Injectable cross-linked polymeric preparations and uses thereof
a cross-linked polymer and injection technology, applied in the direction of drugs, cardiovascular disorders, prosthesis, etc., can solve the problems of early ventricular rupture or aneurysm formation, progressive deterioration of contractile function, heart failure and death, etc., to promote the regeneration of damaged myocardium and increase its function.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
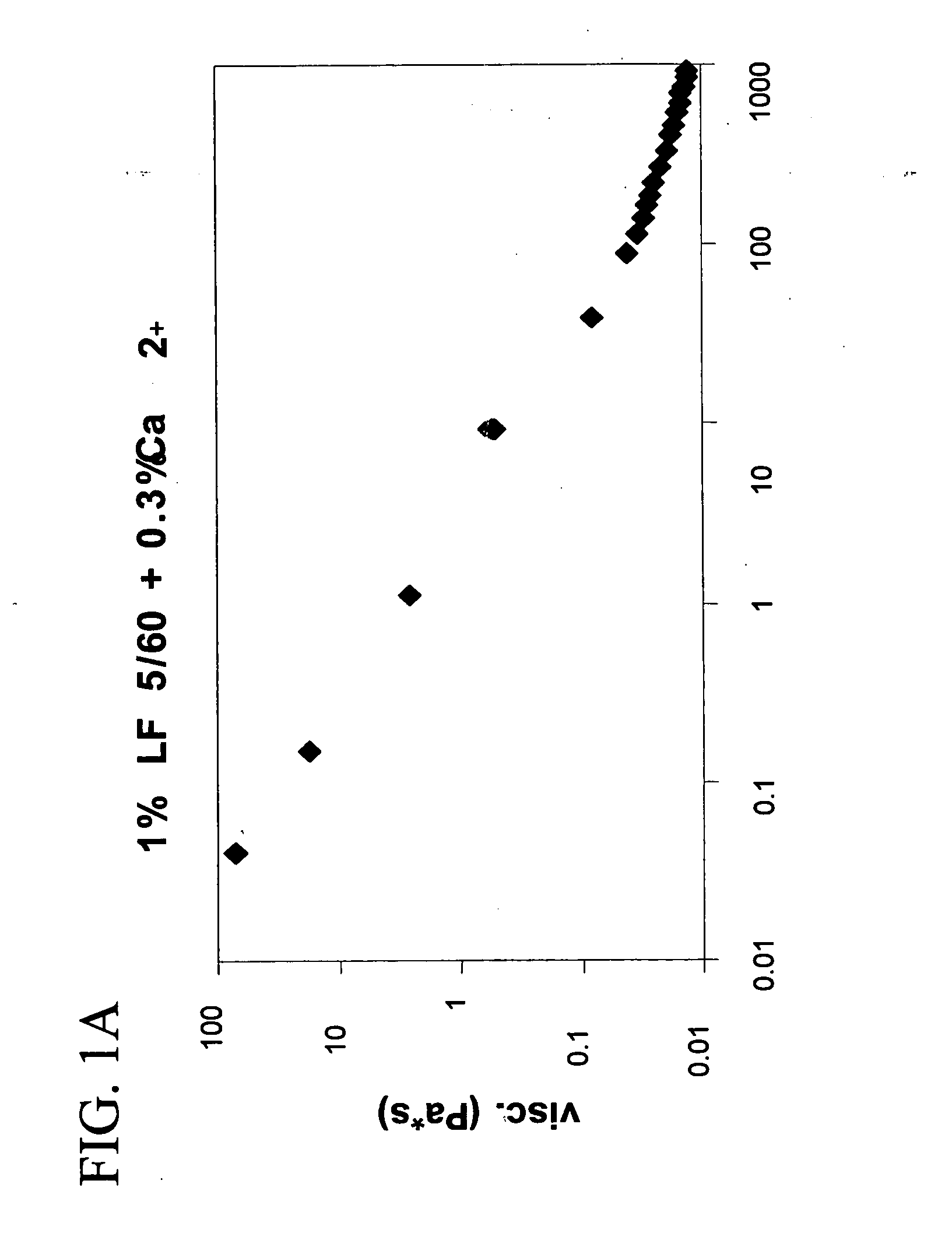
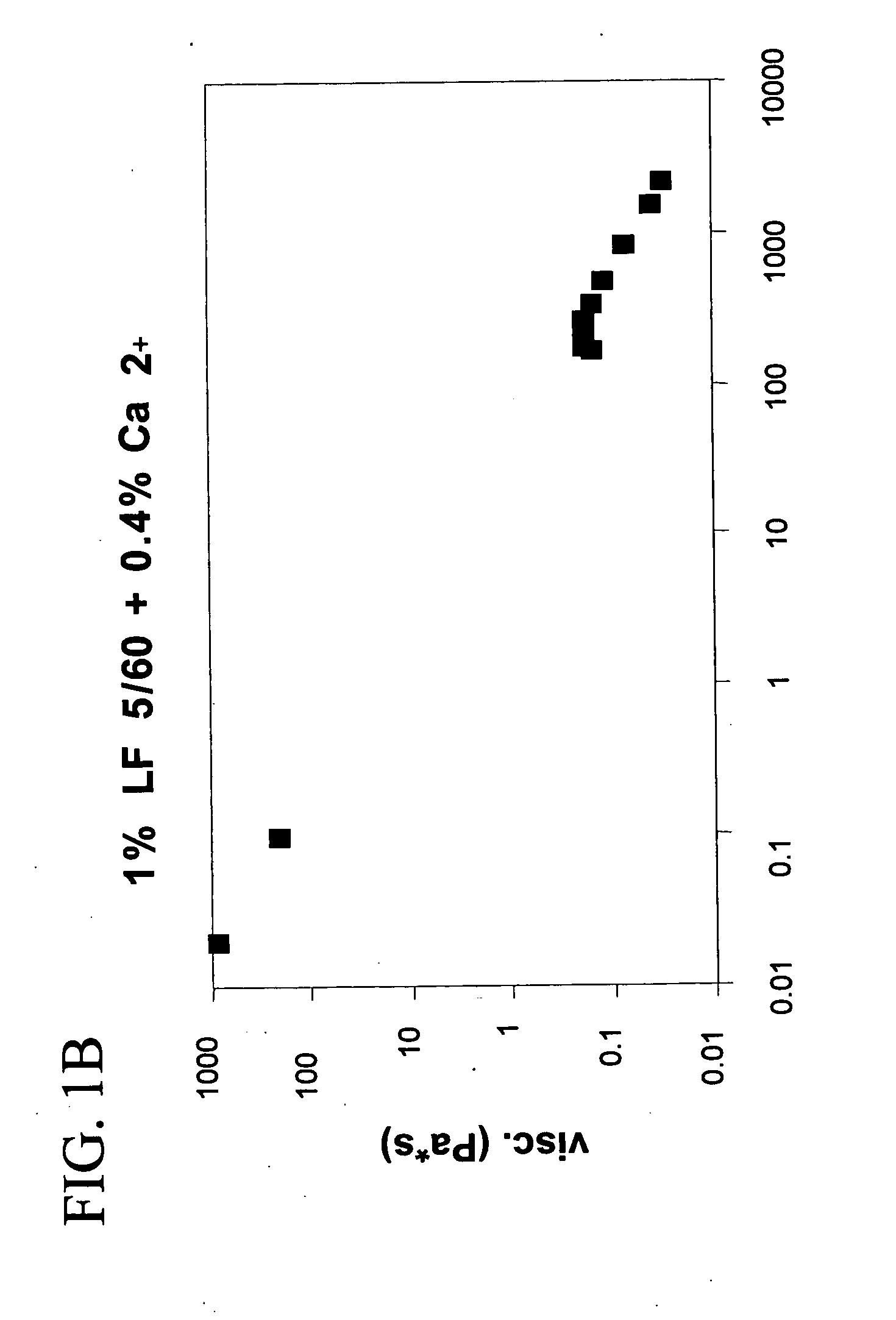
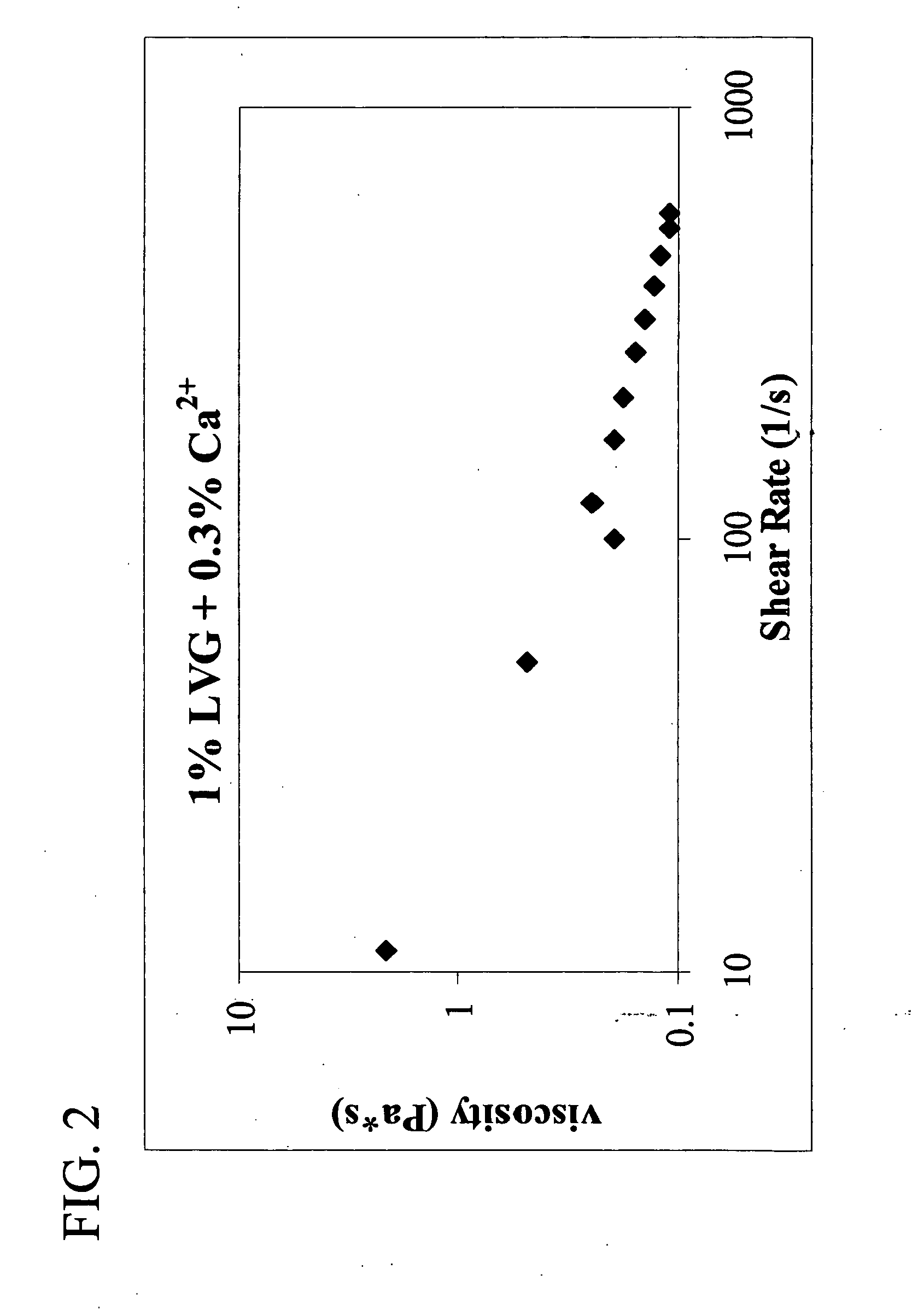
Preparation and Rheological Evaluation of Injectable Cross-Linked Alginate Biomaterial
The molecular weight (Mw) of the biopolymer alginate and its polydispersity (PD) (indication of the distribution range of molecular weight) may have an effect on the formation rate and structure of the resultant entanglement network. Thus, alginates Mw and PD using GPC-MALLS (as described in Experimental Procedures) were characterized (Table 1).
TABLE 1Alginate characterizationAlginateMn (g / mol)Mw (g / mol)PD (Mw / Mn)LF 5 / 602.102e+42.752e+41.309 ± 0.0072.113e+42.656e+41.257 ± 0.01 LVG1.469e+51.667e+51.135 ± 0.0161.385e+51.559e+51.126 ± 0.012MVG2.103e+52.596e+51.235 ± 0.0062.055e+52.385e+51.161 ± 0.007
Notes:
(i) The two numbers given for each measure represent two different batches of the material.
(ii) g / mol = Dalton
The alginate gels fall under the category of physical gels, wherein physical cross-links are formed that are neither permanent nor as strong as covalent cross-links.
Stead...
example 2
Comparing Therapeutic Effects of Alginate Biomaterial to Cardiac Cell Transplantation in a Rat Model of MI
Seven days after extensive MI, rats were randomized to alginate-based biomaterial injections, embryonic cardiomyocyte (1.5×106) implantation, or medium injection into the myocardial scar. The alginate biomaterial was calcium cross-linked, yet it still flowed under injection conditions, as described above. Echocardiography study was performed before and 1 and 2 months after implantation to assess LV remodeling and function. Hearts were harvested two months after implantation for histological evaluation.
Serial echocardiography studies revealed that the cross-linked alginate-based biomaterial injection enhanced scar thickness, prevented LV dilatation and dysfunction, comparable to cardiac cell transplantation, while control animals developed significant LV dilatation accompanied by progressive deterioration in LV contractility. The results are summarized in Table 3.
TABLE 3Al...
example 3
Animal Model of MI Treated with Calcium Cross-Linked Alginate Biomaterial
Overall, 39 rats were included in the study. Thirteen rats died after the surgical procedure to induce MI. Echocardiographic studies and analysis were performed on 24 rats. Fifteen rats were treated with alginate biomaterial injection and the control group (n=9) received injection of serum free culture medium. Two rats received injection of alginate into normal heart to study its safety and effect on normal myocardium.
Echocardiography Functional Study
Alginate biomaterial injection significantly increased scar thickness (Table 4, p<0.0001). Furthermore, it efficiently attenuated the typical course of LV dilatation complicating extensive anterior MI (FIG. 5, Table 4).
Although there was an increase in LV chamber internal diameters and areas, it was significantly less than that observed in control animals (FIG. 5 and Table 4). The beneficial effect of alginate biomaterial on LV remodeling was translat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| deformation oscillatory frequencies | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com