patient tends towards a required concentration [A]des, as a function of [A]post, [A]des, Qpre, and the blood flow rate QB.
Owner:GAMBRO IND
Apparatus and Method for Endoscopic Submucosal Dissection
A kit and a method for delivering an injectable solution to a tissue treatment site are provided. The kit includes a housing having a chamber, a proximal portion and a distal portion. An injectable solution having a viscosity greater than about 10,000 cP is provided in the chamber. A plunger is provided in the proximal portion of the housing. The kit also includes a pressure gauge operably connected to the housing. The kit may also include a handle connected to the housing and a plunger advancing member having a plunger handle connected thereto provided separate from the housing. The kit may also include an inner shaft provided separate from the housing and having a proximal end portion configured for operably connecting with the distal portion of the housing for receiving the injectable solution therethrough and a distal end of the shaft configured for insertion in to the tissue treatment site.
Owner:COOK MEDICAL TECH LLC
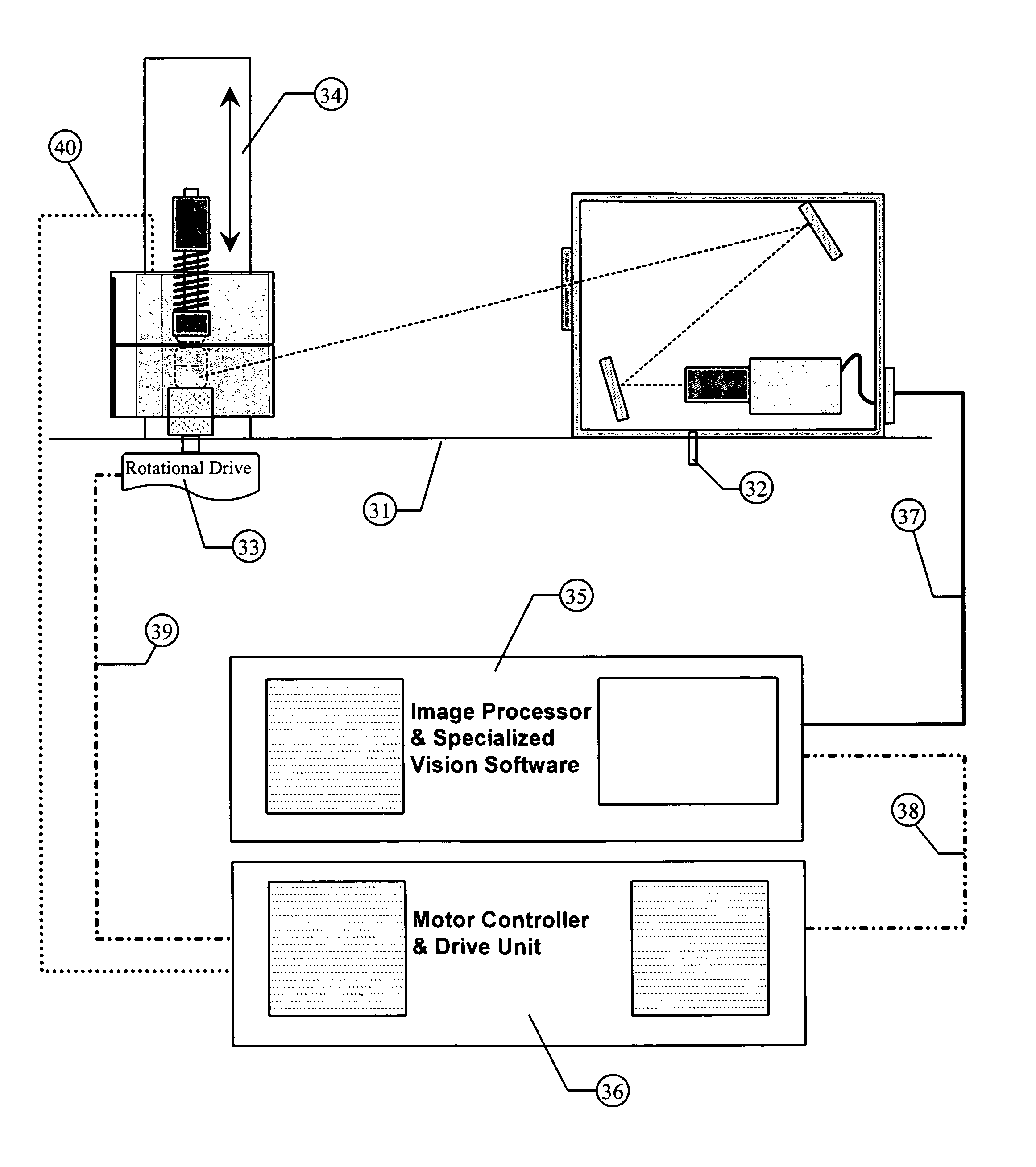
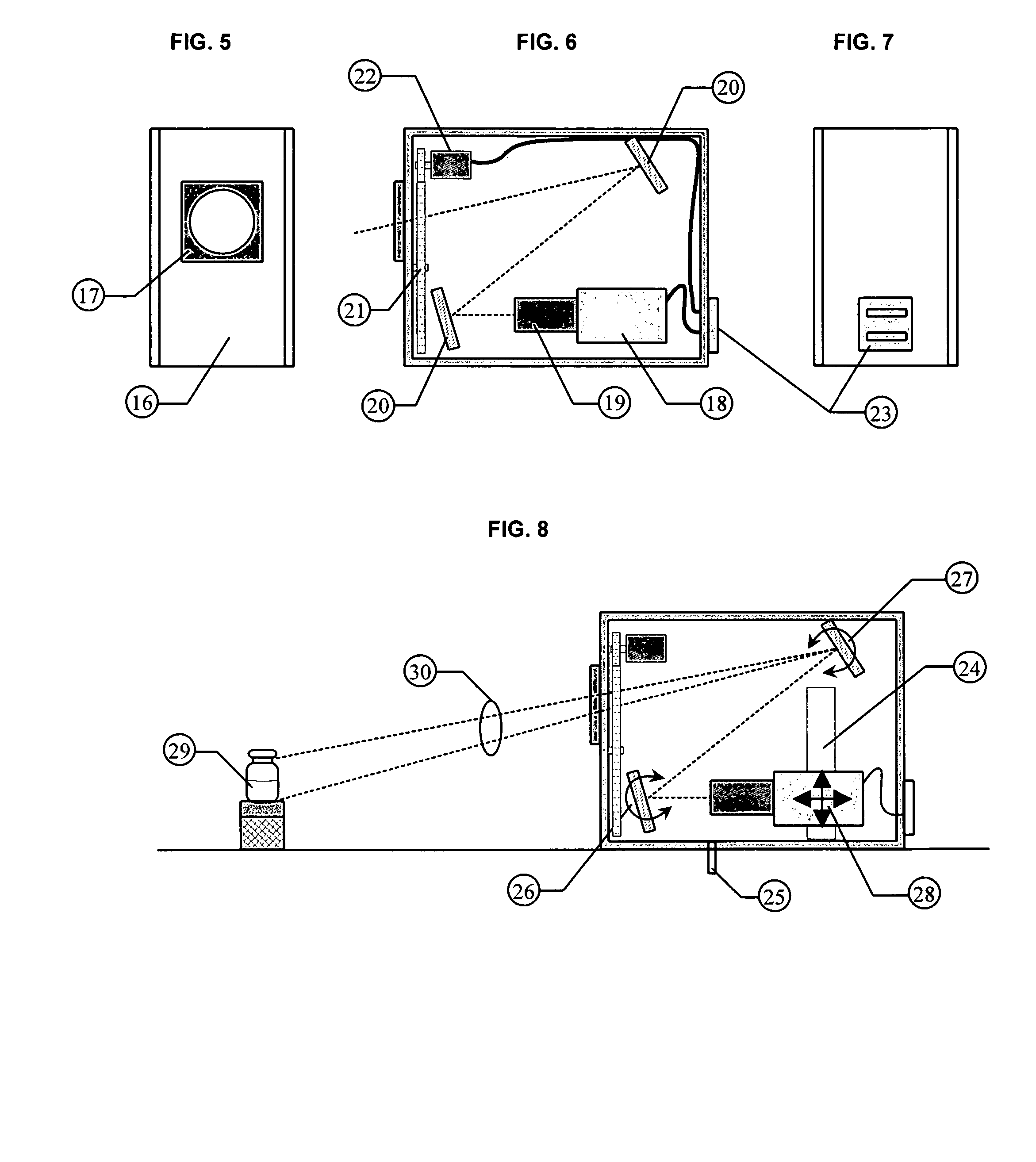
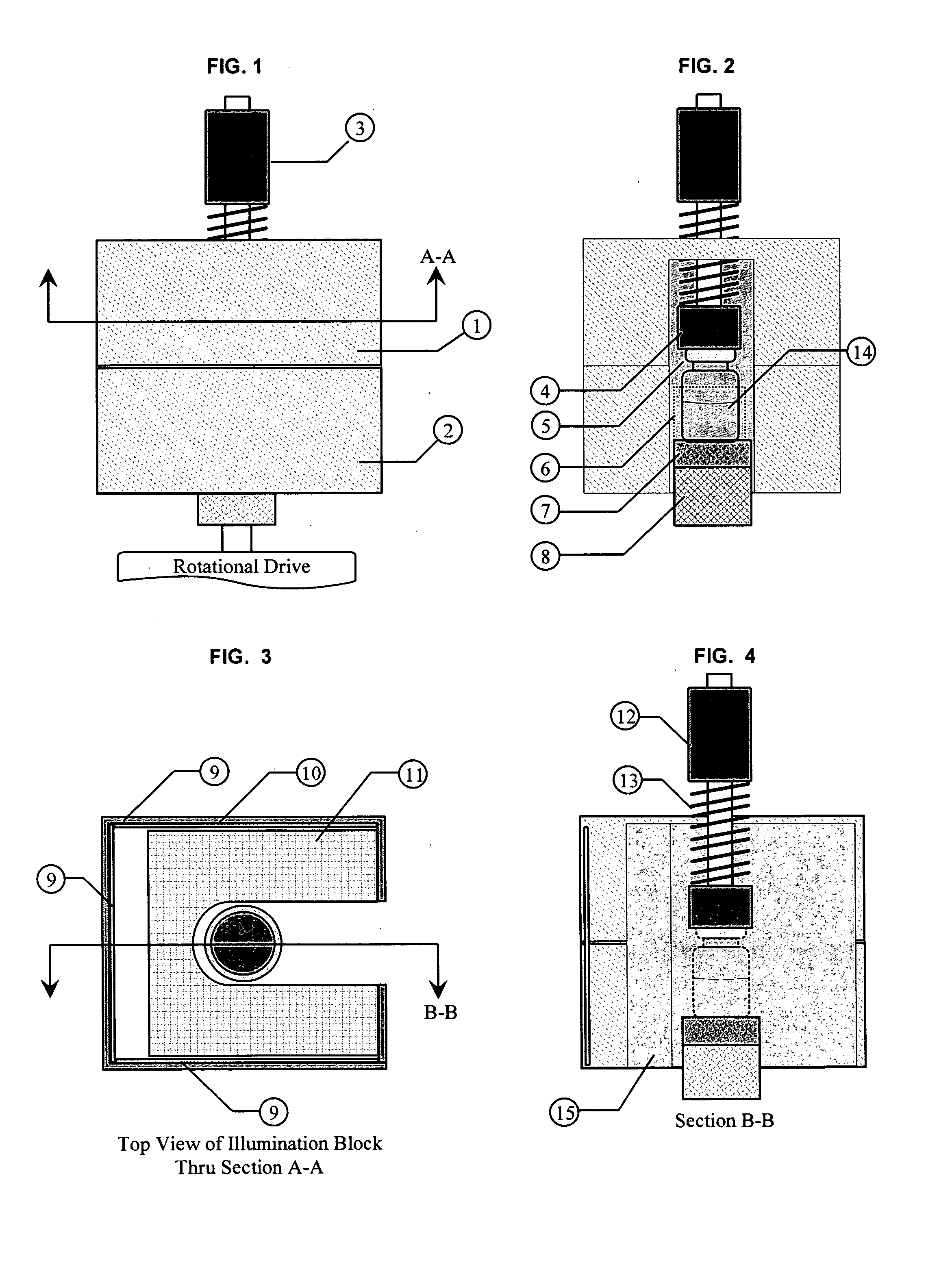
NIST traceable automated visual inspection system for an inspection of particles in solution
ActiveUS7310143B2Improved detection and measurementAccurate determinationOptically investigating flaws/contaminationParticle size analysisInjectable SolutionVisual perception
Owner:BUDD GERALD WALTER
Pharmaceutical compositions comprising docetaxel and methods for preparation thereof
InactiveUS20080306137A1High stability and solubilityEasy to makeBiocideOrganic active ingredientsSolubilityDocetaxel-PNP
A pharmaceutical composition of docetaxel comprising an effective amount of docetaxel, a polysorbate (TWEEN® compound) and a co-solvent, wherein the co-solvent is at least one member selected from the group consisting of glycerol and polyethylene glycol. The composition is an injectable solution or a freeze-dried powder for injection. The solubility of decetaxel is improved by adding a polysorbate and a co-solvent. Methods of preparation of the pharmaceutical composition are also disclosed.
Owner:BEIJING CENTURY BIOCOM PHARMA TECH
Drug-containing nanoparticle, process for producing the same and parenterally administered preparation from the nanoparticle
InactiveCN1917859AGood slow releaseStrong targetingPowder deliveryHydroxy compound active ingredientsNanoparticleIn vivo absorption
An external preparation or injectable solution that exerts the effect of enabling percutaneous or transmucous in vivo absorption of fat-soluble drugs and water-soluble drugs not having been satisfactorily attained hitherto and that contains a highly absorbable fat-soluble / water-soluble drug. The injectable solution especially aims at sustained release and target effects. In particular, drug-containing nanoparticles (secondary nanoparticles) are provided by causing primary nanoparticles containing a fat-soluble drug or fat-solubilized water-soluble drug to act with a divalent or trivalent metal salt. Further, drug-containing nanoparticles (tertiary nanoparticles) are provided by first causing primary nanoparticles containing a fat-soluble drug or fat-solubilized water-soluble drug to act with a divalent or trivalent metal salt to thereby obtain secondary nanoparticles and thereafter causing a monovalent to trivalent basic salt to act on the secondary nanoparticles. Still further, there are provided a process for producing these nanoparticles, and a percutaneous or transmucous external preparation or injectable solution in which these nanoparticles are contained.
Owner:LTT BIO PHARMA
Reconstitutable parenteral composition
InactiveUS7695736B2Equal analgesic effectEqual anti-inflammatory effectPowder deliveryBiocideParecoxib sodiumAdditive ingredient
A pharmaceutical composition comprises, in powder form, (a) at least one water-soluble therapeutic agent selected from selective COX-2 inhibitory drugs and prodrugs and salts thereof, for example parecoxib sodium, in a therapeutically effective total amount constituting about 30% to about 90% by weight, (b) a parenterally acceptable buffering agent in an amount of about 5% to about 60% by weight, and optionally (c) other parenterally acceptable excipient ingredients in a total amount not greater than about 10% by weight, of the composition. The composition is reconstitutable in a parenterally acceptable solvent liquid to form an injectable solution. A lyophilization process is provided for preparation of such a composition.
Owner:PHARMACIA CORP
Ready-to-use paracetamol injection solutions containing propylene glycol as the only cosolvent
The present invention refers to ready-to-use highly stable paracetamol injectable solutions, prepared by mixing paracetamol, wter, propylene glycol, and a citrate buffer. (pH 4.5 to 6.5), and by heating said solution under preset conditions. The resulting solution may be stored for an extended period of time within a wide range of temperatures, with no paracetamol precipitation and / or its chemical modification.
Owner:BAXTER HEALTHCARE SA +1
Reconstitutable parenteral composition containing COX-2 inhibitor
A pharmaceutical composition comprises, in powder form, (a) at least one water-soluble therapeutic agent selected from selective COX-2 inhibitory drugs and prodrugs and salts thereof, for example parecoxib sodium, in a therapeutically effective total amount constituting about 30% to about 90% by weight, (b) a parenterally acceptable buffering agent in an amount of about 5% to about 60% by weight, and optionally (c) other parenterally acceptable excipient ingredients in a total amount not greater than about 10% by weight, of the composition. The composition is reconstitutable in a parenterally acceptable solvent liquid to form an injectable solution. A lyophilization process is provided for preparation of such a composition.
Owner:PHARMACIA CORP
Method and compositions for treatment of chronic neuropathic pain
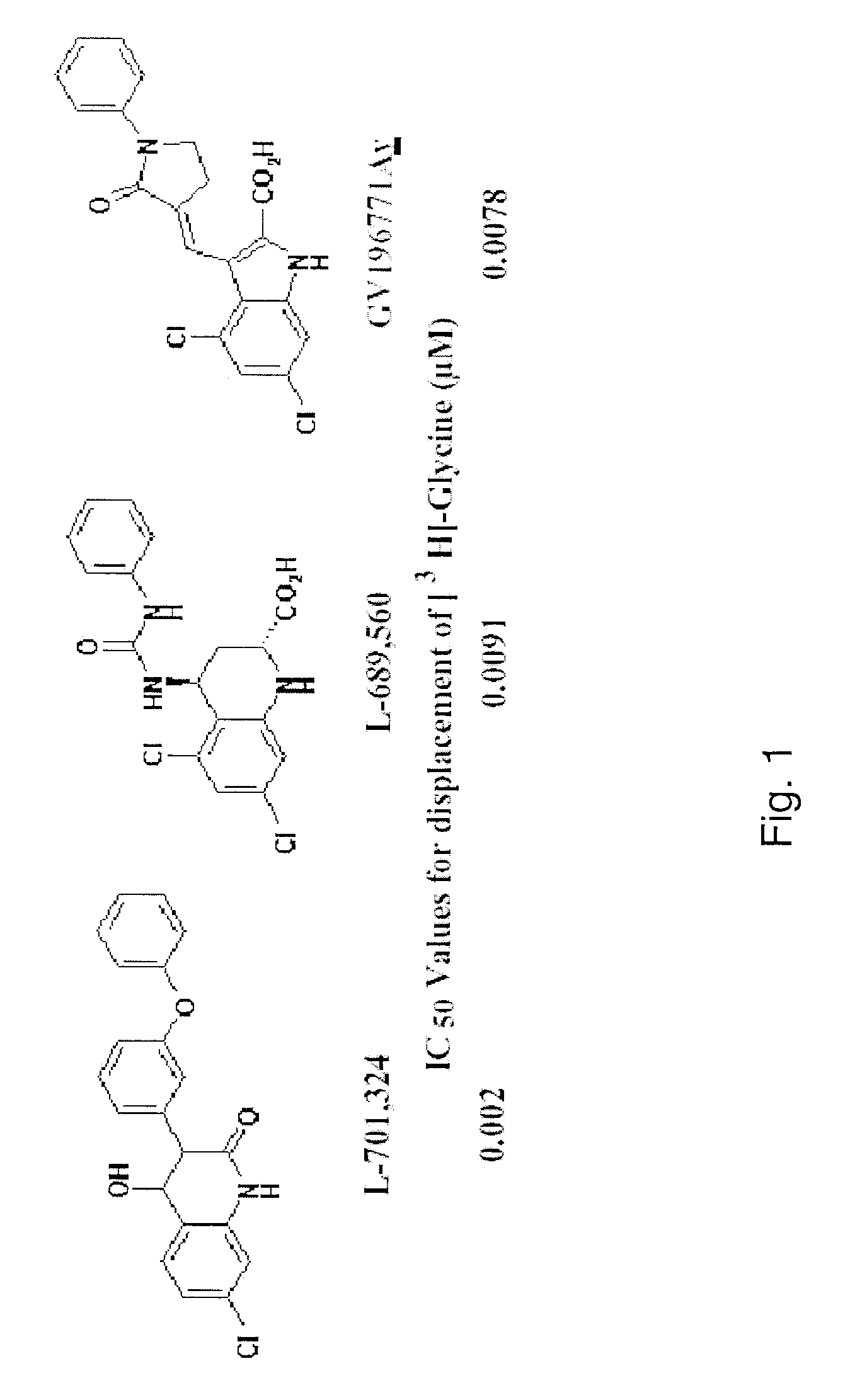
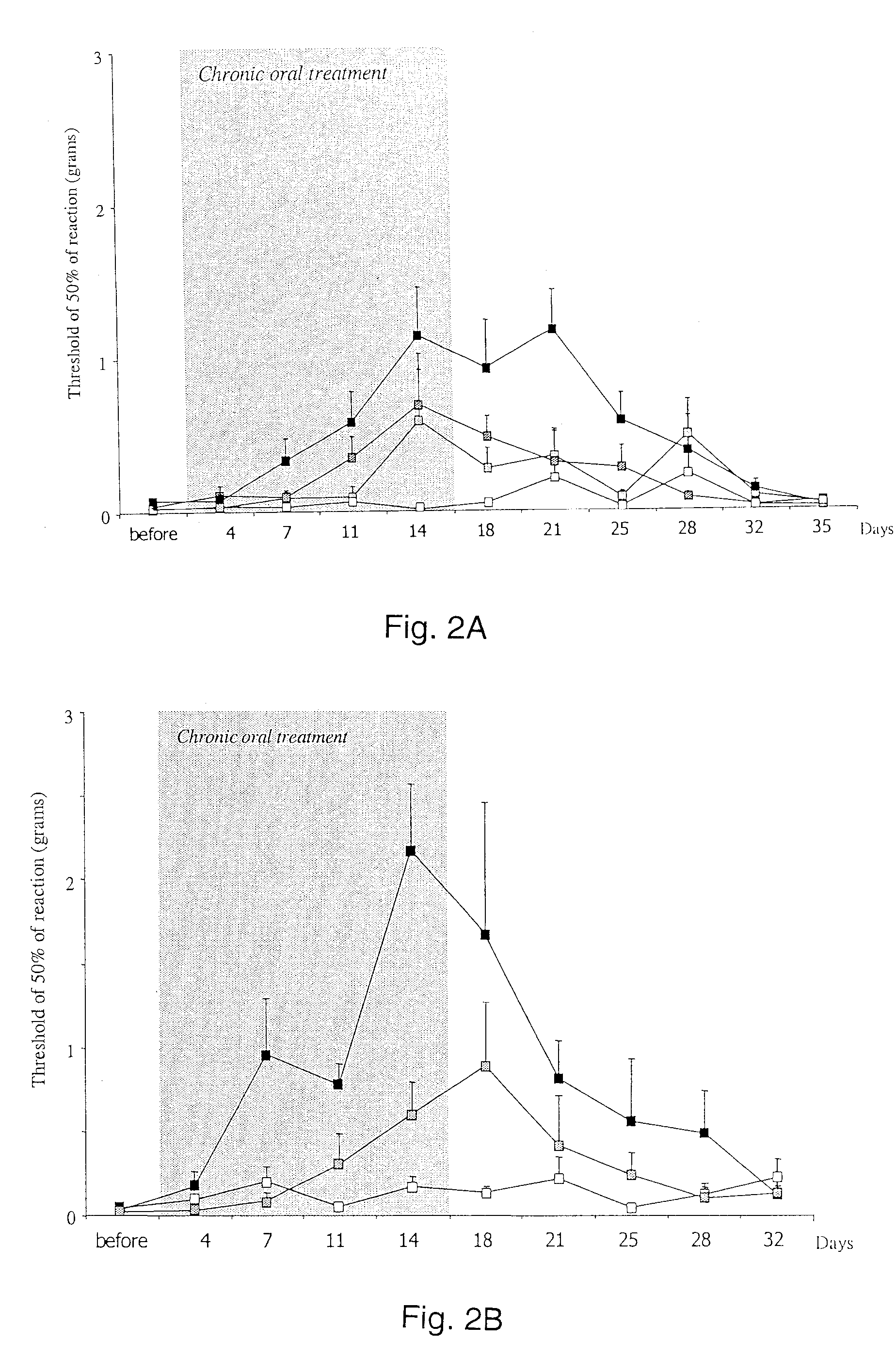
Chronic pain is treated in an individual suffering from chronic pain by administering to the individual an amount of a therapeutic containing a glycine receptor agonist such as D-cycloserine or a GlyT-1 glycine transporter antagonist such as sarcosine in an amount effective to treat the chronic pain. The therapeutic may also contain a secondary analgesic such as opiates, NSAIDs or cox-2 inhibitors. The analgesic can be formulated in a pharmaceutical composition in the form of an injectable solution that contains at least two different analgesics, at least one of the analgesics of which is a glycine receptor agonist or a GlyT-1 glycine transporter antagonist. Suitable pharmaceutical compositions contain D-cycloserine and / or sarcosine, optionally in combination with opiates, NSAIDs or cox-2 inhibitors.
Owner:APKARIAN TECH
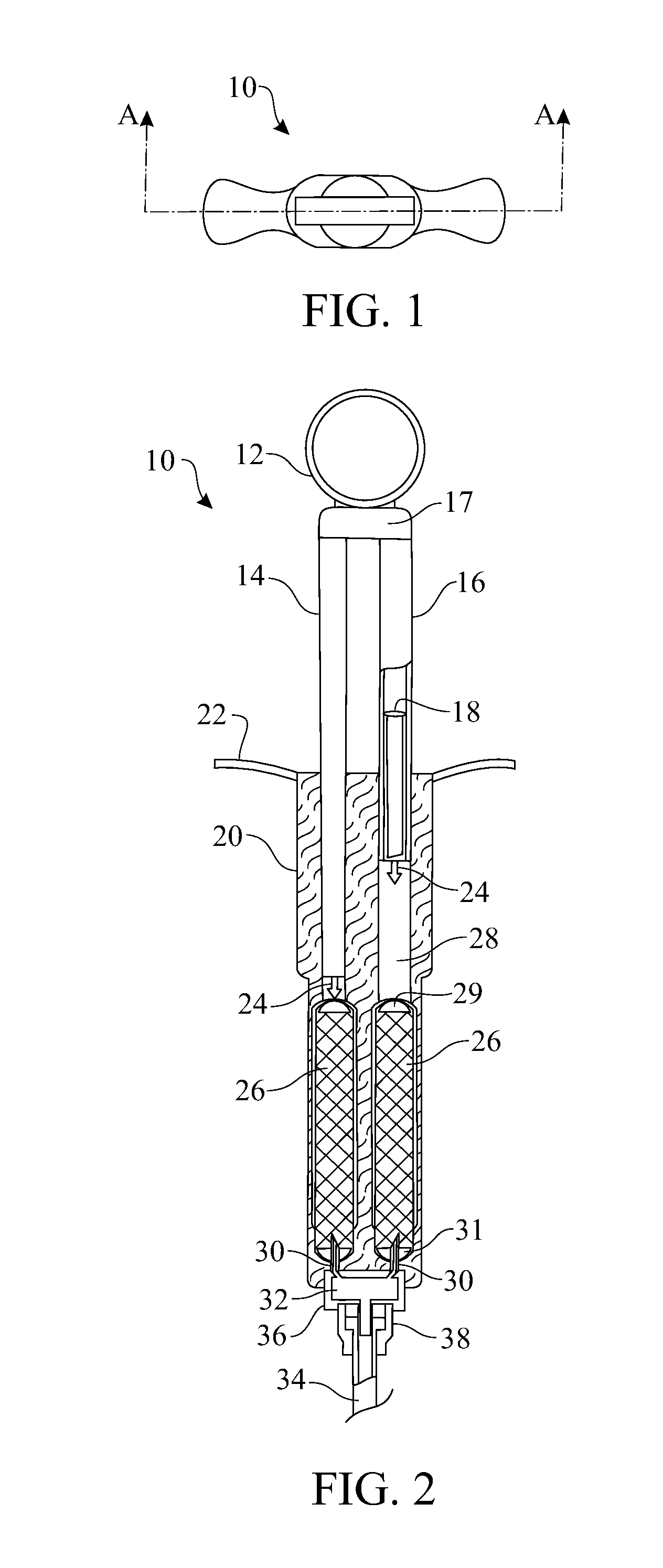
Double-chamber mixing syringe and method of use
ActiveUS20150065993A1Easy to useImprove efficiencyAmpoule syringesMedical devicesEngineeringInjectable Solution
Described is a syringe housing at least one removable carpule containing at least two injectable solutions and having a mixing chamber for mixing two injectable solutions during administration of the solutions, and methods of using the syringe.
Owner:PHARMA PHD II LLC
NIST traceable automated visual inspection system for an inspection of particles in solution
ActiveUS20050099625A1Accurate measurementImproved detection and measurementOptically investigating flaws/contaminationParticle size analysisInjectable SolutionVisual perception
A method for the substantially complete detection and measurement of all particles, within a predetermined size, range, contained in an injectable solution comprising the steps of: a) rotation of the container causes substantially all of the particles in the injectable solution in the container to be set in motion; b) uniformly illuminating the background around the container with light; and c) detecting at least one of light scatter, light reflection and light extinction caused by said particles, with detectors having a depth of focus of detection in a specified volume of the container. Wherein the detectors are positioned, relative to the container whereby the optical path and field of view allows the sensor sufficient focus to view substantially all of the bottom interior surface of the container and substantially all of the solution volume within the container. The method and apparatus produces a geometric representation of the particles in the detection region, whereby the size of detected particles can be is accurately adjusted to an actual size by either calculation or by calculated offset to allow accurate measurement of particle dimensions.
Owner:BUDD GERALD WALTER
Process for producing a stable low concentration, injectable solution of noradrenaline
ActiveUS20170049720A1Prevent oxidationReduce occurrenceOrganic active ingredientsNervous disorderAntioxidantPreservative
In a first aspect, the present invention relates to a process for producing a stable, injectable solution with low content of noradrenaline, which includes dissolving noradrenaline and optionally an excipient in deoxygenated or degassed water, filtrating the resulting noradrenaline solution in a nitrogen current, distributing the solution in a nitrogen current, and sterilization, preferably hot. The invention further provides a stable, injectable solution with low content of noradrenaline, substantially free of anti-oxidizing and preservative agents, as well as uses thereof in the medical and pharmaceutical fields.
Owner:SINTETICA SA
Pharmaceutical compositions comprising docetaxel and methods for preparation thereof
InactiveUS8044093B2High stability and solubilityEasy to makeOrganic active ingredientsBiocideDocetaxel-PNPInjectable Solution
A pharmaceutical composition of docetaxel comprising an effective amount of docetaxel, a polysorbate (TWEEN® compound) and a co-solvent, wherein the co-solvent is at least one member selected from the group consisting of glycerol and polyethylene glycol. The composition is an injectable solution or a freeze-dried powder for injection. The solubility of decetaxel is improved by adding a polysorbate and a co-solvent. Methods of preparation of the pharmaceutical composition are also disclosed.
Owner:BEIJING CENTURY BIOCOM PHARMA TECH
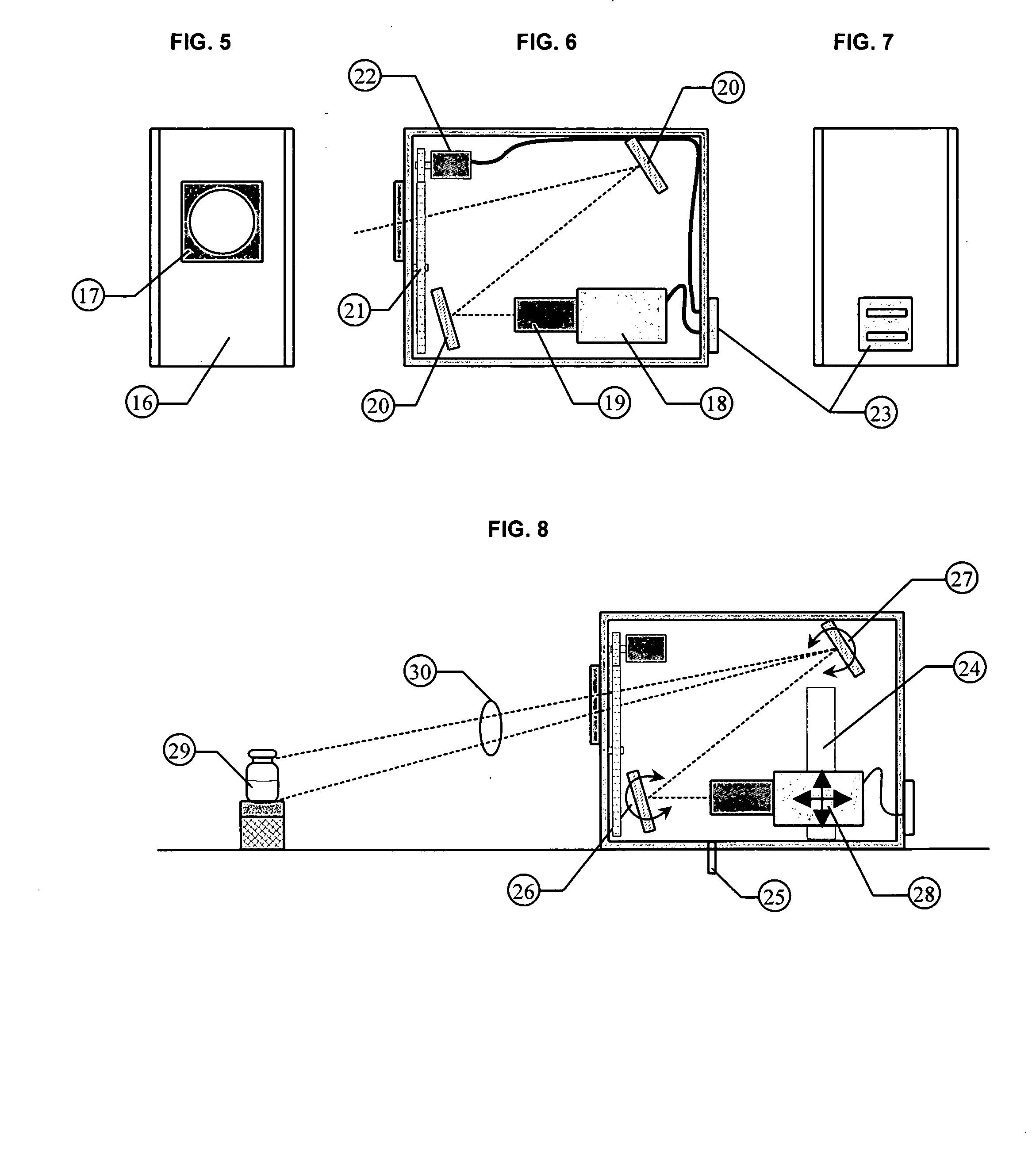
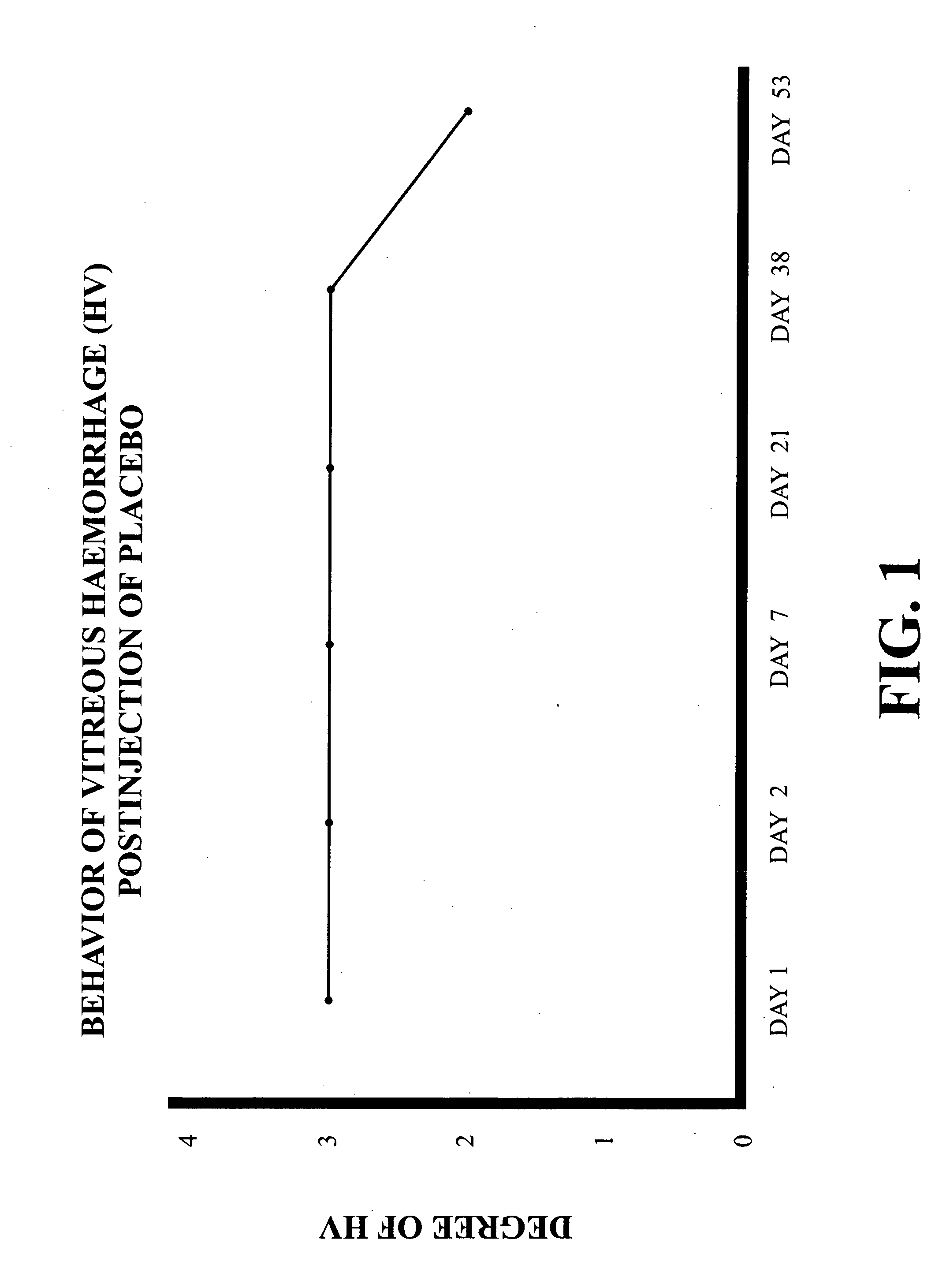
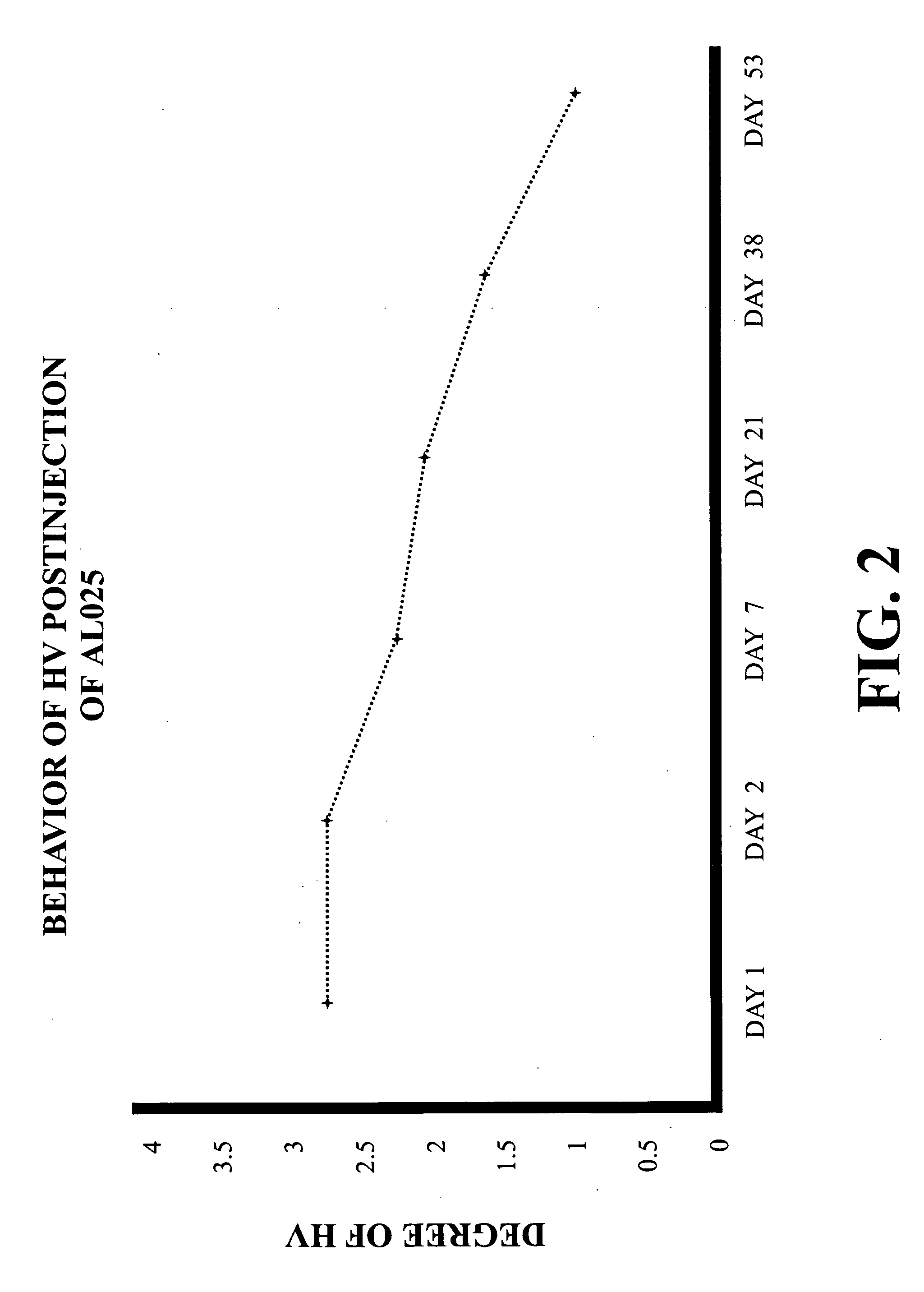
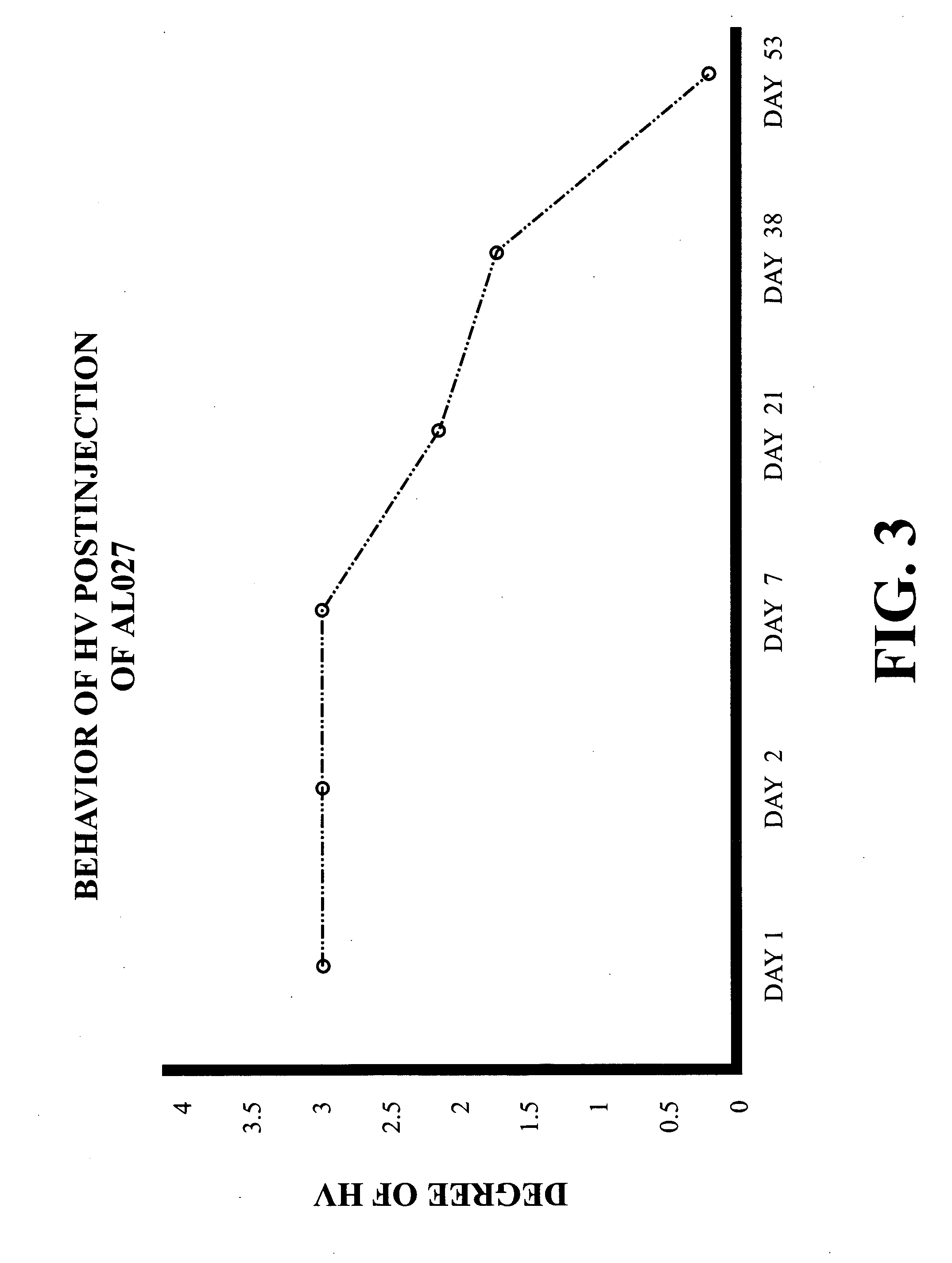
Intravitreally-injectable solution for the treatment of vitreous hemorrhages
InactiveUS20060116428A1Shorten clarification timeShorten the timeBiocideSenses disorderVitreous HumorsVitreous Bodies
The present invention relates to an ophthalmic solution for the clarification of vitreous hemorrhages. More specifically, it relates to a pharmaceutically acceptable intraocular injectable solution, for the treatment of vitreous hemorrhages, whereby the reabsorption of such hemorrhage is encouraged. It enables the clarification of the vitreous hemorrhage in a significantly short period of time to allow for the timely diagnosis of the lesion and the repair of the damage the hemorrhage has caused to the vitreous body. The ophthalmic solution of the present invention is injected at least once into the vitreous humor of the patient in a therapeutically effective dose to obtain the desired result.
Owner:JIMENEZ BAYARDO ARTURO +4
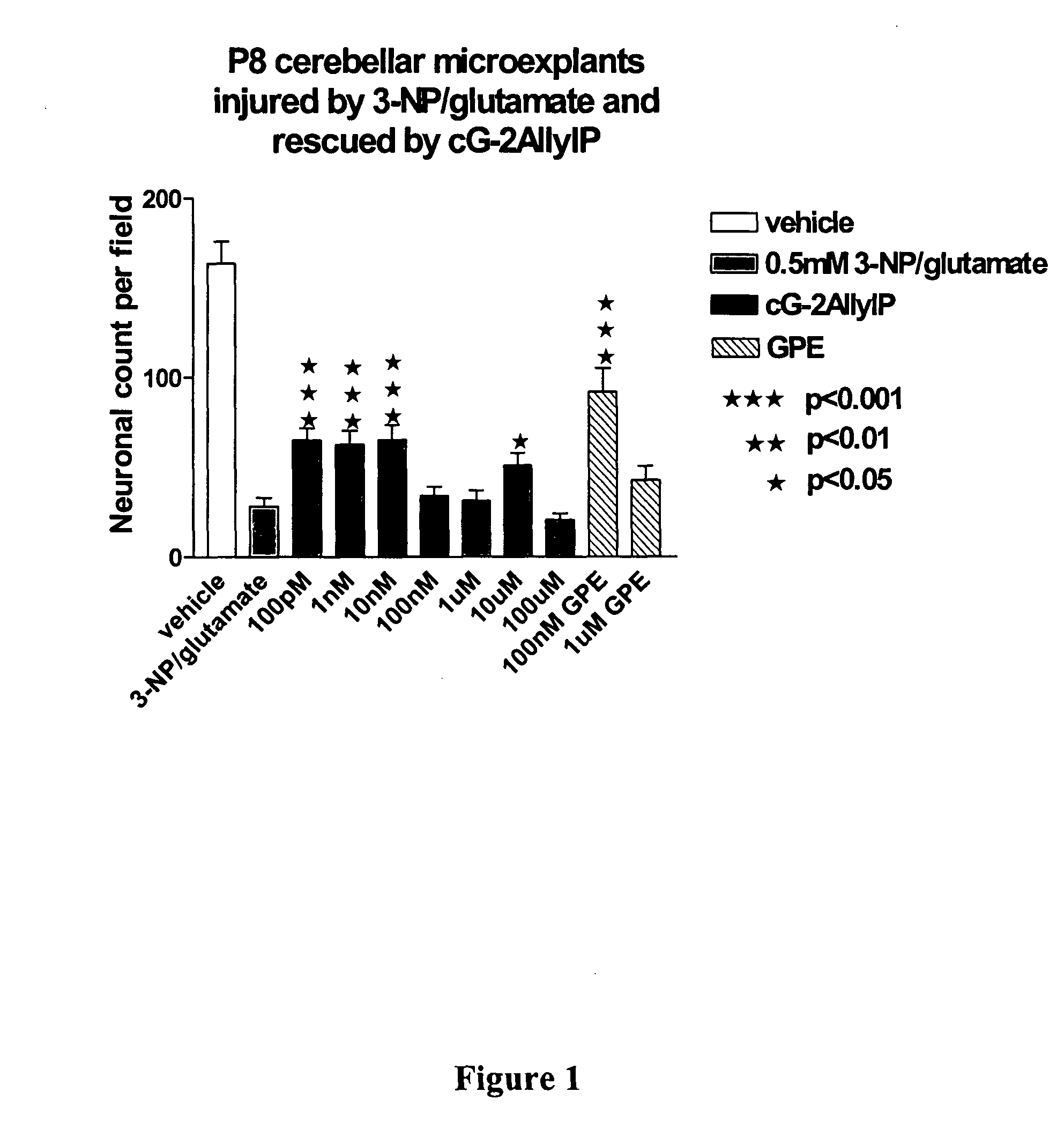
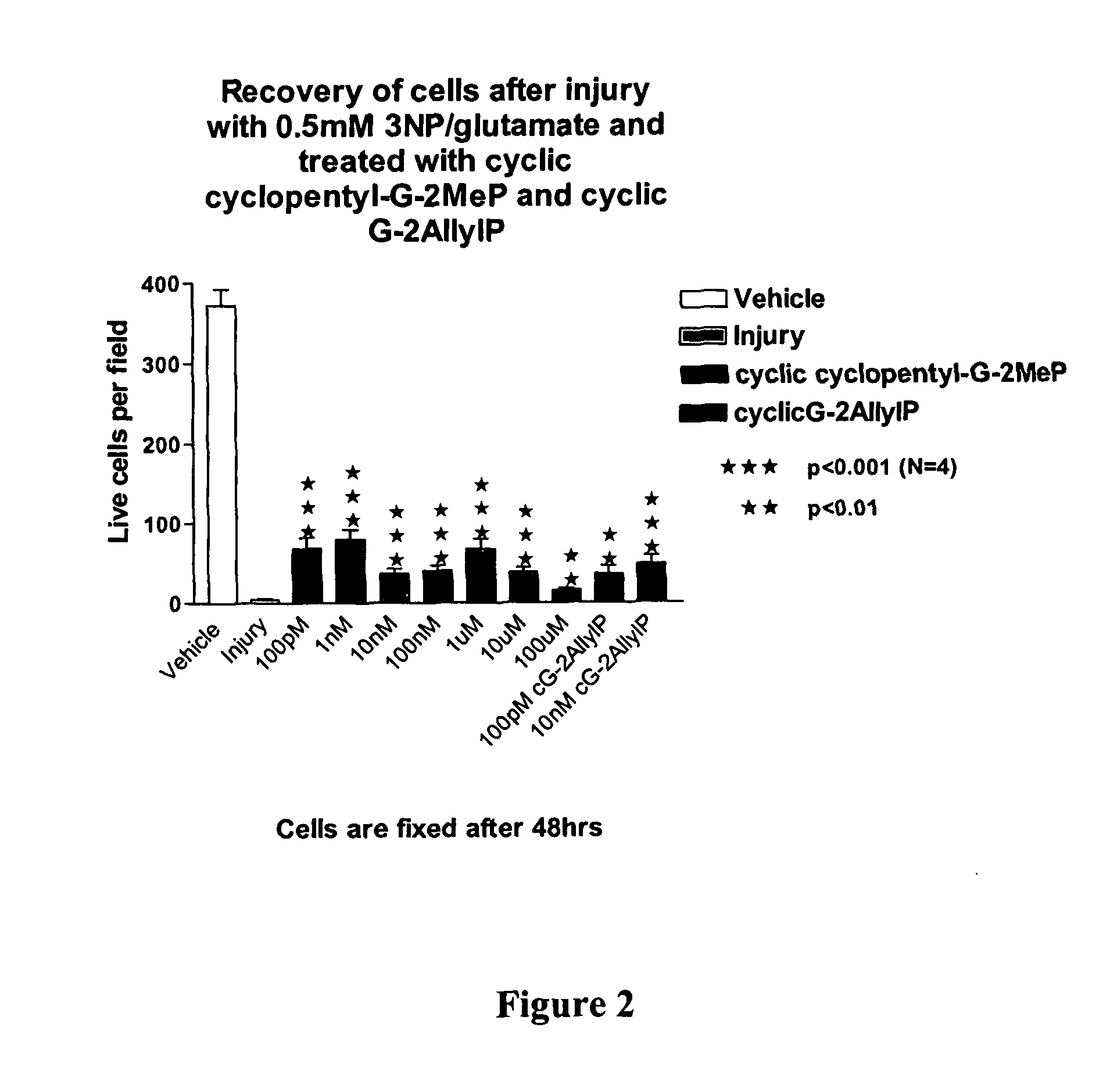
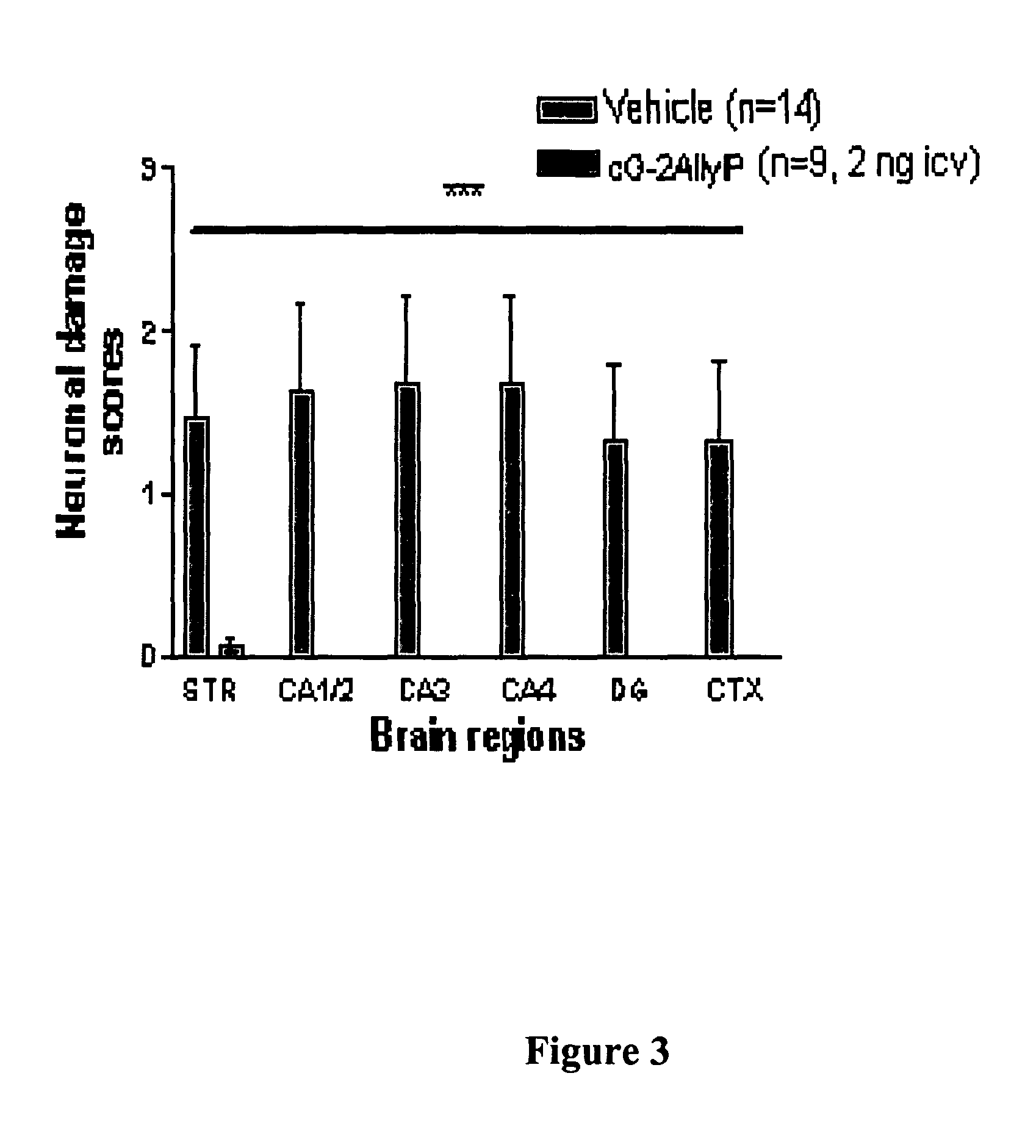
Cyclic G-2Allylproline in treatment of Parkinson's disease
Embodiments of this invention provide methods for thereapeutic use of cyclic G-2-Allyl Proline to treat disorders of dopaminergic neurons, including Parkinson's disease. Cyclic G-2Allyl P is neuroprotective and has utility as a therapeutic agent for treatment of diseases and other conditions characterised by degeneration and / or death of dopaminergic neurons and the adverse symptoms of such degeneration and / or death. Such symptoms include loss of cognition and motor function. Compounds are also useful for manufacture of medicaments including tablets, capsules and injectable solutions that are useful for treatment of such conditions.
Owner:NEUREN PHARMA LTD
Process for producing a stable low concentration, injectable solution of noradrenaline
ActiveUS10251848B2Prevent oxidationReduce occurrenceOrganic active ingredientsNervous disorderAntioxidantNitrogen
In a first aspect, the present invention relates to a process for producing a stable, injectable solution with low content of noradrenaline, which includes dissolving noradrenaline and optionally an excipient in deoxygenated or degassed water, filtrating the resulting noradrenaline solution in a nitrogen current, distributing the solution in a nitrogen current, and sterilization, preferably hot. The invention further provides a stable, injectable solution with low content of noradrenaline, substantially free of anti-oxidizing and preservative agents, as well as uses thereof in the medical and pharmaceutical fields.
Owner:SINTETICA SA
Stabilized single-liquid pharmaceutical composition containing docetaxel
InactiveUS20100267818A1Avoid disintegrationExcellent long-term pharmaceutical storage stabilityOrganic active ingredientsBiocidePolyoxyethylene castor oilDocetaxel-PNP
This invention relates to a single-liquid pharmaceutical composition for injection containing docetaxel. The composition includes (A) docetaxel and pharmaceutically acceptable salts thereof, (B) a surfactant selected from the group consisting of polysorbate, polyoxyethylene glycol ester and polyoxyethylene castor oil derivatives, (C) a solvent comprising anhydrous ethanol in a concentration range of 100 to 800 mg / ml, in an injectable solution, and (D) a pH adjuster of an amount suitable for adjusting the pH of the liquid composition to 5 or less. The composition may be directly diluted in a perfusion liquid even without the use of an intermediary dilute solution in case the composition is used for injectable preparations since the composition is in a single liquid phase. Furthermore, the composition is suitable for effective administration of docetaxel since the pharmaceutical stability of the composition is significantly improved.
Owner:DONG A PHARMA
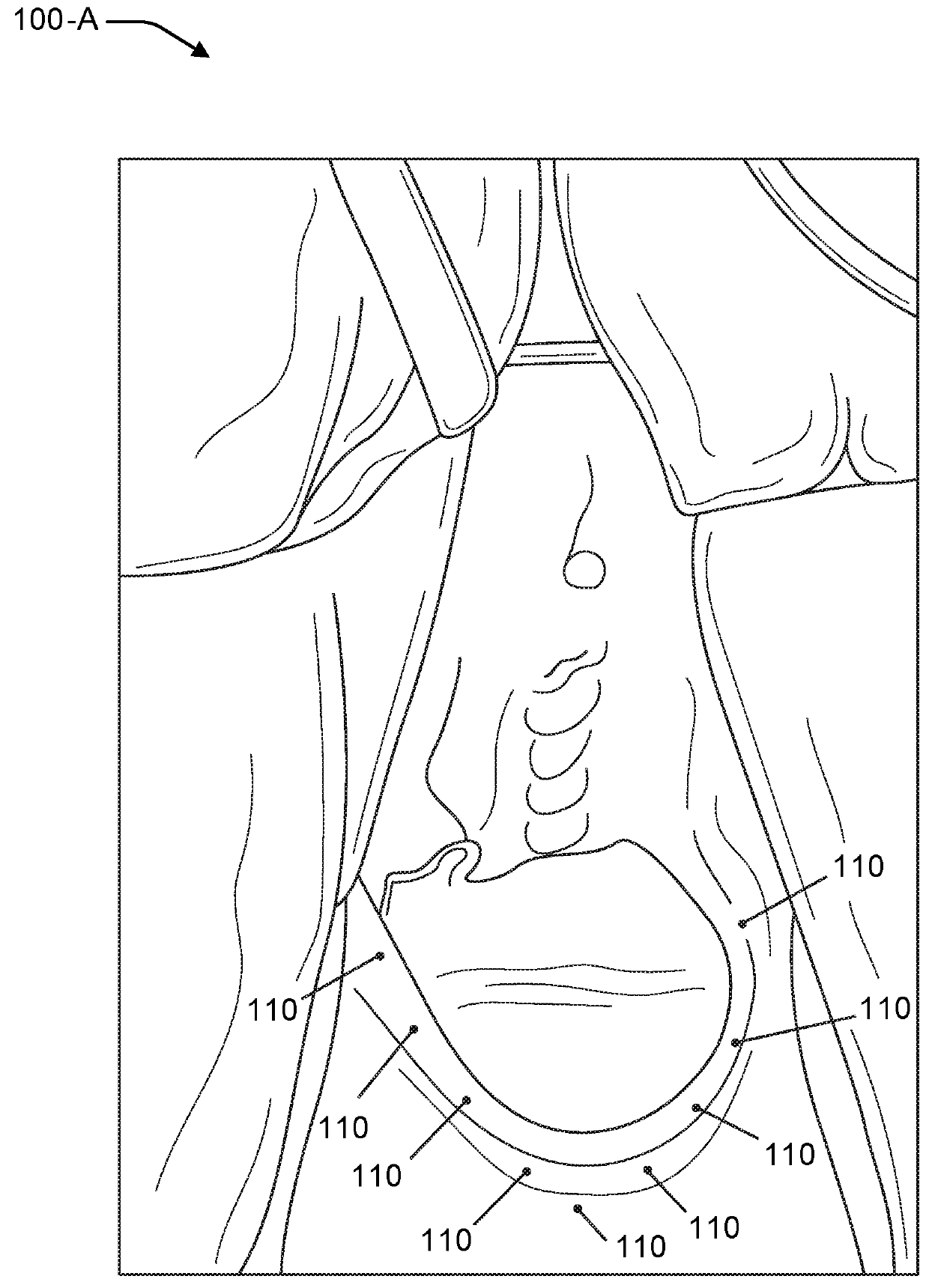
Vaginal rejuvenation methods and devices
InactiveUS20180177989A1Reduce slackPharmaceutical delivery mechanismMedical devicesVaginal epitheliumVaginal canal
Methods and apparatuses for vaginal rejuvenation are provided. A method of vaginal rejuvenation includes providing an injectable solution of amniotic membrane allograft. The solution is injected into a human female in a plurality of locations in a vaginal canal and introitus area to a depth immediately below a vaginal epithelium. A speculum includes at least one longitudinal member extending from an opening along a longitudinal axis and having a wall that curves about the axis having an inner surface and an outer surface, wherein the longitudinal member comprises multiple spaced portals extending through the wall. A kit includes the speculum and the injectable solution of amniotic membrane allograft.
Owner:REGEN MEDICAL INC
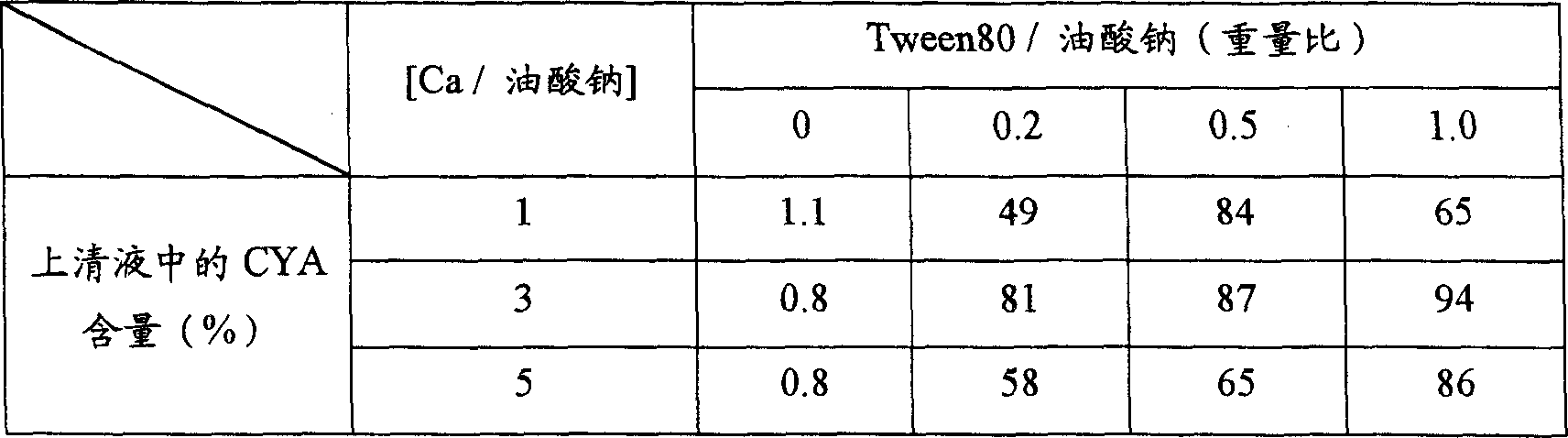
Method For Dissolving Hyaluronic Acid And/or A Salt Thereof
ActiveCN102812051ASmall molecular weightInhibit aggregationAntipyreticRotary stirring mixersPhosphoric acidInjectable Solution
Disclosed is a method for dissolving hyaluronic acid and / or a salt thereof, in which a stirring tank provided with a stirring blade selected from a turbine-type serrated blade, a dispersion-type serrated blade, a dispersion-turbine-type serrated blade, an anchor-type serrated blade and a paddle-blade equipped serrated blade is used when dissolving hyaluronic acid and / or a salt thereof in an injectable solution selected from injection grade water, physiological saline, and phosphate buffered saline. The method for dissolving hyaluronic acid and / or a salt thereof enables large-scale production of injectable solutions of hyaluronic acid with a molecular weight of 1,500,000 to 4,000,000.
Owner:DENKA CO LTD
Method for depositing hard metallic coatings
InactiveUS8431190B2Drawback can be obviatedFurnaces without endless coreVacuum evaporation coatingSusceptorDecomposition
A method for depositing a hard metallic chrome coating or similar metal by chemical vapor deposition on a metallic substrate, includes: a) preparing a solution containing, in an oxygen-free solvent, i) a molecular compound of the bis(arene) family that's a precursor of the deposited metal with a decomposition temperature 300° C.-550° C., and ii) a chlorinated additive; b) introducing the solution as aerosol into a heated evaporator at a temperature between the solvent boiling temperature and the precursor decomposition temperature (PDT); and c) driving the vaporized aerosol from the evaporator towards a CVD reactor including a susceptor carrying the substrate, heated above the PDT, up to 550° C., the evaporator and CVD reactor being subjected to atmospheric pressure. This DLI-CVD method performed at low temperature and atmospheric pressure enables continuous industrial treatment of large metallic plates, producing hard, monolayer or nanostructured multilayer metallic coatings. An appropriate injectable solution is also described.
Owner:INST NAT POLYTECHNIQUE DE TOU LOUSE
Pharmaceutical compostions comprising kisspeptin or derivatives thereof
InactiveUS20160074320A1Peptide/protein ingredientsPharmaceutical delivery mechanismImmediate releaseSilicone Elastomers
The present invention relates to pharmaceutical compositions comprising a peptide that stimulates the release of gonadotropins and sexual steroids. More specifically, the present invention provides pharmaceutical compositions comprising kisspeptin, preferably in the kp-10 form, or derivatives thereof, for use in ovulation cycle inducing and / or infertility treatment programs. The formulations according to the present invention belong to two main groups: injectable solutions and implantable formulations. The injectable solutions according to the present invention can be divided into immediate release solutions and prolonged action solutions. The implantable formulations according to the present invention can be prepared using an RTV silicone elastomer, a rapid vulcanization silicone elastomer or a rapid vulcanization silicone elastomer with a release modulator.
Owner:OURO FINO SAUDE ANIMAL +1
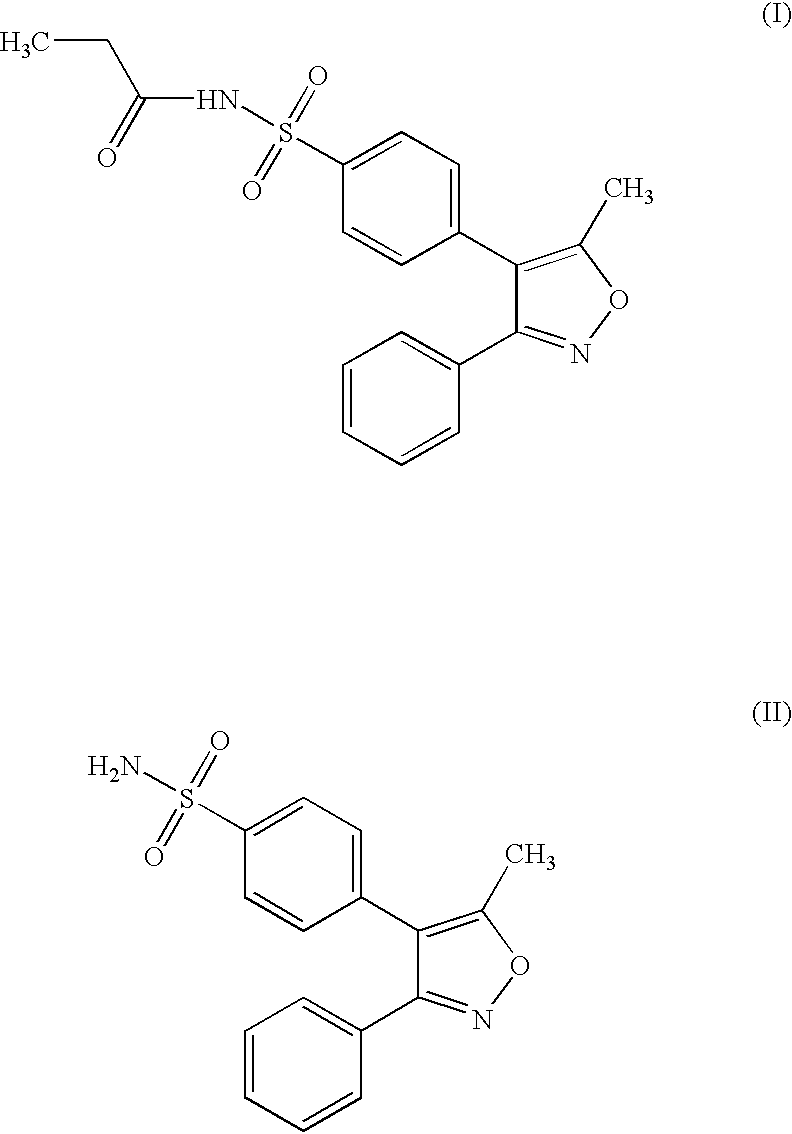
Process For The Preparation Of Anhydrous And Hydrated Active Pharmaceutical Ingredients (Apis); Stable Pharmaceutical Compositions Prepared From The Same And Uses Of Said Compositions
InactiveUS20080051450A1Reduction in yieldReduce in quantityBiocideOrganic chemistryDrugInjectable Solution
This invention describes a process for the production of ANHYDROUS active pharmaceutical ingredients (APIs); a process for the preparation of HYDRATED active pharmaceutical ingredients, a process for the preparation of sterile and stable injectable solutions, and their use, more specifically, APIs which are taxane derivatives, especially (2R,3S) 4-acetoxy-2-α-benzoyloxy-5β-20-epoxy-1,7-β-10-β-tri-hydroxy-9-oxo-tax-11-en-13α-il 3-tert-butoxycarbonylamino-2-hydroxy-3-phenylpropionate (I); 4-acetoxy-2-α-benzoyloxy-5-β-20-epoxy-1, 7β-10-β-tri-hidroxy-9-oxo-tax-11-en-13α-il (2R,3S) 3-benzoylamino-2-hydroxy-3-phenylpropionate (II), and particularly 4-acetoxy-2-α-benzoyloxy-5β-20-epoxy-1,7-β-10-β-tri-hidroxy-9-oxo-tax-11-en-13α-il (2R,3S) 3-tert-butoxycarbonylamino-2-hydroxy-3-phenylpropionate tri-hydrate (III).
Owner:QUIRAL QUIMICA DO BRASIL +1
Use of buffers for radionuclide complexation
InactiveCN102341127AIn-vivo radioactive preparationsGroup 3/13 element organic compoundsCarboxylic acidInjectable Solution
The present invention relates to a method for complexation of a chelate with a radionuclide, advantageously gallium, the complexation being carried out advantageously at ambient temperature without heating, by adding the radionuclide to the chelate in a buffer solution, the buffer of this solution comprising between two and five functions for coordination with the radionuclide, each coordination function being independently chosen from a carboxylic acid function and a hydroxyl function, on the condition that the buffer comprises at least one carboxylic acid function and at most two carboxylic acid functions. It also relates to the injectable solution obtained.
Owner:GUERBET SA
Drug injection device
ActiveCN105705178ASmall sizeReduce thicknessAmpoule syringesAnaesthesiaDrug injectionInjectable Solution
A drug injection device is disclosed. A drug injection device, according to the present invention, comprises: an injection unit which is provided with an ampoule accommodation portion for accommodating an ampoule that contains an injectable solution, and injects the injectable solution into a person being operated on; and an injection speed adjustment unit which is connected to the injection unit, applies pressure to the injectable solution, and adjusts the injection speed of the injectable solution, being injected into the body of the person being operated on, by selectively adjusting the speed of the pressure applied to the injectable solution, wherein the injection speed adjustment unit comprises: an injection unit attachment portion to which the ampoule accommodation portion is detachably attached; a pressurizing plunger which is connected to the ampoule accommodated in the ampoule accommodation portion and applies pressure to the injectable solution; and a pressurizing plunger up / down driving module which is provided on the same axis as the pressurizing plunger and drives the pressurizing plunger up or down.
Owner:MEGAGEN IMPLANT
Novel biological material with controlled release of nitrogen monoxide under catalysis of enzyme, as well as preparation method and application thereof
ActiveCN103342759AGood biocompatibilityPromote degradationOrganic active ingredientsSurgeryDiseaseFiber
The invention discloses a novel biological material with controlled release of nitrogen monoxide under catalysis of an enzyme, as well as a preparation method and application thereof. The novel biological material with the controlled release of the nitrogen monoxide under the catalysis of the enzyme is prepared by connecting a nitrogen monoxide donor compound with stable glycosylation with a natural polymer, namely chitosan through covalent bonds. The material has good processability and can be prepared into injectable solutions, thin films, porous supports, electrostatic spinning fiber films and other various products. The CS-NO and a composite material thereof can be used for treating diabetic lower limb ischemia, skin injures and myocardial infarction disease. As the nitrogen monoxide can be released in a controlled manner according to requirements, the material shows a great effect in treatment.
Owner:NANKAI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com