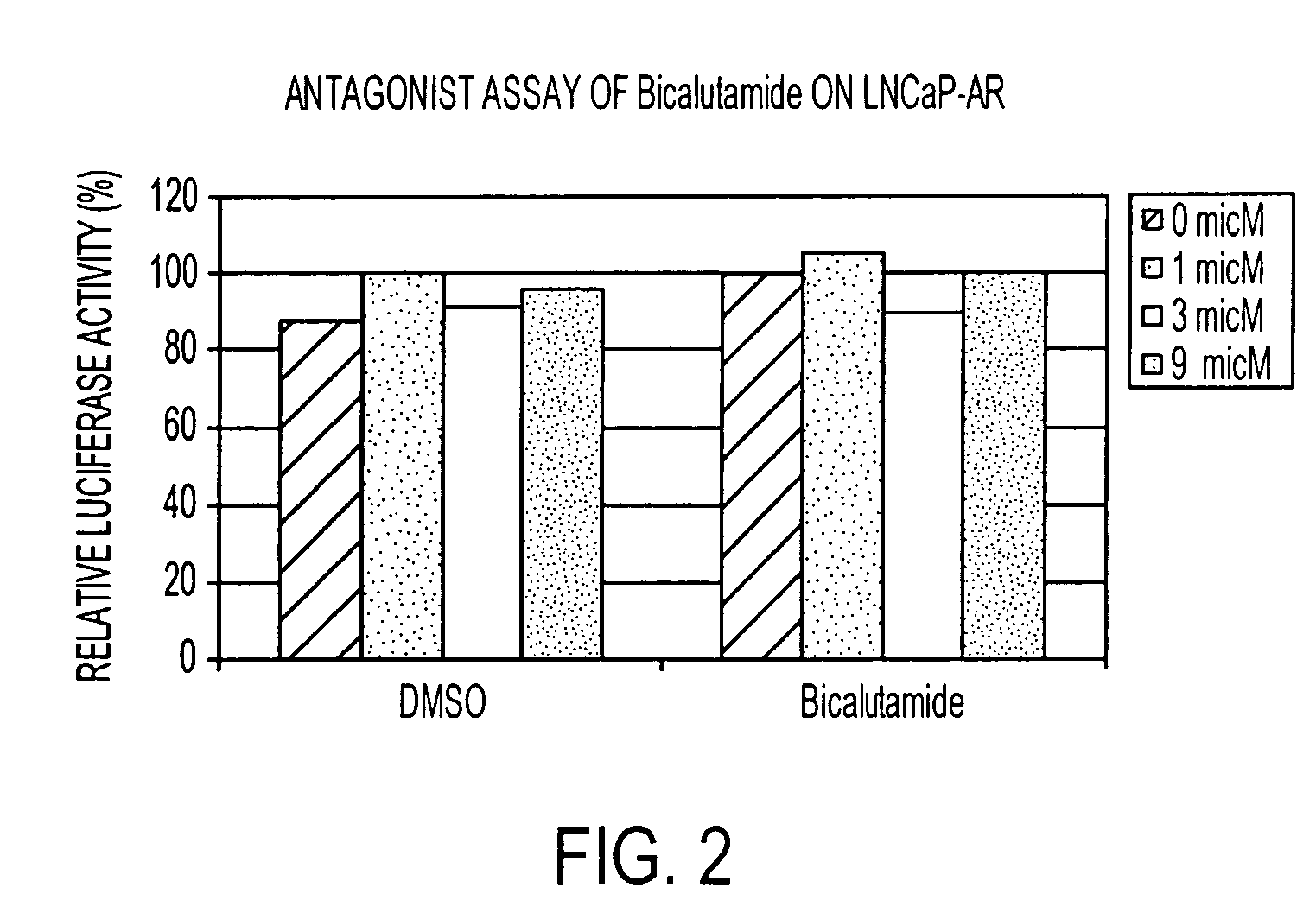
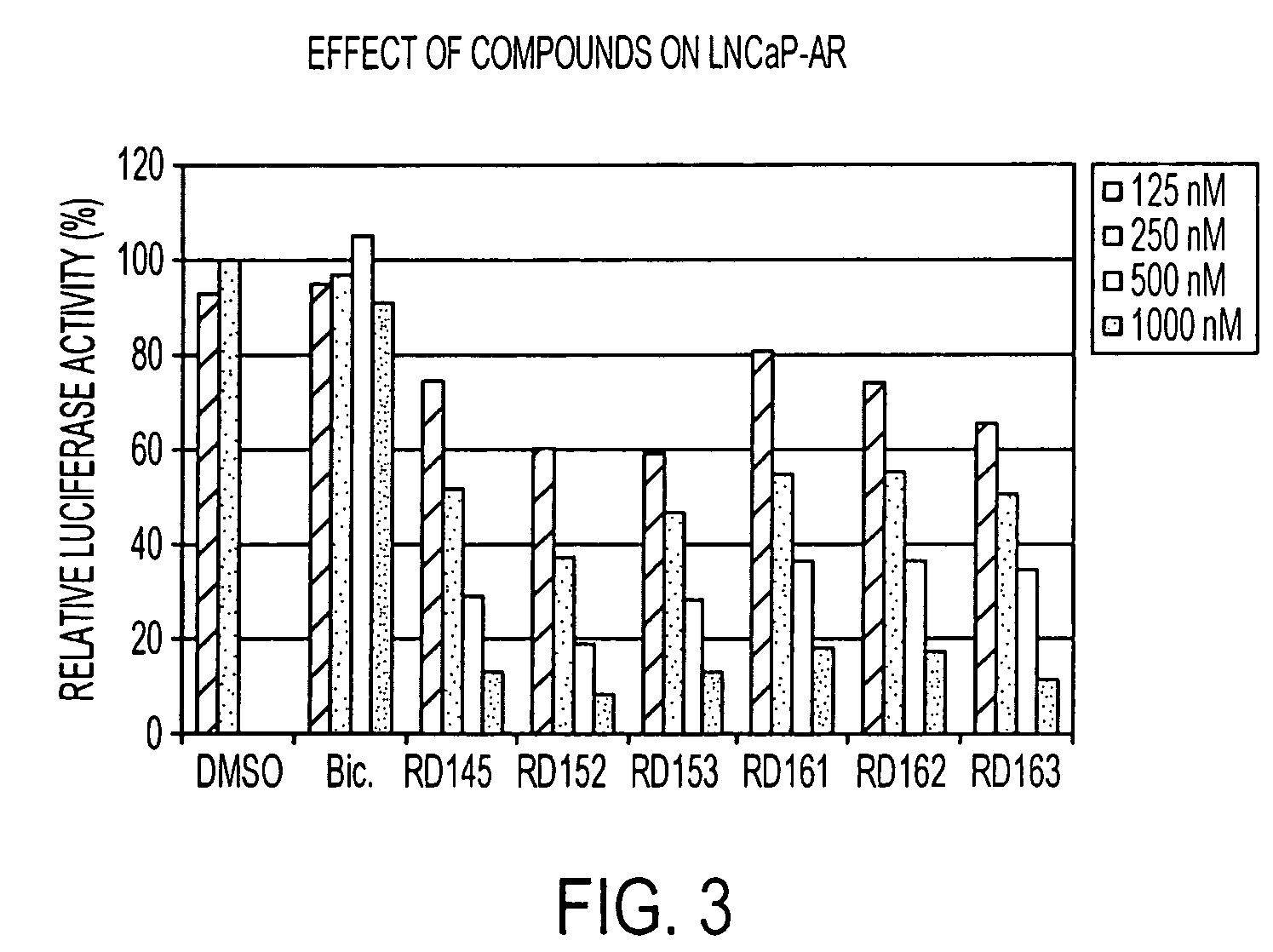
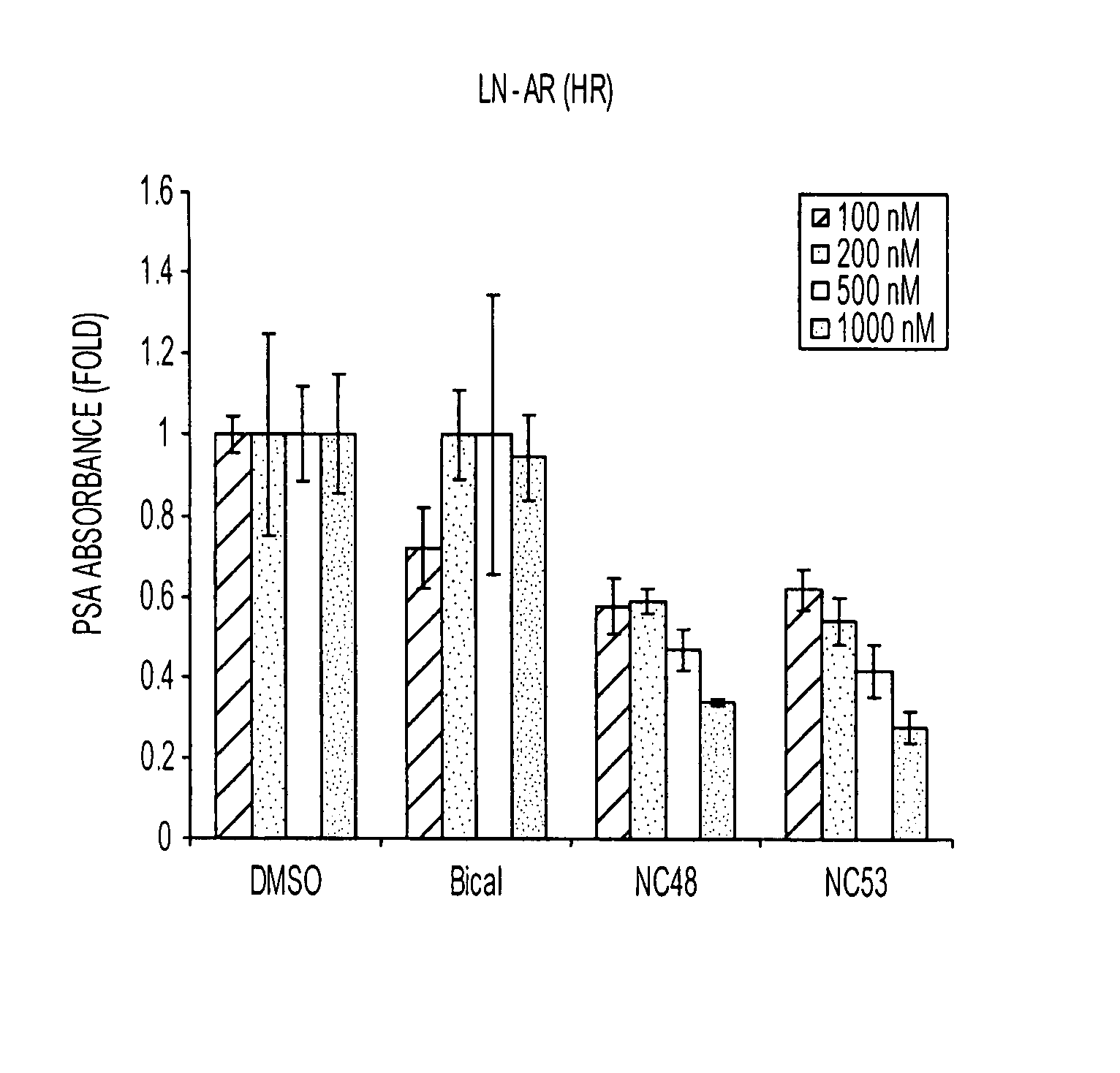
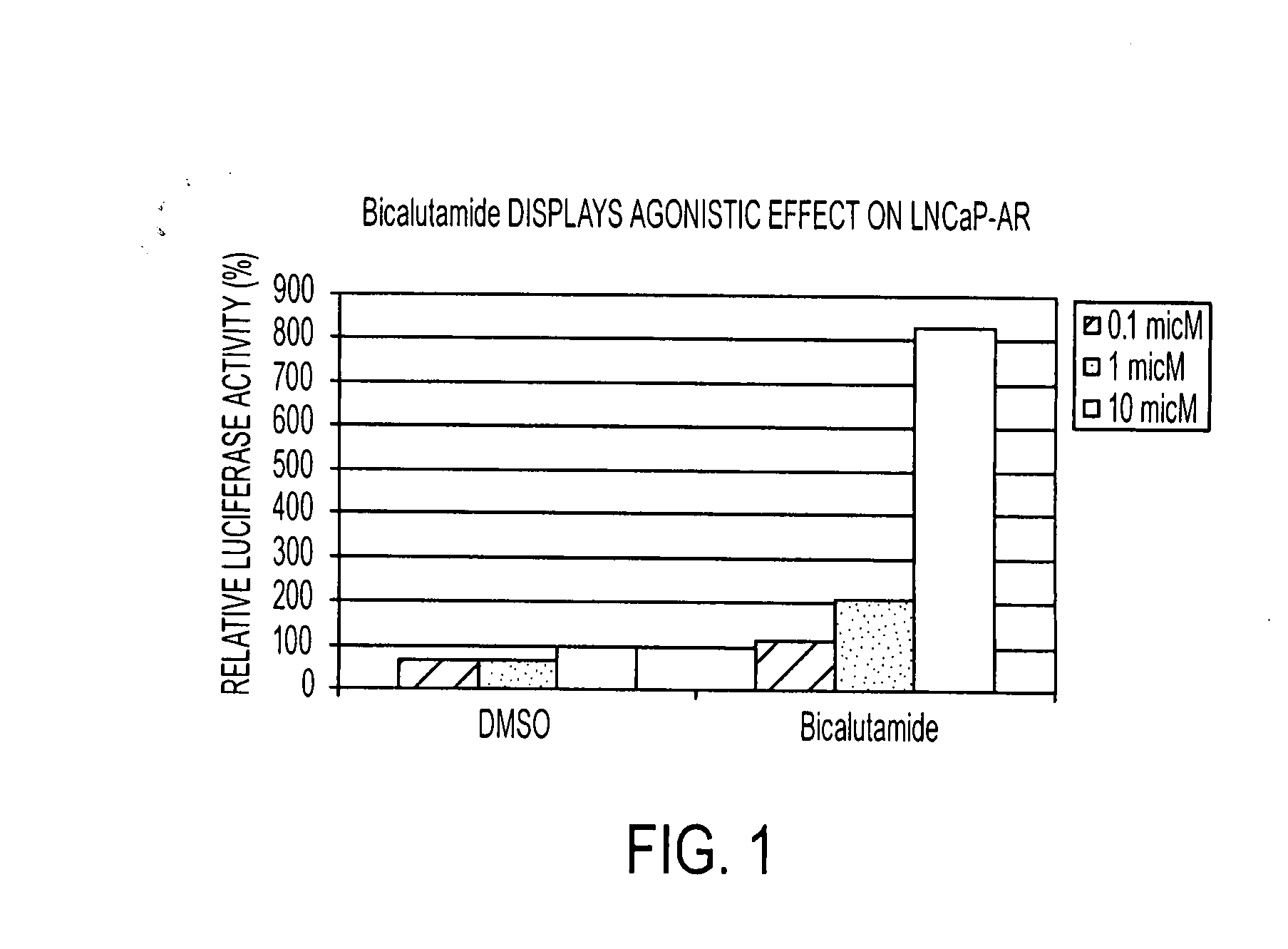
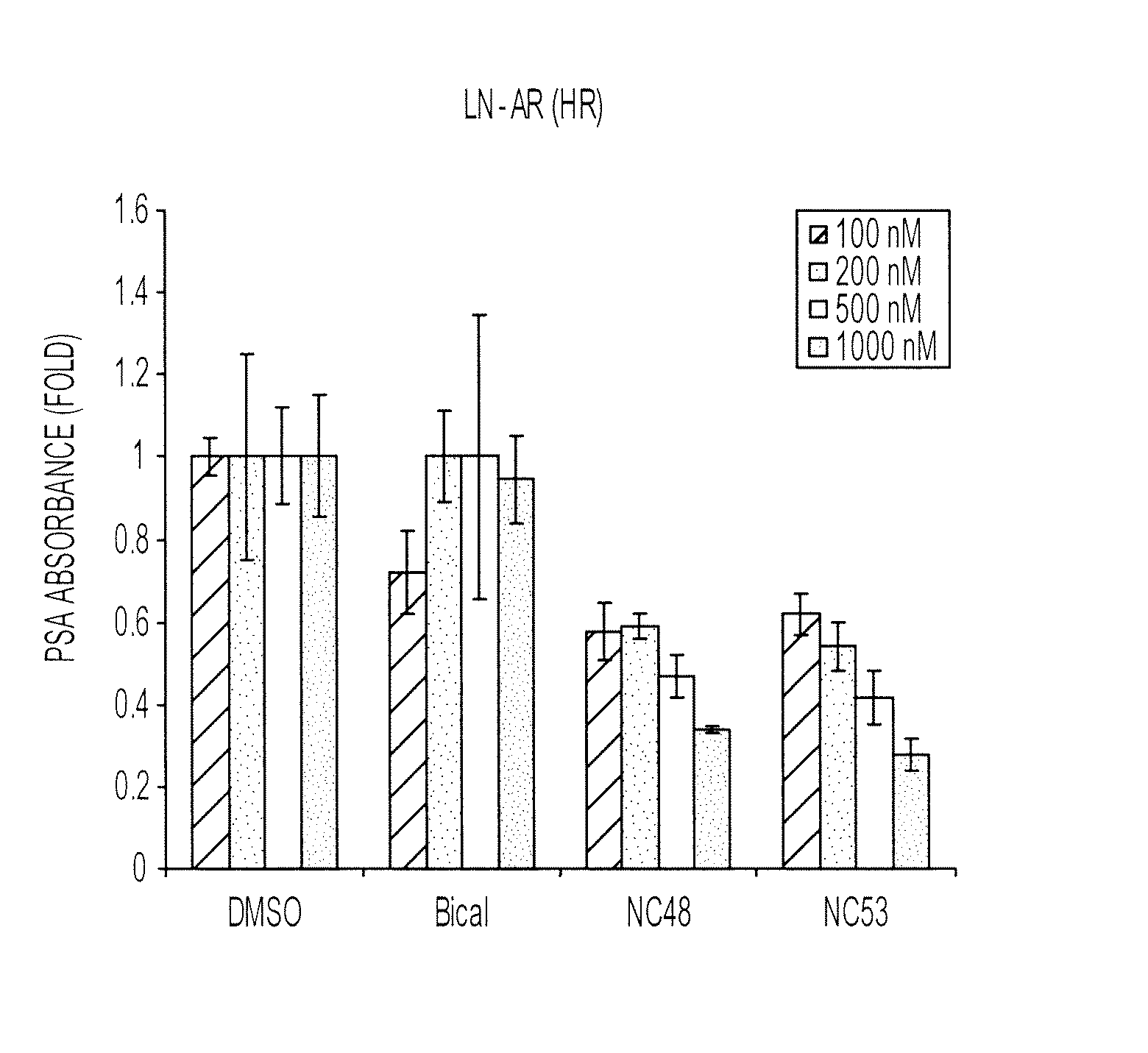
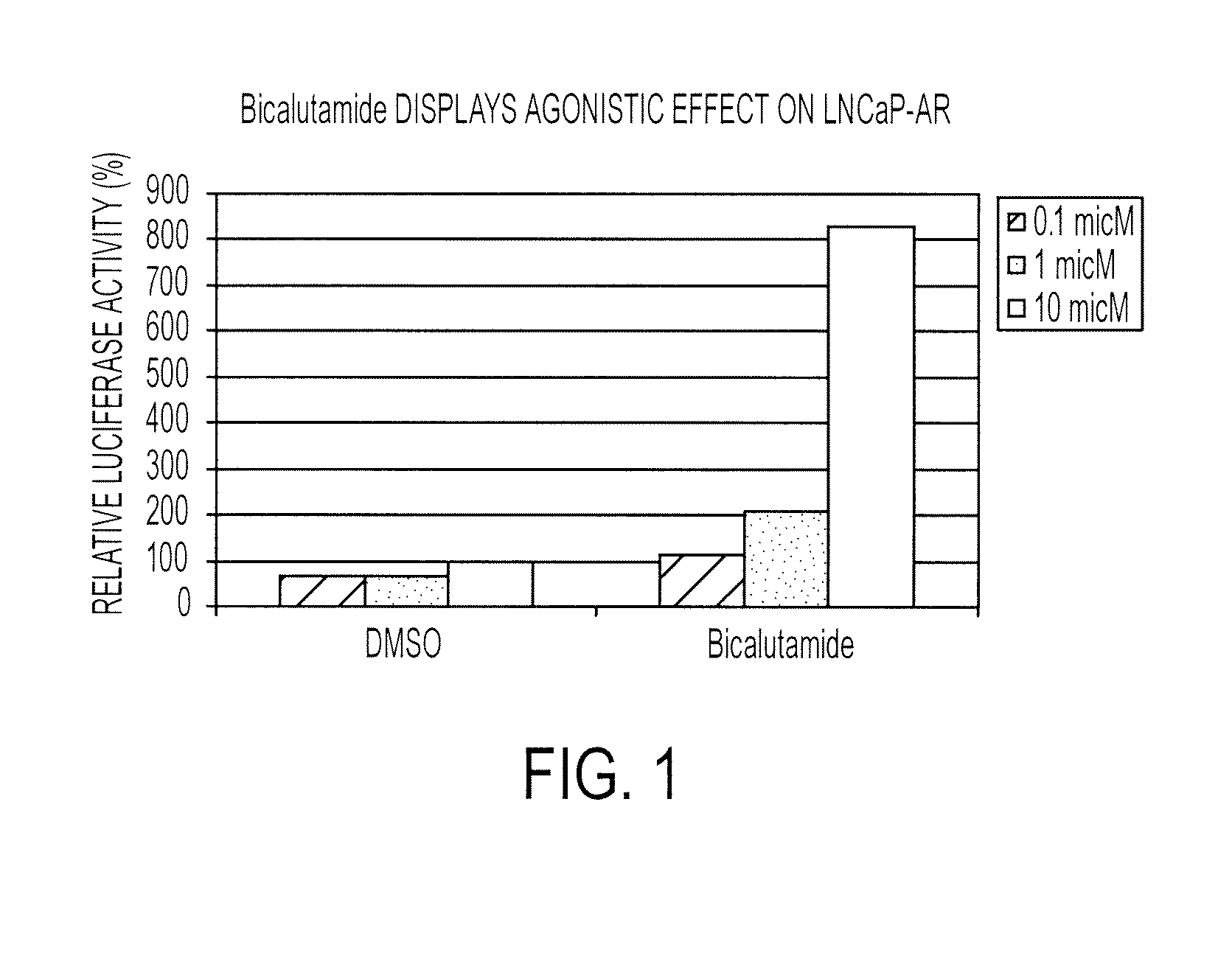
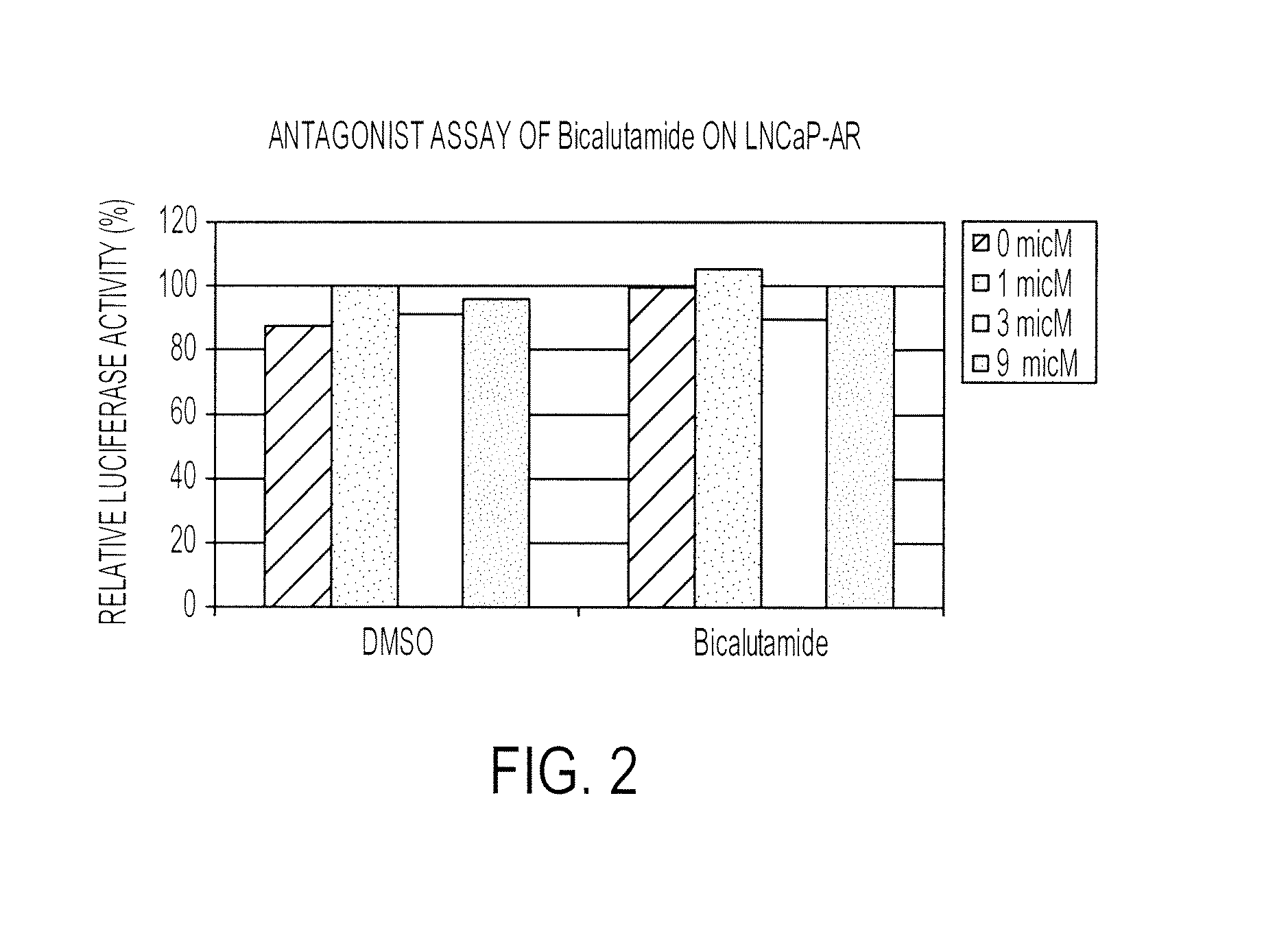
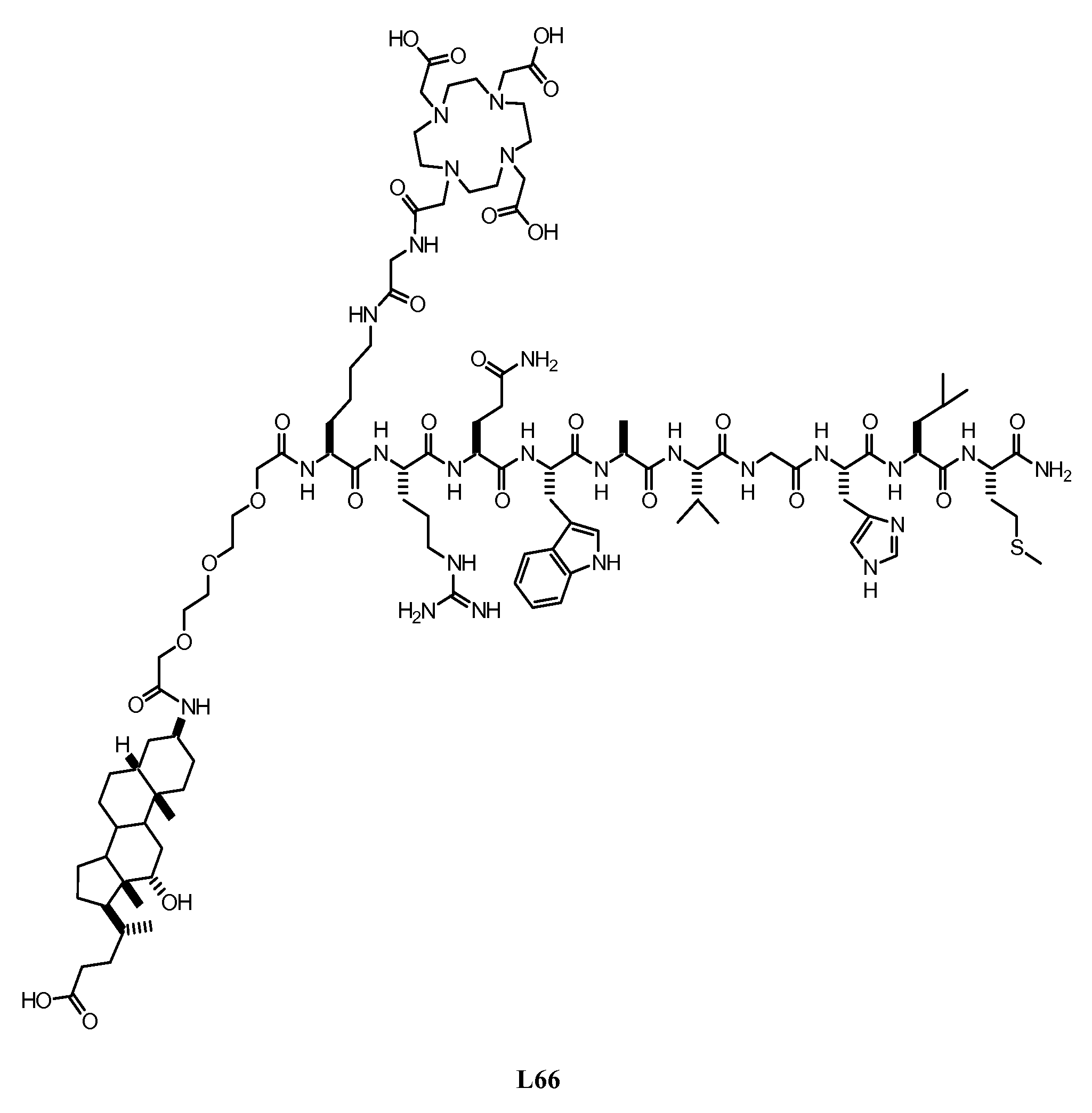
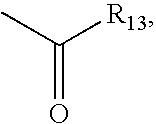
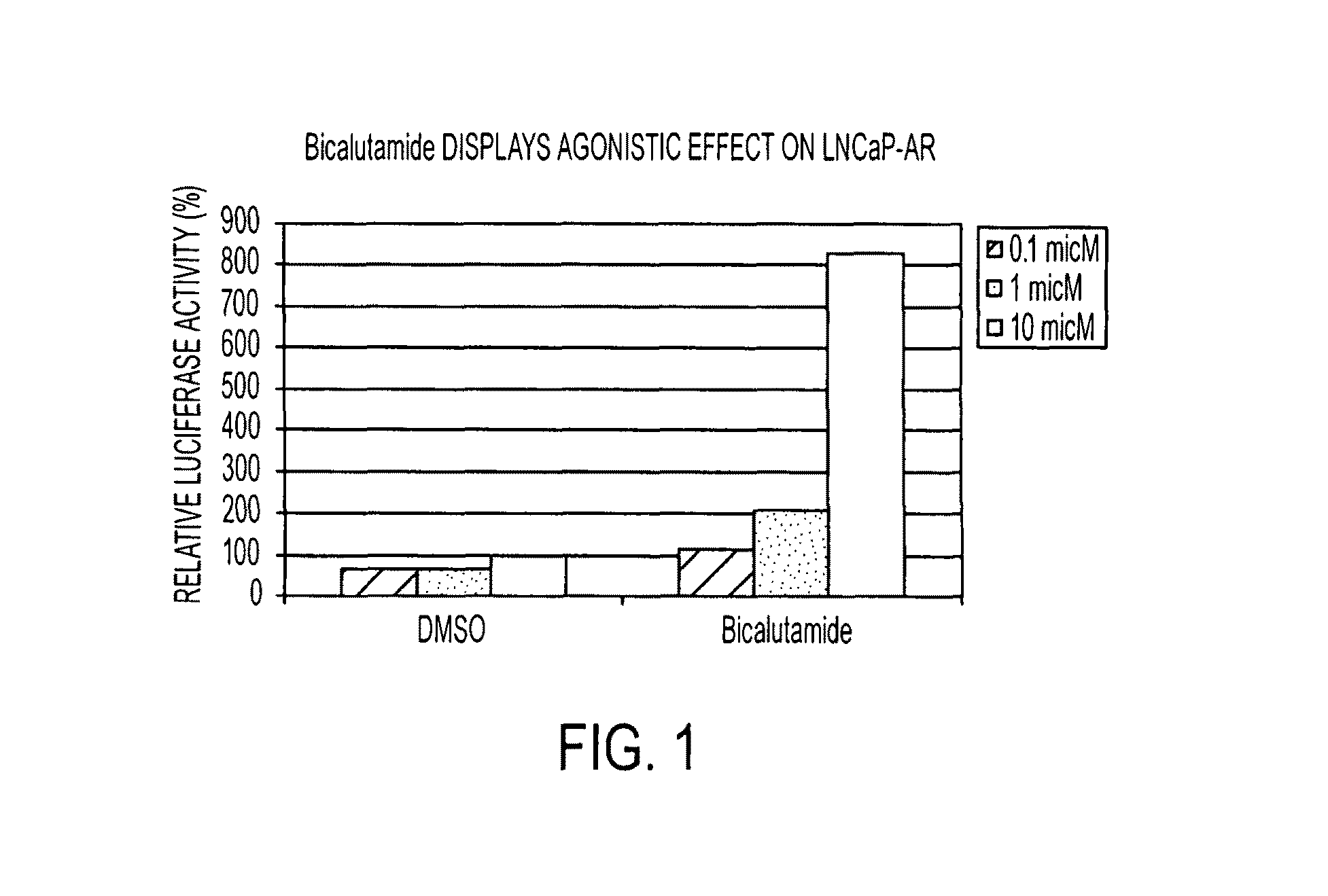
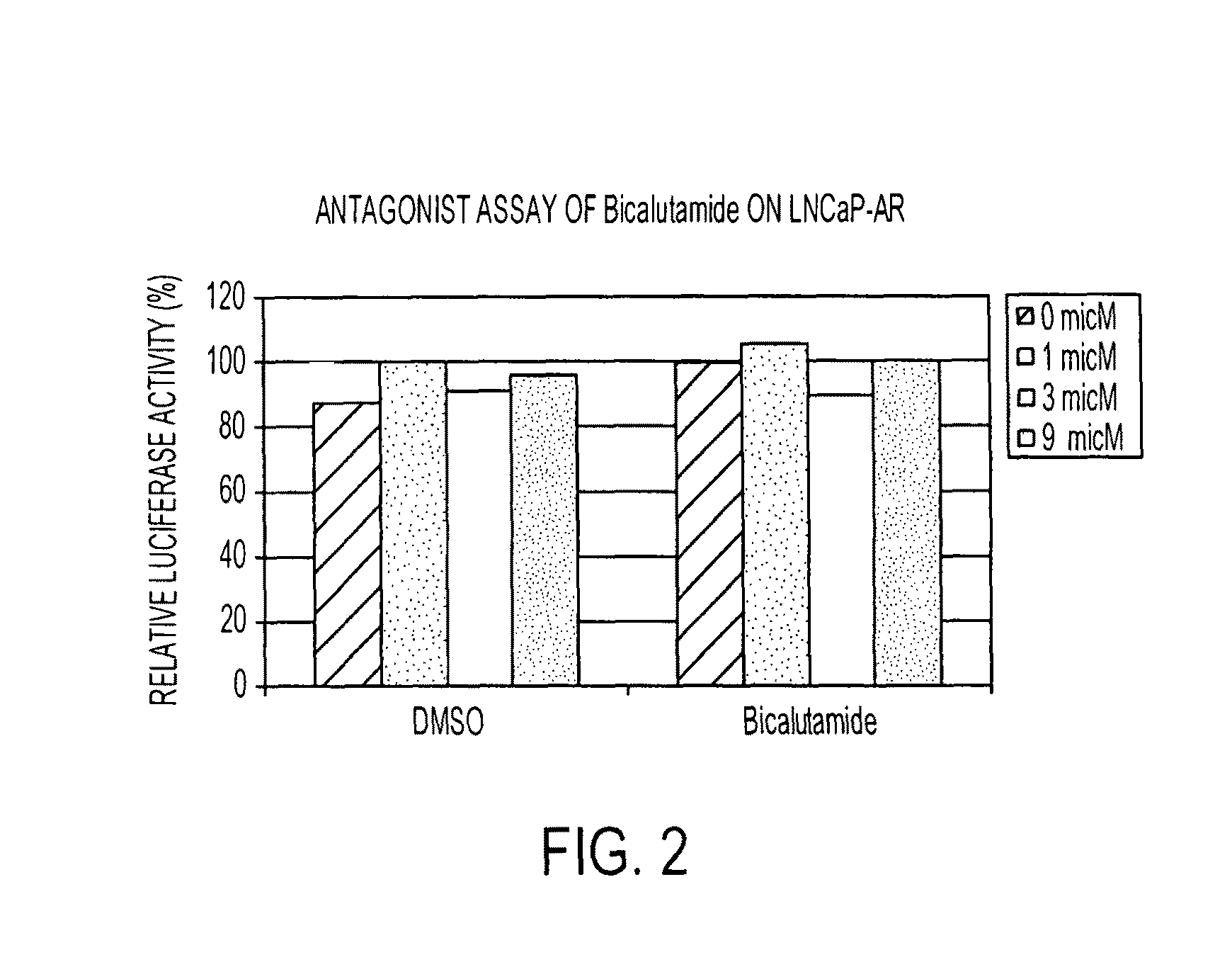
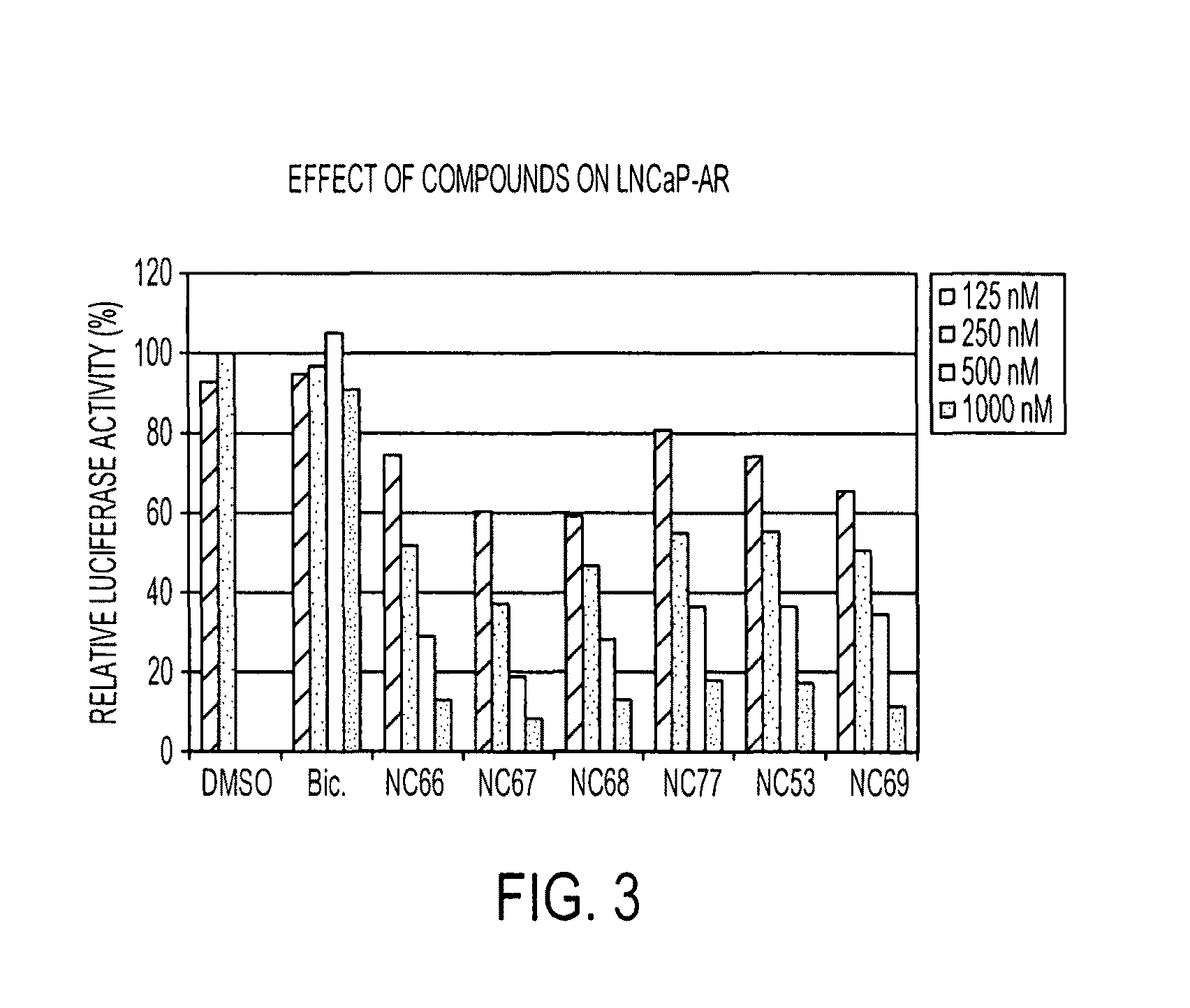
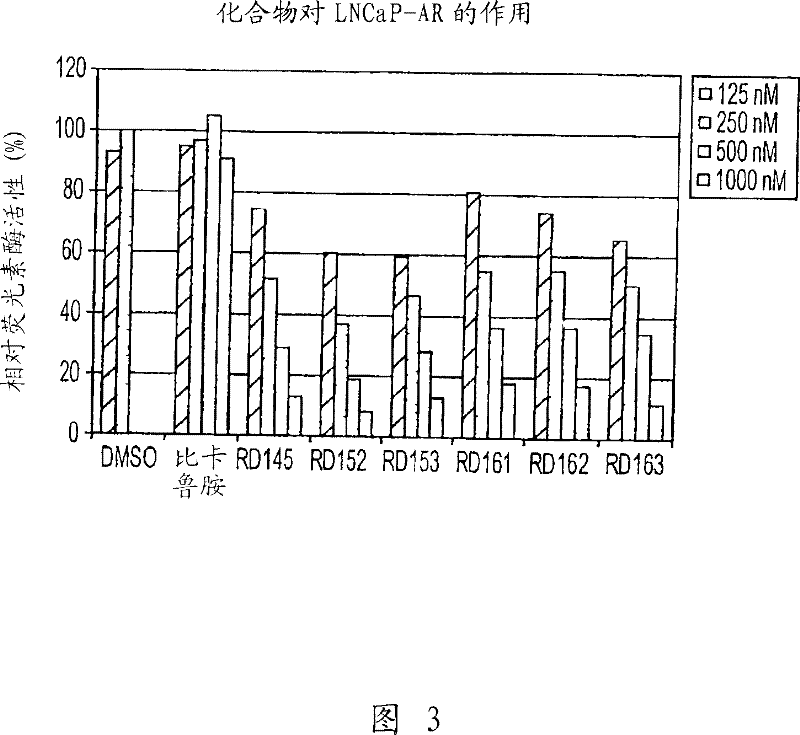
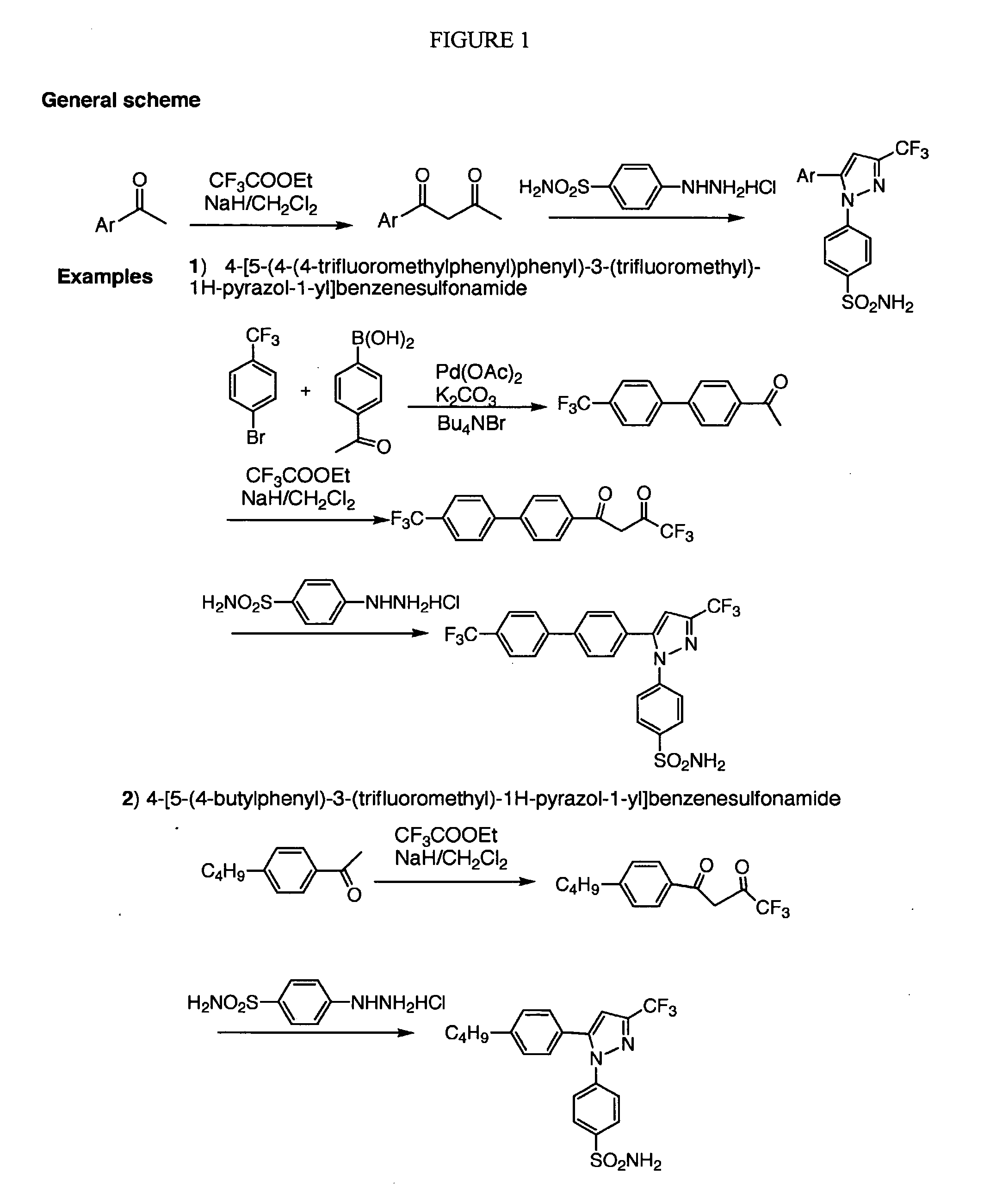
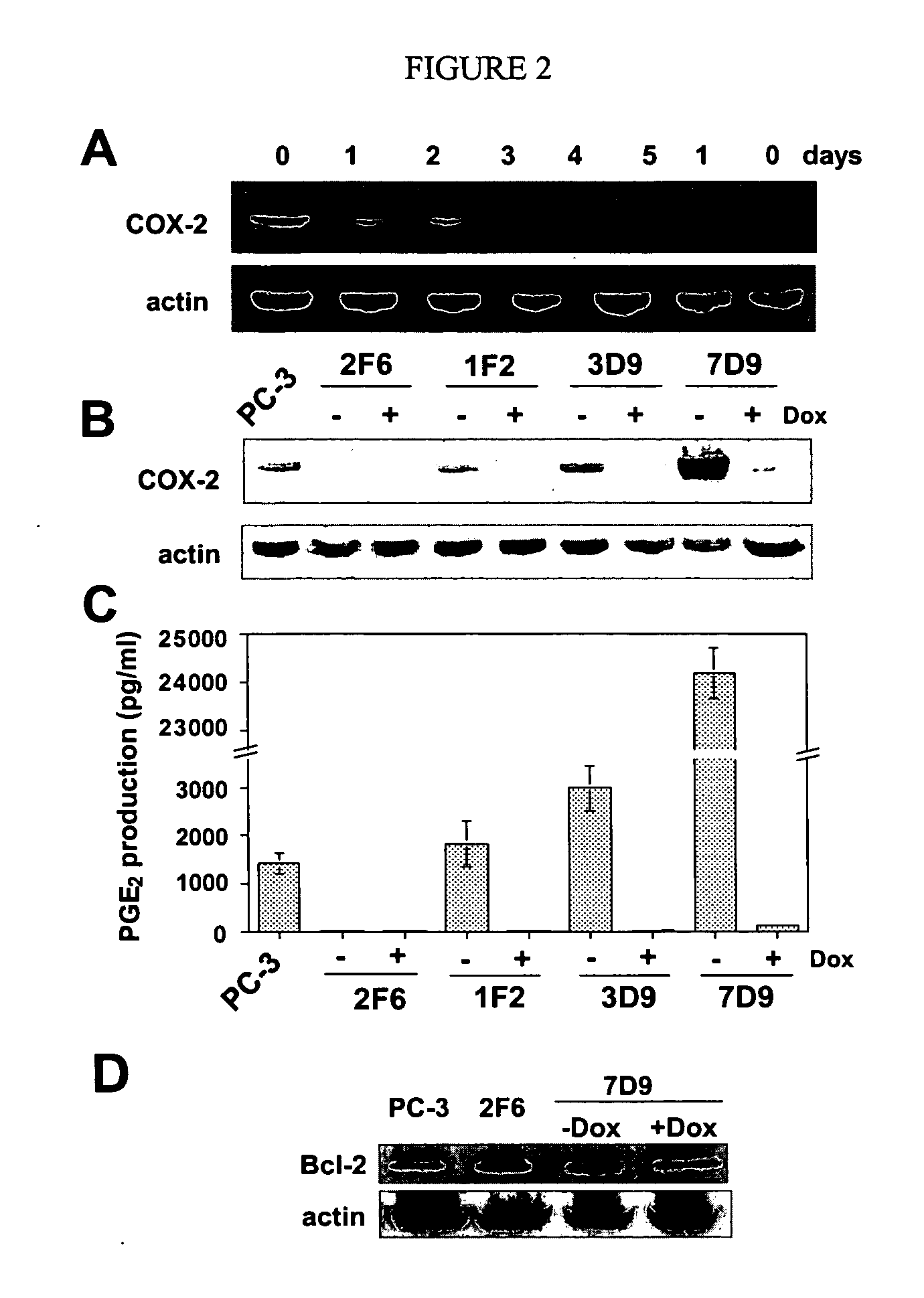
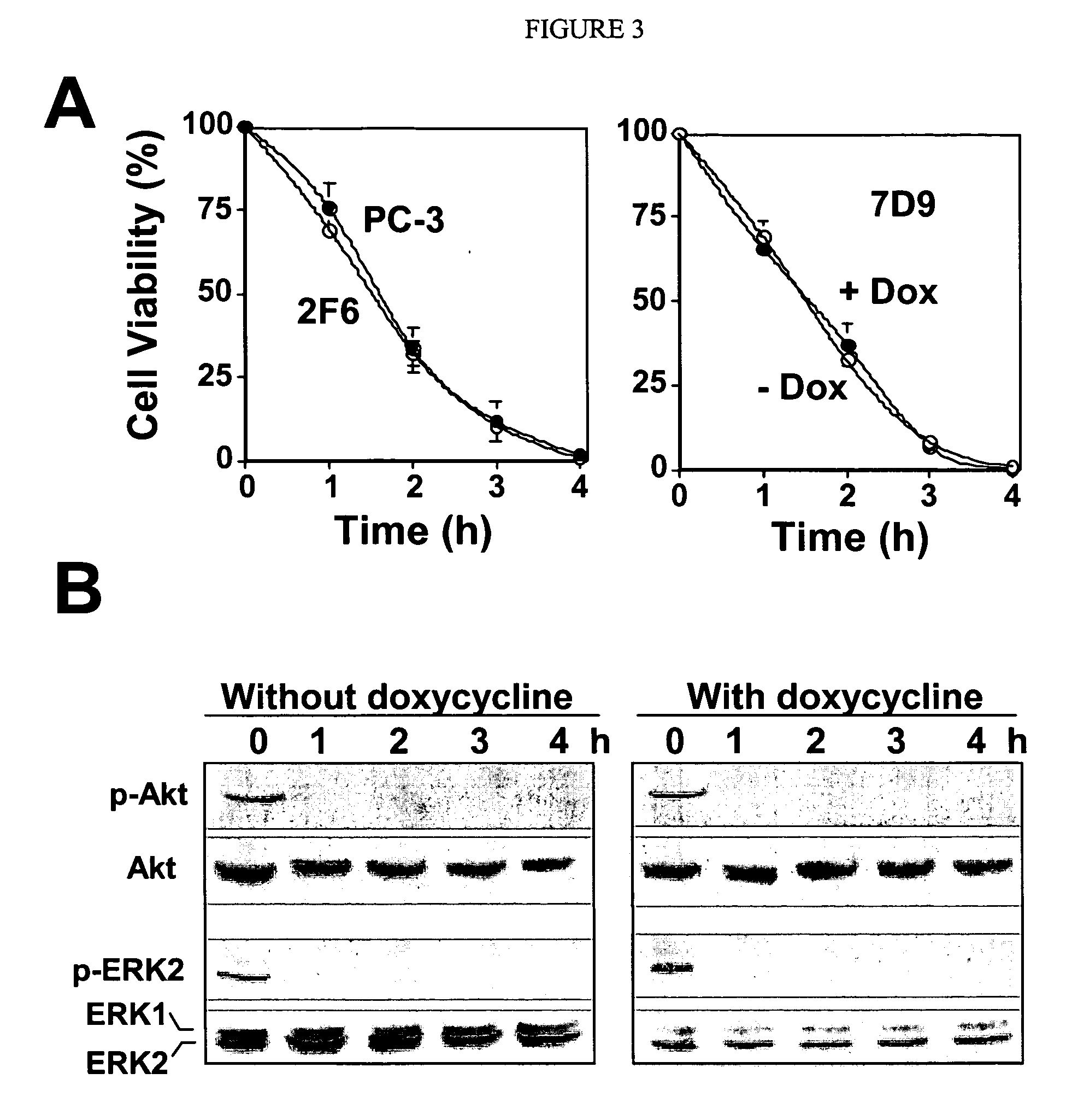
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
38 results about "Hormone refractory prostate cancer" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hormone-refractory prostate cancer is a disease that includes a variety of patients and represents a treatment dilemma for the practicing physician. Because of the diversity of this group, management strategies must be targeted to the clinical situations of the individual patients and their wishes.
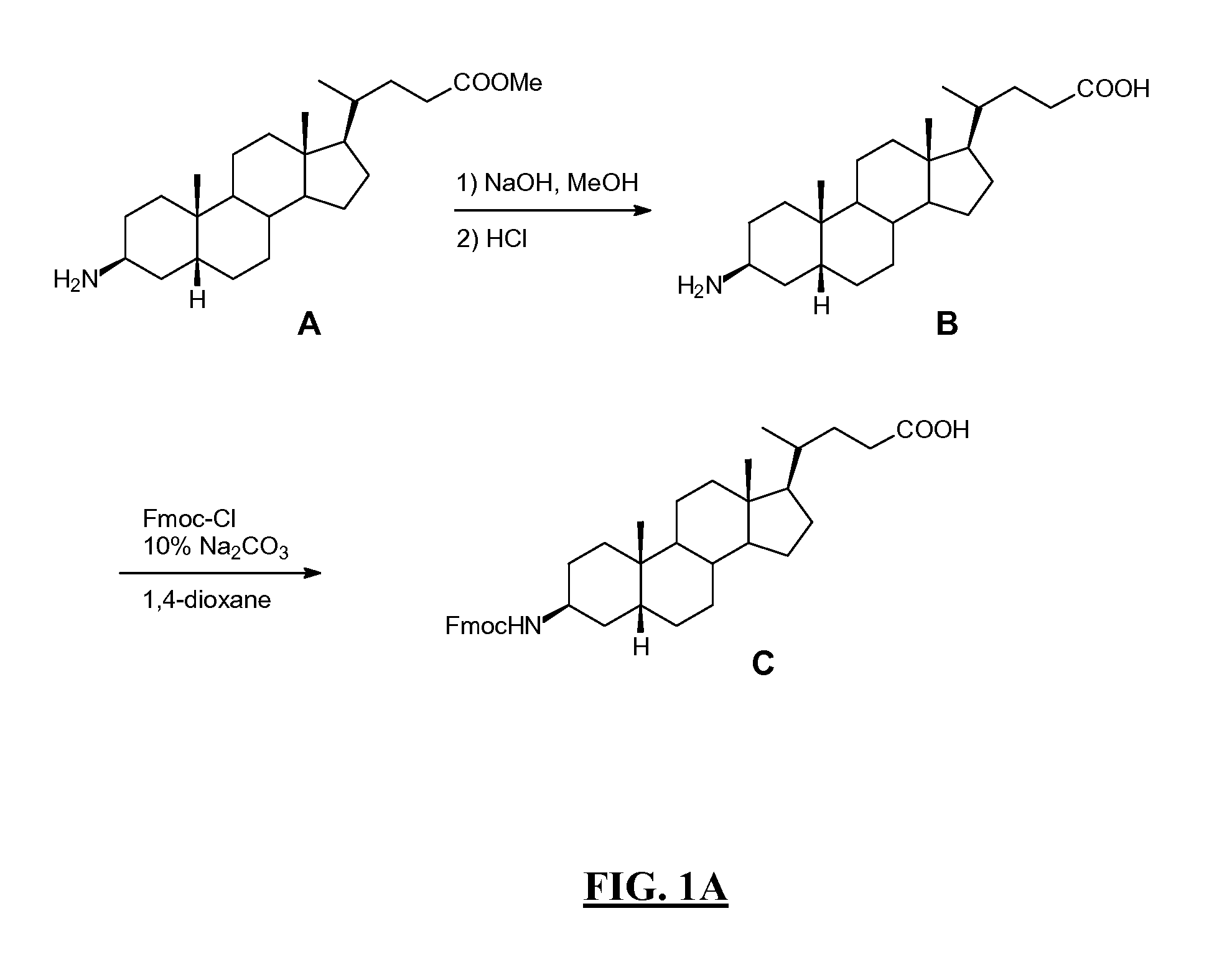
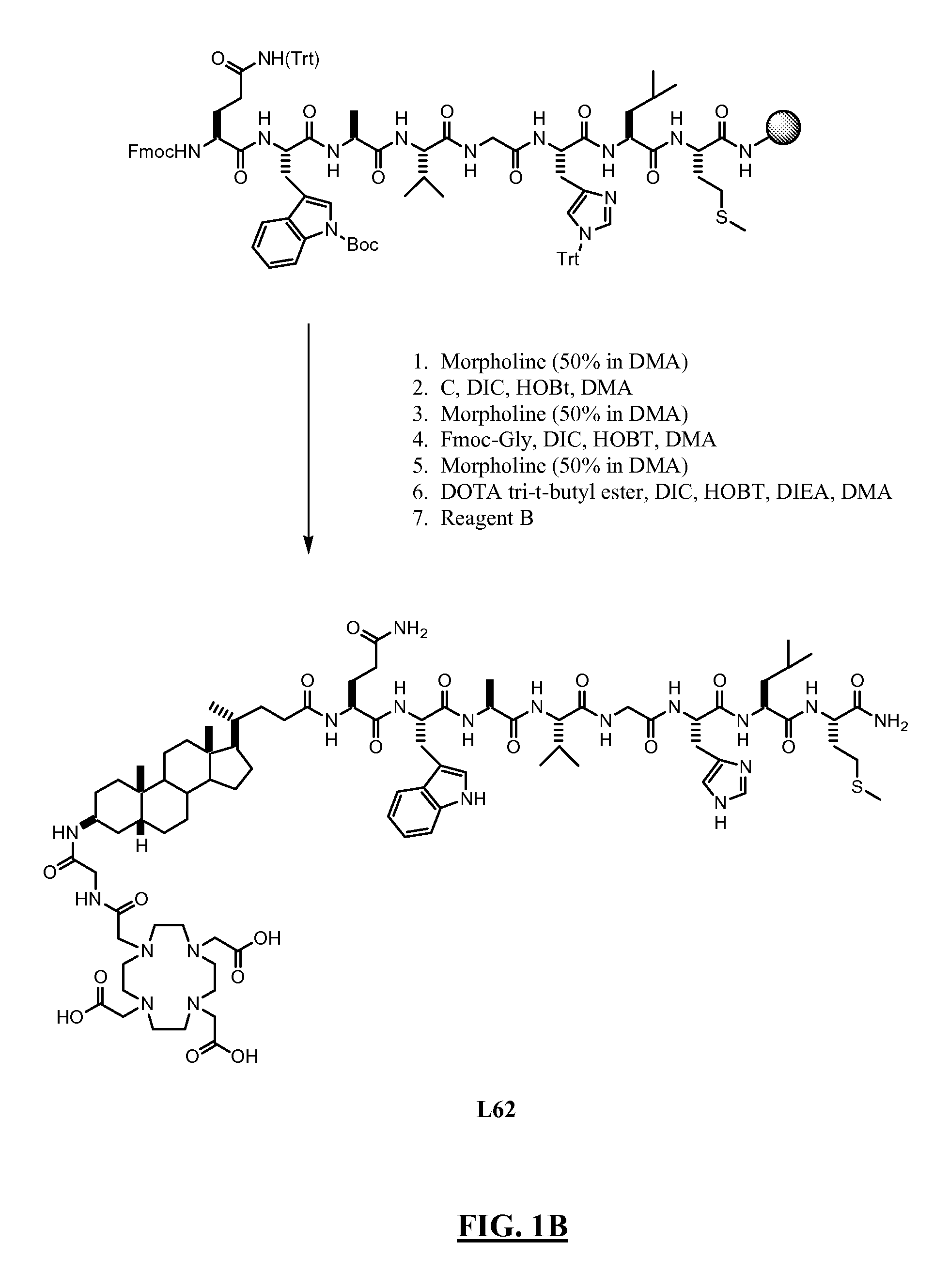
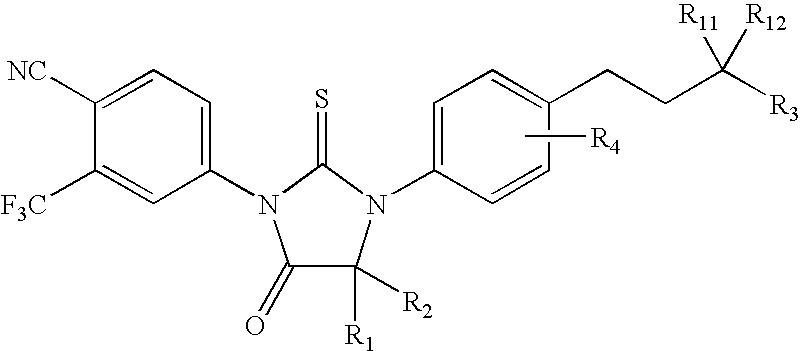
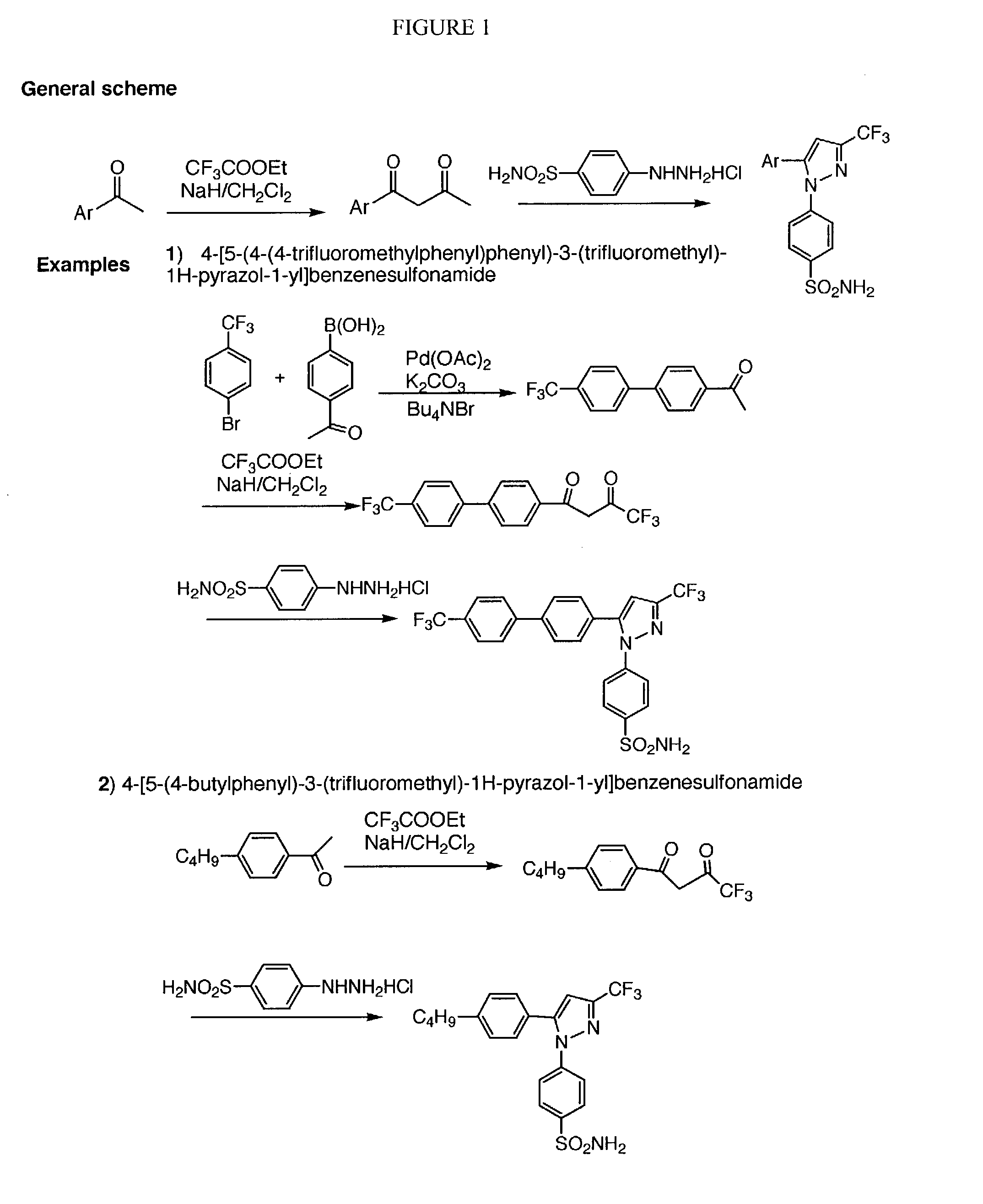
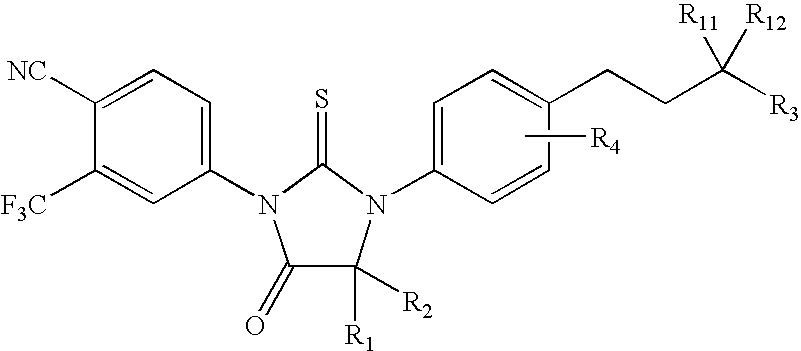
Diarylhydantoin compounds
The present invention relates to diarylhydantoin compounds, including diarylthiohydantoins, and methods for synthesizing them and using them in the treatment of hormone refractory prostate cancer.
Owner:RGT UNIV OF CALIFORNIA
Diarylhydantoin compounds
The present invention relates to diarylhydantoin compounds, including diarylthiohydantoins, and methods for synthesizing them and using them in the treatment of hormone refractory prostate cancer.
Owner:RGT UNIV OF CALIFORNIA
Diarylthiohydantoin compounds
ActiveUS20070254933A1Avoid problemsPrevent nuclear translocationOrganic active ingredientsBiocideHormone refractory prostate cancerPharmacology
The present invention relates to diarylthiohydantoin compounds and methods for synthesizing them and using them in the treatment of hormone refractory prostate cancer.
Owner:RGT UNIV OF CALIFORNIA
E25A protein, methods for producing and use thereof
InactiveUS7030232B1Useful in detectionUseful in therapyCell receptors/surface-antigens/surface-determinantsBacteriaDiseaseCancer cell
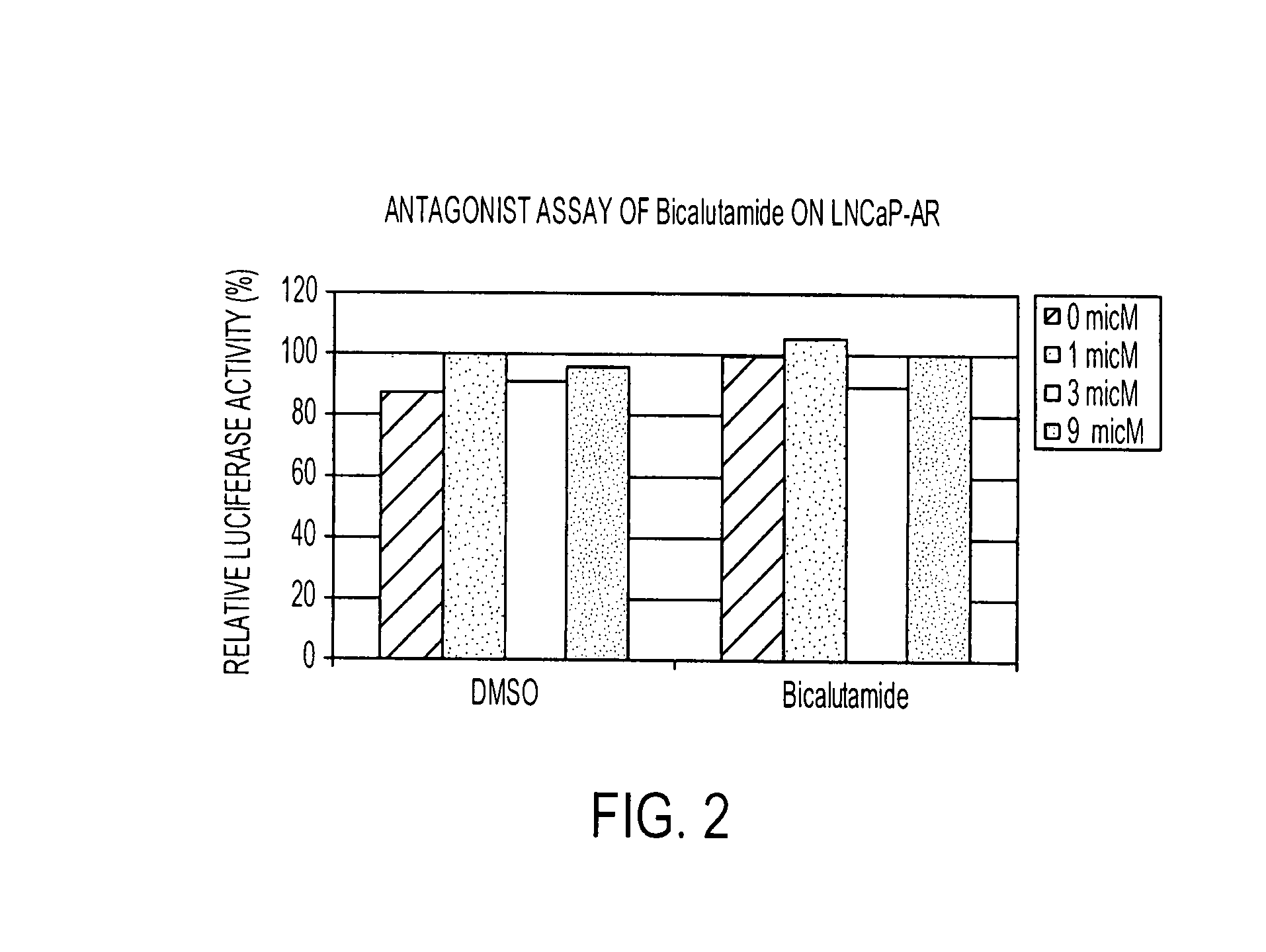
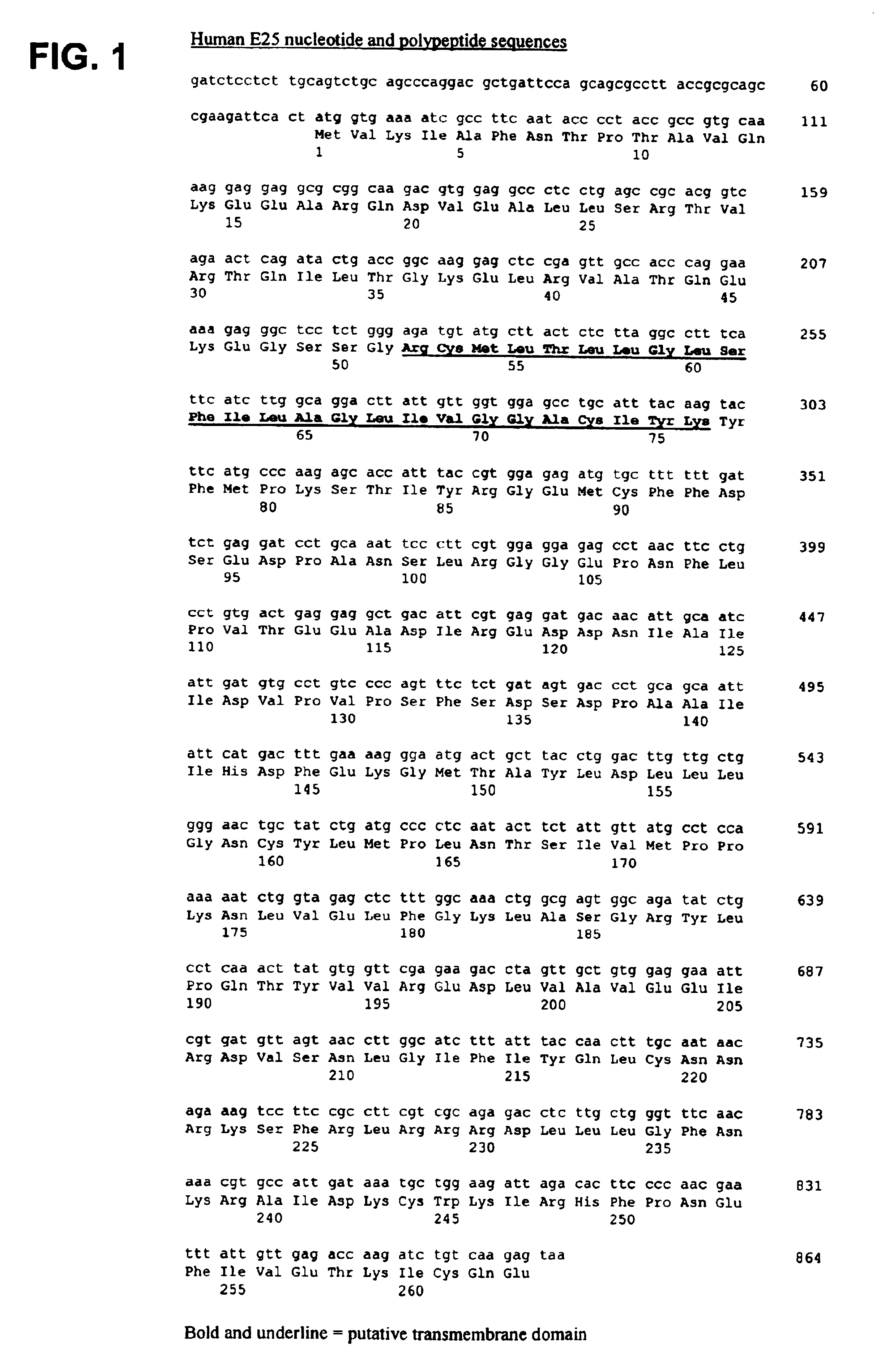
The invention provides a human E25a protein which is upregulated in cancerous cells, including those of hormone refractory prostate cancer, colon cancer, breast cancer or other cancers of epithelial origin. The invention also provides nucleic acid molecules encoding E25a protein, nucleic acid probes which hybridize with nucleic acid molecules encoding E25a protein, and antibodies which bind E25a protein. E25a protein and its related molecules can be useful as diagnostic markers of cancer, including hormone refractory prostate cancer, and as specific therapeutic targets in this disease. The invention also provides methods for diagnosing cancer, including hormone refractory prostate cancer.
Owner:RGT UNIV OF CALIFORNIA
Diarylthiohydantoin compounds
ActiveUS20080139634A2Avoid translocationDestabilize an androgen receptor proteinOrganic active ingredientsBiocideHormone refractory prostate cancerPharmacology
Owner:RGT UNIV OF CALIFORNIA
Gastrin Releasing Peptide Compounds
InactiveUS20080008649A1Improve targetingDecreasing aberrant vascular permeabilityRadioactive preparation carriersGastrin releasing peptideCholic acidTherapeutic Hormone
New and improved compounds for use in diagnostic imaging or therapy having the formula M-N—O—P-G, wherein M is a metal chelator having the structure: wherein R1-R5 and FG are as defined herein (in the form complexed with a metal radionuclide or not), N—O—P is the linker containing at least one non-alpha amino acid with a cyclic group, at least one substituted bile acid or at least one non-alpha amino acid, and G is the GRP receptor targeting peptide. In the preferred embodiment, M is an Aazta metal chelator or a derivative thereof. Methods for imaging a patient and / or providing radiotherapy or phototherapy to a patient using the compounds of the invention are also provided. Methods and kits for preparing a diagnostic imaging agent from the compound is further provided. Methods and kits for preparing a radiotherapeutic agent are further provided. Novel methods of treating prostate tumors or of delaying the progression of prostate tumors are also provided, including, methods of treating bone or soft tissue metastases of prostate cancer, methods for treating hormone sensitive and hormone refractory prostate cancer, methods for delaying the progression of hormone sensitive prostate cancer, for facilitating combination therapy in patients with hormone sensitive prostate cancer and for decreasing aberrant vascular permeability in patients with hormone sensitive prostate cancer.
Owner:BRACCO IMAGINIG SPA
Diarylhydantoin compounds
ActiveUS20090111864A1High antagonistic activityMinimal agonistic activityOrganic active ingredientsBiocideHormone refractory prostate cancerMedicine
The present invention relates to diarylhydantoin compounds and methods for synthesizing them and using them in the treatment of hormone refractory prostate cancer.
Owner:RGT UNIV OF CALIFORNIA
MSMB-gene based diagnosis, staging and prognosis of prostate cancer
InactiveUS20090203010A1Loss of gene functionHigh degreeSugar derivativesMicrobiological testing/measurementAndrogen sensitivityRegulatory region
This invention relates generally to a method of diagnosis for distinguishing between a benign prostate hyperplasia and a prostate cancer and between a hormone-sensitive and a hormone-refractory prostate cancer condition and specifically to identification of a hypermethylated (on CpG and non-CpG dinucleotides) CpG island in the beta-microseminoprotein (MSMB) regulatory regions surrounding the transcriptional start site of the MSMB gene as a diagnostic indicator of prostate cancer (PrCa) and for distinguishing androgen-refractory from androgen-sensitive prostate cancer.
Owner:KATHOLIEKE UNIV LEUVEN
Diarylthiohydantoin compounds
ActiveUS8110594B2Avoid translocationDestabilize an androgen receptor proteinBiocideOrganic active ingredientsHormone refractory prostate cancerPharmacology
The present invention relates to diarylthiohydantoin compounds and methods for synthesizing them and using them in the treatment of hormone refractory prostate cancer.
Owner:RGT UNIV OF CALIFORNIA
Compounds and methods for inducing apoptosis in proliferating cells
InactiveUS7026346B2Treating and inhibiting and delaying onsetBiocideOrganic active ingredientsMelanomaApoptosis
Compounds useful for inducing apoptosis in proliferative cells, particularly cancer cells, including but not limited to prostate cancer, leukemia, non-smalll cell lung cancer, colon cancer, CNS cancer, melanoma, ovarian cancer, renal cancer, bladder cancer, lymphoma, and breast cancer. These compounds are particularly useful in the treatment of androgen-independent cancers, including hormone-refractory prostate cancer. Further provided are methods of treating cancer in a subject in need of such treatment using the compounds of the present invention. Further provided are methods for using the compounds of the present invention to treat, inhibit, or delay the onset of cancer in a subject. Further provided are methods of inducing apoptosis in rapidly proliferating cells, particularly, though not necessarily cancer cells, using the compounds of the present invention.
Owner:THE OHIO STATE UNIV RES FOUND
Methods and related compositions for the treatment of cancer
ActiveUS20110224141A1Increased intracellular granularitySufficient amountBiocidePeptide/protein ingredientsProstate cancer cellCancer cell
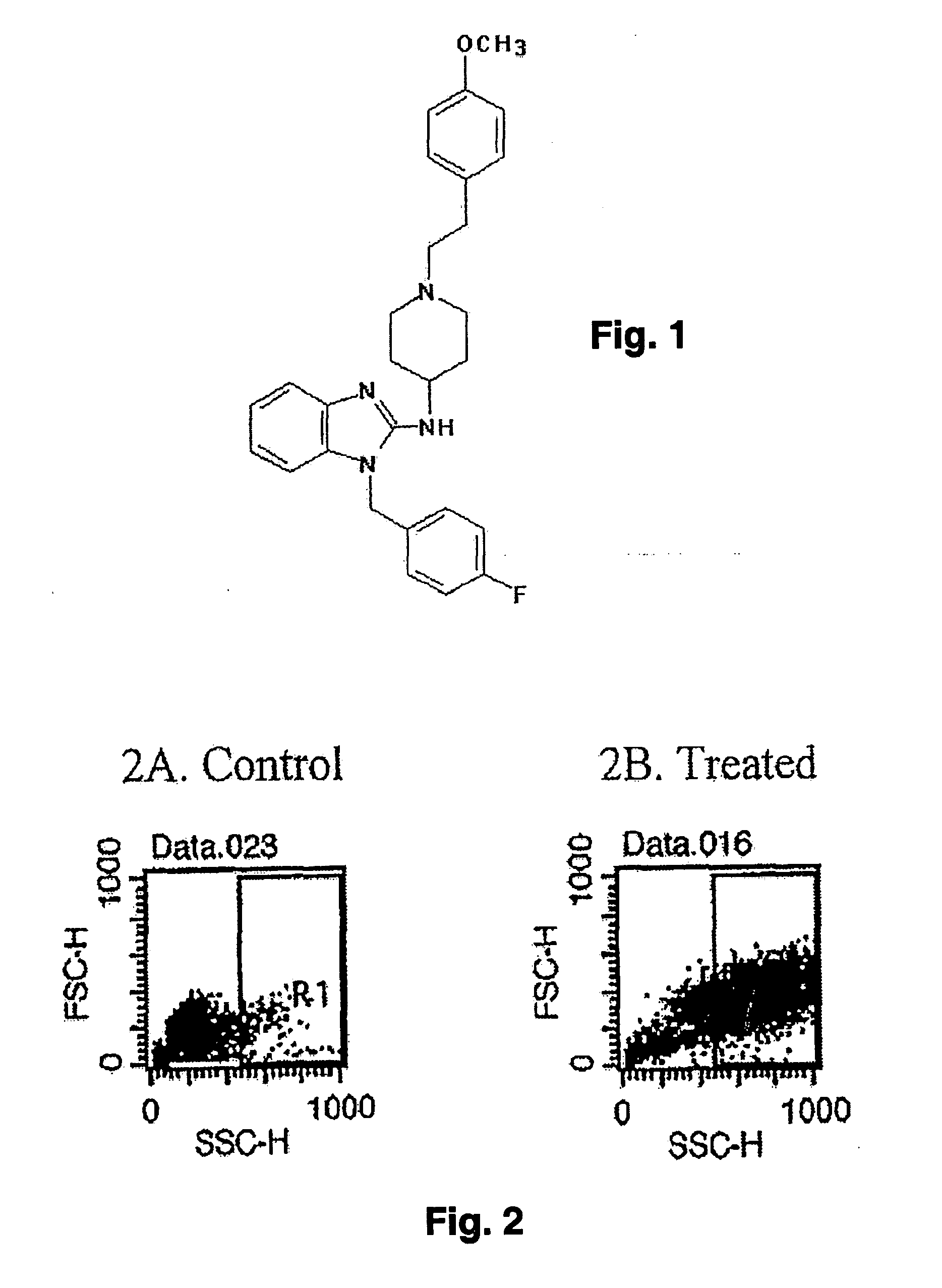
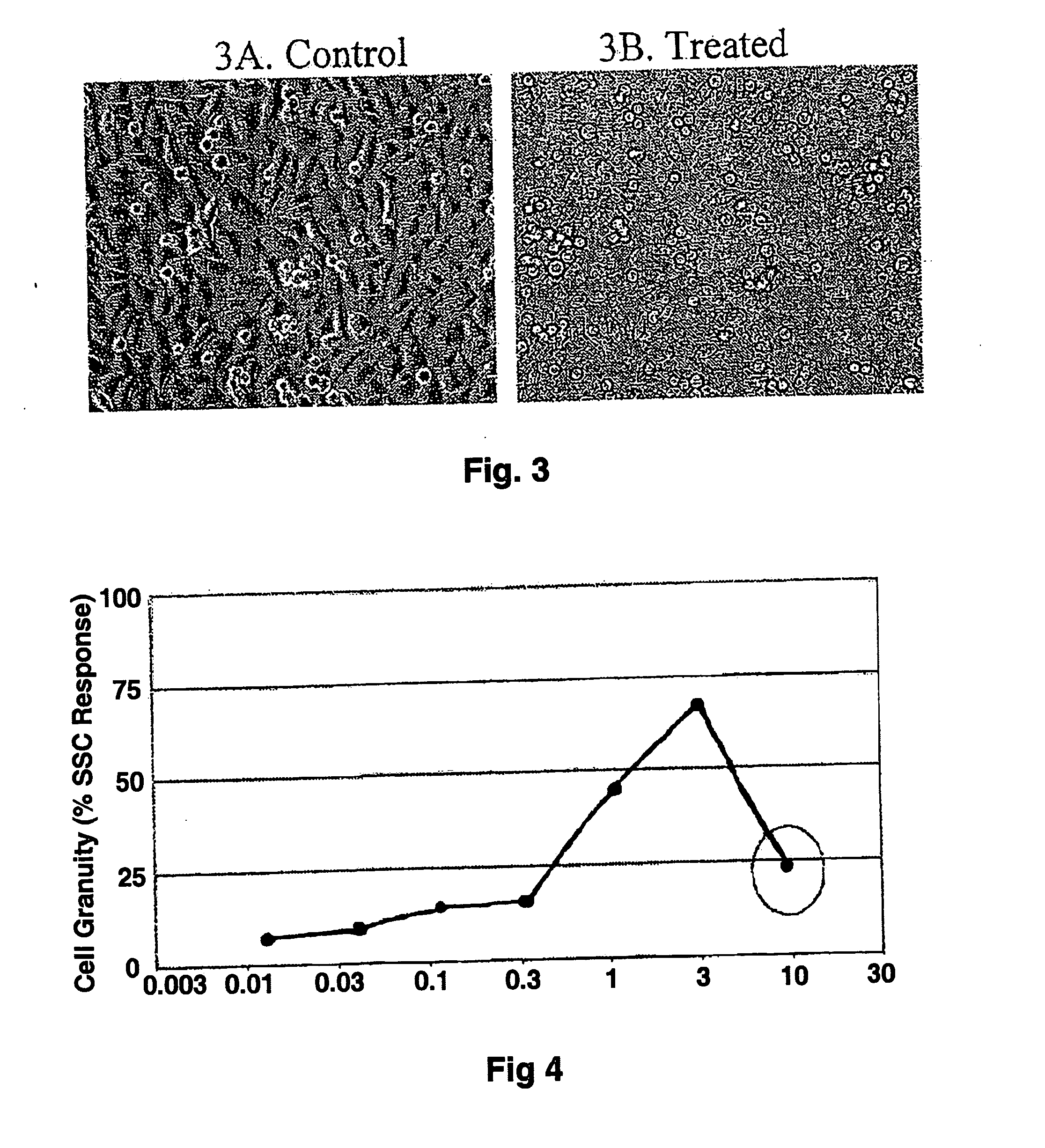
A method of treatment and / or prevention of cancer comprises administering agents which cause increased intracellular granularity in cancer cells, at least in an amount sufficient to inhibit proliferation of such cells and preferably in an amount sufficient to lead to cancer cell death. The method is particularly directed to refractory cancer, particularly hormone refractory prostate cancer. The agents identified cause increased intracellular granularity in the cancer cells, and also convert adherent cancer cells to non-adherent cancer cells, leading to cancer cell death. Using the present invention, cancer cells undergo increased intracellular granularity at relatively low agent concentrations, while also inhibiting cell proliferation. Increased concentrations lead to conversion of adherent cancer cells to non-adherent cancer cells, then to cell death. While the exact mechanism of cancer cell degradation and death is not completely understood, the treated cancer cells, including refractory prostate cancer cells, give indications of cell death through an autophagic mechanism. Pharmaceutical compositions related to the presently disclosed methods are also disclosed.
Owner:STC UNM
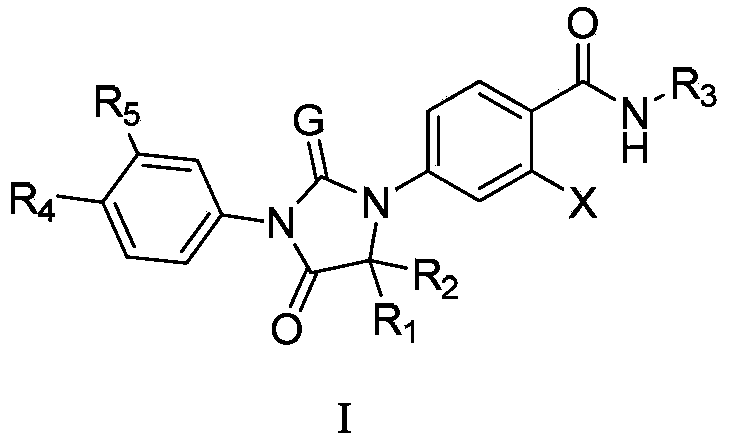
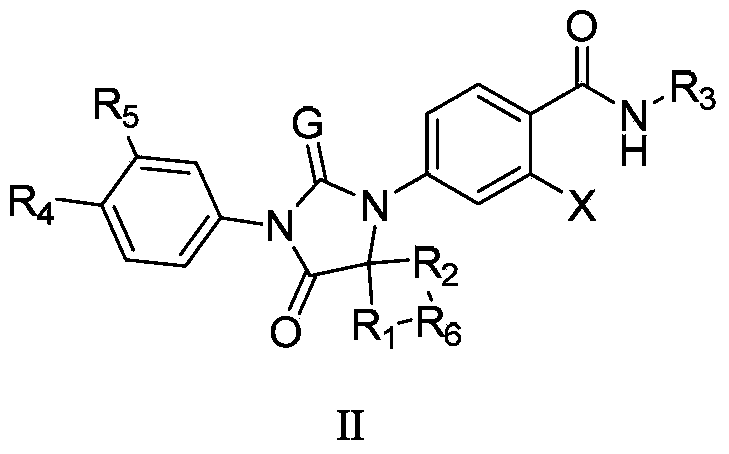
Diaryl hydantoin compound as androgen receptor antagonist and applications of diaryl hydantoin compound
InactiveCN104341352AOrganic active ingredientsOrganic chemistryHormone refractory prostate cancerNK1 receptor antagonist
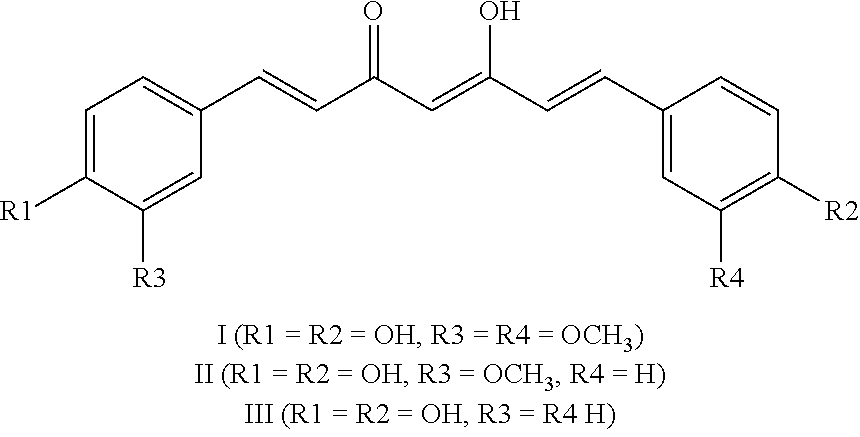
The invention relates to diaryl hydantoin compounds of general formula (I), and derivatives, pharmaceutically acceptable salts and solvates of the diaryl hydantoin compounds, as well as applications thereof in treatment of hormone refractory prostate cancer and breast cancer. The structural formula is as shown in the description, wherein R1-R5, G and X are as defined in the description.
Owner:NANJING HENGJIE BIOTECH
Prognostic Marker for Endometrial Carcinoma
InactiveUS20110217701A1Microbiological testing/measurementDisease diagnosisNon small lung cancerRegimen
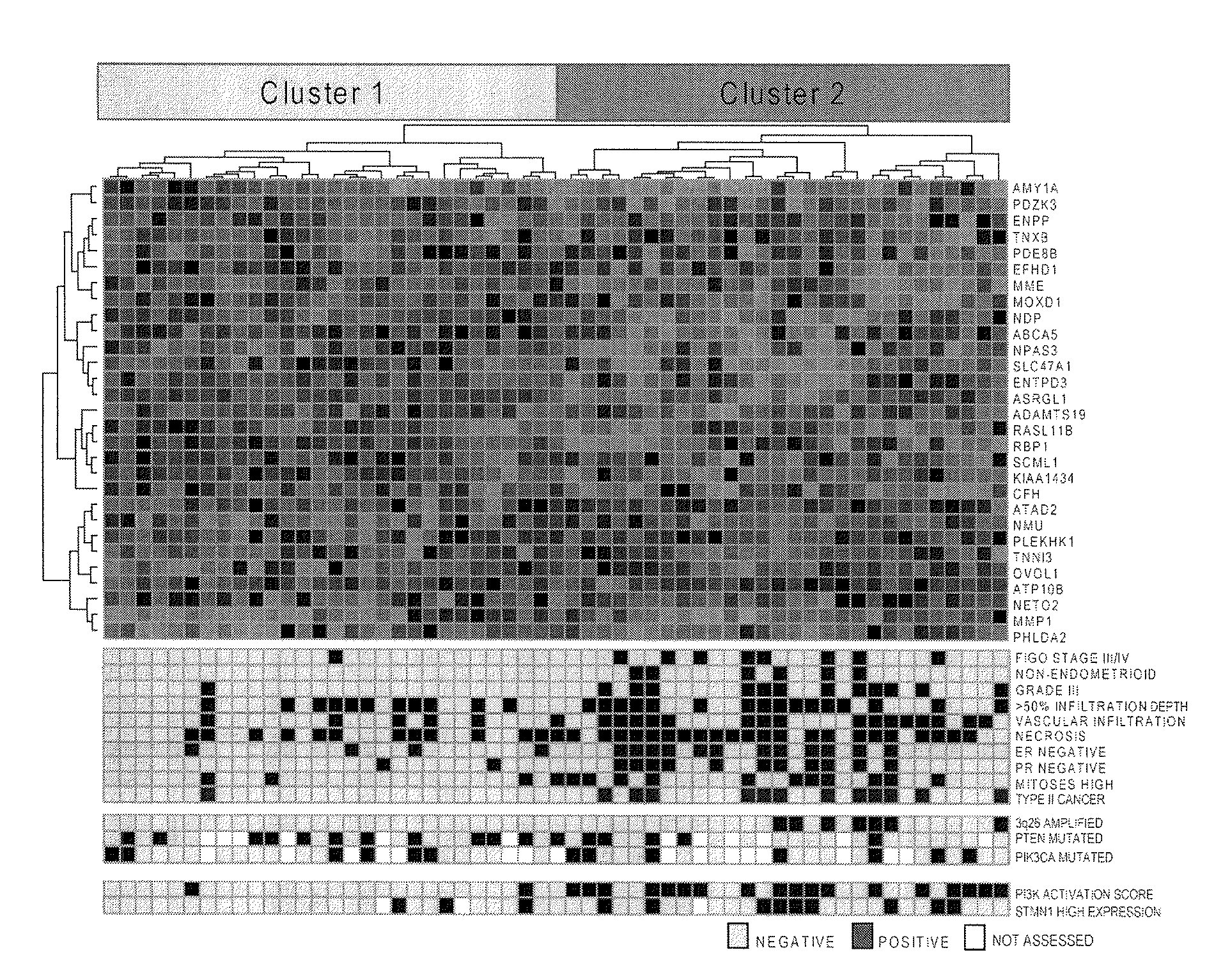
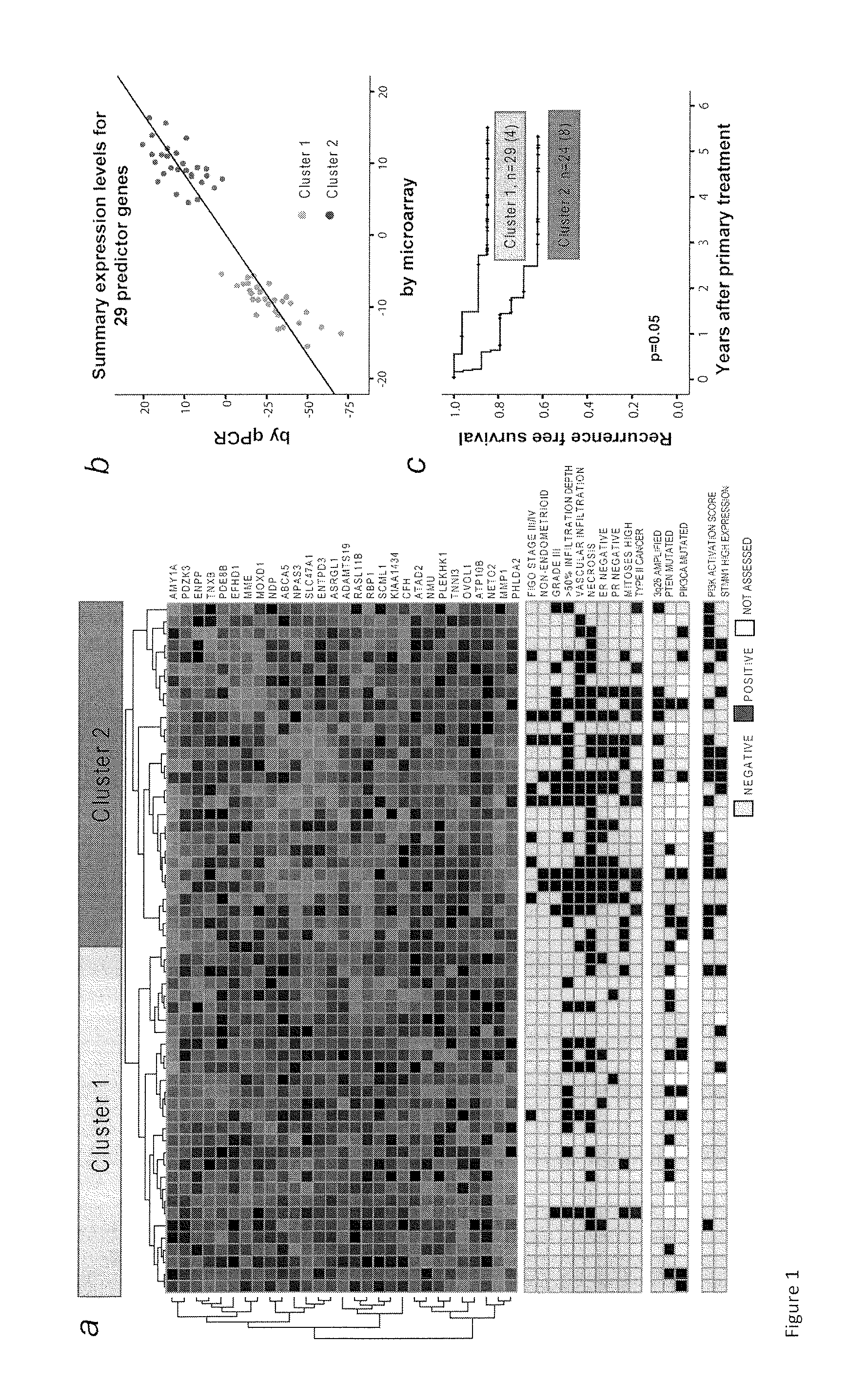
The present invention relates to a method for diagnosis of different stages of endometrial cancer in an individual. Further, the present invention relates to a method for evaluating the probability of survival for an individual suffering from endometrial carcinoma. In another aspect, the present invention relates to the stratification of therapy regimen of endometrial tumor, ovarian cancer, breast cancer, non-small lung cancer or hormone refractory prostate cancer therapy in an individual or monitoring therapeutic efficacy in an individual suffering from the same based on the expression status of STMN1 gene or protein. Moreover, the present invention relates to a kit for use in any of the above referenced methods comprising a means for determining amplifications and deletions of chromosomal regions 3q26.32 and 12p12.1, determining alterations of the gene expression profile of the genes (gene signature): upregulation of the genes PLEKHK1, ATP10B, NMU, MMP1, ATAD2, NETO2, TNNI3, PHLDA2, OVOL1 and down-regulation of the genes: NDP, KIAA1434, MME, CFH, MOXD1, SLC47A1, RBP1, PDE8B, ASRGL1, ADAMTS19, EFHD1, ABCA5, NPAS3, SCML1, TNXB, ENTPD3, AMY1A, ENPP, RASL11B, PDZK3, or the expression status of the STMN1 gene or protein, respectively. Finally, the present invention provides a method for predicting the response to taxanes in an individual suffering from a disease treated with the taxanes based on the expression status of the STMN1 gene or protein.
Owner:BERGEN TEKNOLOGIOVERFORING
Diarylhydantoin compounds
ActiveCN101222922BOrganic active ingredientsOrganic chemistryMedicineHormone refractory prostate cancer
The present invention relates to diarylhydantoin compounds, including diarylthiohydantoins, and methods for syntheszing them and using them in the treatment of hormone refractory prostate cancer.
Owner:RGT UNIV OF CALIFORNIA
Compounds and methods for inducing apoptosis in proliferating cells
InactiveUS20060142368A1Treating and inhibiting and delaying onsetBiocideOrganic active ingredientsMelanomaApoptosis
Compounds useful for inducing apoptosis in proliferative cells, particularly cancer cells, including but not limited to prostate cancer, leukemia, non-small cell lung cancer, colon cancer, CNS cancer, melanoma, ovarian cancer, renal cancer, bladder cancer, lymphoma, and breast cancer. These compounds are particularly useful in the treatment of androgen-independent cancers, including hormone-refractory prostate cancer. Further provided are methods of treating cancer in a subject in need of such treatment using the compounds of the present invention. Further provided are methods for using the compounds of the present invention to treat, inhibit, or delay the onset of cancer in a subject. Further provided are methods of inducing apoptosis in rapidly proliferating cells, particularly, though not necessarily cancer cells, using the compounds of the present invention.
Owner:THE OHIO STATE UNIV RES FOUND
Methods and pharmaceutical compositions for the treatment of hormone-refractory prostate cancers
The present invention relates to a combination of a curcuminoid and a taxane for use in the treatment of a hormone-refractory prostate cancer (HRPC) in a patient in need thereof.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
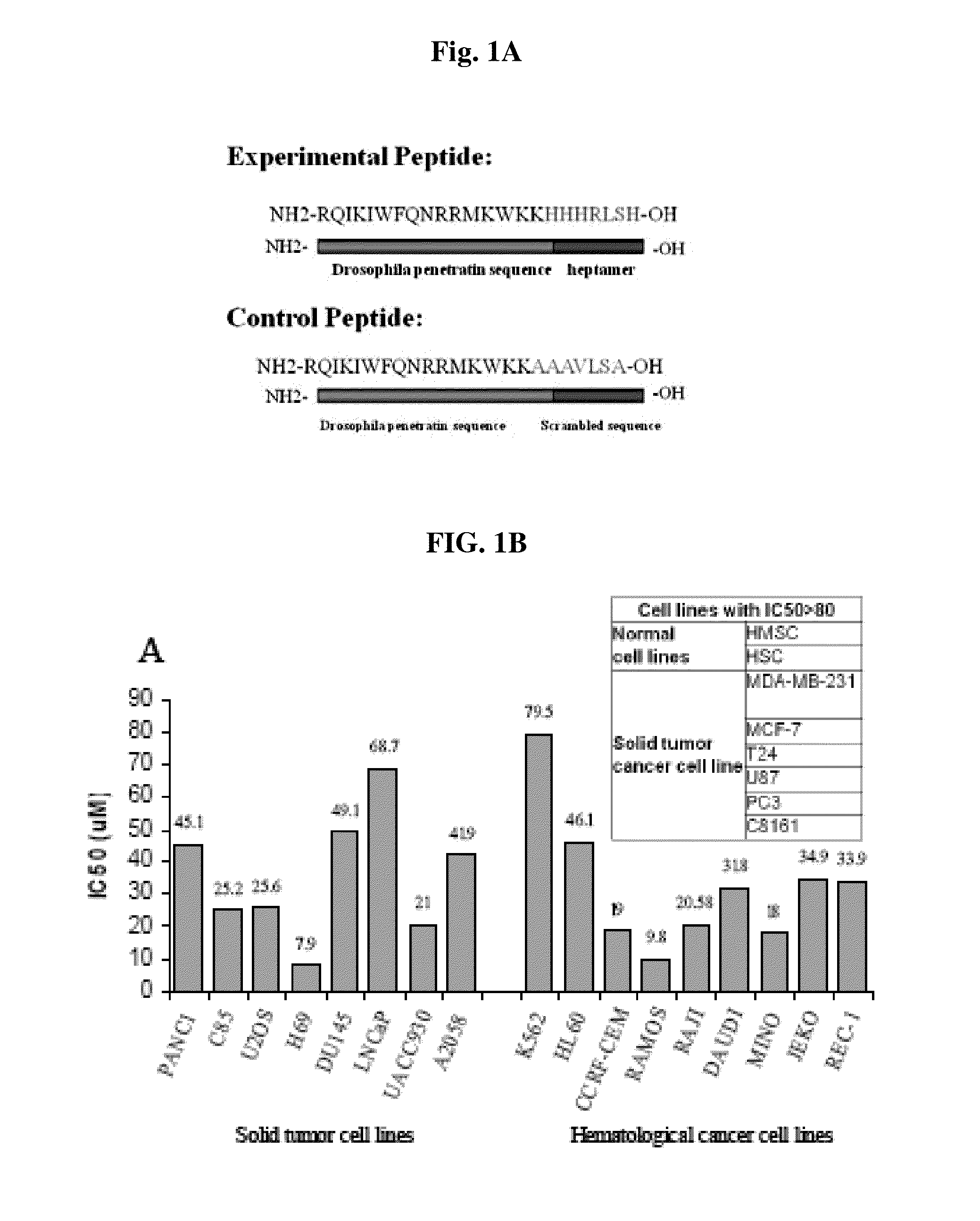
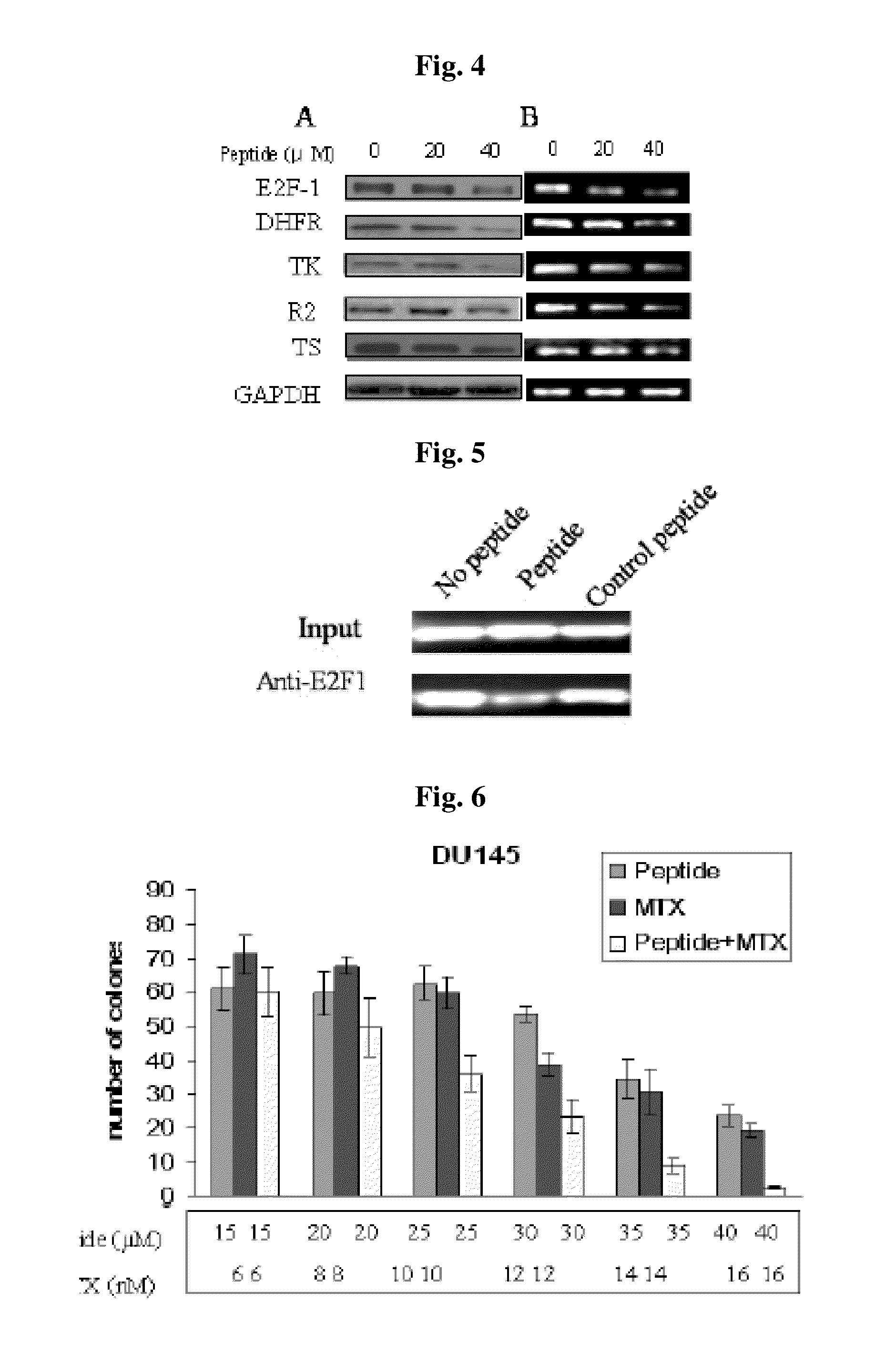
E2f as a target of hormone refractory prostate cancer
InactiveUS20120093919A1Useful in treatmentOrganic active ingredientsFungiHormone refractory prostate cancerDNA
The instant invention provides amino acid sequences competing with E2F for DNA binding. Methods of using said amino acid sequences for treatment of hormone-refractory prostate cancer are also provided.
Owner:RUTGERS THE STATE UNIV
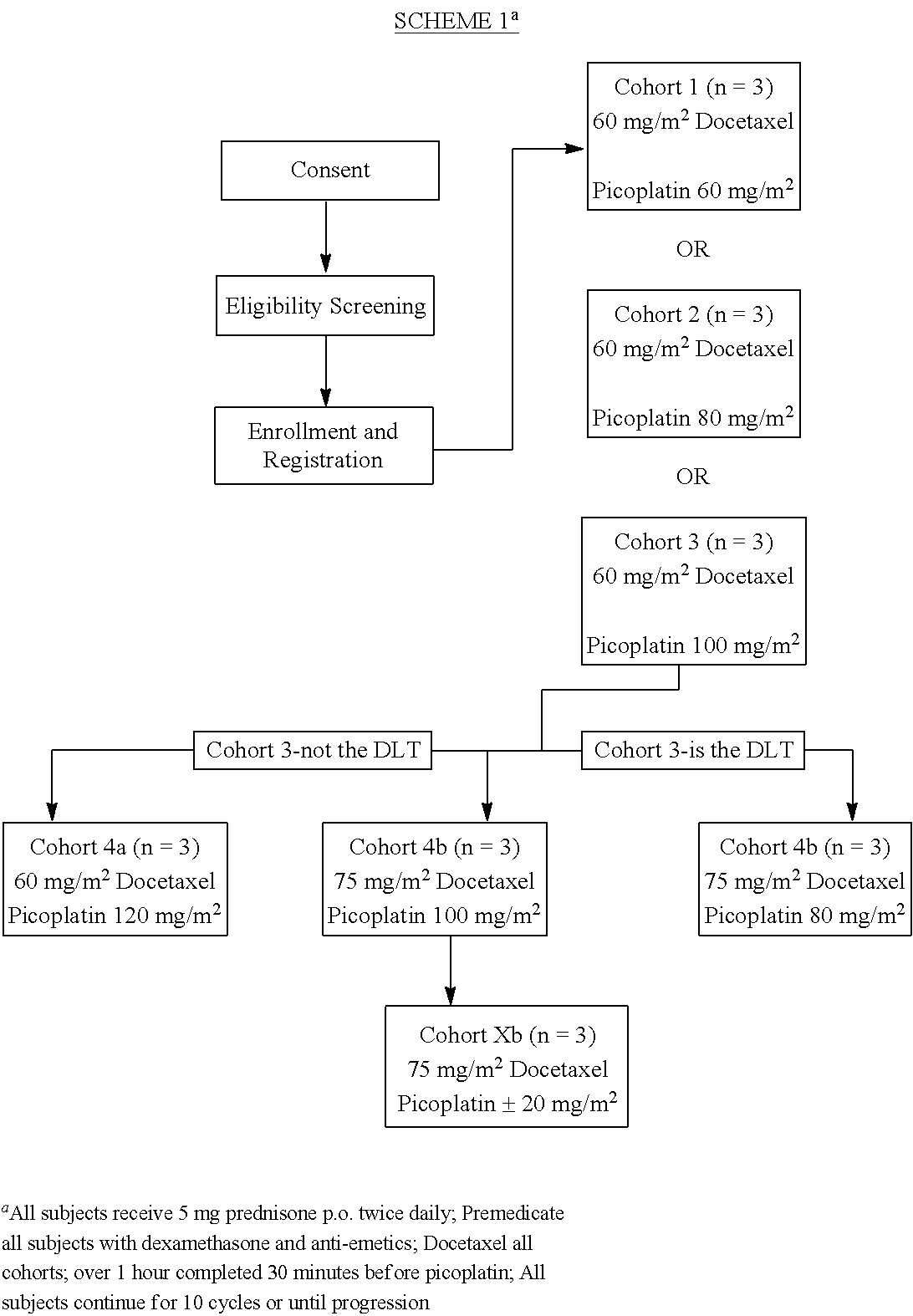
Use of picoplatin to treat prostate cancer
InactiveUS20120122825A1Improve the quality of lifeLower Level RequirementsBiocideOrganic active ingredientsRegimenDocetaxel-PNP
The invention provides a method of treatment of metastatic hormone-refractory prostate cancer involving substantially as concurrent administration of picoplatin and docetaxel. Prednisone may also be administered. Dosages and dosing regimens are provided.
Owner:SCHWEGMAN LUNDBERG& WOESSNER P A
Compositions and methods of use of epb1, and erbb3 binding protein
InactiveUS20080269133A1Facilitating cessationHighly effectiveBiocidePeptide/protein ingredientsProstate cancer cellRadical radiotherapy
Inhibition of the proliferation of hormone refractory prostate cancer cells is achieved by administering EPB1, an ErbB3 binding protein, in combination with another anti-proliferation therapy such as administration of antiandrogens, other anticancer agents, radiation therapy, or surgery. Administration of EPB1 reverses the phenotype of hormone-resistant prostate cancer cells to hormone-sensitive prostate cancer cells.
Owner:UNIV OF MARYLAND
Methods and related compositions for the treatment of cancer
A method of treatment and / or prevention of cancer comprises administering agents which cause increased intracellular granularity in cancer cells, at least in an amount sufficient to inhibit proliferation of such cells and preferably in an amount sufficient to lead to cancer cell death. The method is particularly directed to refractory cancer, particularly hormone refractory prostate cancer. The agents identified cause increased intracellular granularity in the cancer cells, and also convert adherent cancer cells to non-adherent cancer cells, leading to cancer cell death. Using the present invention, cancer cells undergo increased intracellular granularity at relatively low agent concentrations, while also inhibiting cell proliferation. Increased concentrations lead to conversion of adherent cancer cells to non-adherent cancer cells, then to cell death. While the exact mechanism of cancer cell degradation and death is not completely understood, the treated cancer cells, including refractory prostate cancer cells, give indications of cell death through an autophagic mechanism. Pharmaceutical compositions related to the presently disclosed methods are also disclosed.
Owner:STC UNM
Diarylhydantoin compounds
InactiveCN102584712AOrganic active ingredientsOrganic chemistryMedicineHormone refractory prostate cancer
The present invention relates to diarylhydantoin compounds, including diarylthiohydantoins, and methods for syntheszing them and using them in the treatment of hormone refractory prostate cancer.
Owner:RGT UNIV OF CALIFORNIA
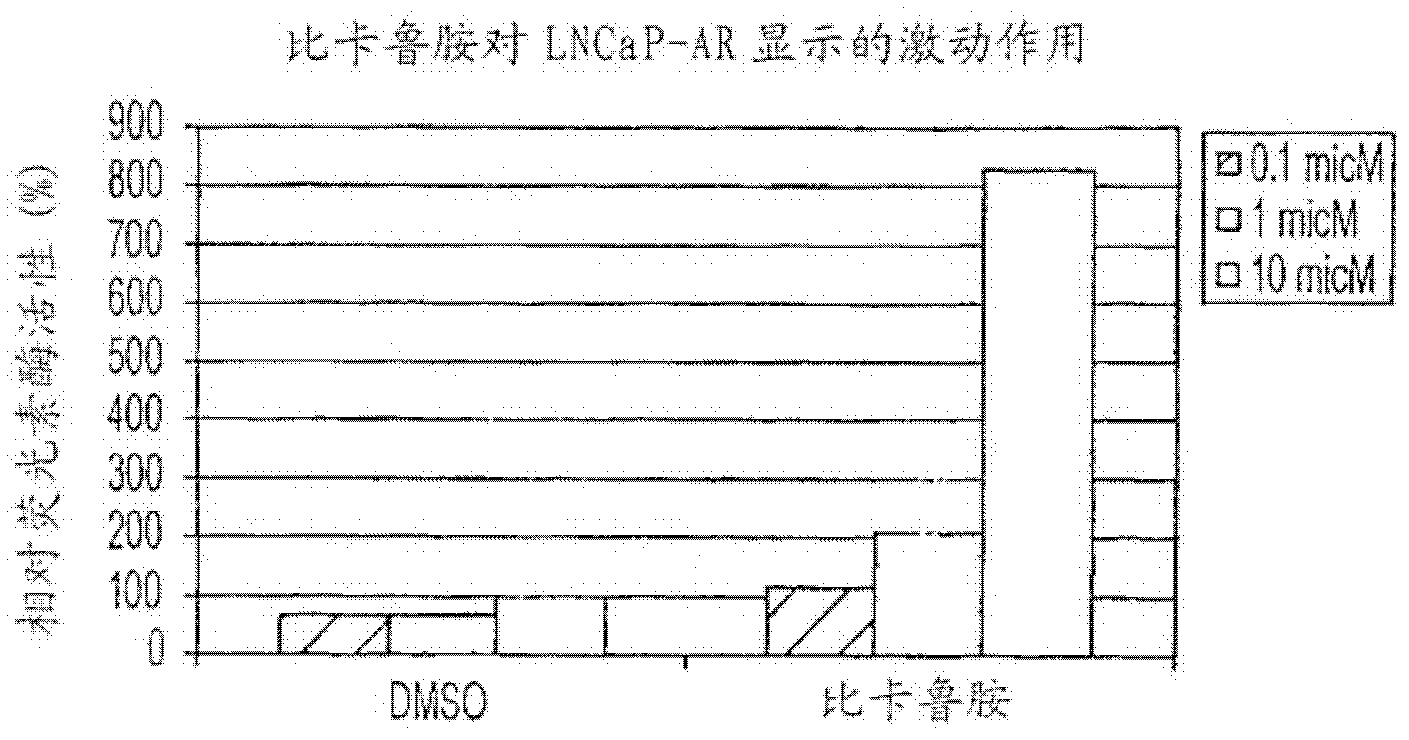
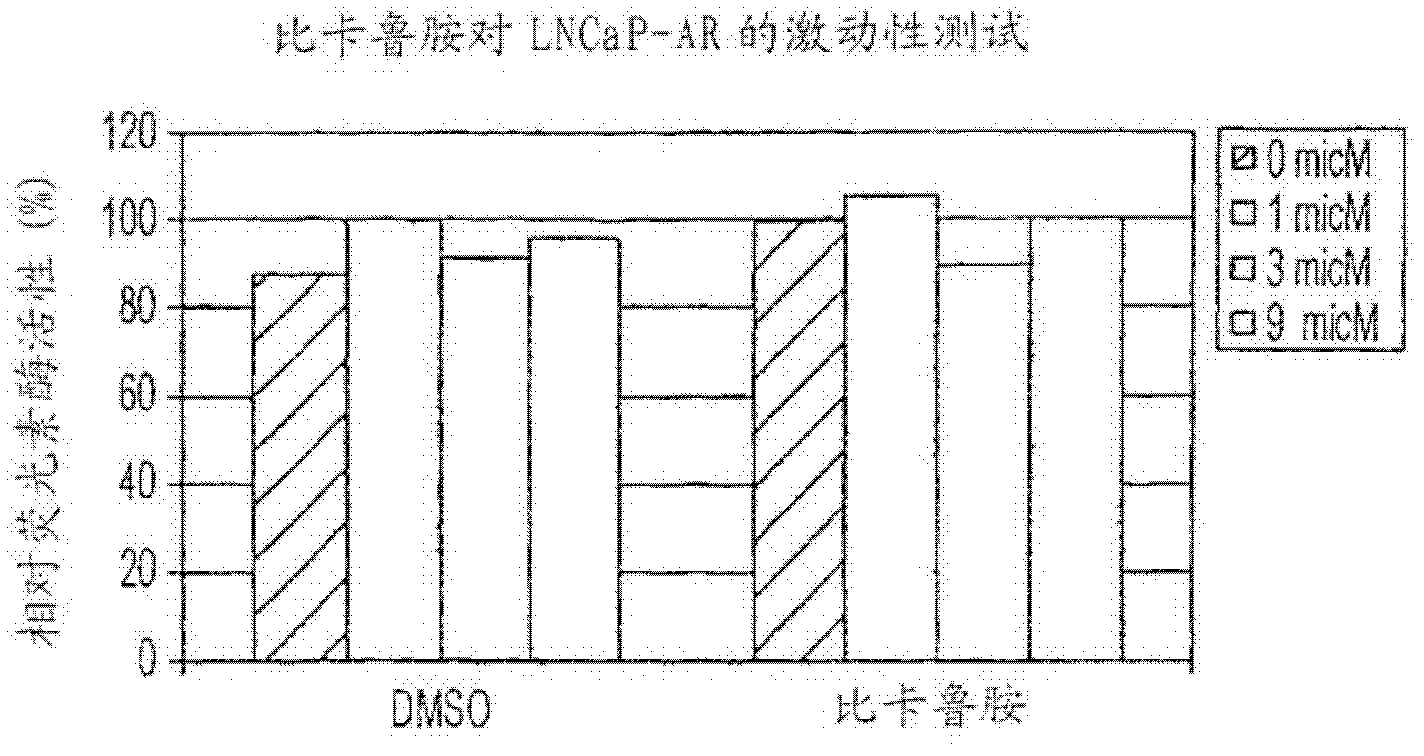
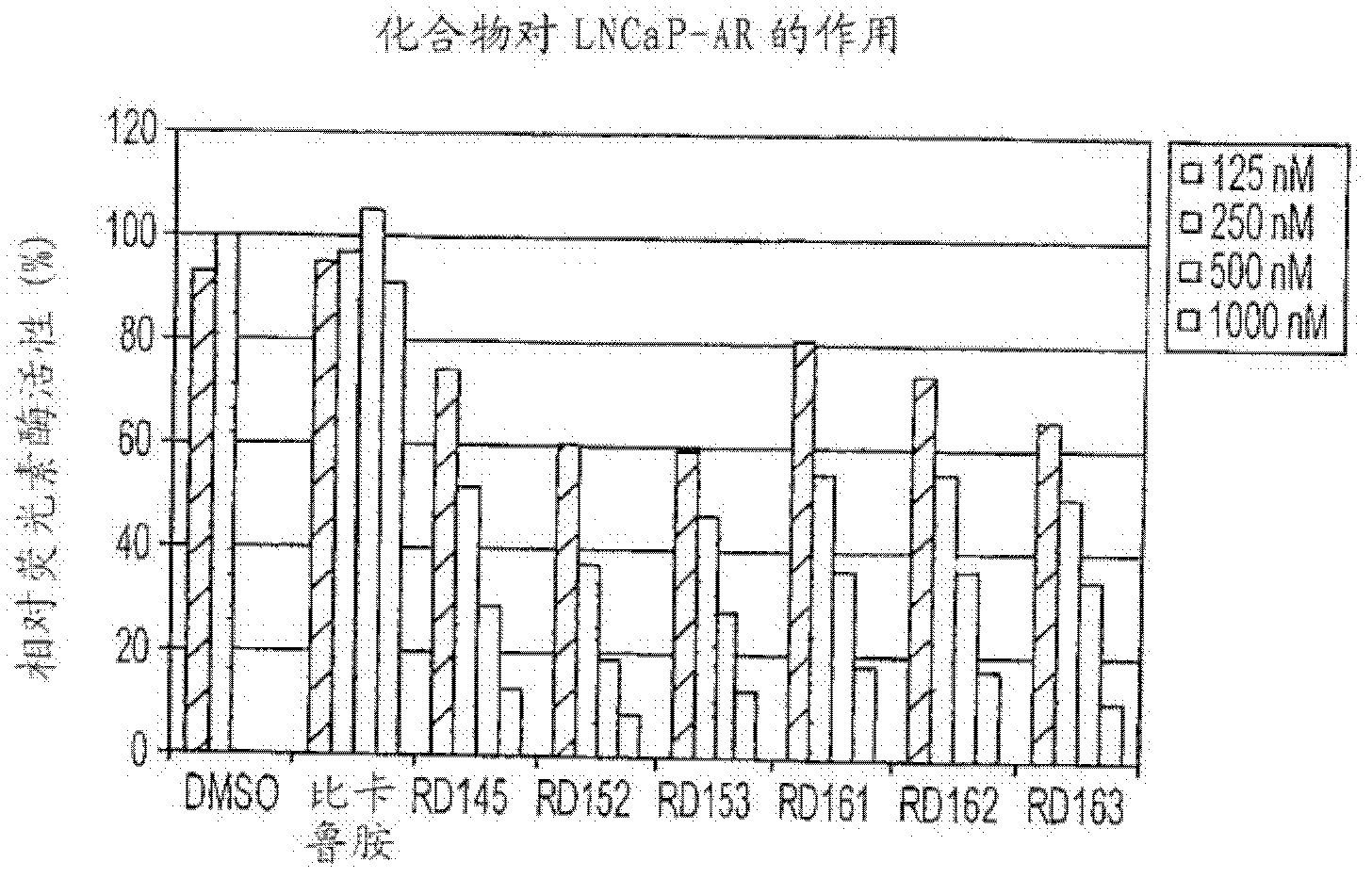
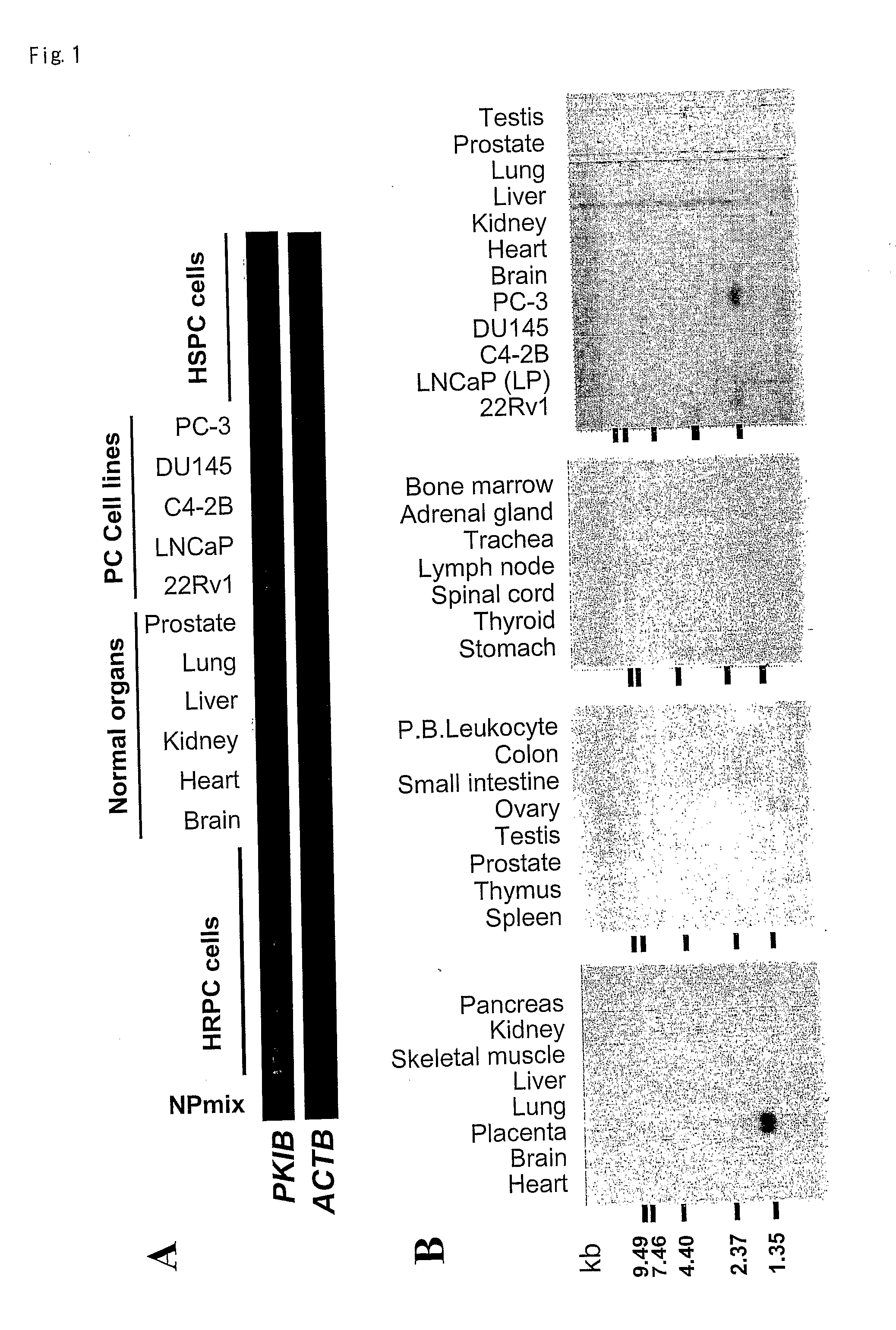
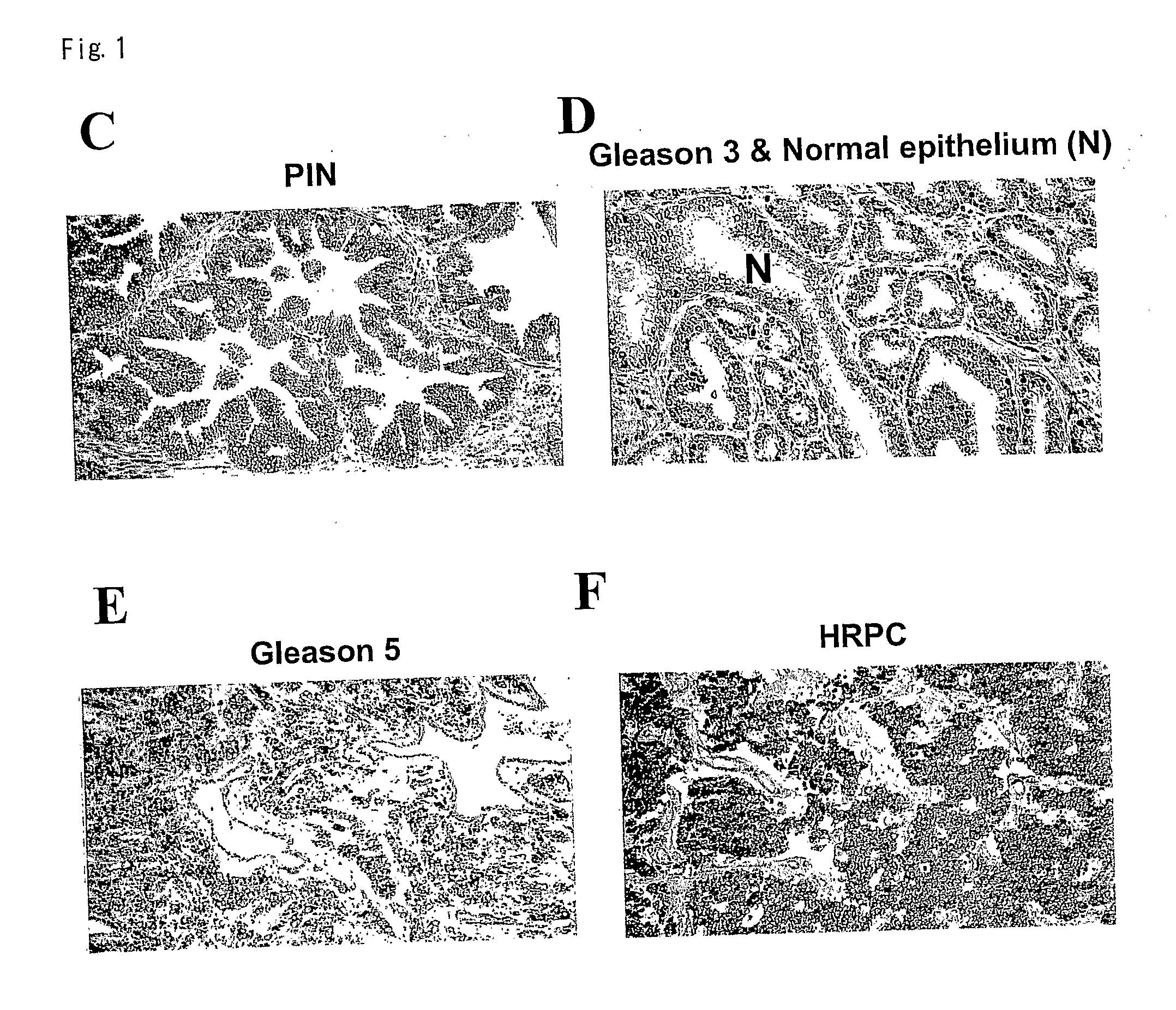
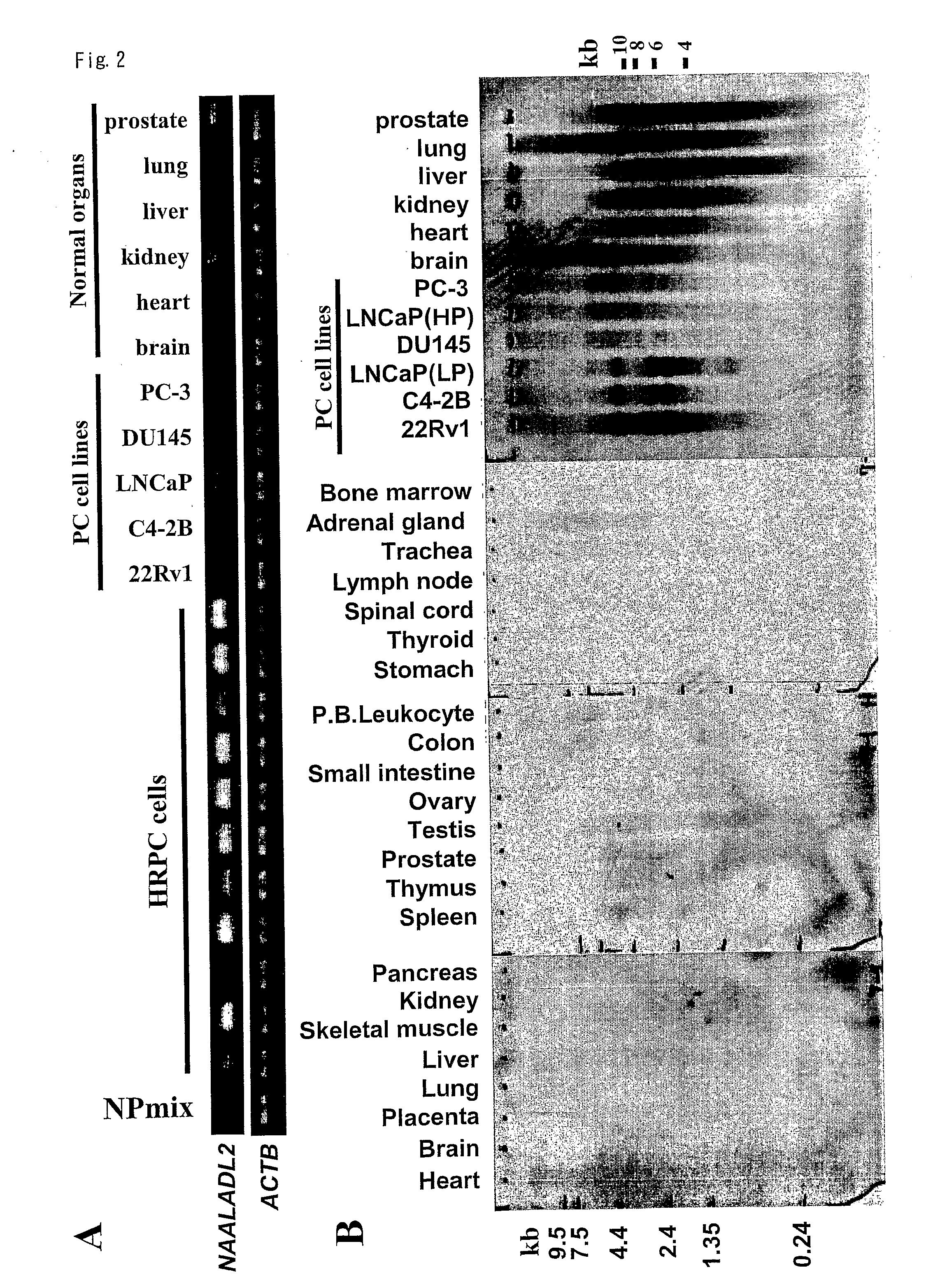
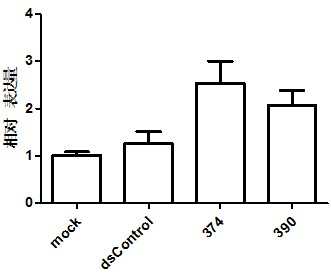
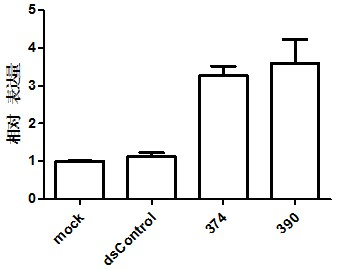
PKIB and NAALADL2 for Target Genes of Prostate Cancer Therapy and Diagnosis
InactiveUS20120022128A1Inhibit cell growthReduced expression levelGenetic material ingredientsLibrary screeningHormone refractory prostate cancerCastration resistant
The invention features methods for detecting prostate cancer, especially hormone-refractory prostate cancer (HRPC) or castration-resistant prostate cancer (CRPC), by detecting over-expression of PKIB or NAALADL2 compared the normal organs. Also disclosed are methods of identifying compounds for treating and preventing prostate cancer including HRPC, based on the over-expression of PKIB or NAALADL2 in the prostate cancer, the cell proliferation function of PKIB or NAALADL2, the intracellular localization of PKIB or NAALADL2 or the interaction between PKIB and PKA-C. Also, provided are a method for treating prostate cancer by administering a double-stranded molecule against the PKIB or NAALADL2 gene. The invention also provides products, including the double-stranded molecules and vectors encoding them, as well as compositions comprising the molecules or vectors, useful in the provided methods.
Owner:ONCOTHERAPY SCI INC
Marker for carcinoma
InactiveUS20130267440A1Microbiological testing/measurementLibrary screeningNon small lung cancerRegimen
The present invention relates to a method for diagnosis of different stages of endometrial cancer in an individual. Further, the present invention relates to a method for evaluating the probability of survival for an individual suffering from endometrial carcinoma. In another aspect, the present invention relates to the stratification of therapy regimen of endometrial tumor, ovarian cancer, breast cancer, non-small lung cancer or hormone refractory prostate cancer therapy in an individual or monitoring therapeutic efficacy in an individual suffering from the same based on the expression status of STMN1 gene or protein. Moreover, the present invention relates to a kit for use in any of the above referenced methods comprising a means for determining amplifications and deletions of chromosomal regions 3q26.32 and 12p12.1, determining alterations of the gene expression profile of the genes (gene signature): upregulation of the genes PLEKHK1, ATP10B, NMU, MMP1, ATAD2, NETO2, TNNI3, PHLDA2, OVOL1 and down-regulation of the genes: NDP, KIAA1434, MME, CFH, MOXD1, SLC47A1, RBP1, PDE8B, ASRGL1, ADAMTS19, EFHD1, ABCA5, NPAS3, SCML1, TNXB, ENTPD3, AMY1A, ENPP, RASL11B, PDZK3, or the expression status of the STMN1 gene or protein, respectively. Finally, the present invention provides a method for predicting the response to taxanes in an individual suffering from a disease treated with the taxanes based on the expression status of the STMN1 gene or protein.
Owner:BERGEN TEKNOLOGIOVERFORING AS
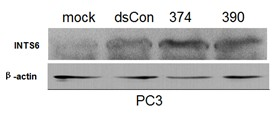
Application of small activating RNA (Ribonucleic Acid) of INTS6 (Homo Sapiens Integrator Complex Subunit 6) gene to preparation of prostate cancer fighting medicament
InactiveCN102657878AEasy to operateLow costGenetic material ingredientsAntineoplastic agentsProstate cancer cellIntegrator Complex Subunit 6
The invention provides application of small RNA (Ribonucleic Acid) of a targeted up-regulation INTS6 (Homo Sapiens Integrator Complex Subunit 6) gene to preparation of a hormone refractory prostate cancer fighting medicament. A nucleotide sequence of the small RNA consists of four pieces of positive-sense strands and anti-sense strands of 21 nucleotides; two nucleotides, which are generally dTdT, are overhung at the end 3' of each strand; and the middle 19 nucleotides are paired. According to the application, the expression of INTS6 gene in a hormone refractory prostate cancer cell is induced by the small double-stranded RNA of a specific sequence, so that the proliferation capacity and the movement capacity of tumor cells are inhibited, and the aim of inhibiting tumor growth and development is fulfilled. The small RNA is easy to operate, low in cost and less in using amount, and a good activation effect can be achieved with 20 nM transfection concentration; and the activation effect is clear, and the target gene mRNA (micro Ribonucleic Acid) level and the protein level are both raised. In addition, the saRNA (small activating RNA) induced gene expression belongs to apparent genetic regulation, the integrity of a genome is not damaged, and thus the application is safe.
Owner:ZHEJIANG UNIV
E2F as a target of hormone refractory prostate cancer
InactiveUS8822421B2Organic active ingredientsPeptide/protein ingredientsHormone refractory prostate cancerDNA
The instant invention provides amino acid sequences competing with E2F for DNA binding. Methods of using said amino acid sequences for treatment of hormone-refractory prostate cancer are also provided.
Owner:RUTGERS THE STATE UNIV
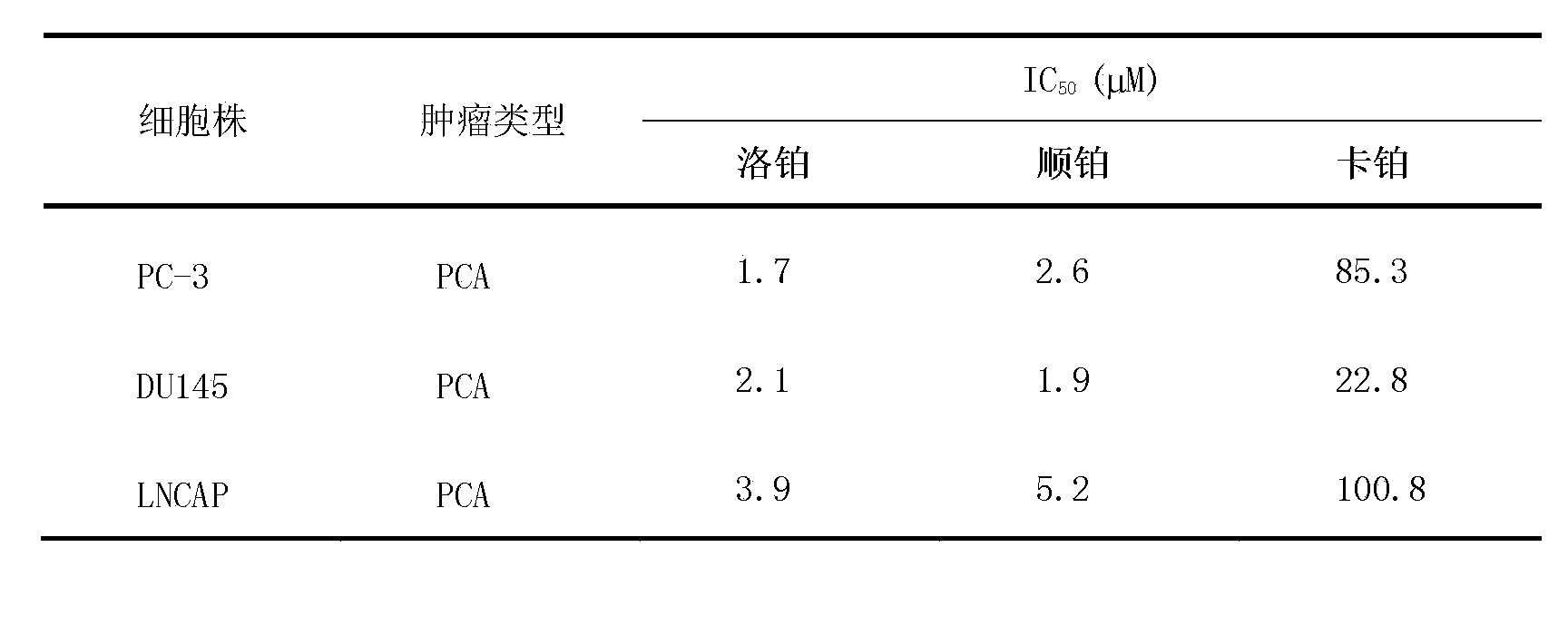
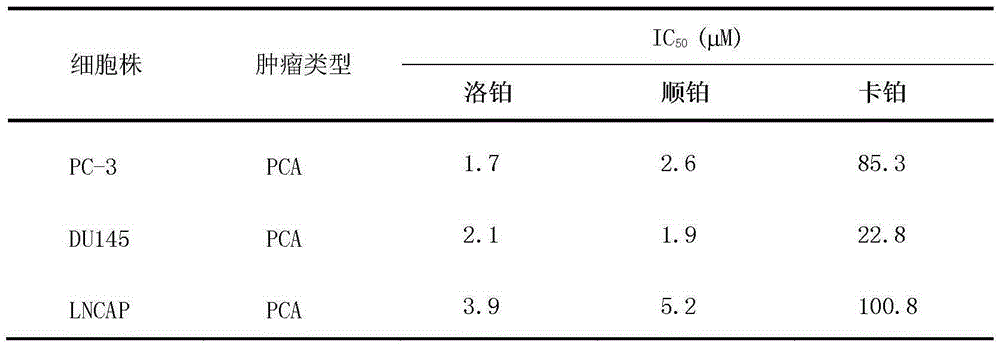
Application of lobaplatin in preparation of drugs used for treating prostate cancer
ActiveCN103505450AAntineoplastic agentsHeavy metal compound active ingredientsHormone refractory prostate cancerHormone dependence
The invention relates to application of lobaplatin in preparation of drugs used for treating prostate cancer, especially to application of lobaplatin in preparation of drugs used for treating hormone refractory prostate cancer. An effective therapeutic dose of lobaplatin is 20 to 50 mg per square of a body surface area. According to the invention, a novel therapeutic drug is provided for treatment of prostate cancer, and clinical application of lobaplatin and a preparation thereof is broadened.
Owner:GUIZHOU YIBAI PHARMA CO LTD
Application of lobaplatin in the preparation of drugs for treating prostate cancer
ActiveCN103505450BAntineoplastic agentsHeavy metal compound active ingredientsHormone refractory prostate cancerHormone dependence
The present invention relates to the application of lobaplatin in the preparation of drugs for treating prostate cancer, especially the application in the preparation of drugs for treating non-hormone-dependent prostate cancer. The effective therapeutic dose of lobaplatin is 20-50 mg / m2 body surface area. The present invention It provides new therapeutic drugs for prostate cancer and expands the clinical application of lobaplatin and its preparations.
Owner:GUIZHOU YIBAI PHARMA CO LTD
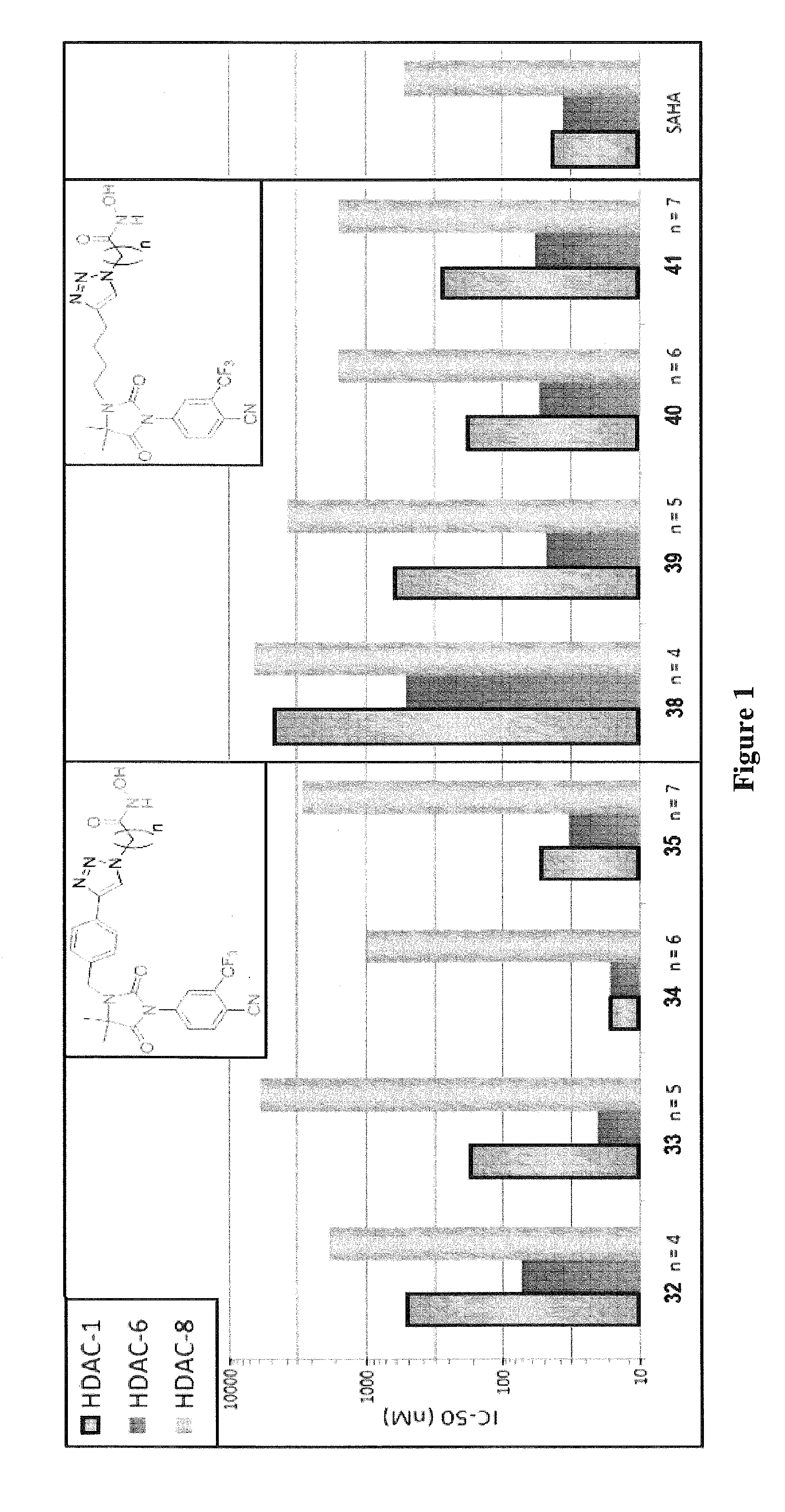
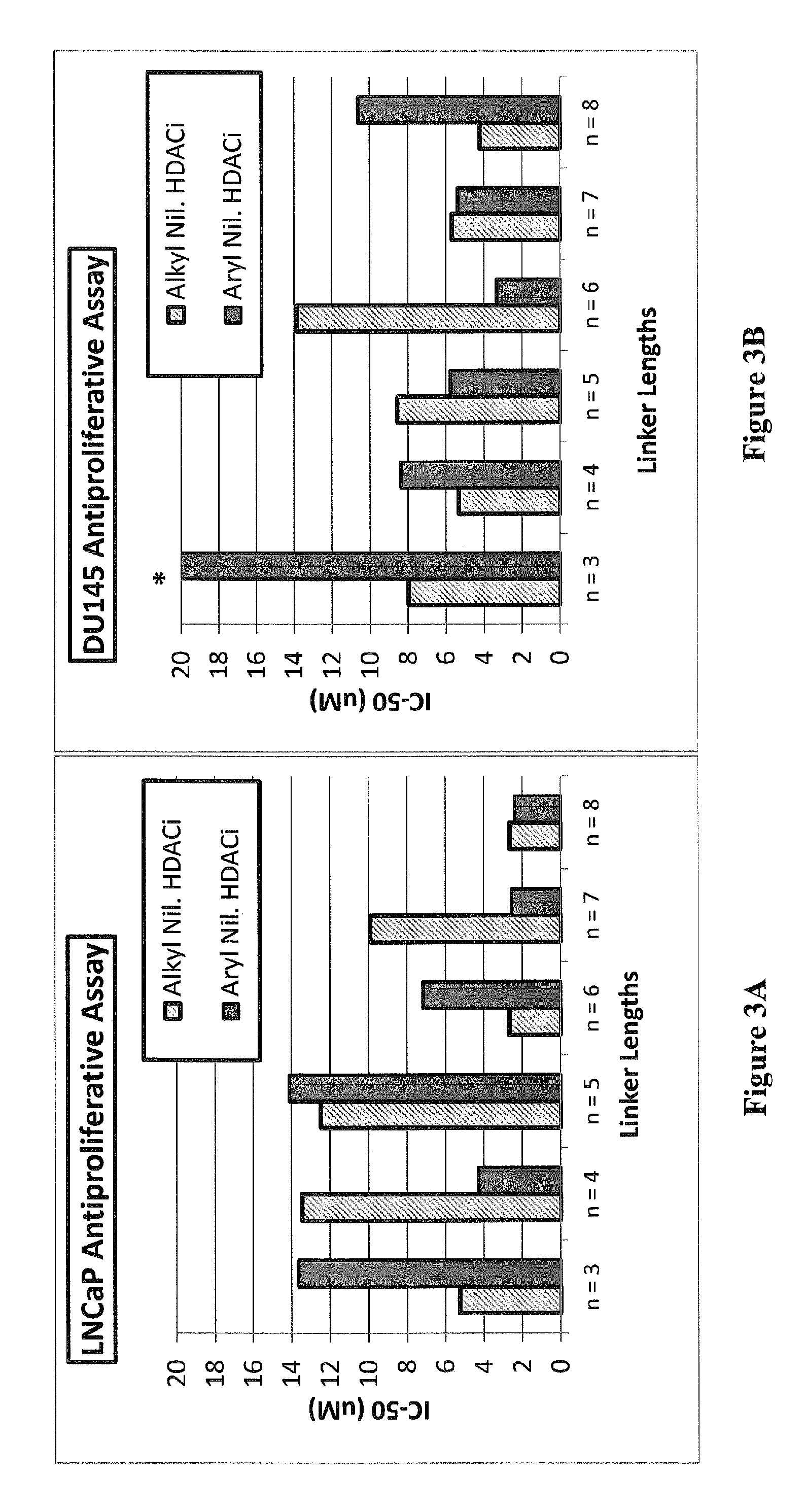
Histone deacetylase (HDAC) inhibitors targeting prostate tumors and methods of making and using thereof
Compounds of Formula (I), and methods of making and using thereof, are described herein; wherein AR is an aryl group, ZBG is a Zinc Binding Group, and other substituents are as defined herein. The compounds can be administered as a pharmaceutically acceptable salt, prodrug, or solvate. The compounds may be useful to treat and / or prevent hyperproliferative disorders which may include hormone sensitive and hormone refractory prostate cancers. The compounds can be formulated with a pharmaceutically acceptable carrier and, optionally one or more pharmaceutically acceptable excipients, for enteral or parenteral administration.
Owner:GEORGIA TECH RES CORP
Diarylhydantoin compounds
The present invention relates to diarylhydantoin compounds and methods for synthesizing them and using them in the treatment of hormone refractory prostate cancer.
Owner:RGT UNIV OF CALIFORNIA
Gastrin Releasing Peptide Compounds
InactiveUS20090175786A1Improve targetingDecreasing aberrant vascular permeabilityPeptide/protein ingredientsRadioactive preparation carriersGastrin-releasing peptideImaging agent
New and improved compounds for use in diagnostic imaging or therapy having the formula M-N—O—P-G, wherein M is a metal chelator having the structure:wherein R1-R5 and FG are as defined herein (in the form complexed with a metal radionuclide or not), N—O—P is the linker containing at least one non-alpha amino acid with a cyclic group, at least one substituted bile acid or at least one non-alpha amino acid, and G is the GRP receptor targeting peptide. In the preferred embodiment, M is an Aazta metal chelator or a derivative thereof.Methods for imaging a patient and / or providing radiotherapy or phototherapy to a patient using the compounds of the invention are also provided. Methods and kits for preparing a diagnostic imaging agent from the compound is further provided. Methods and kits for preparing a radiotherapeutic agent are further provided.Novel methods of treating prostate tumors or of delaying the progression of prostate tumors are also provided, including, methods of treating bone or soft tissue metastases of prostate cancer, methods for treating hormone sensitive and hormone refractory prostate cancer, methods for delaying the progression of hormone sensitive prostate cancer, for facilitating combination therapy in patients with hormone sensitive prostate cancer and for decreasing aberrant vascular permeability in patients with hormone sensitive prostate cancer.
Owner:BRACCO IMAGINIG SPA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com