Method and compositions for treatment of chronic neuropathic pain
a neuropathic pain and composition technology, applied in the field of chronic neuropathic pain treatment methods and compositions, can solve the problems of no scientifically validated treatment for chronic pain, increased incidence of chronic pain, and huge burden on society in both health care and productivity loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
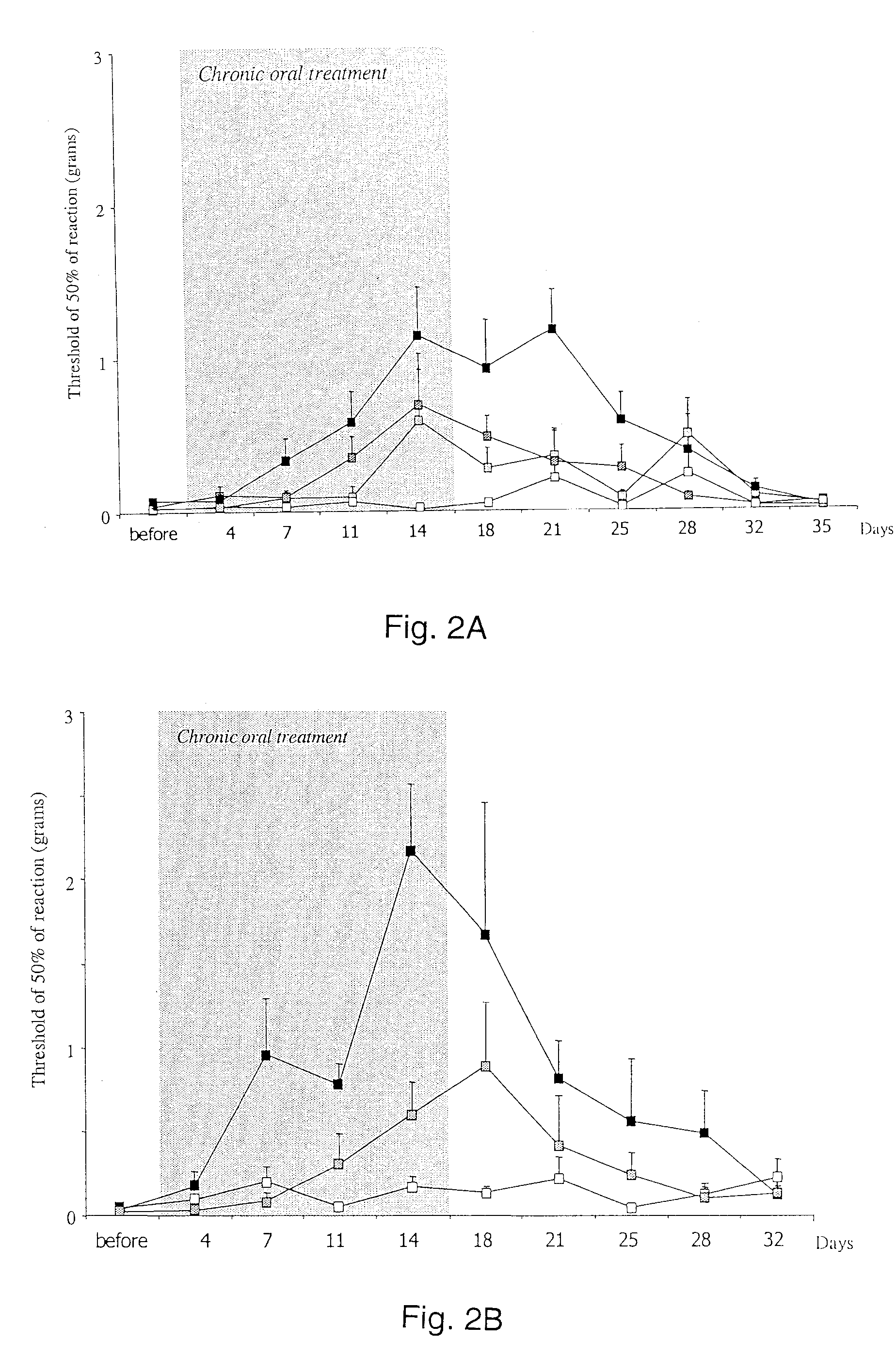
[0054] Rats with mechanically injured paws were used as models of neuropathic pain. In the test, a weight is applied to the injured paw, and the pain response is assessed. More effective pain control allows the rat to tolerate a greater weight. Rats (groups or 8-10 rats per treatment) were treated with oral saline (control) or 3, 10 or 30 mg / kg of D-cycloserine for a period of two weeks. Pain was monitored from two days before the treatment commenced for a period of 35 days total.
[0055]FIG. 2A shows a graphical representation of the results of this study, with the period of oral treatment with D-cycloserine shown in the shaded region. The open squares represent the control. The black squares represent 30 mg / kg dosage. The two intermediate lines are 3 (lighter) and 10 mg / kg (darker). As can be seen, during the time of treatment, there is a generally dose-dependent response. Post-treatment, the animals show long-lasting analgesia, with a return to initial neuropathy levels at day 35....
example 2
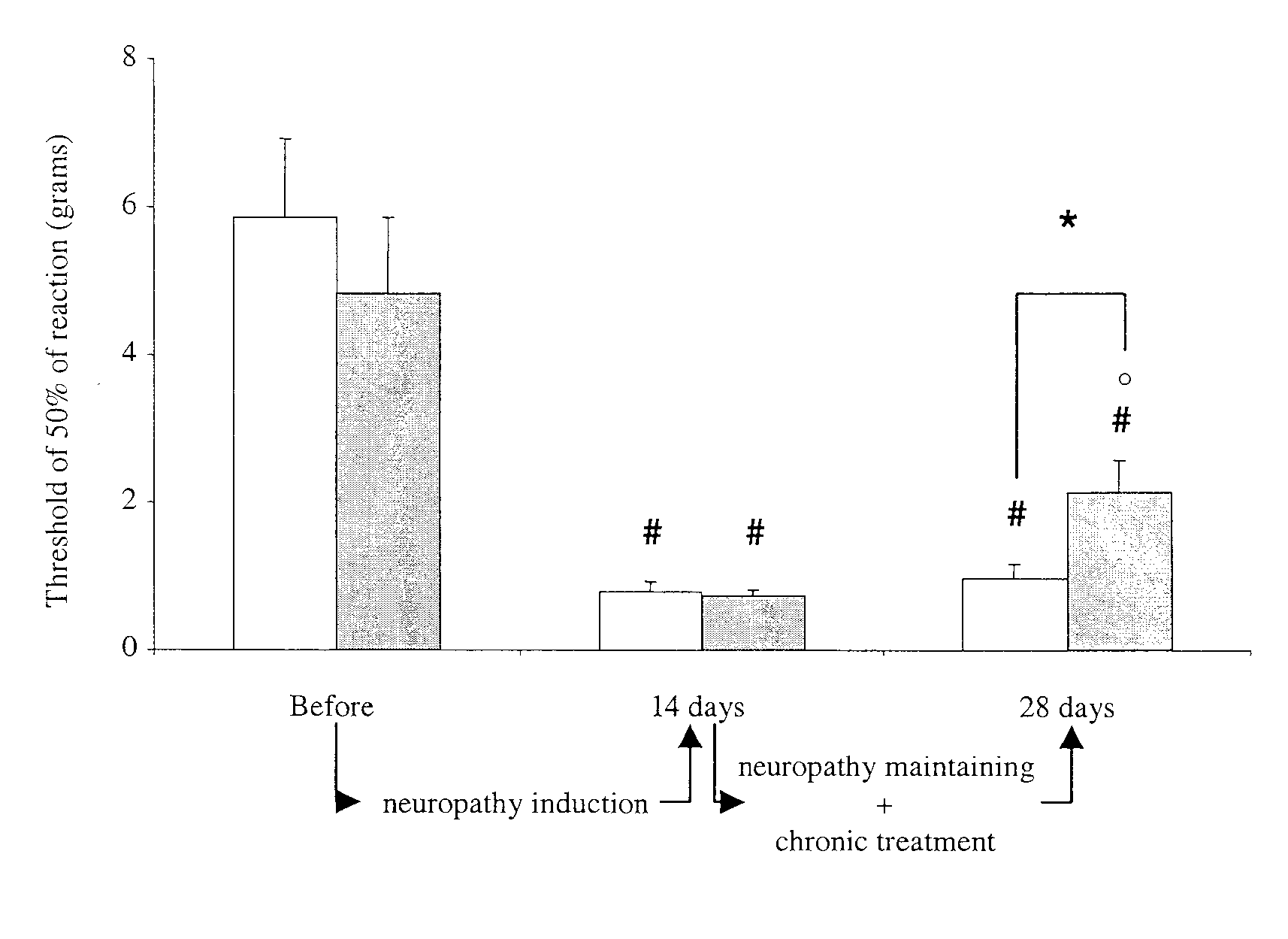
[0060] To model drug-induced pain, rats were treated with cisplatin (2 mg / kg), a common chemotherapy drug. As shown graphically in FIG. 4, after 14 days of treatment, the rats had developed mechanical sensitivity. In the next 14 days of treatment with cisplatin, oral treatment with 30 mg / kg of D-cycloserine, two treatments per day, result in a partial reversal of the mechanical sensitivity (gray bars). In contrast, sensitivity was maintained in rats treated with a saline control (white bars). Thus, in rat models of cisplatin-induced neuropathy, pain behavior decreases 50% in two weeks in animals treated with cycloserine compared with animals treated with placebo.
example 3
[0061]FIG. 5 shows the effects of infusing D-cycloserine (50 μg) into the medial prefrontal cortex (black squares), bilateral amygdala (black triangles), and visual cortex (white triangles) as compared to a saline infusion in the medial prefrontal cortex (white squares) in a rat exhibiting mechanical sensitivity Only medial prefrontal cortex and amygdala infusions result in analgesia.
PUM
| Property | Measurement | Unit |
|---|---|---|
| healing time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| soluble | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com