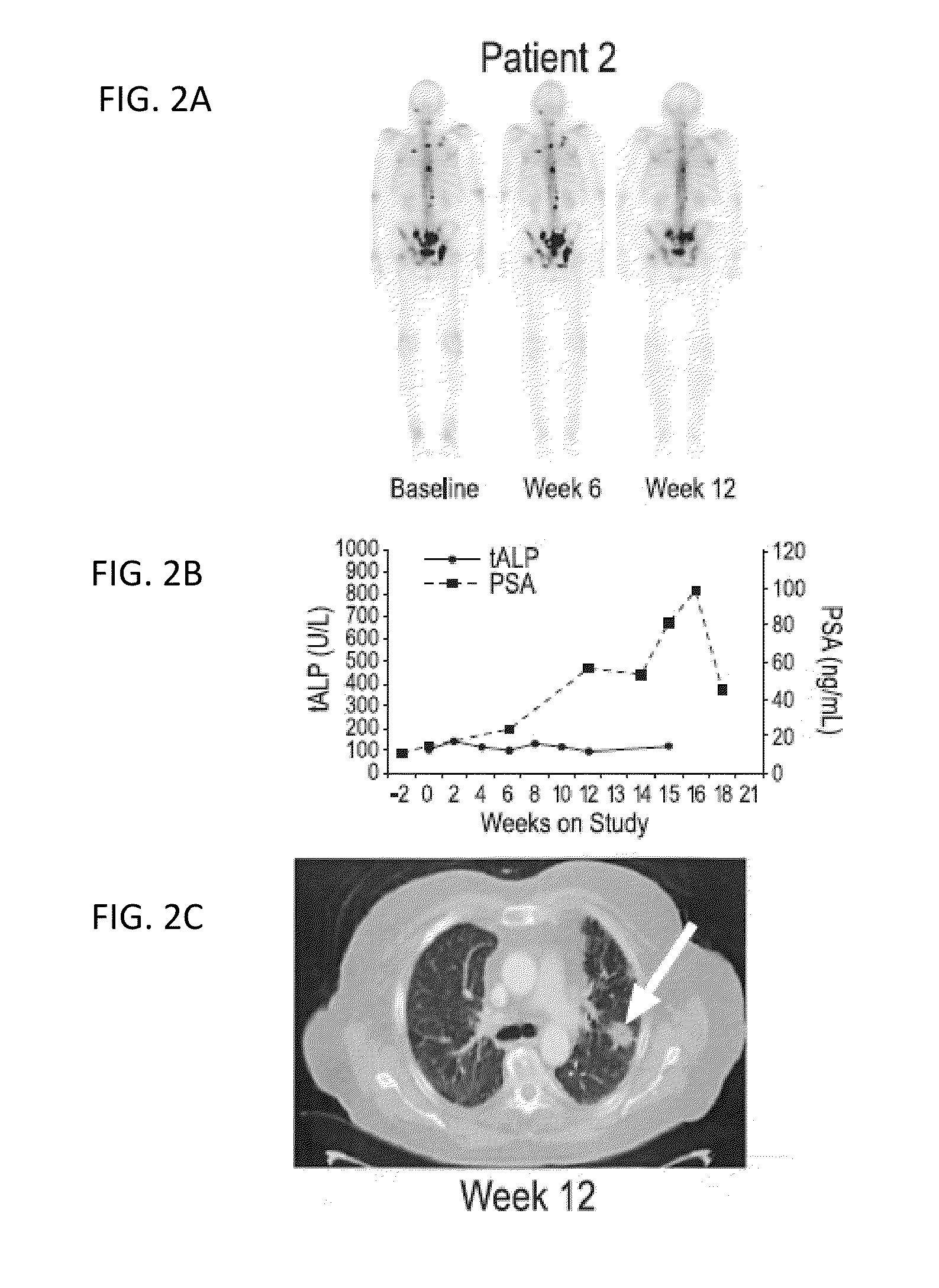
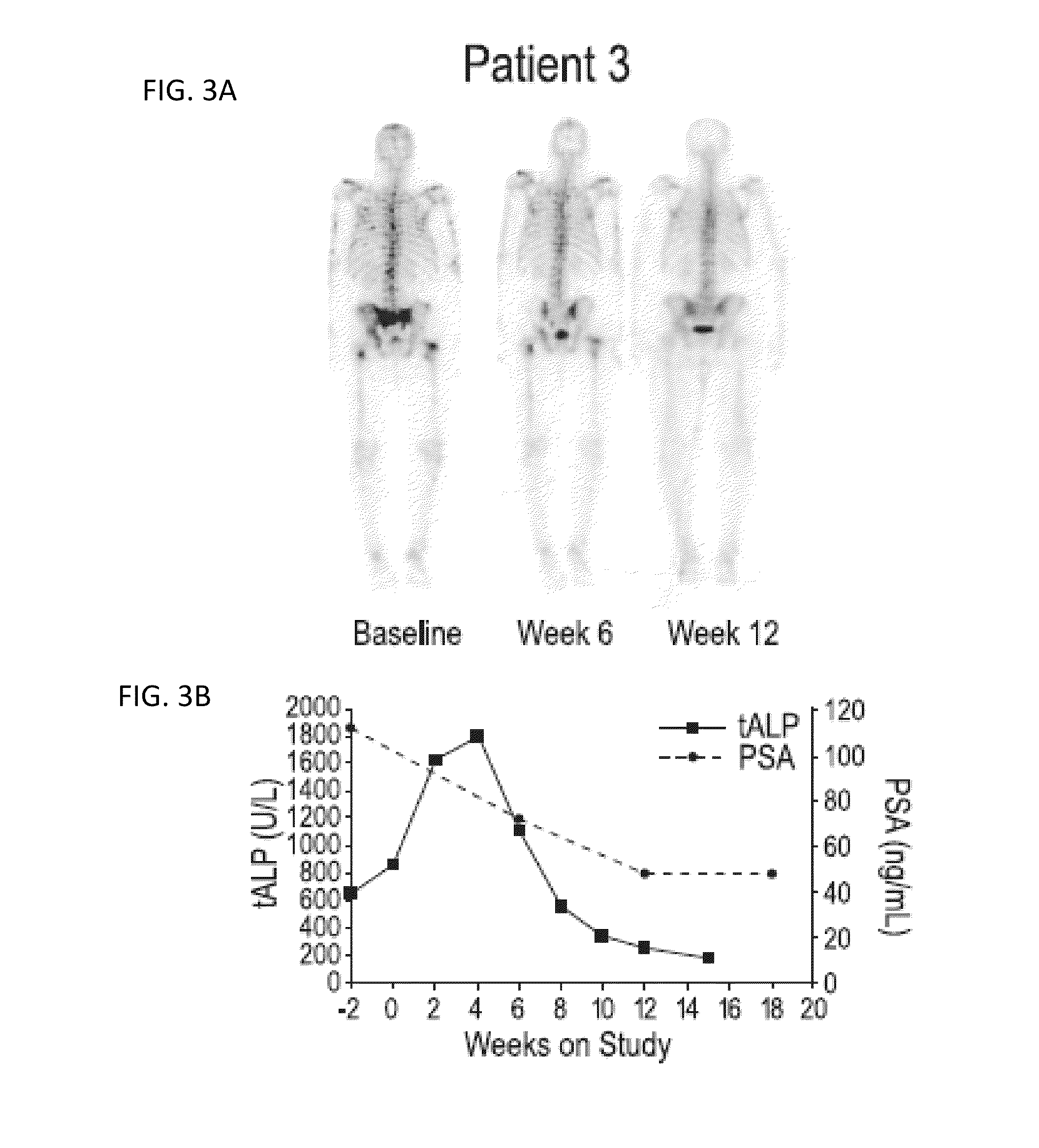
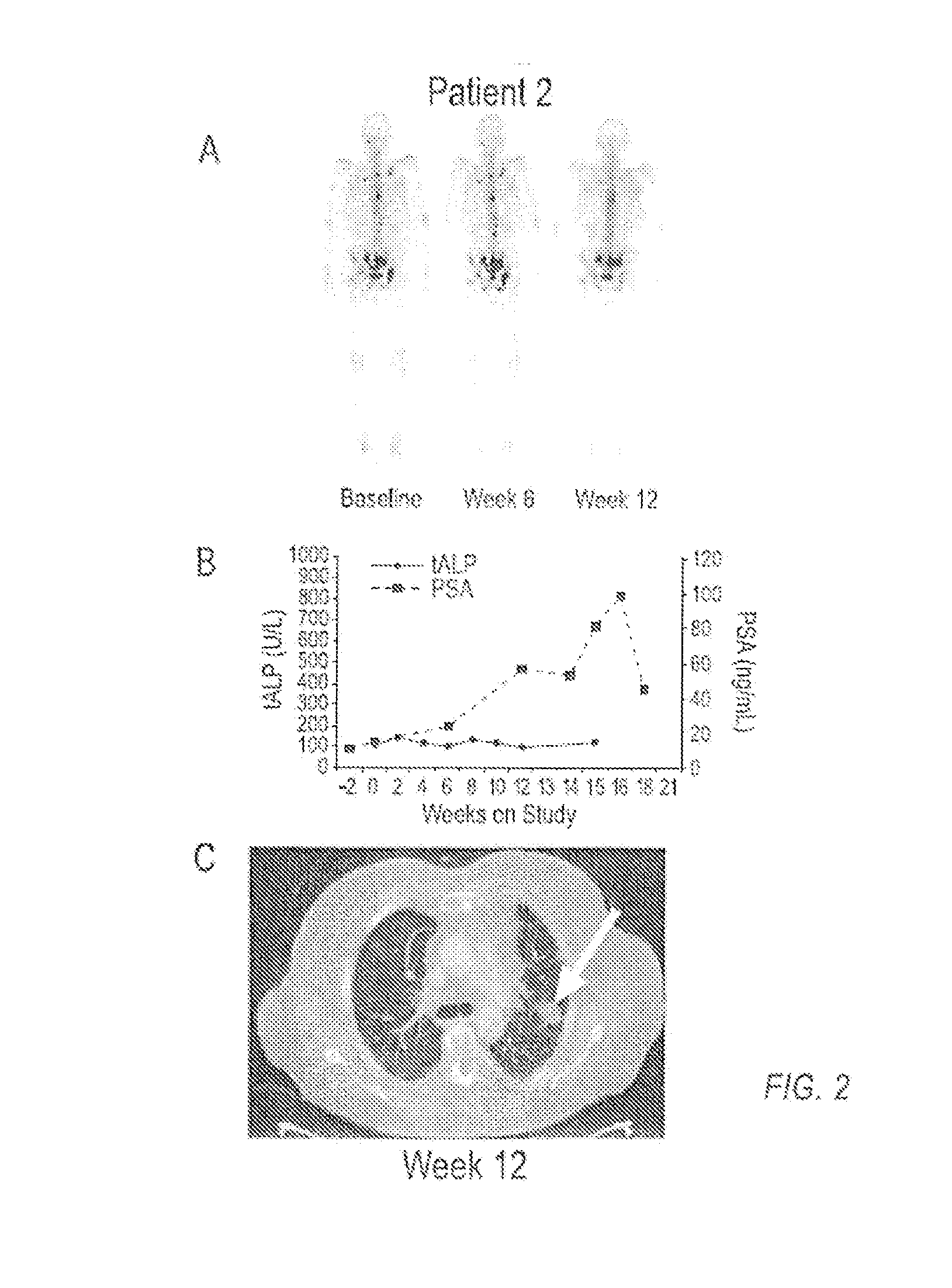
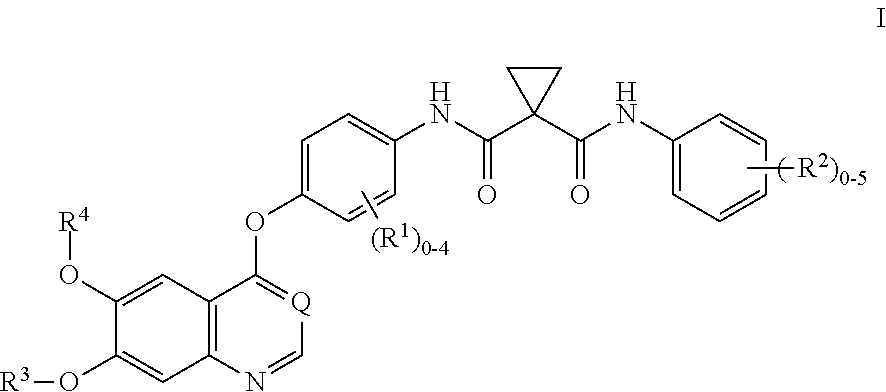
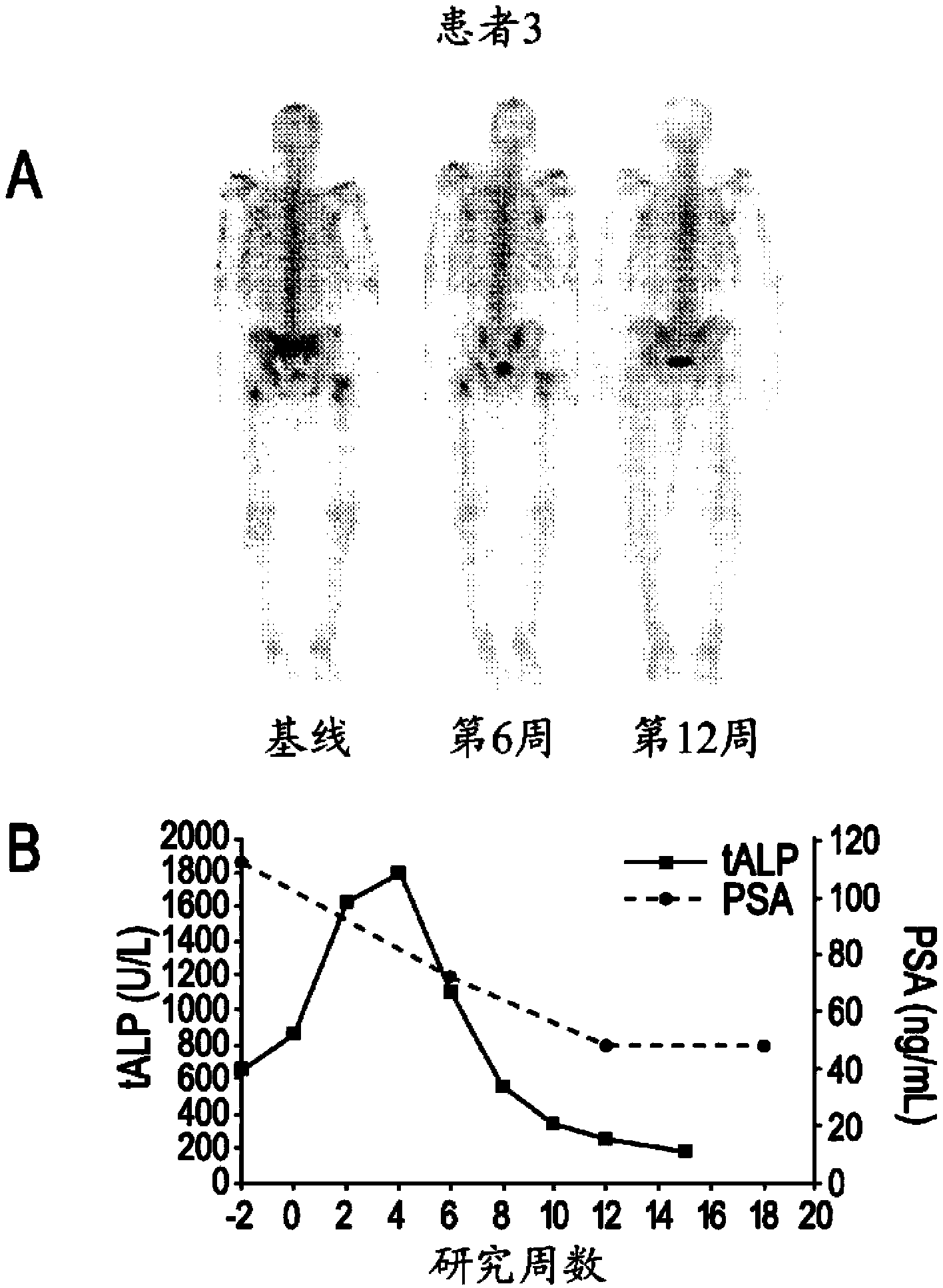
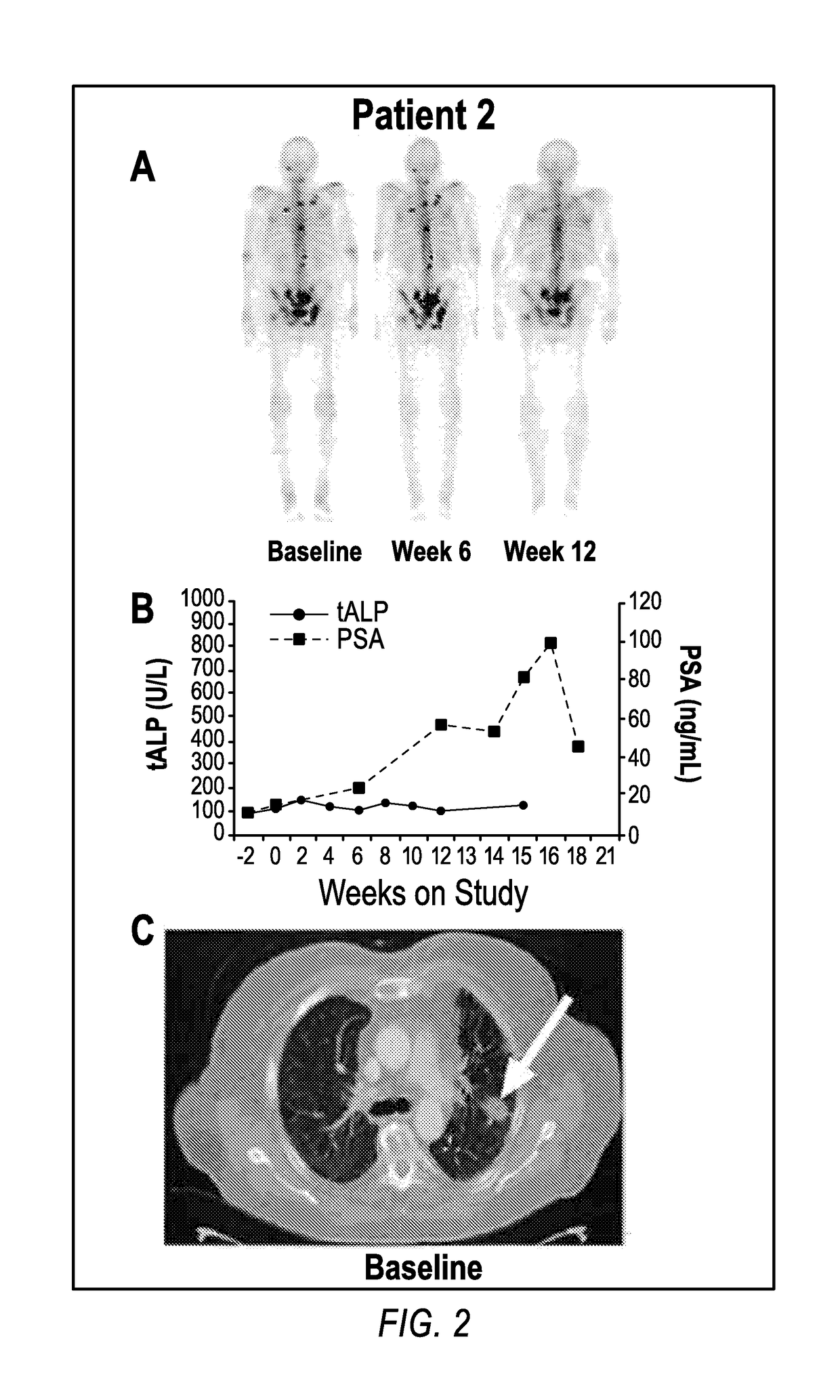
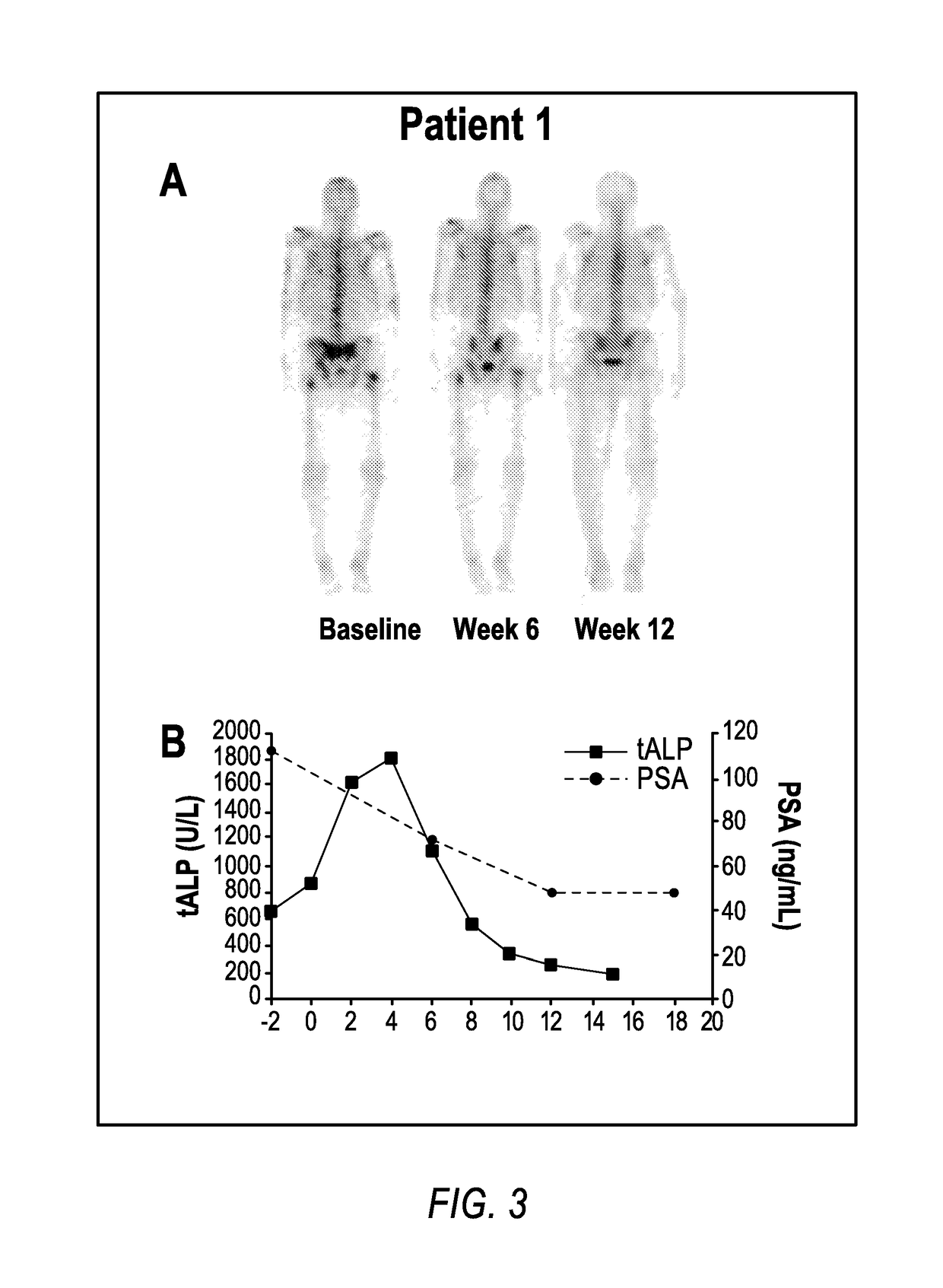
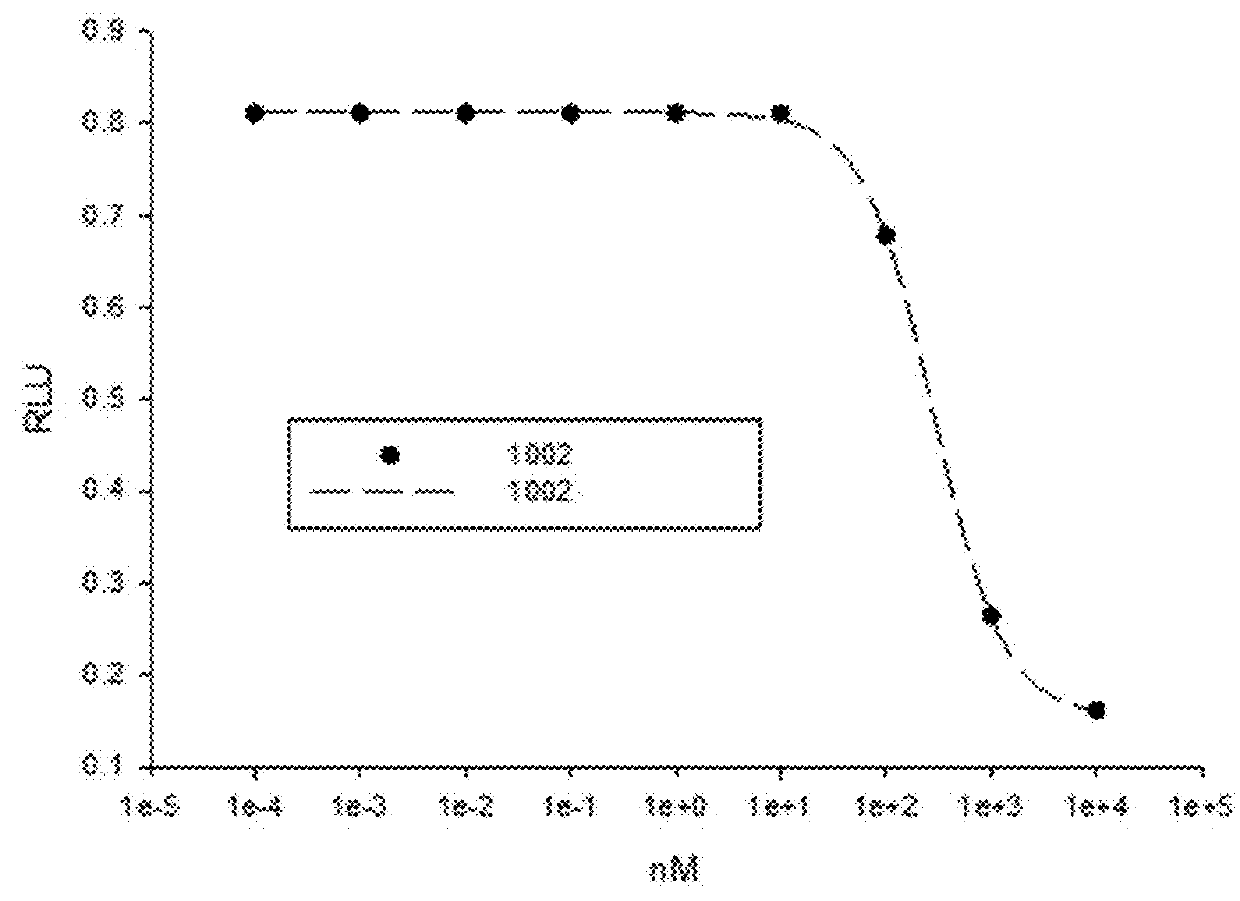
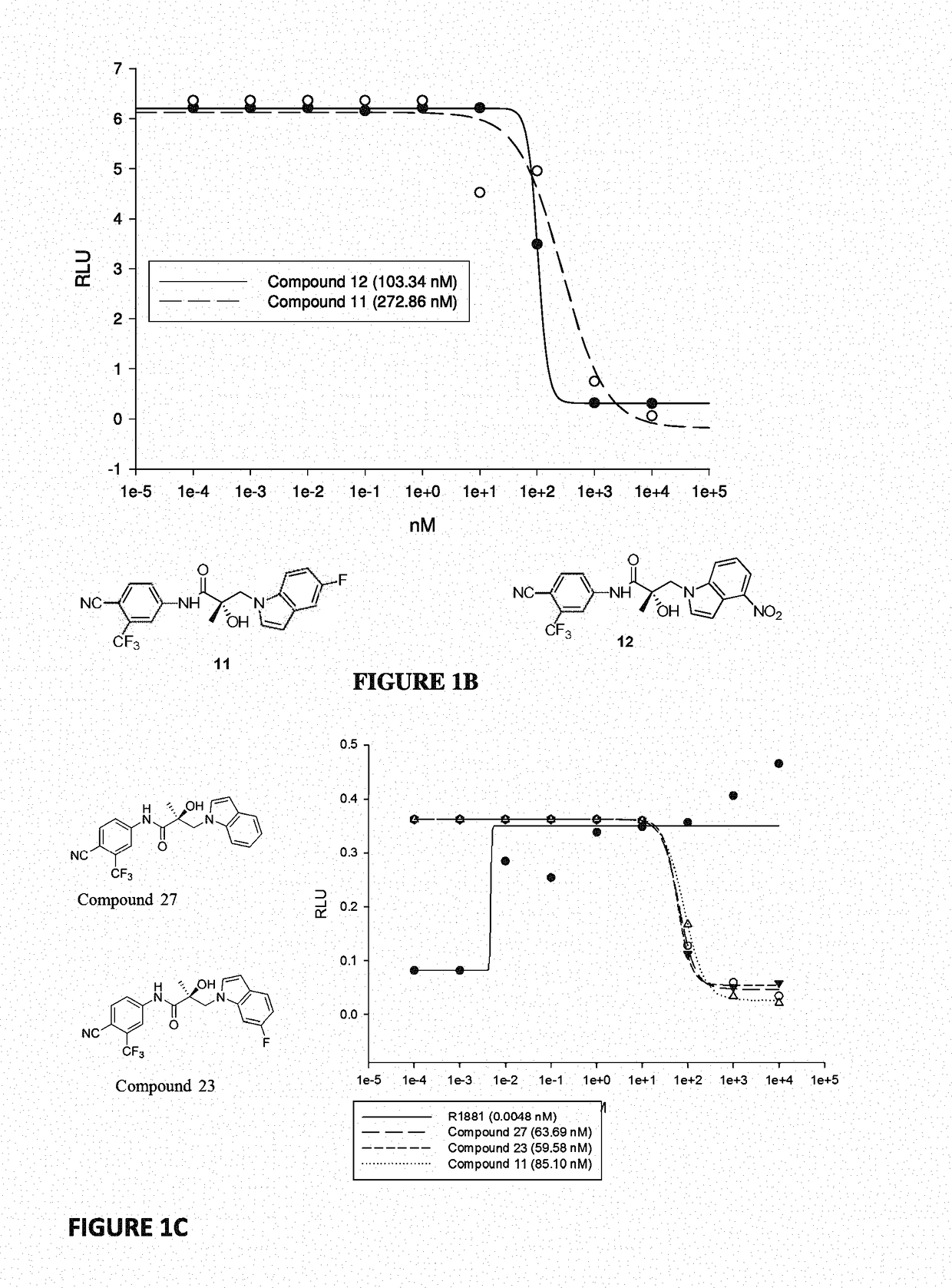
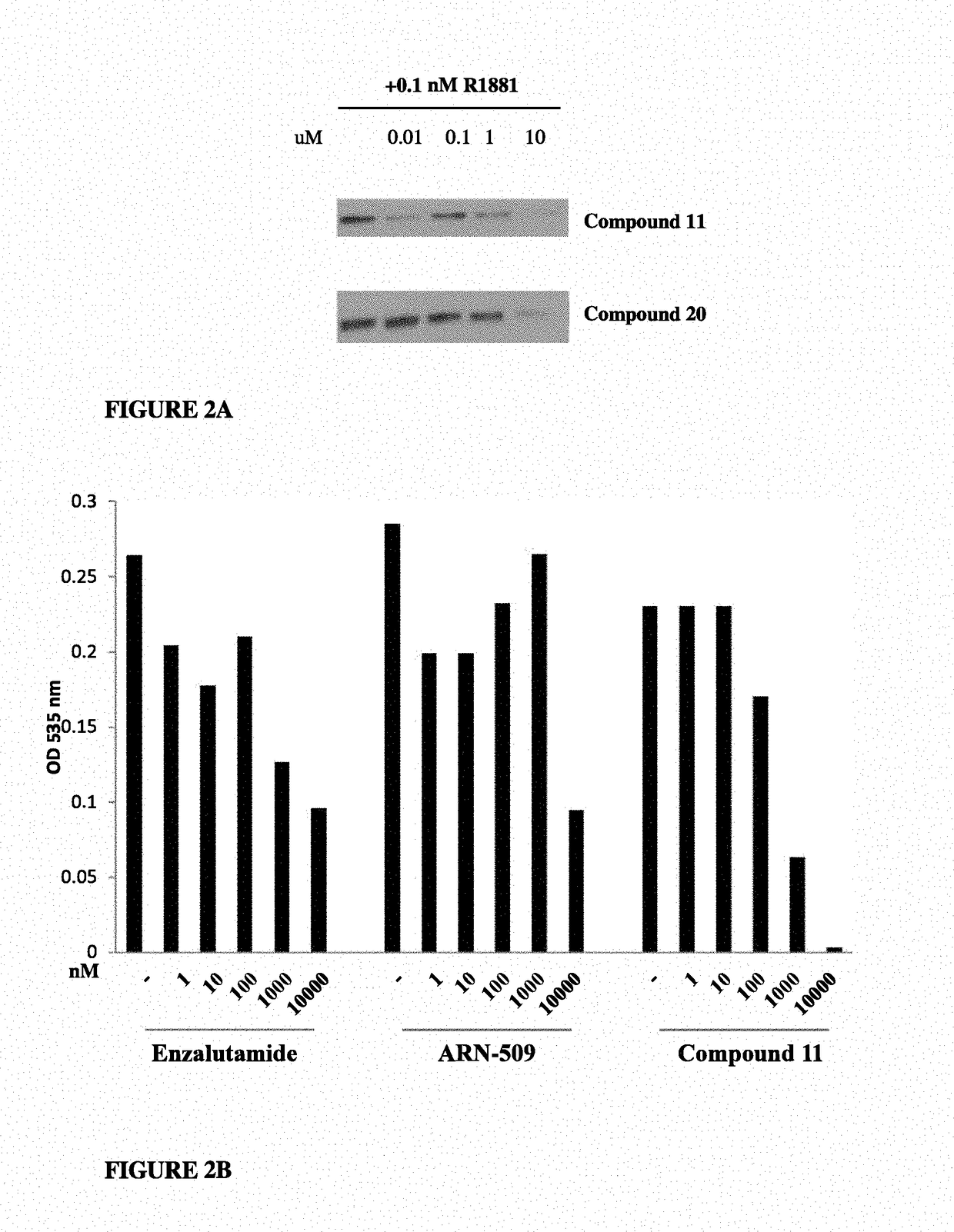
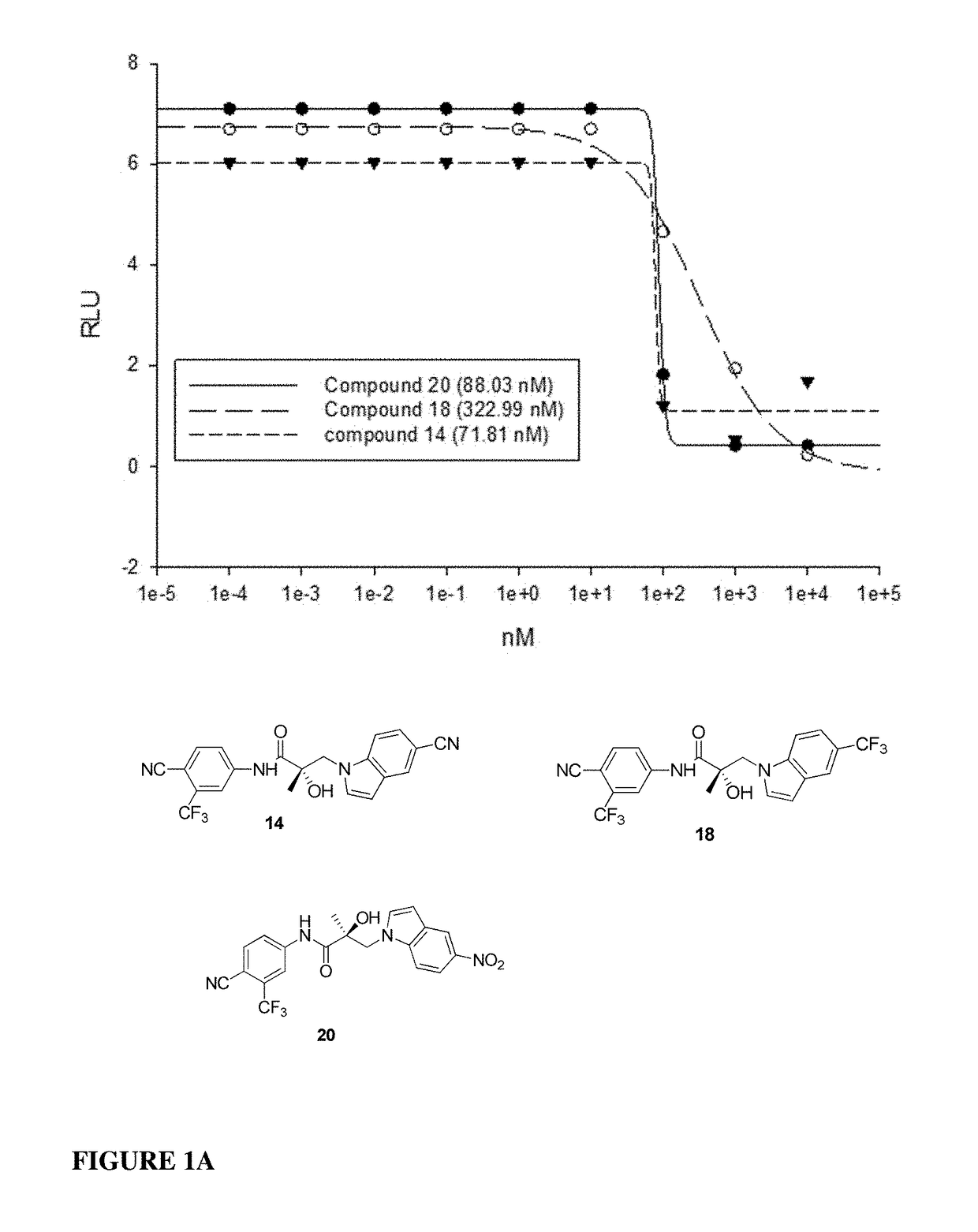
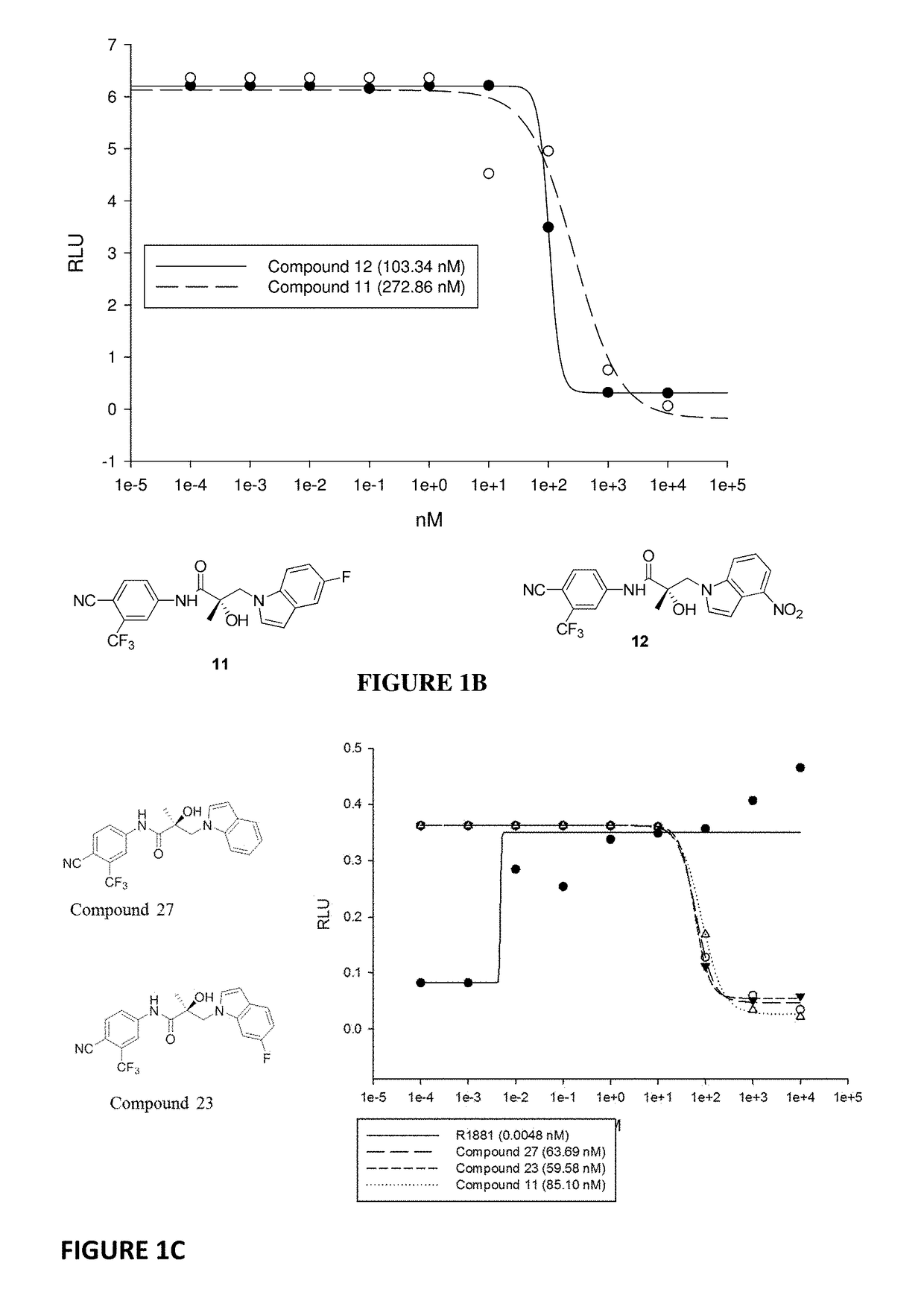
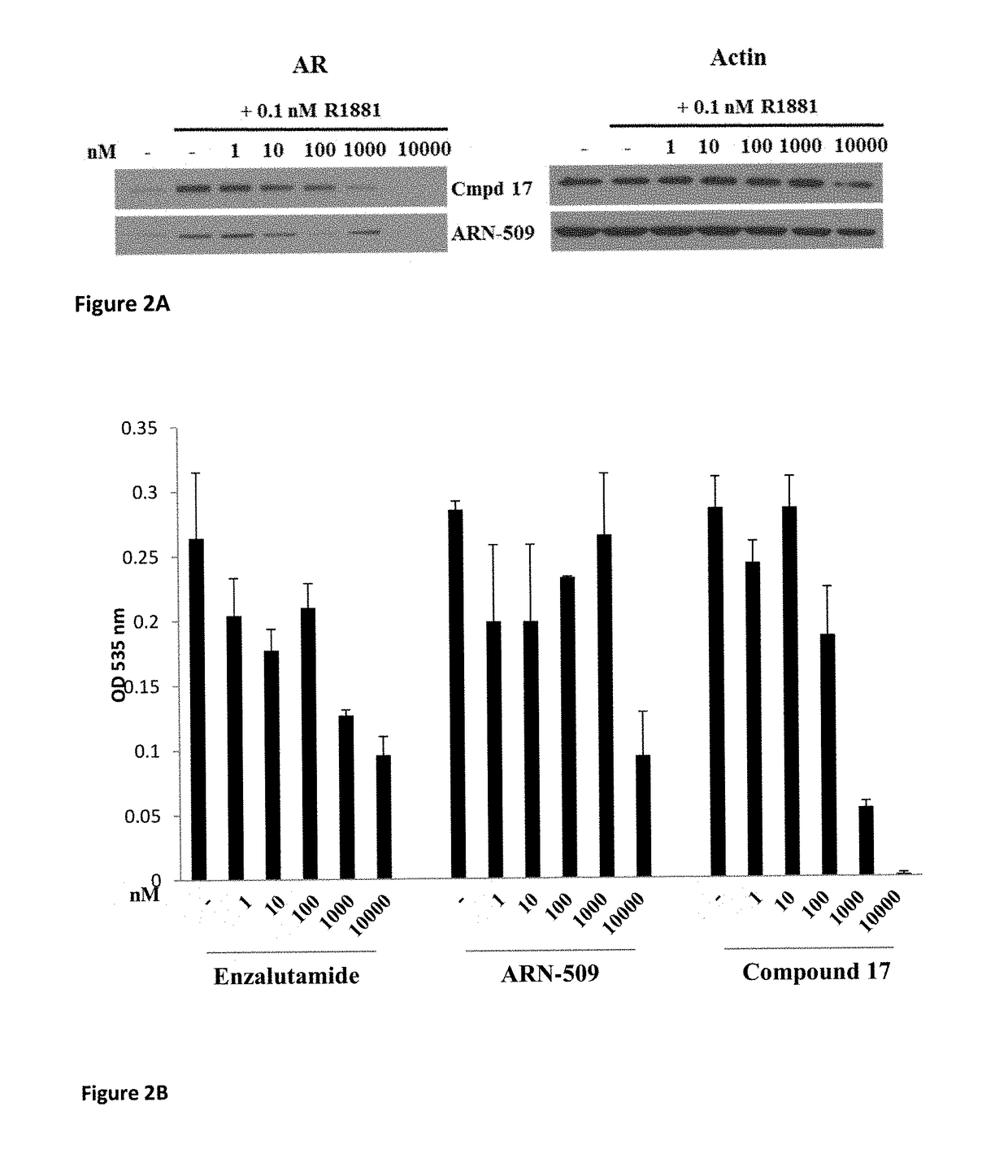
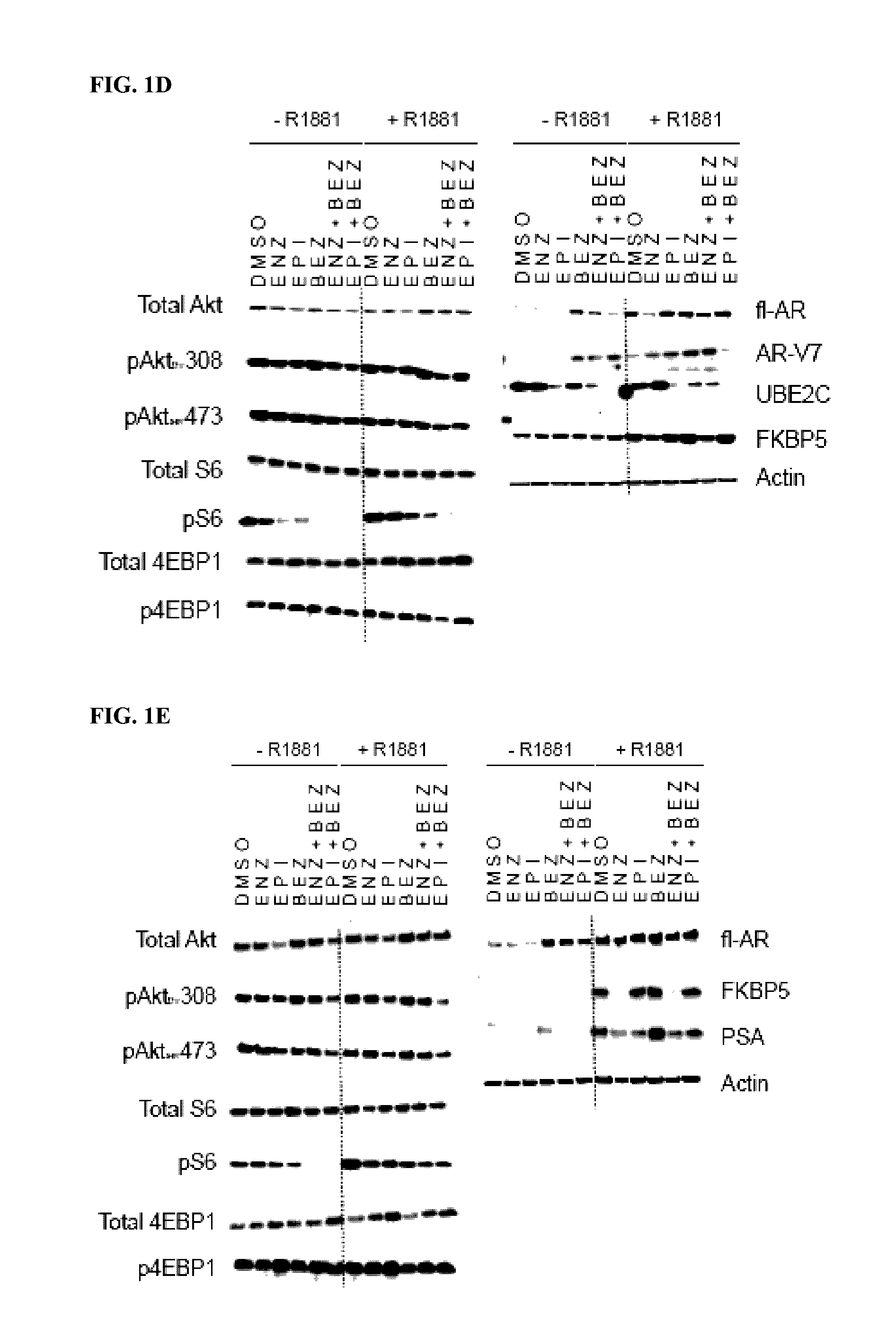
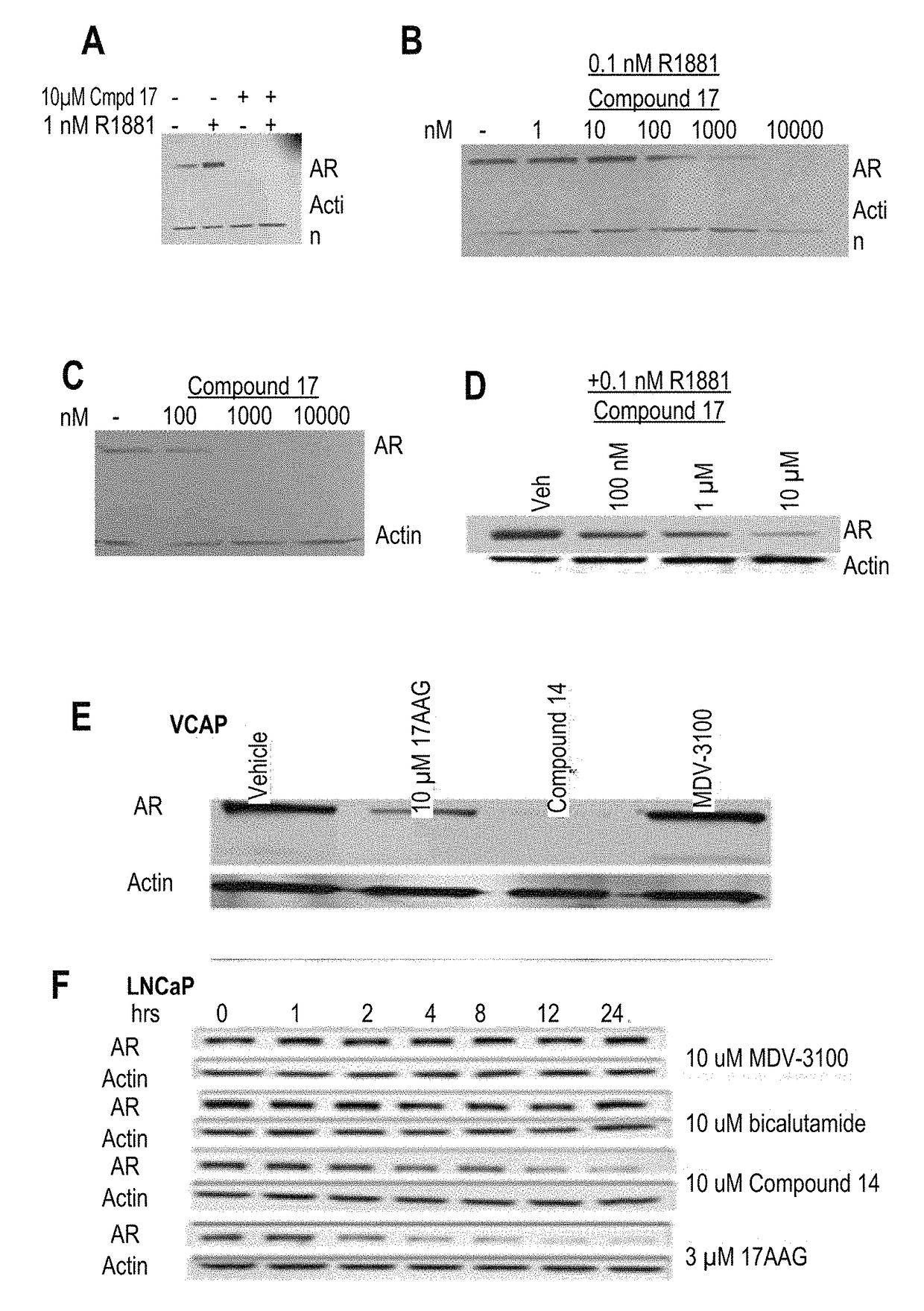
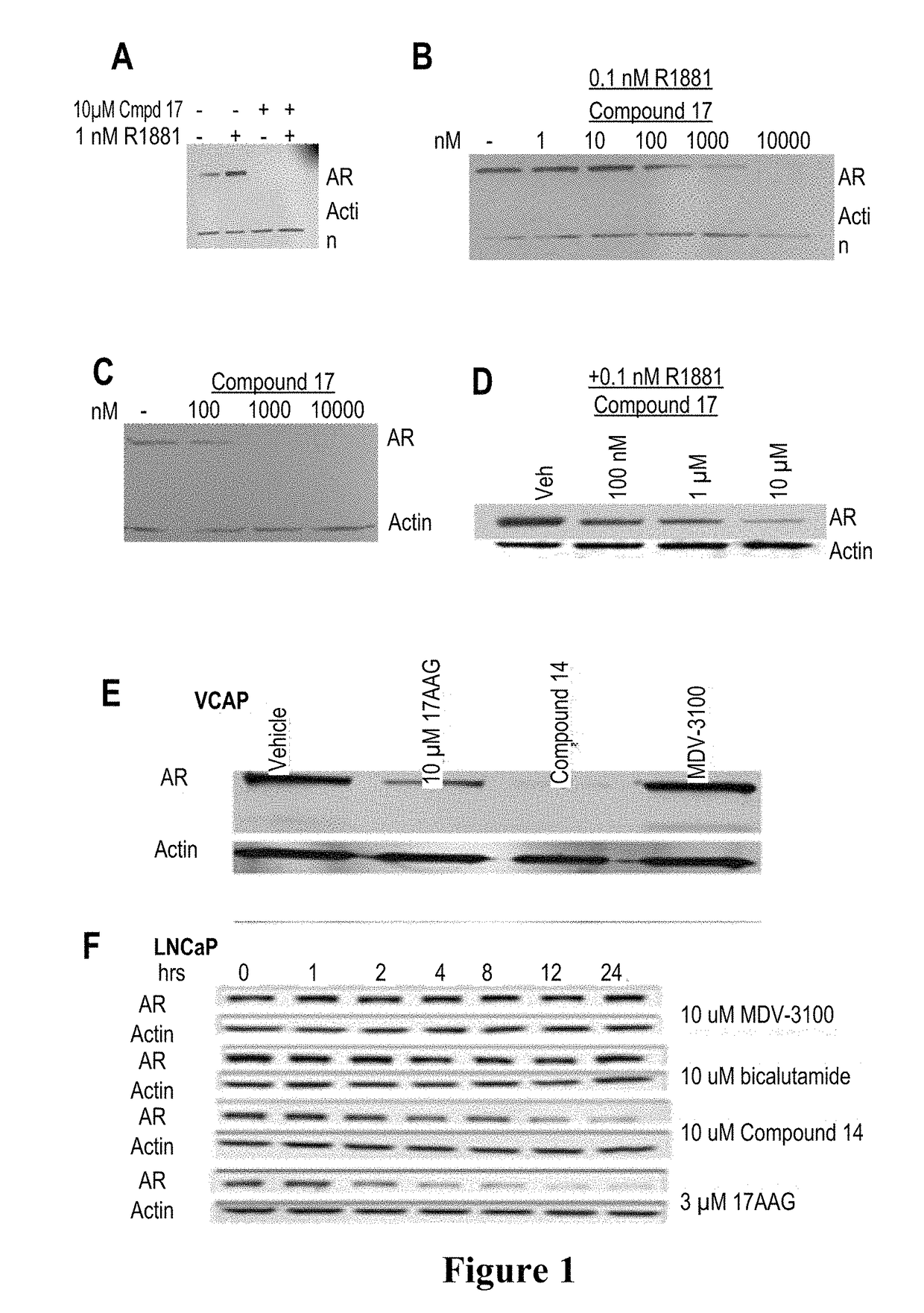
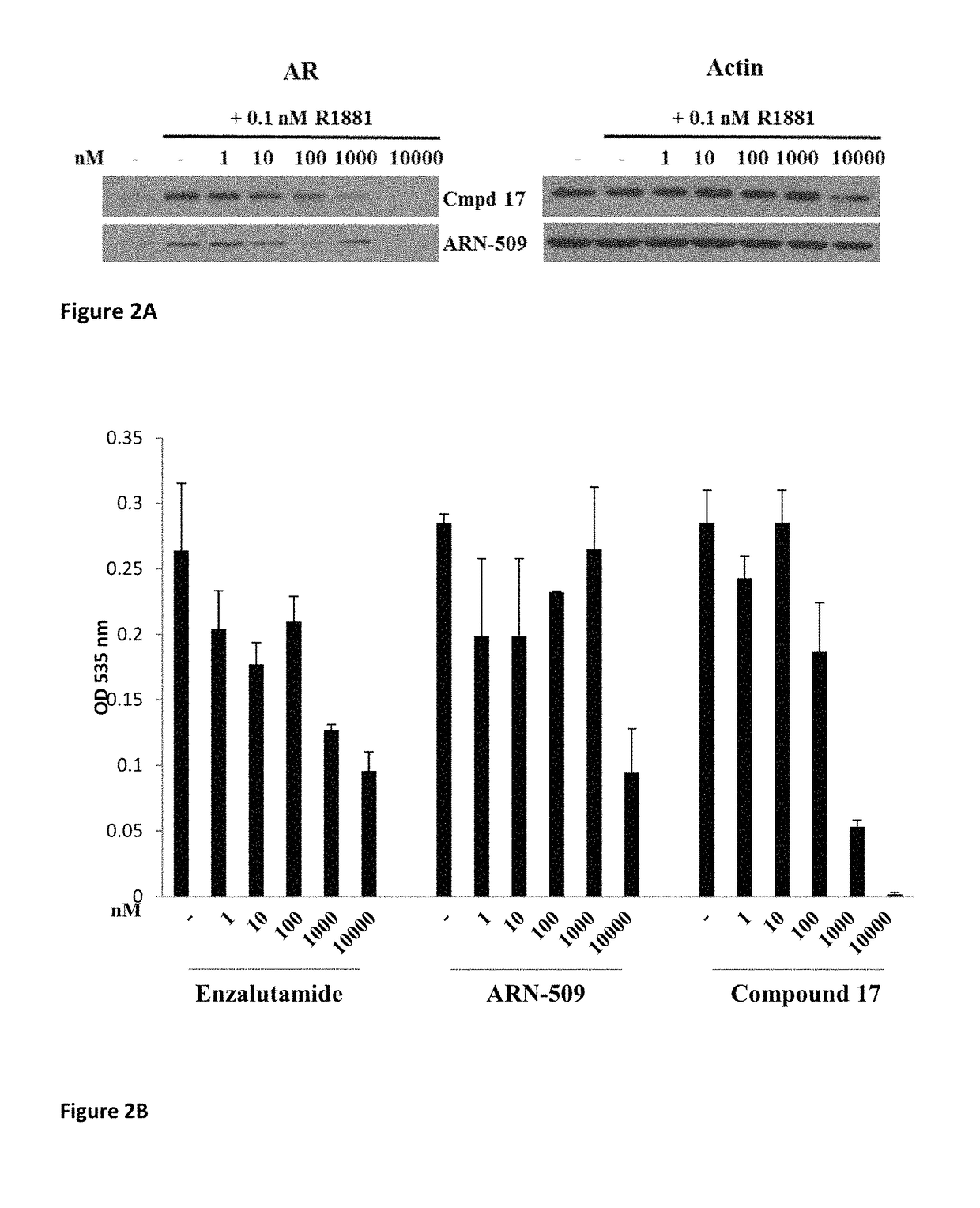
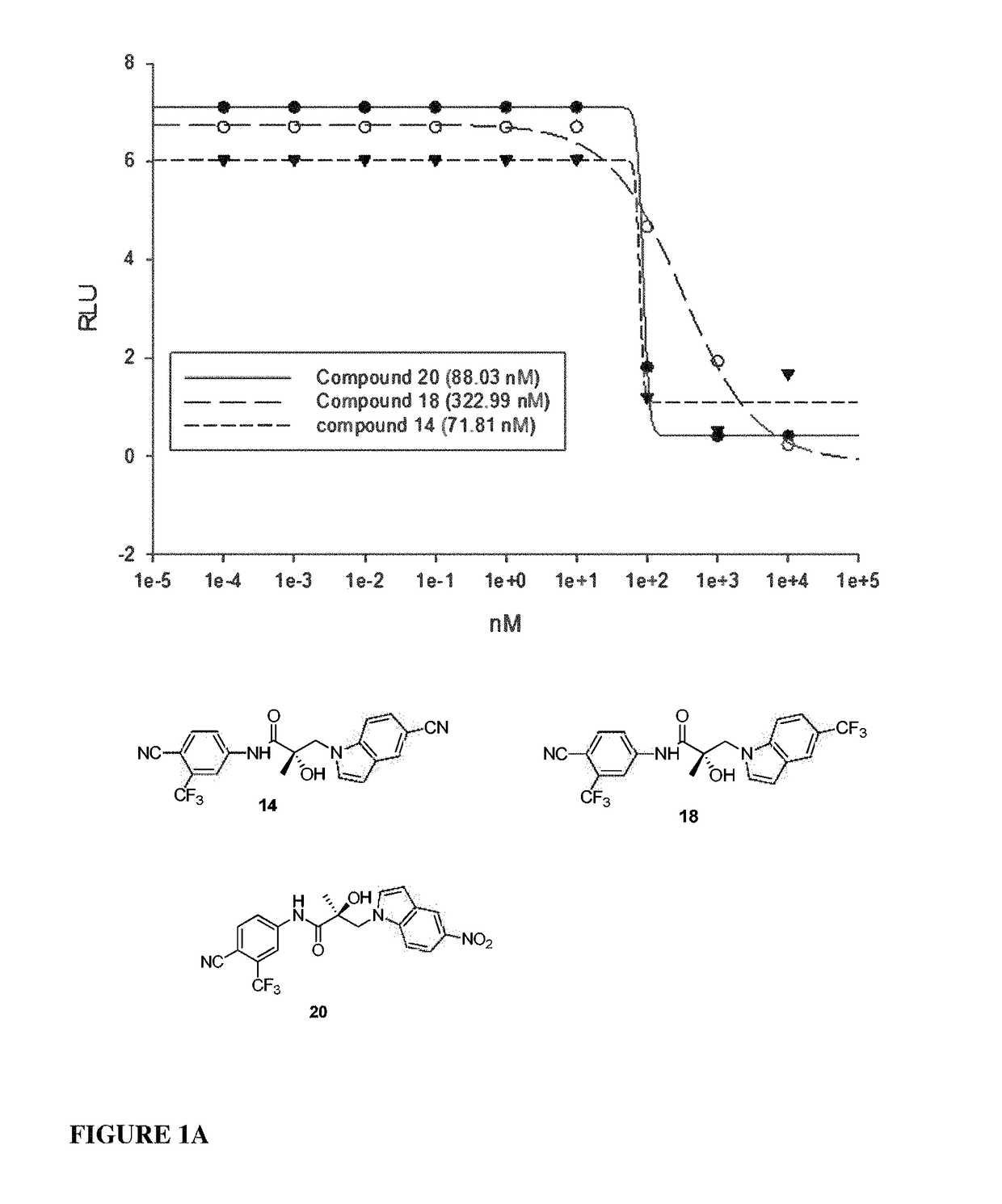
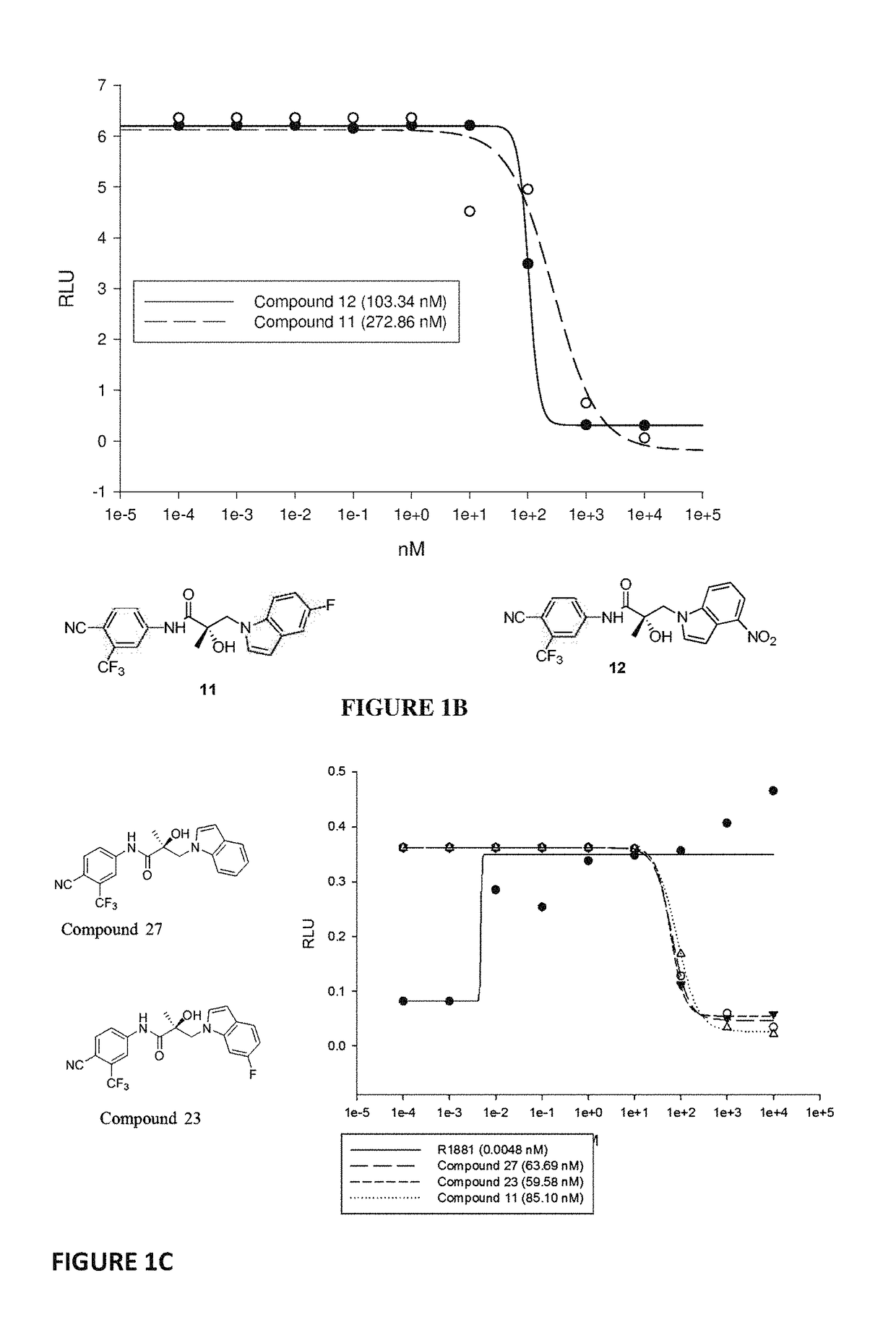
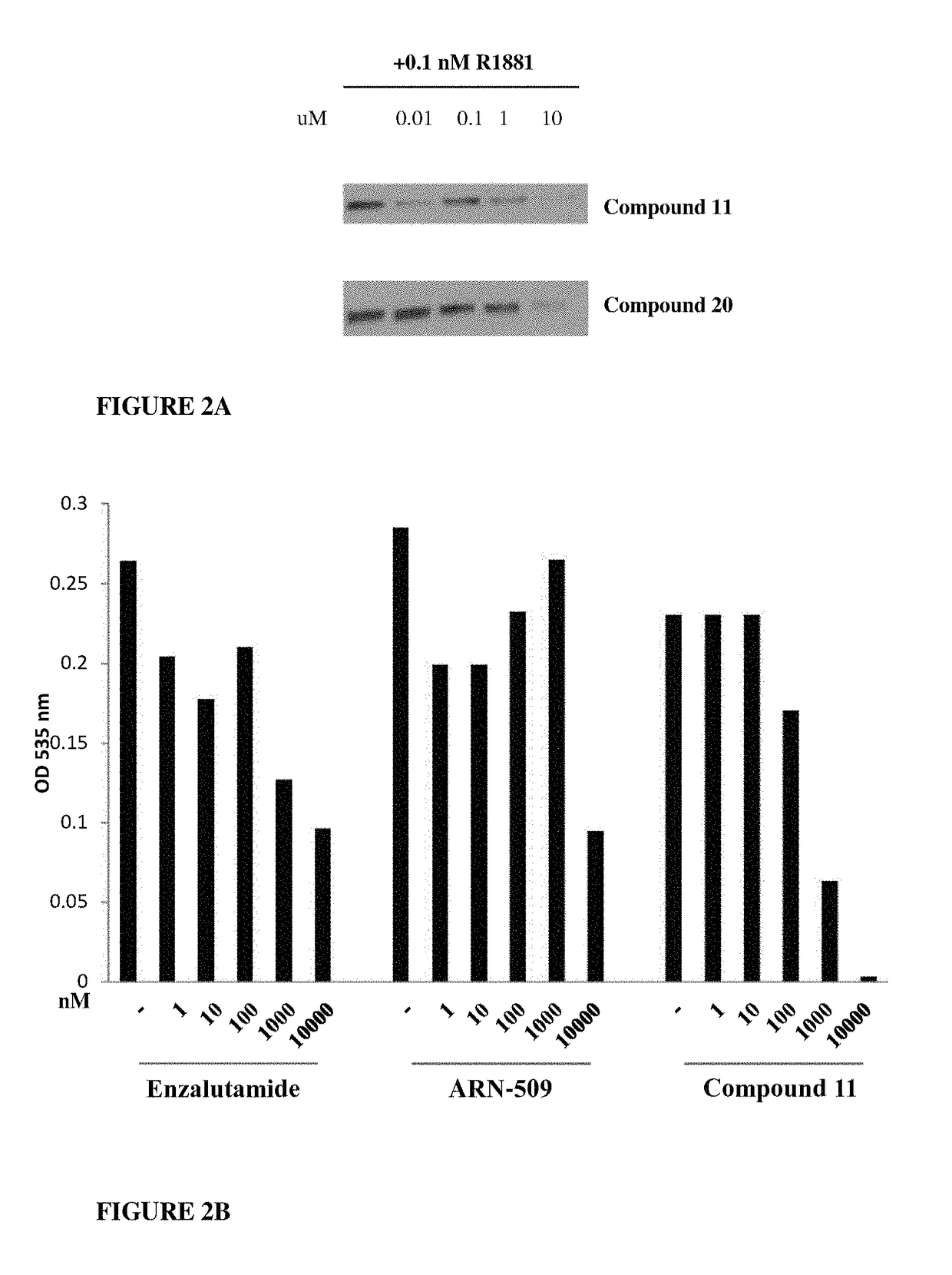
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
137 results about "Castration resistant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
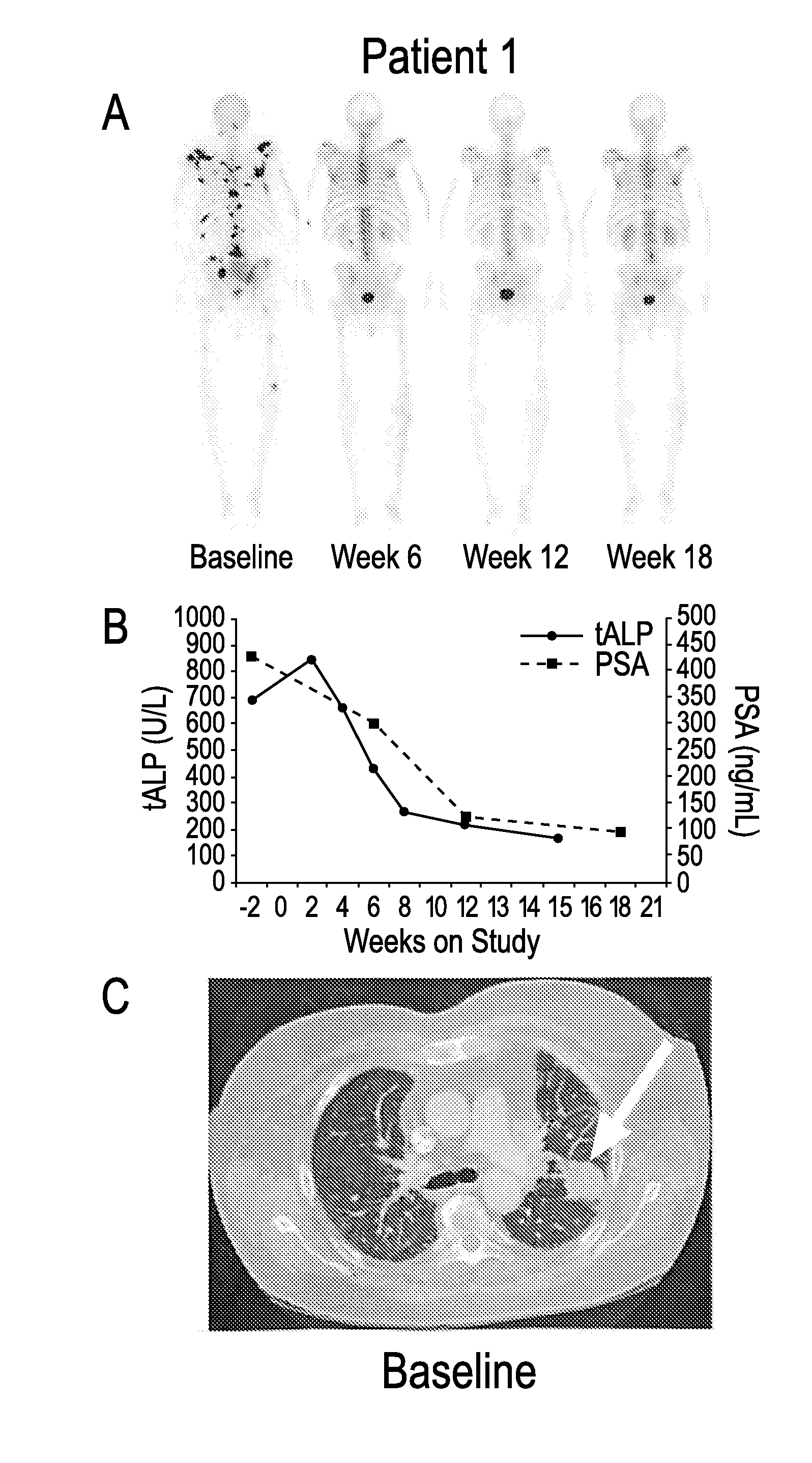
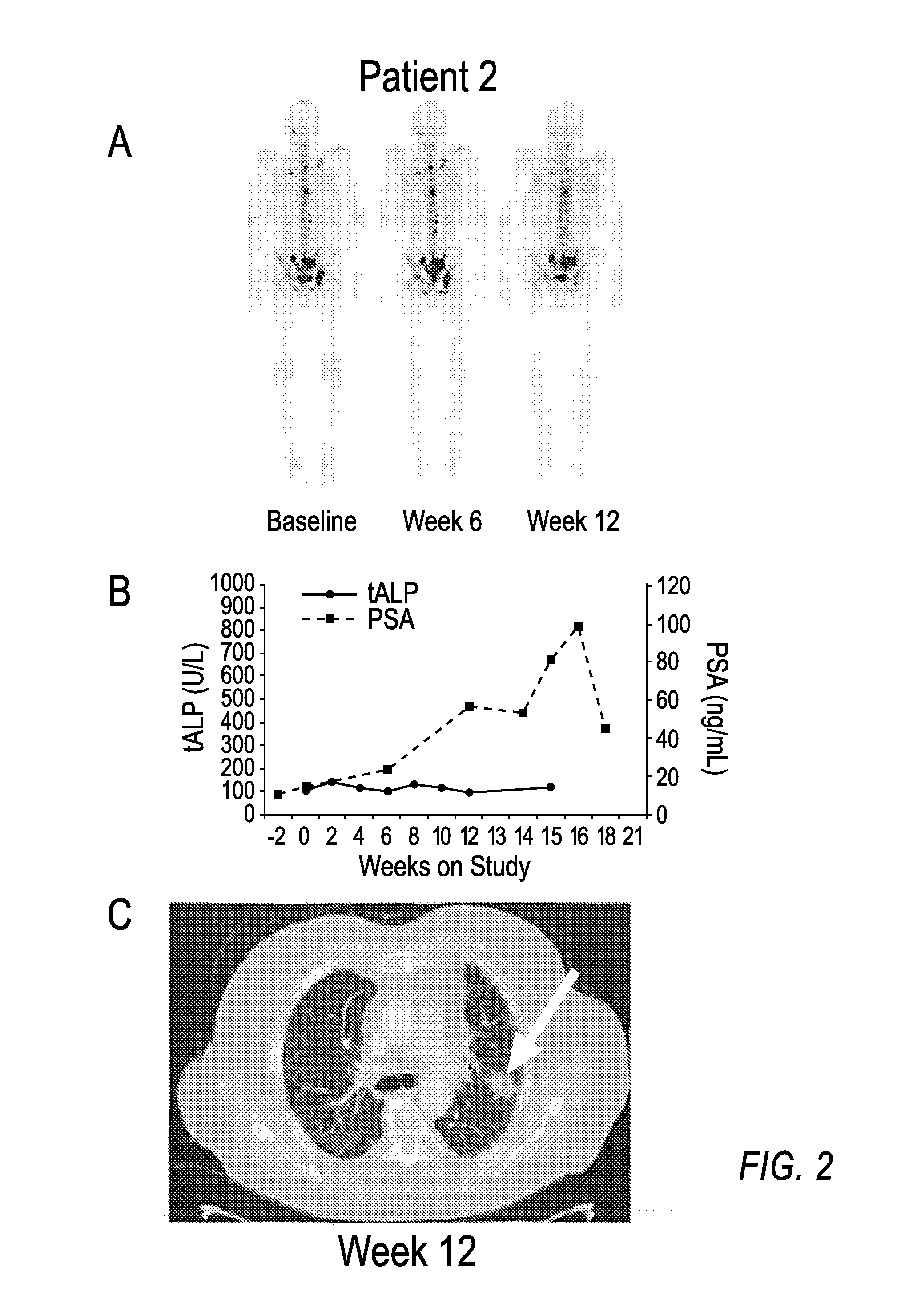
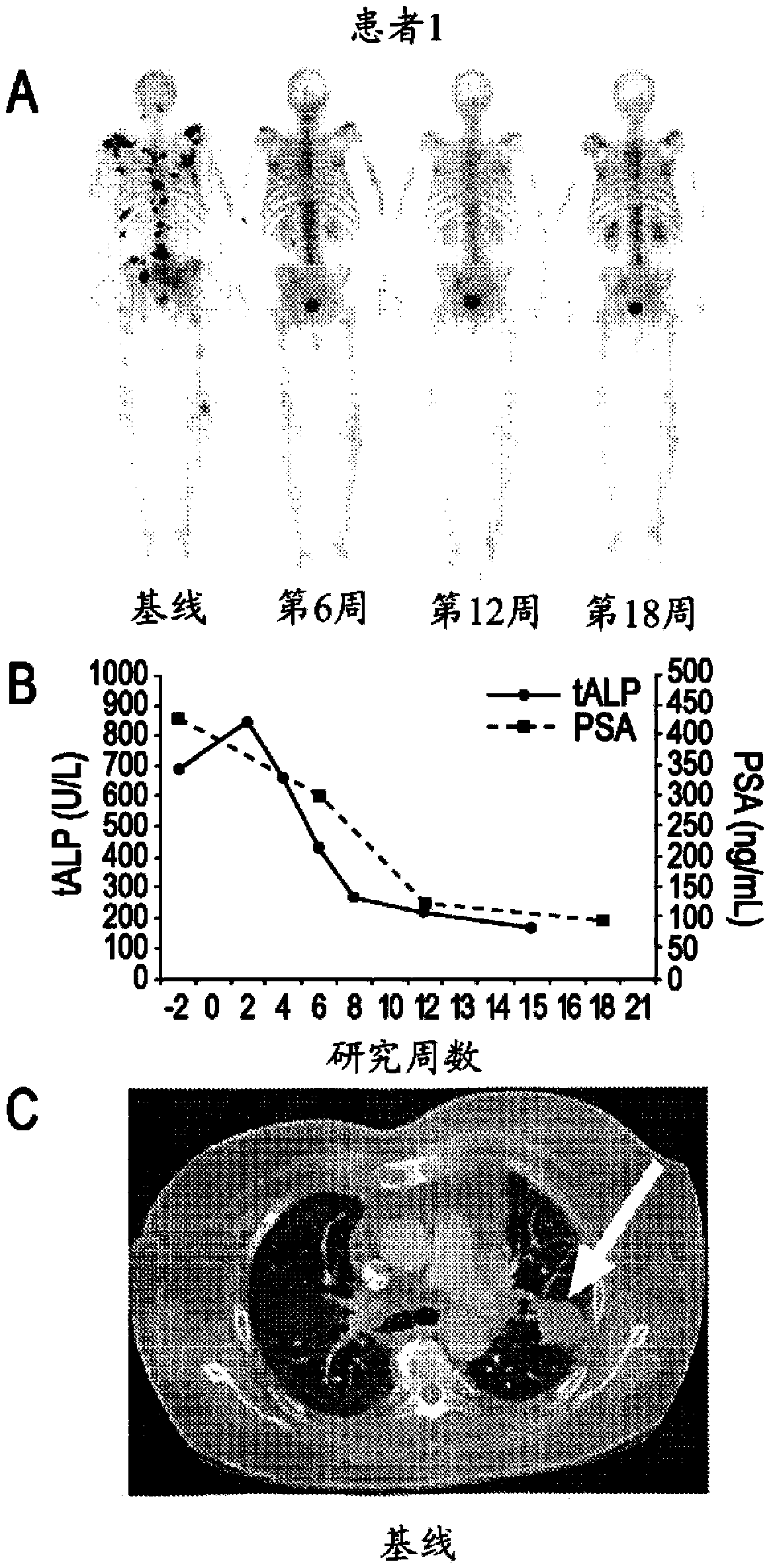
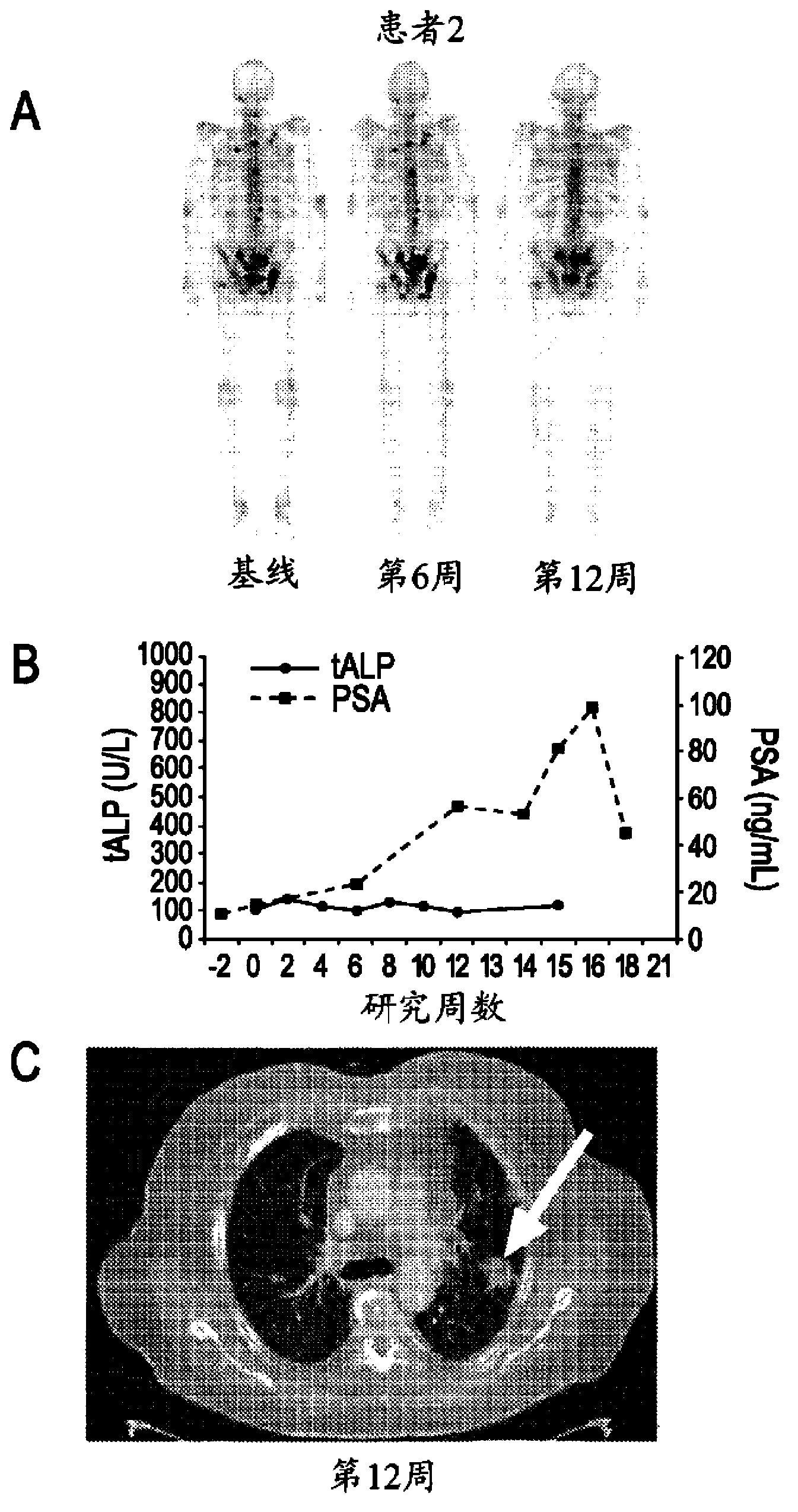
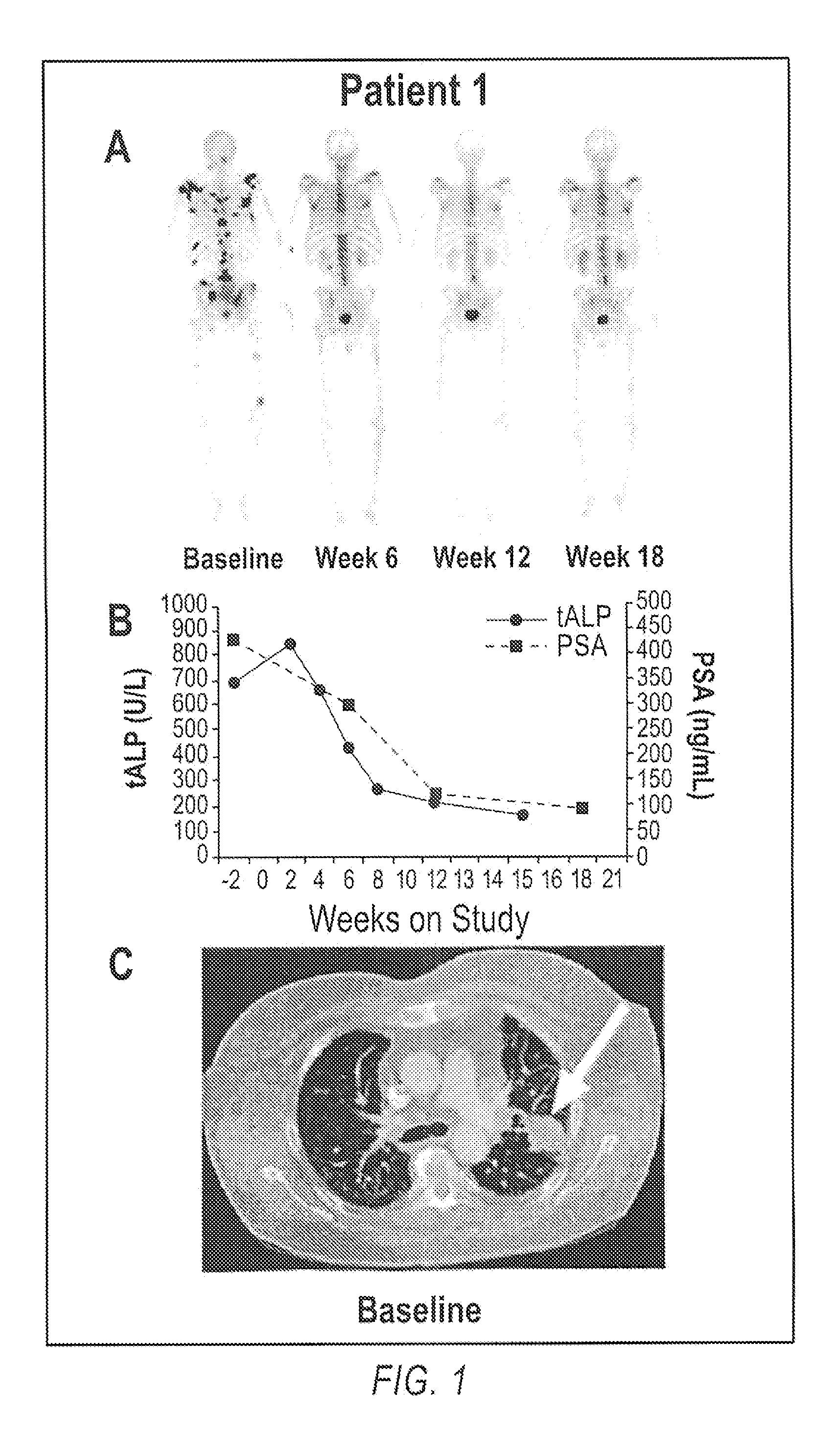
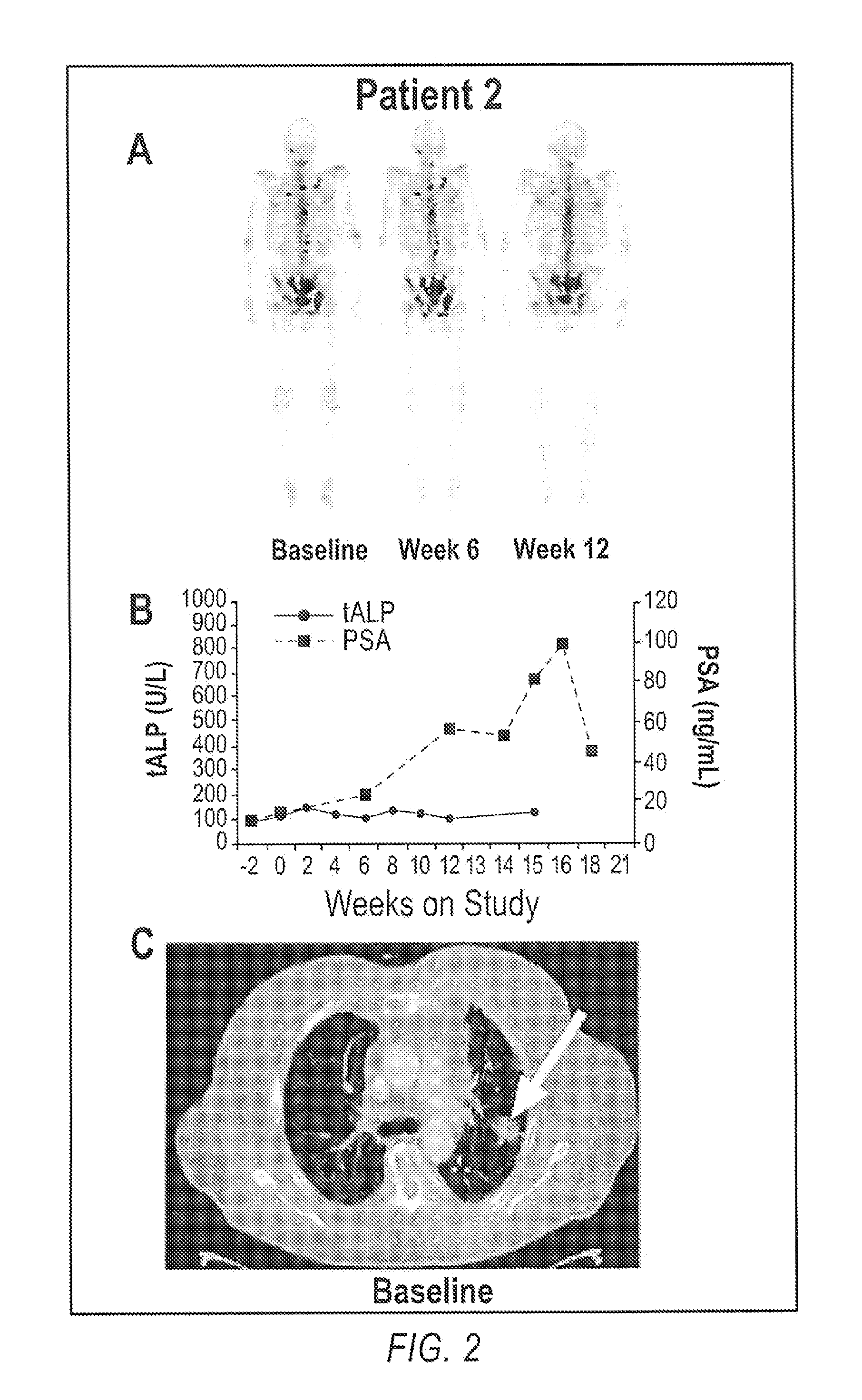
Advanced castration resistant to prostate cancer (CRPC) defined by progression of the disease, despite the treatment of androgenic deprivation (ADT) and may be present one or a combination of a constant increase in serum levels of the specific prostatic antigen (PSA) , the development of pre-existing diseases, or the emergence of new metastases.
Method of Treating Cancer
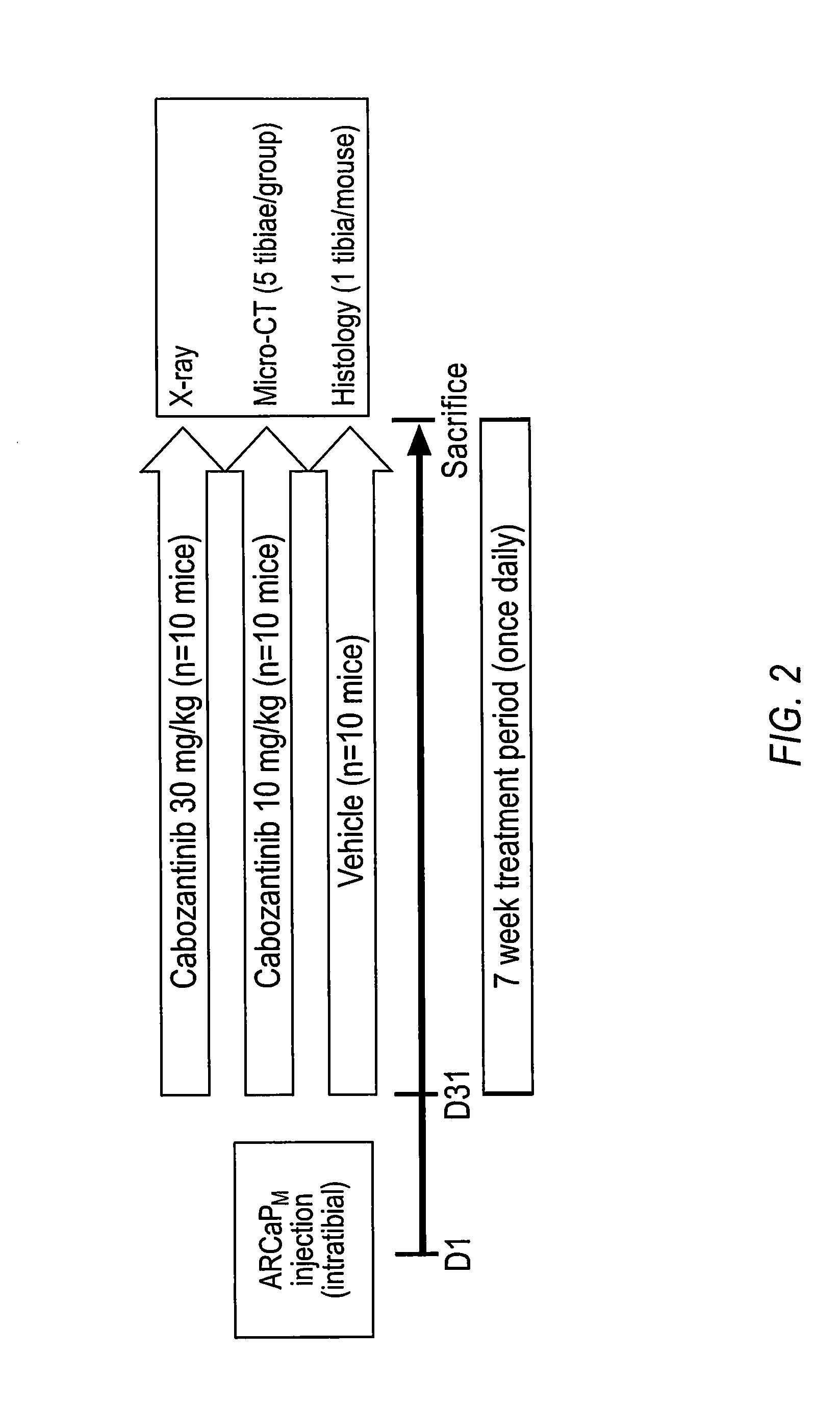
InactiveUS20160220554A1Relieve painImprove survivalOrganic chemistrySkeletal disorderOsteoblastDual inhibitor
Owner:EXELIXIS INC
Method of Treating Cancer
This invention is directed to the treatment of cancer, particularly castration-resistant prostate cancer and osteoblastic bone metastases, with a dual inhibitor of MET and VEGF.
Owner:EXELIXIS INC
Method of Treating Cancer
InactiveUS20140066444A1Relieve painImprove survivalAntineoplastic agentsHeterocyclic compound active ingredientsOsteoblastDual inhibitor
This invention is directed to the treatment of cancer, particularly castration-resistant prostate cancer and osteoblastic bone metastases, with a dual inhibitor of MET and VEGF.
Owner:EXELIXIS INC
Method of Treating Cancer
InactiveUS20140057908A1Organic chemistryAntipyreticCastration resistantCastrate-resistant prostate cancer
This invention is directed to the treatment of cancer, particularly castration-resistant prostate cancer and osteoblastic bone metastases, with a dual inhibitor of MET and VEGF.
Owner:EXELIXIS INC
Method of Treating Cancer
InactiveUS20140323522A1Improve survivalRelieve painBiocideOrganic chemistryDual inhibitorCastration resistant
This invention is directed to the treatment of cancer, particularly castration-resistant prostate cancer and bone metastases, with a dual inhibitor of MET and VEGF.
Owner:EXELIXIS INC
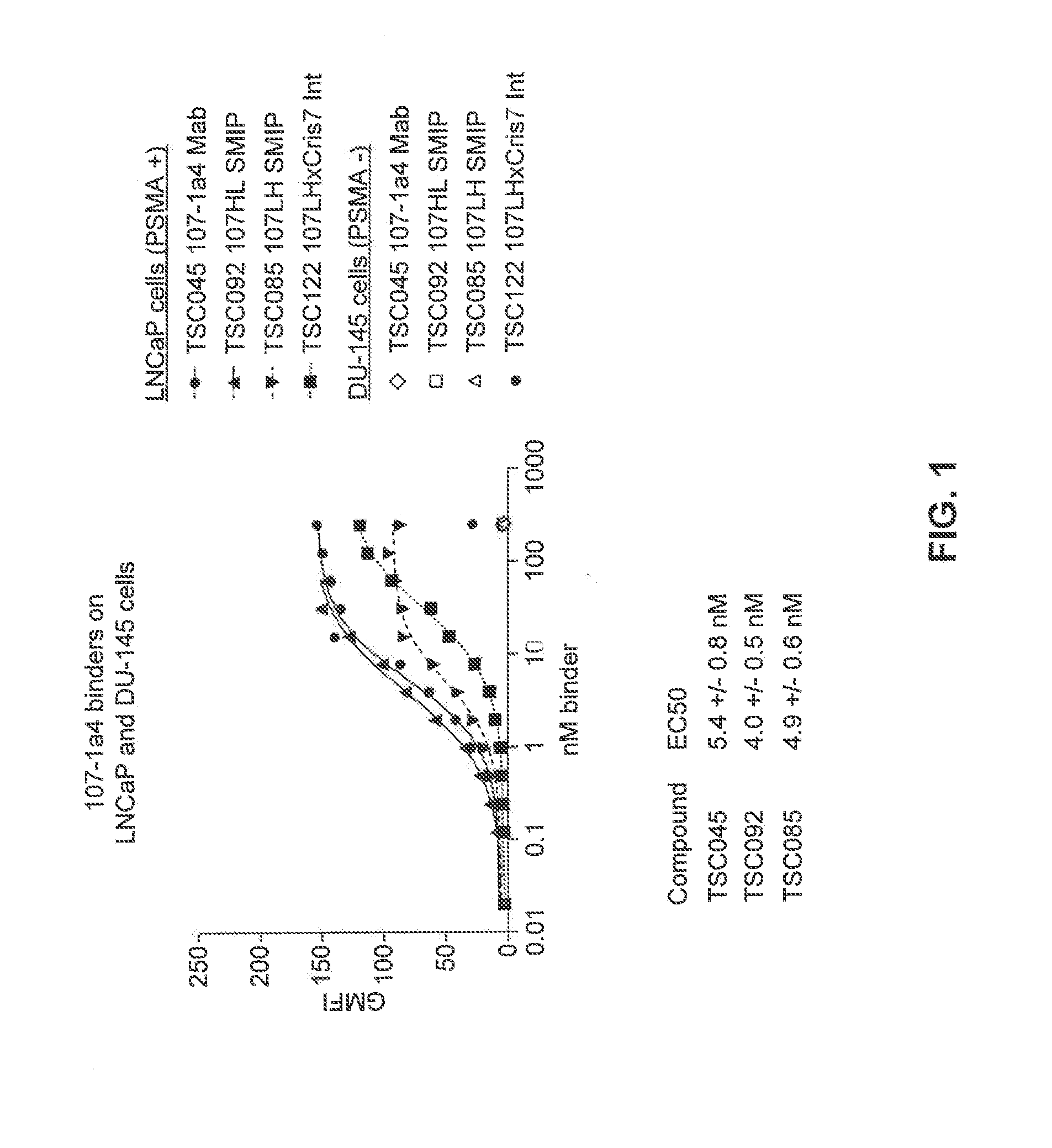
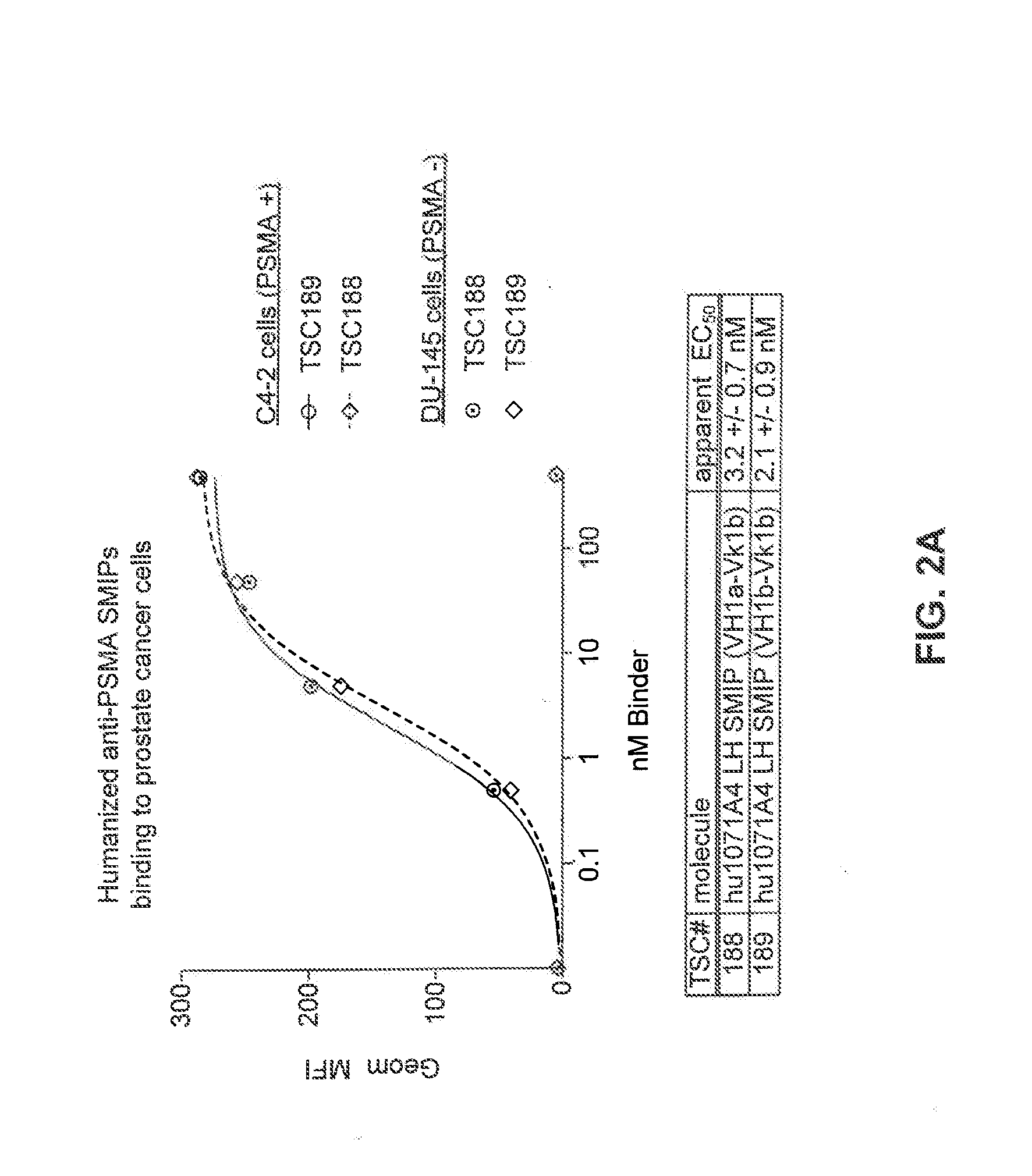
Prostate-Specific Membrane Antigen Binding Proteins and Related Compositions and Methods
InactiveUS20140161800A1Extended half-lifeDecreased internalizationBacteriaImmunological disordersAntigen bindingT cell
The present invention relates to mono-specific and multi-specific polypeptide therapeutics that specifically target cells expressing prostate-specific membrane antigen (PSMA) and are useful for the treatment of prostate cancer (e.g., castrate-resistant prostate cancer), tumor-related angiogenesis or benign prostatic hyperplasia (BPH). In one embodiment, the multi-specific polypeptide therapeutics bind both PSMA-expressing cells and the T-cell receptor complex on T cells to induce target-dependent T-cell cytotoxicity, activation and proliferation.
Owner:APTEVO RES & DEV LLC
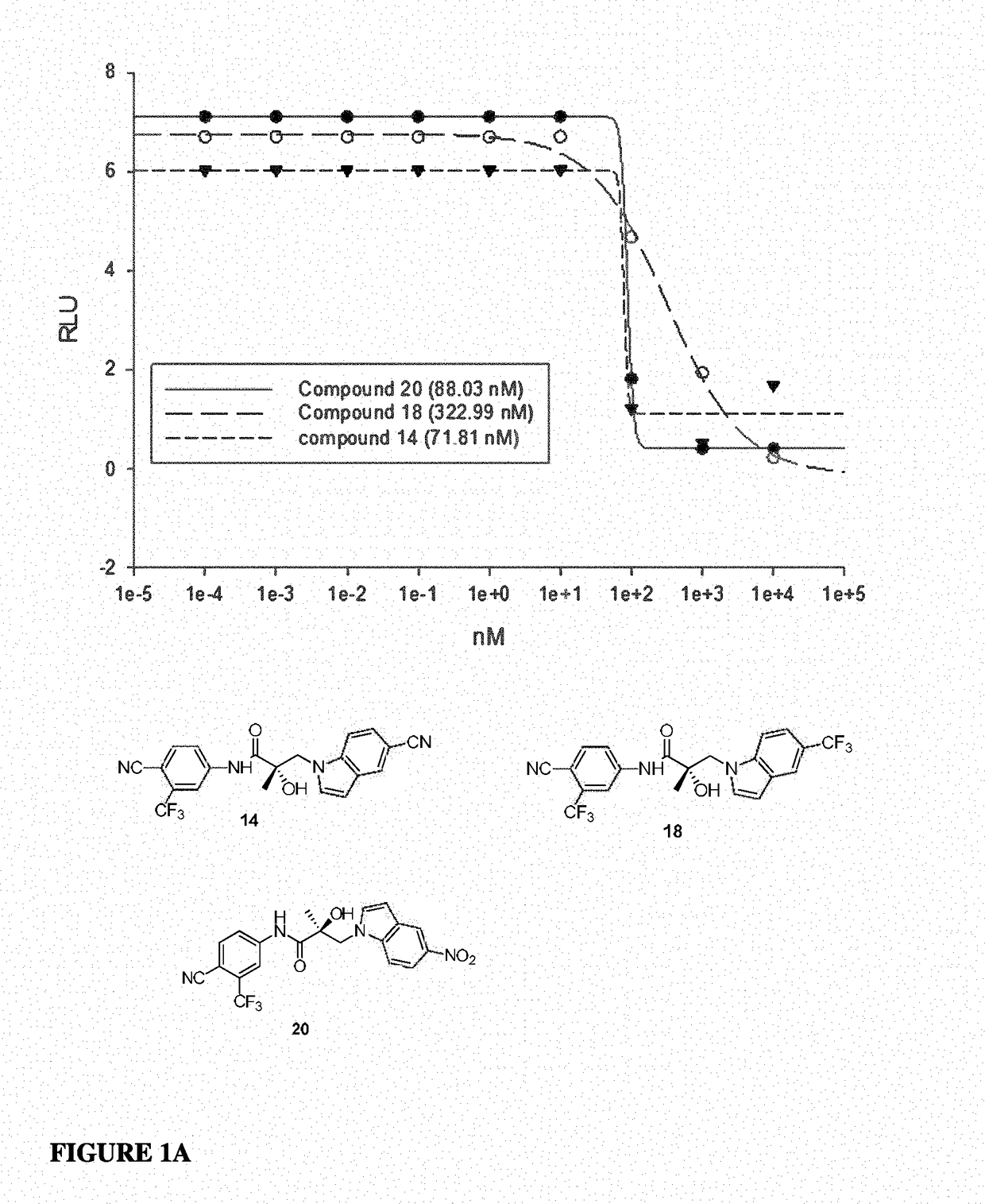
Diaryl hydantoin derivative, as well as preparation method, medicine composition and application thereof
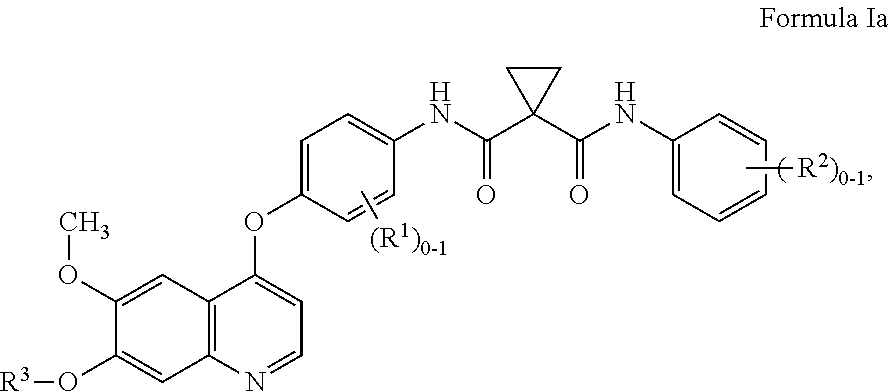
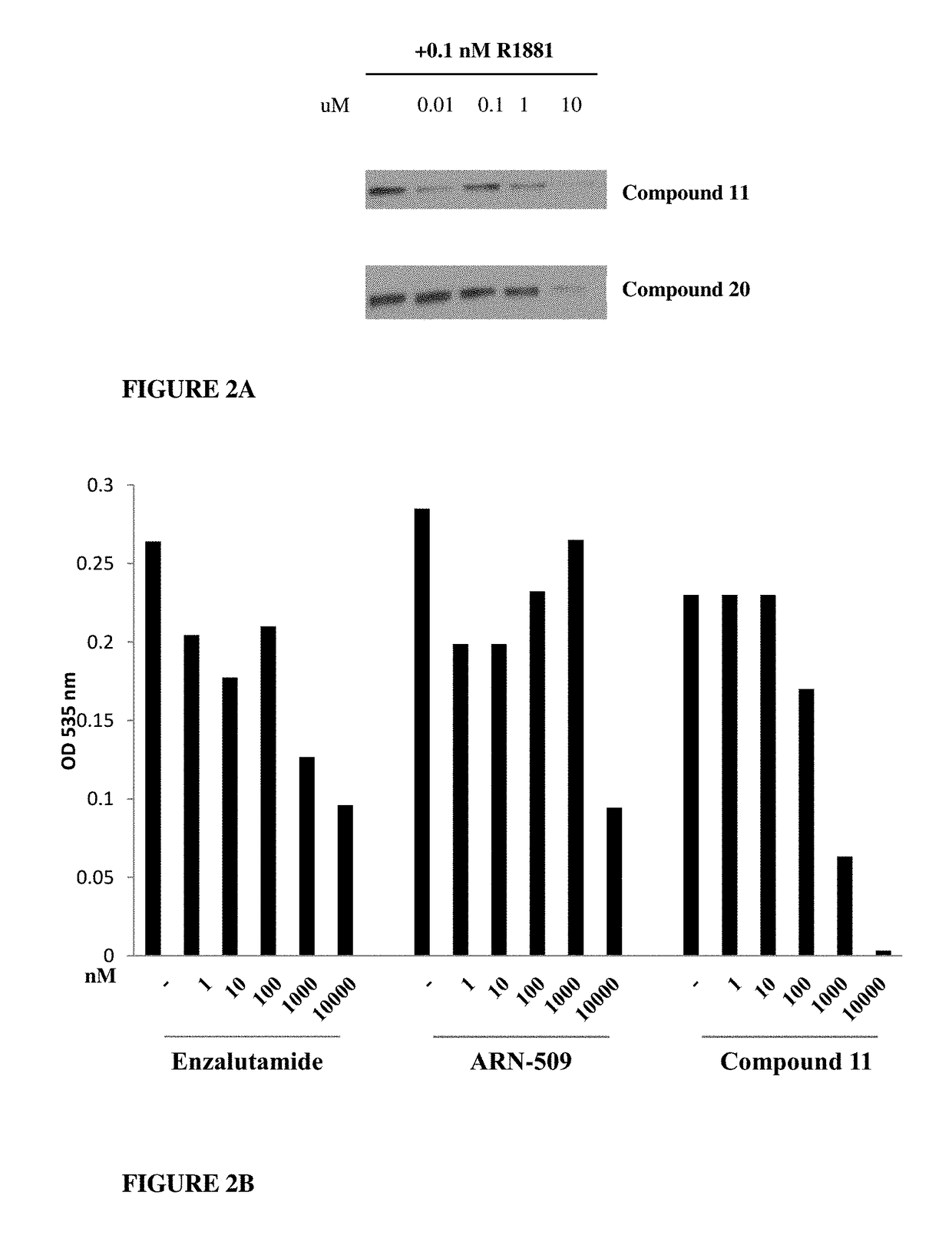
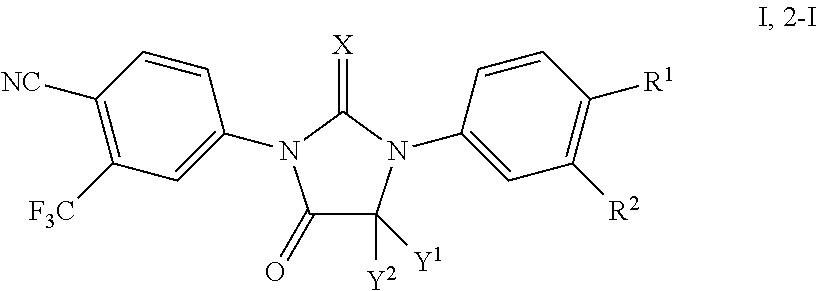
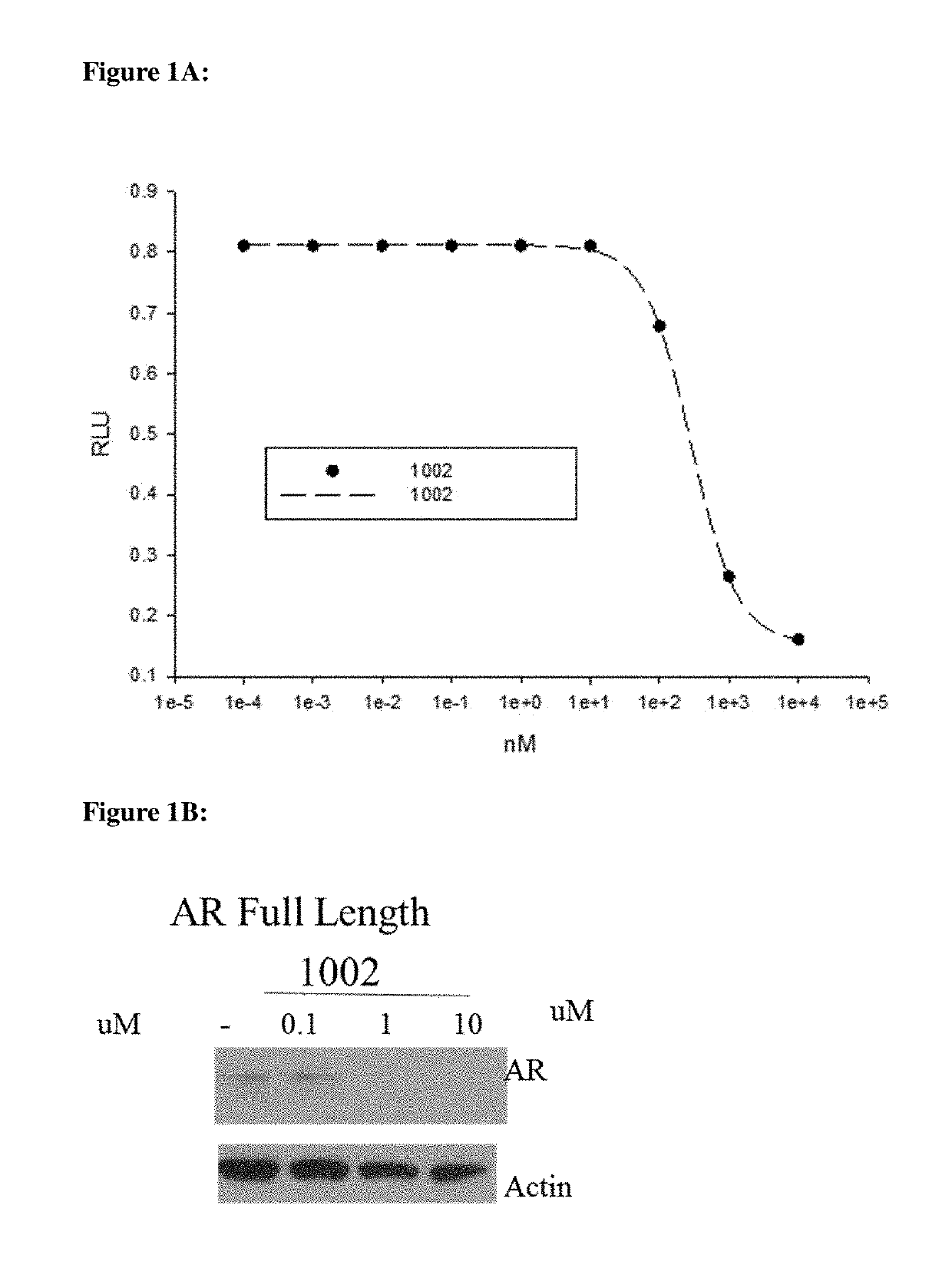
The present invention discloses a diarylhydantoin derivative, and preparation method, pharmaceutical composition, and application thereof. The present invention provides a diarylhydantoin derivative as represented by formula I, a pharmaceutically acceptable salt, solvate, metabolite, stereoisomer, tautomer, polymorph, or prodrug thereof; the diarylhydantoin derivative of the present invention has good performance in treatment of diseases related to androgen receptors, in particular, castration-resistant prostate cancer.
Owner:SHANGAI PHARMA GRP CO LTD +1
Selective androgen receptor degrader (SARD) ligands and methods of use thereof
ActiveUS20170095446A1Improve survivalReduce morbidityOrganic chemistryPharmaceutical delivery mechanismResistance mutationGlutamine
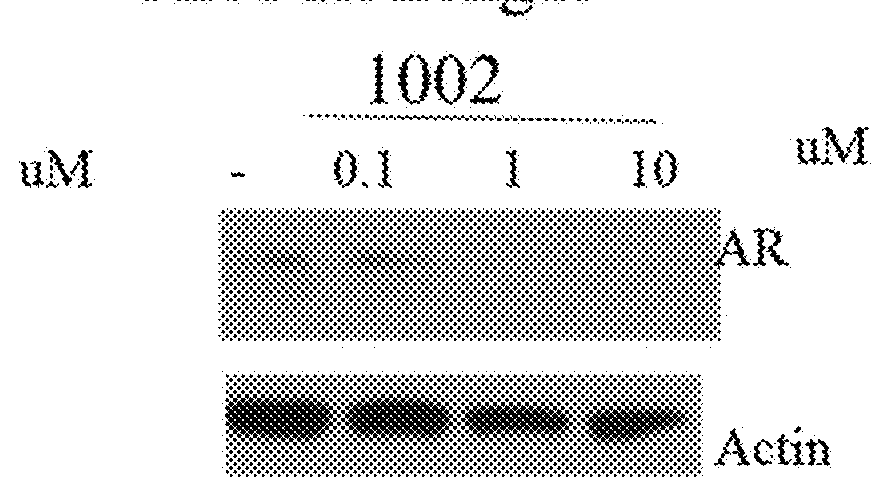
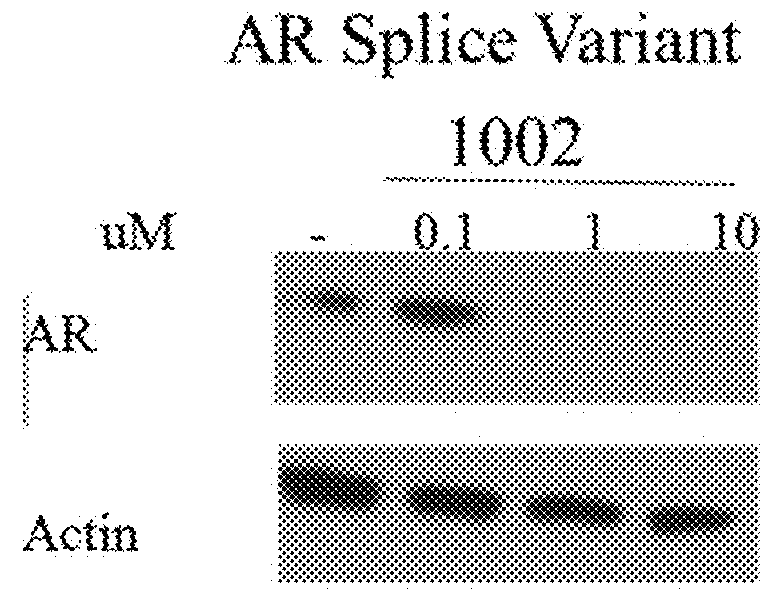
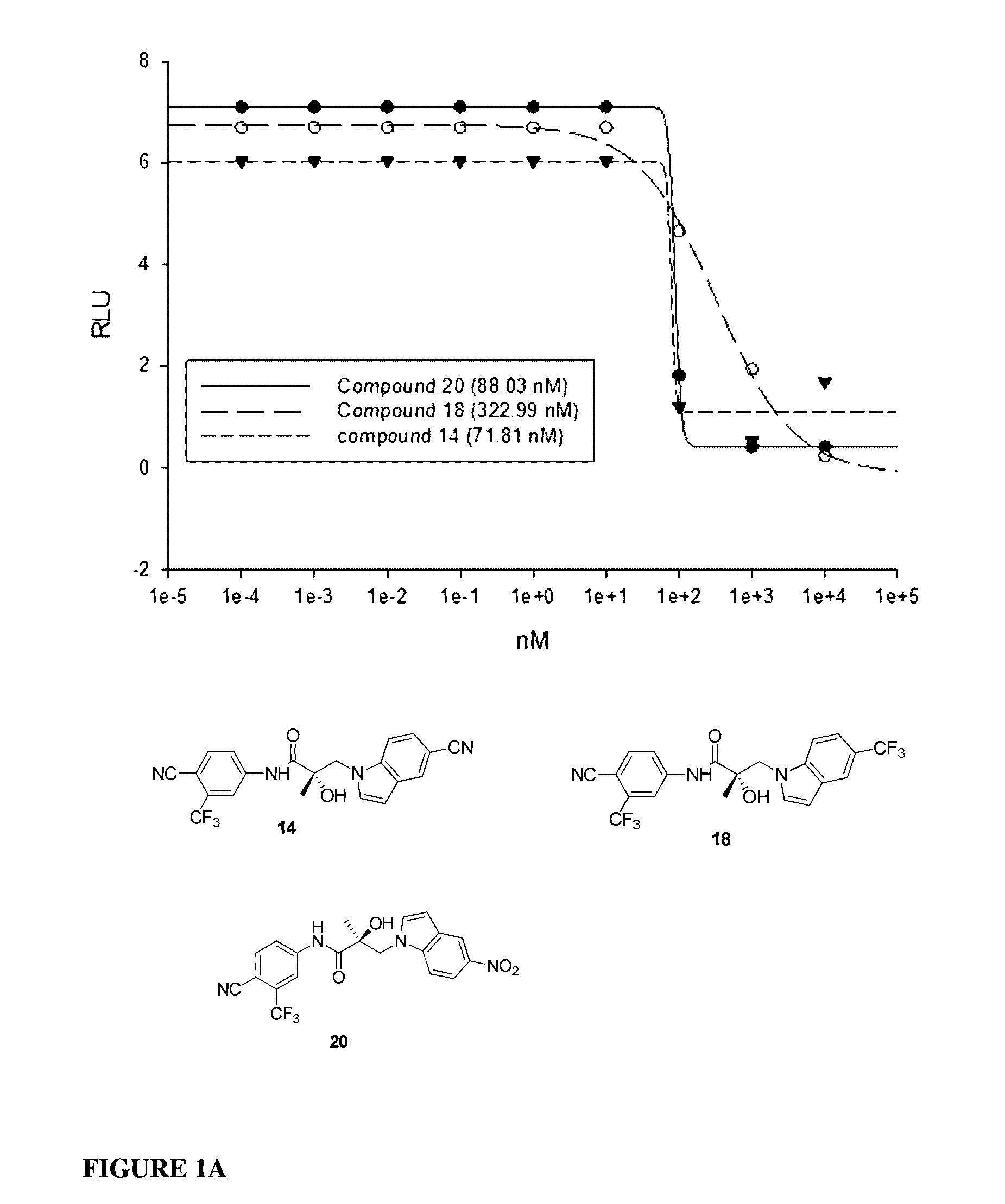
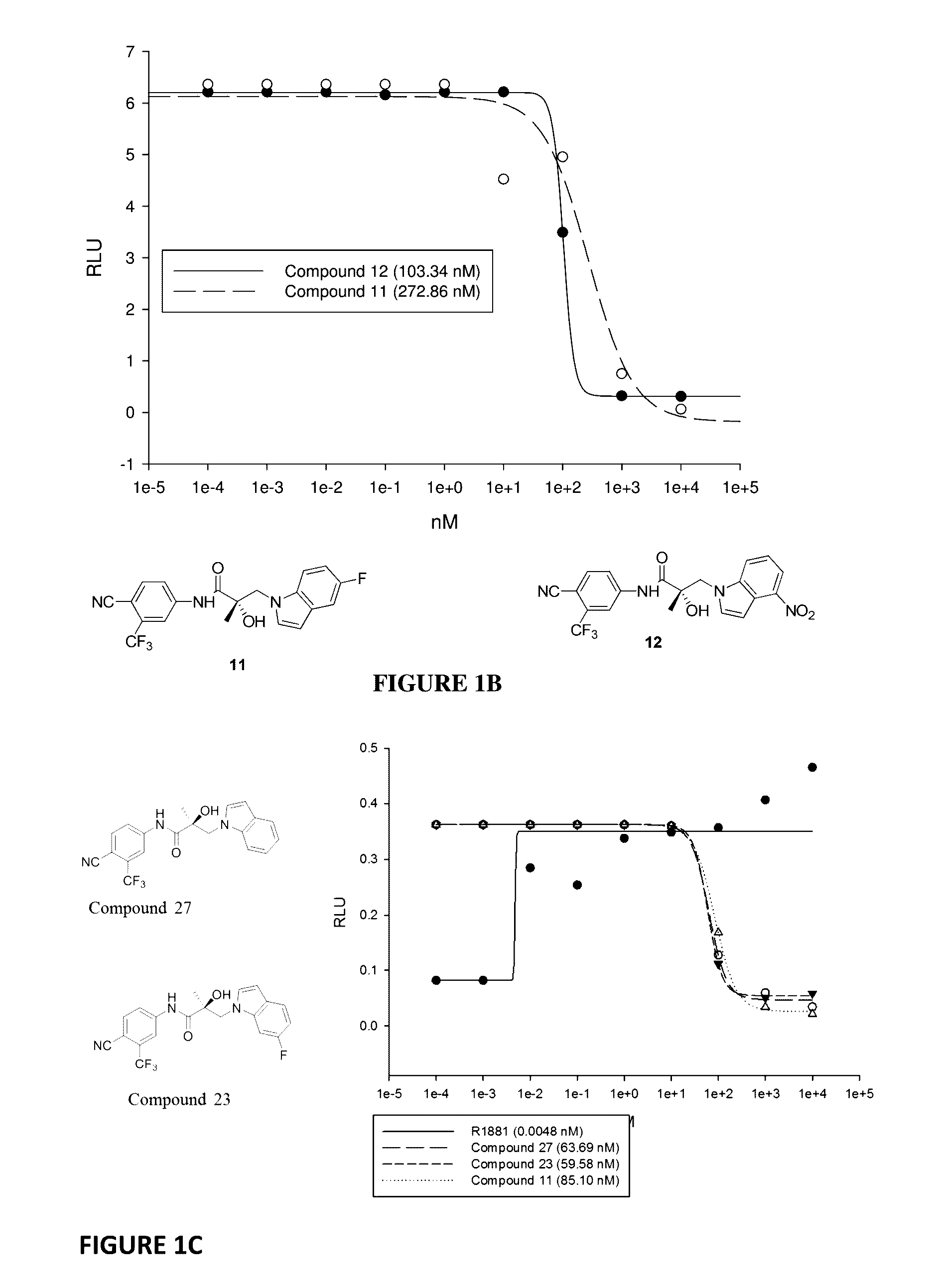
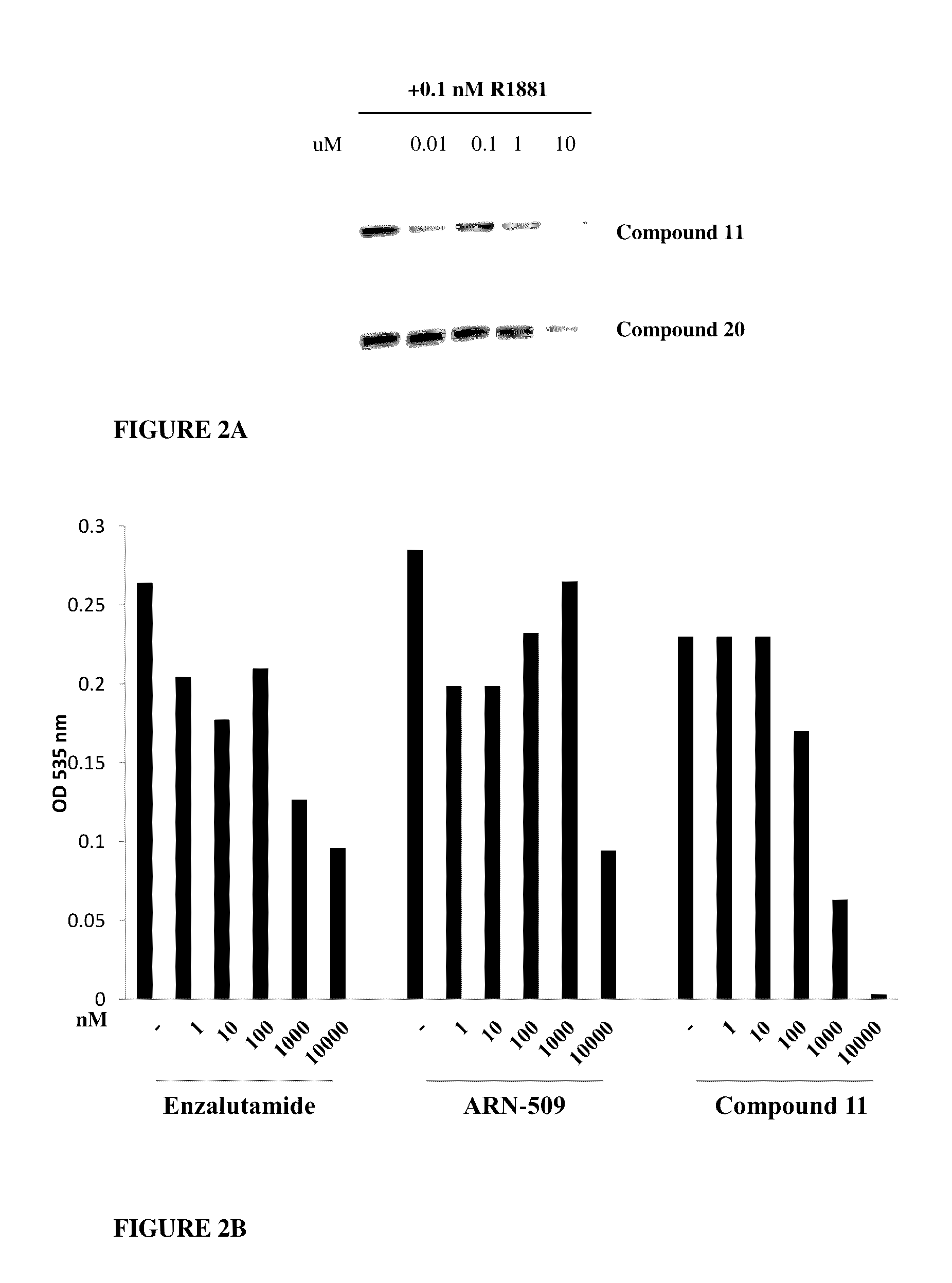
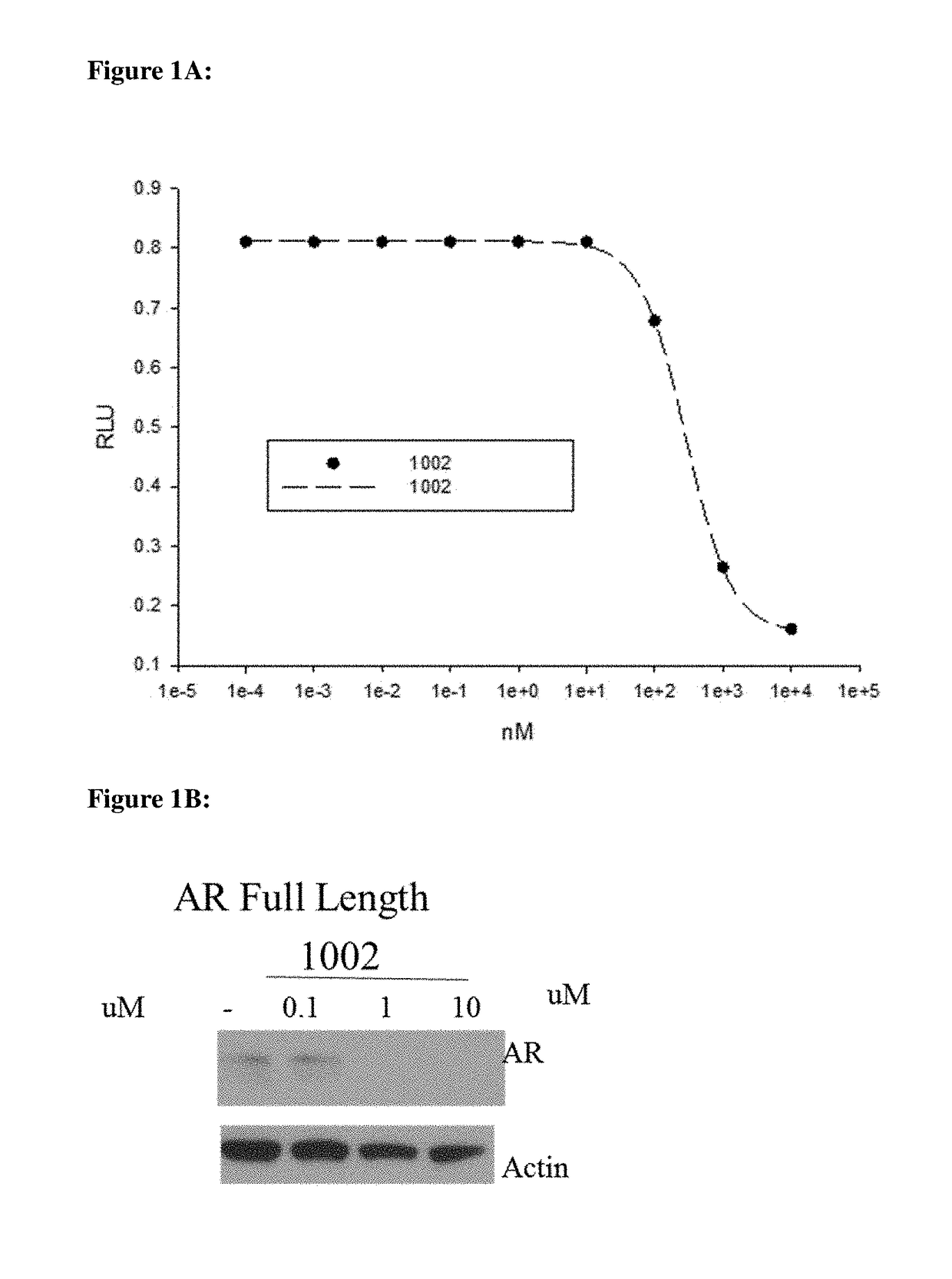
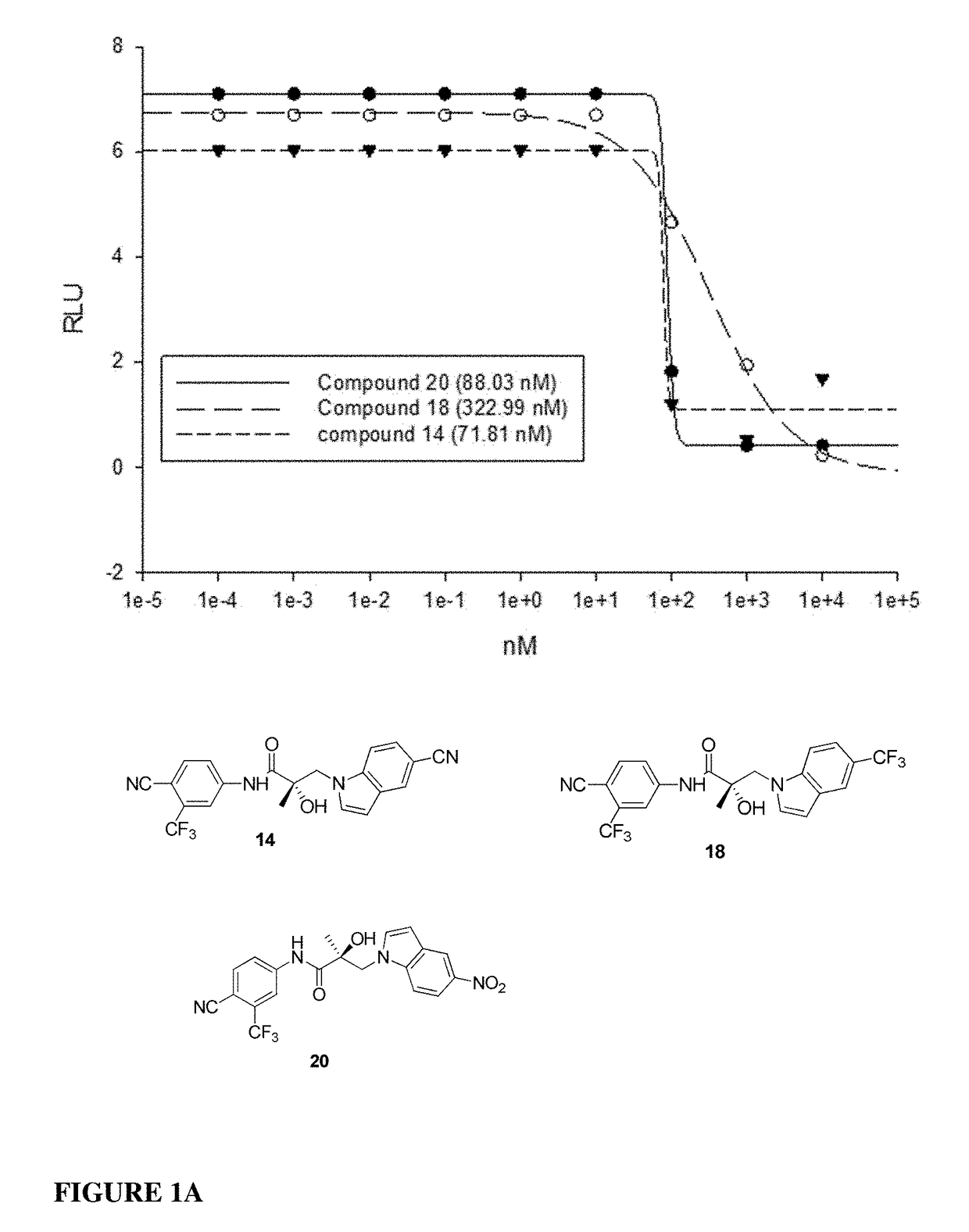
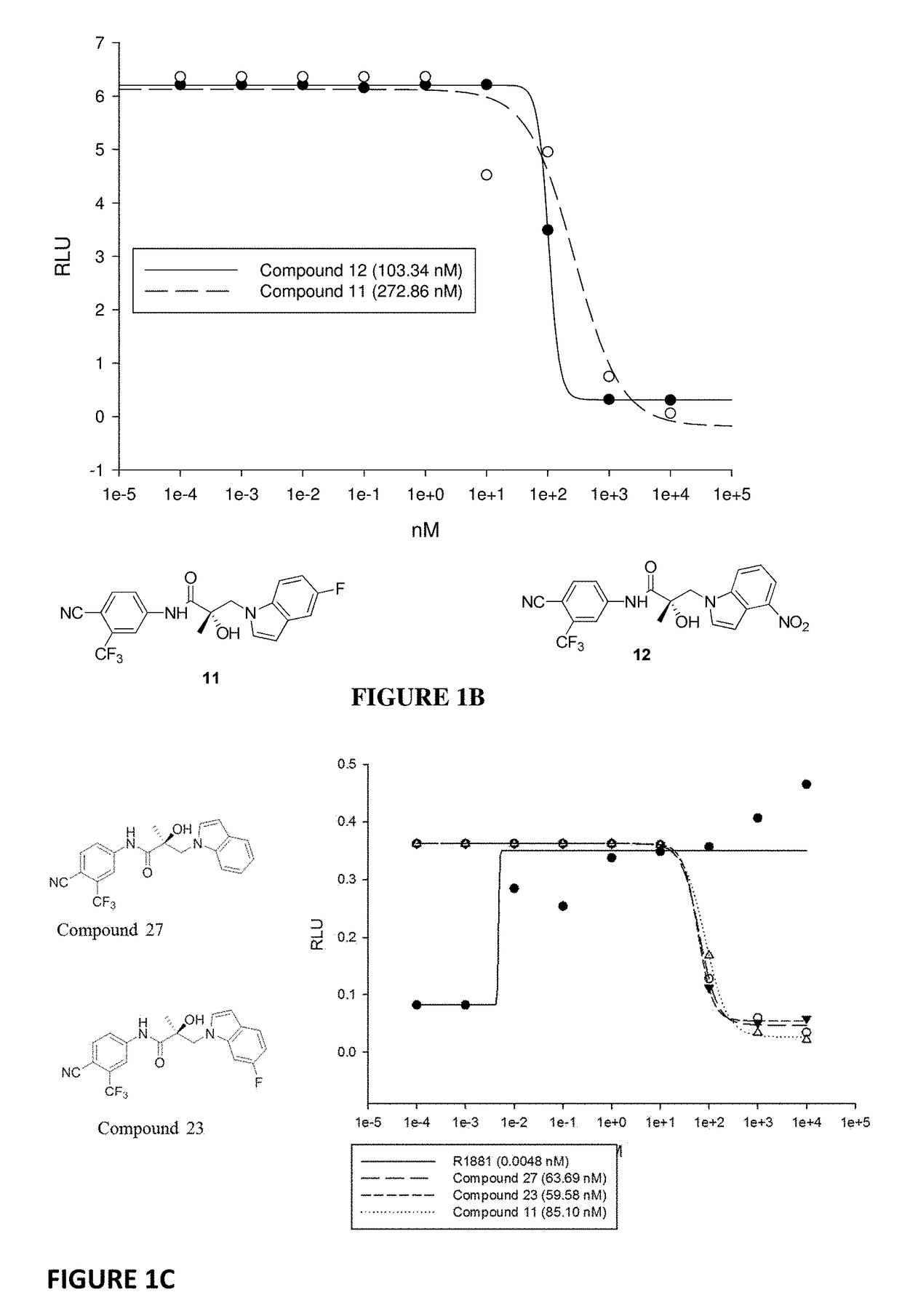
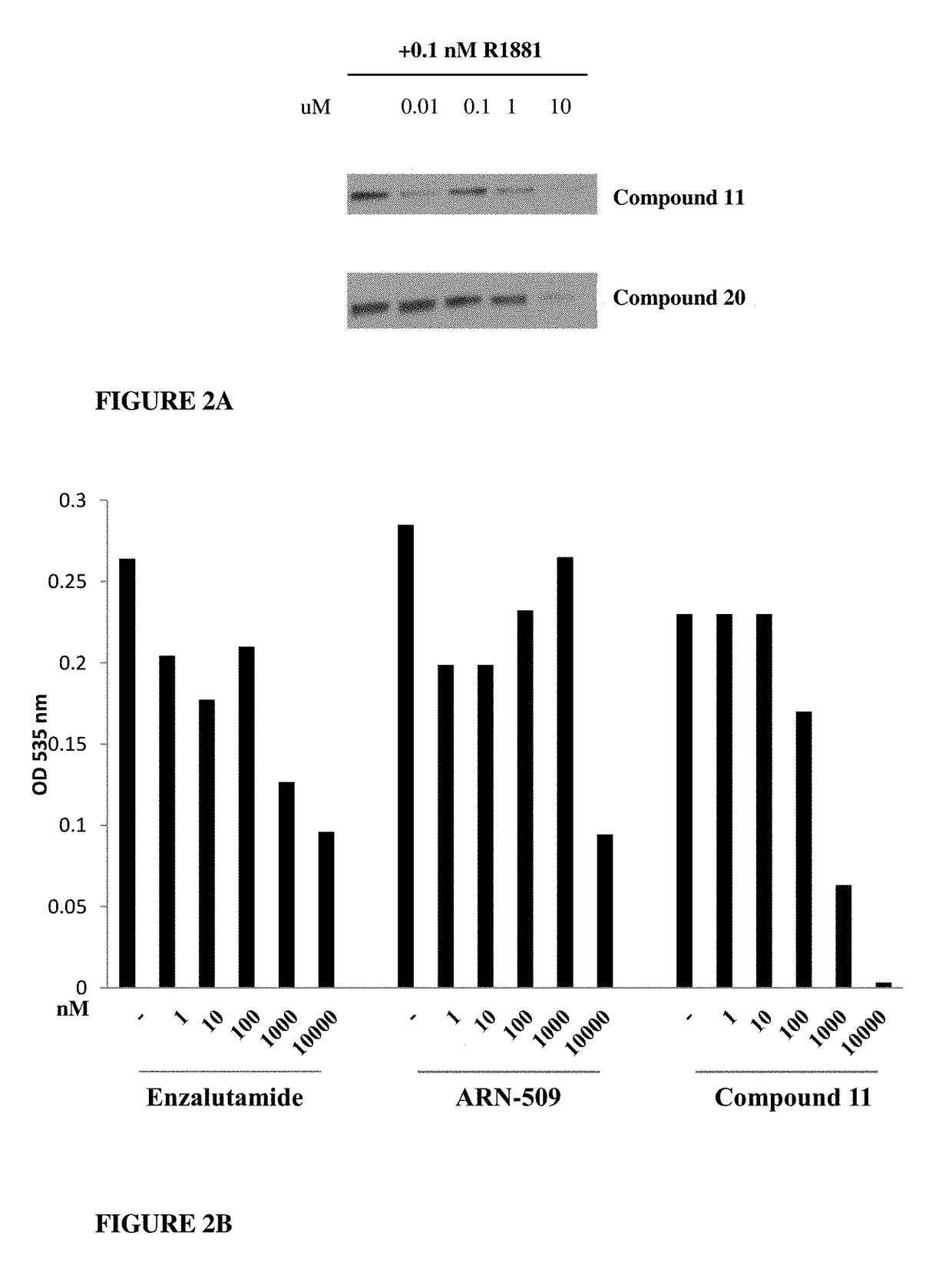
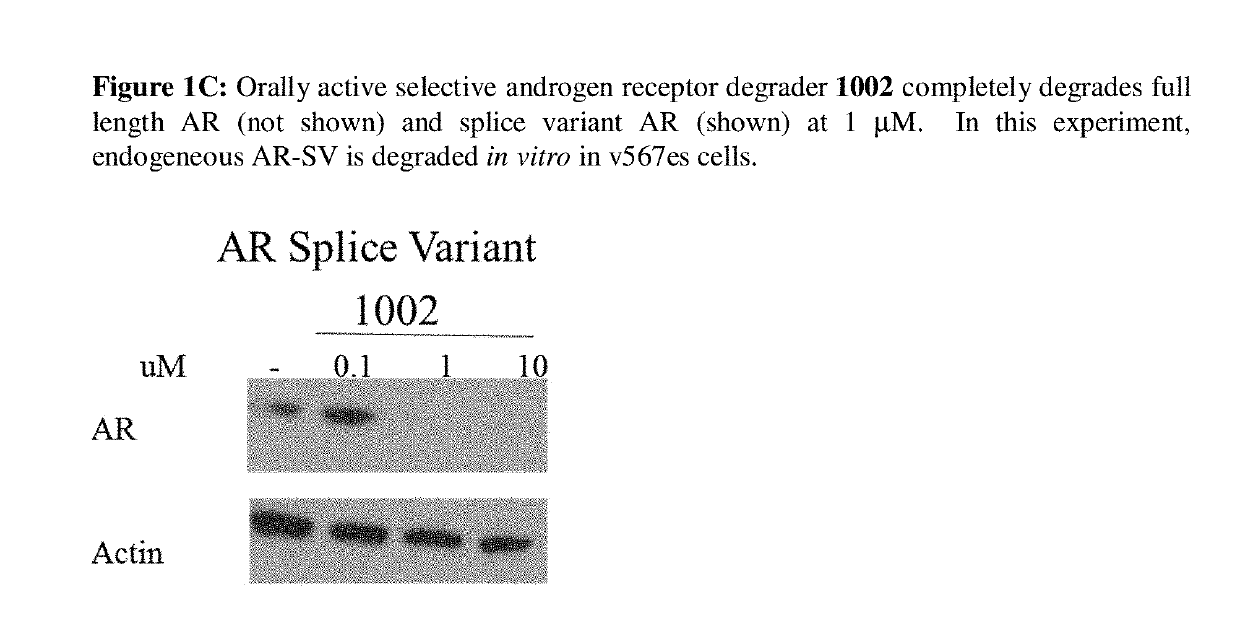
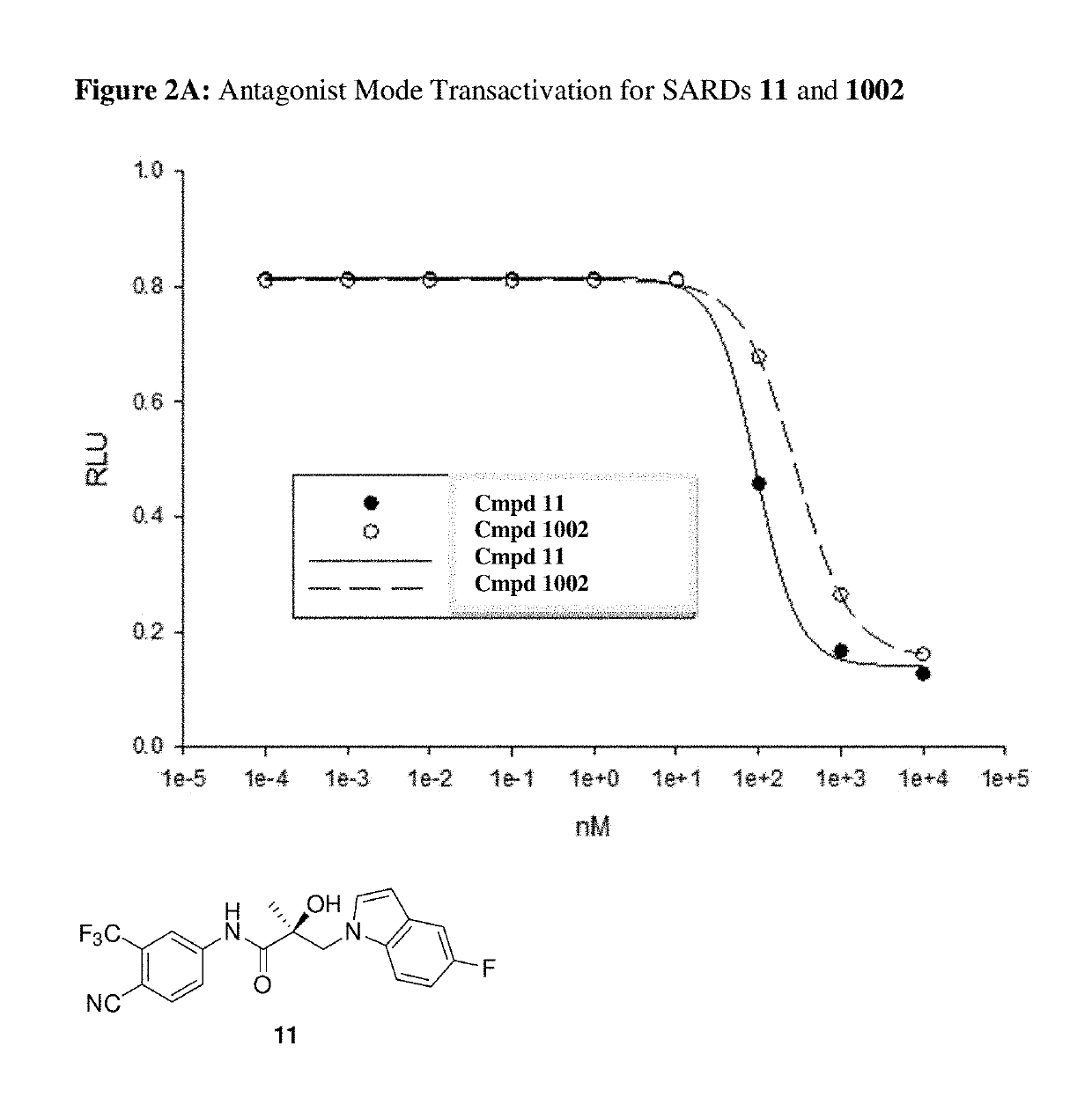
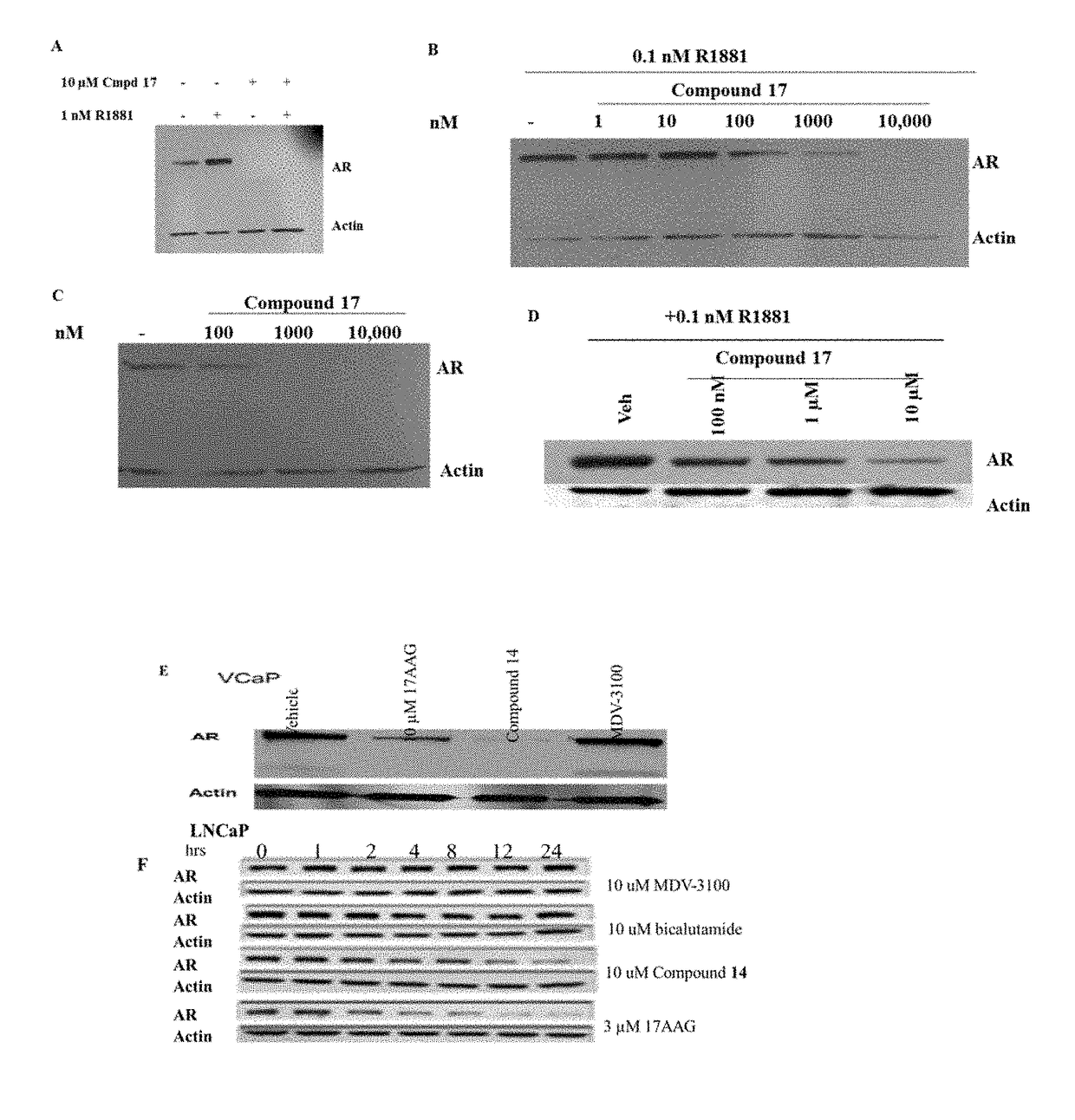
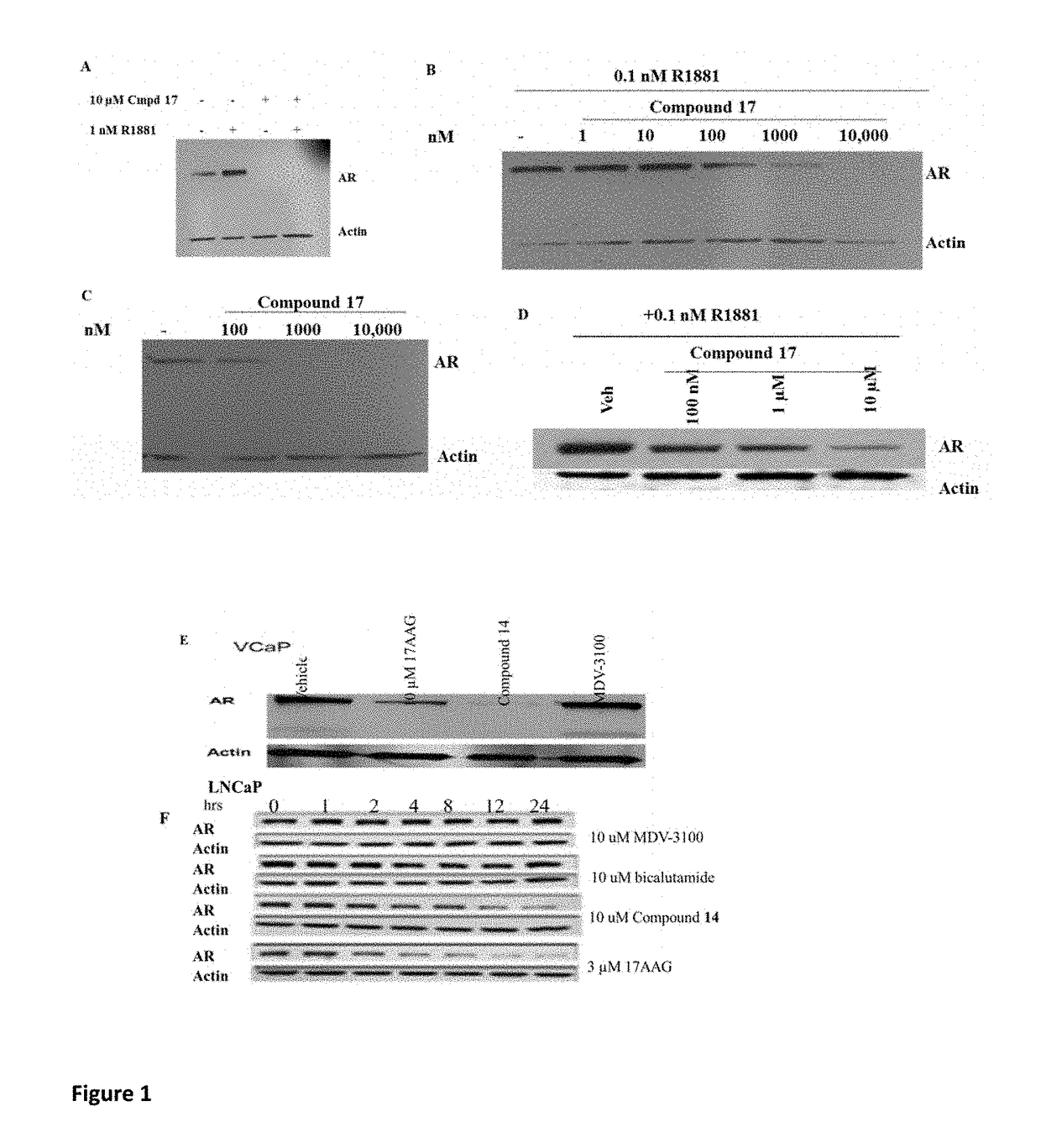
This invention provides novel indole, indazole, benzimidazole, indoline, quinolone, isoquinoline, and carbazole selective androgen receptor degrader (SARD) compounds, pharmaceutical compositions and uses thereof in treating prostate cancer, advanced prostate cancer, castration resistant prostate cancer, androgenic alopecia or other hyper androgenic dermal diseases, Kennedy's disease, amyotrophic lateral sclerosis (ALS), and uterine fibroids, and to methods for reducing the levels of androgen receptor-full length (AR-FL) including pathogenic and / or resistance mutations, AR-splice variants (AR-SV), and pathogenic polyglutamine (polyQ) polymorphisms of AR in a subject.
Owner:UNIV OF TENNESSEE RES FOUND +1
Dual inhibitors of met and vegf for the treatment of castration resistant prostate cancer and osteoblastic bone metastases
InactiveCN103391773AOrganic chemistryAntipyreticDual inhibitorCastration-Resistant Prostate Carcinoma
This invention is directed to the treatment of cancer, particularly castration- resistant prostate cancer and osteoblastic bone metastases, with a dual inhibitor of MET and VEGF.
Owner:EXELIXIS INC
Method of treating cancer
ActiveUS9861624B2Relieve painImprove survivalSkeletal disorderUrinary disorderDual inhibitorOncology
This invention is directed to the treatment of cancer, particularly castration-resistant prostate cancer and osteolytic bone metastases, with a dual inhibitor of MET and VEGF.
Owner:EXELIXIS INC
Selective androgen receptor degrader (SARD) ligands and methods of use thereof
ActiveUS20180273487A1Lower Level RequirementsShorten the lengthOrganic chemistryPharmaceutical delivery mechanismMorpholineResistance mutation
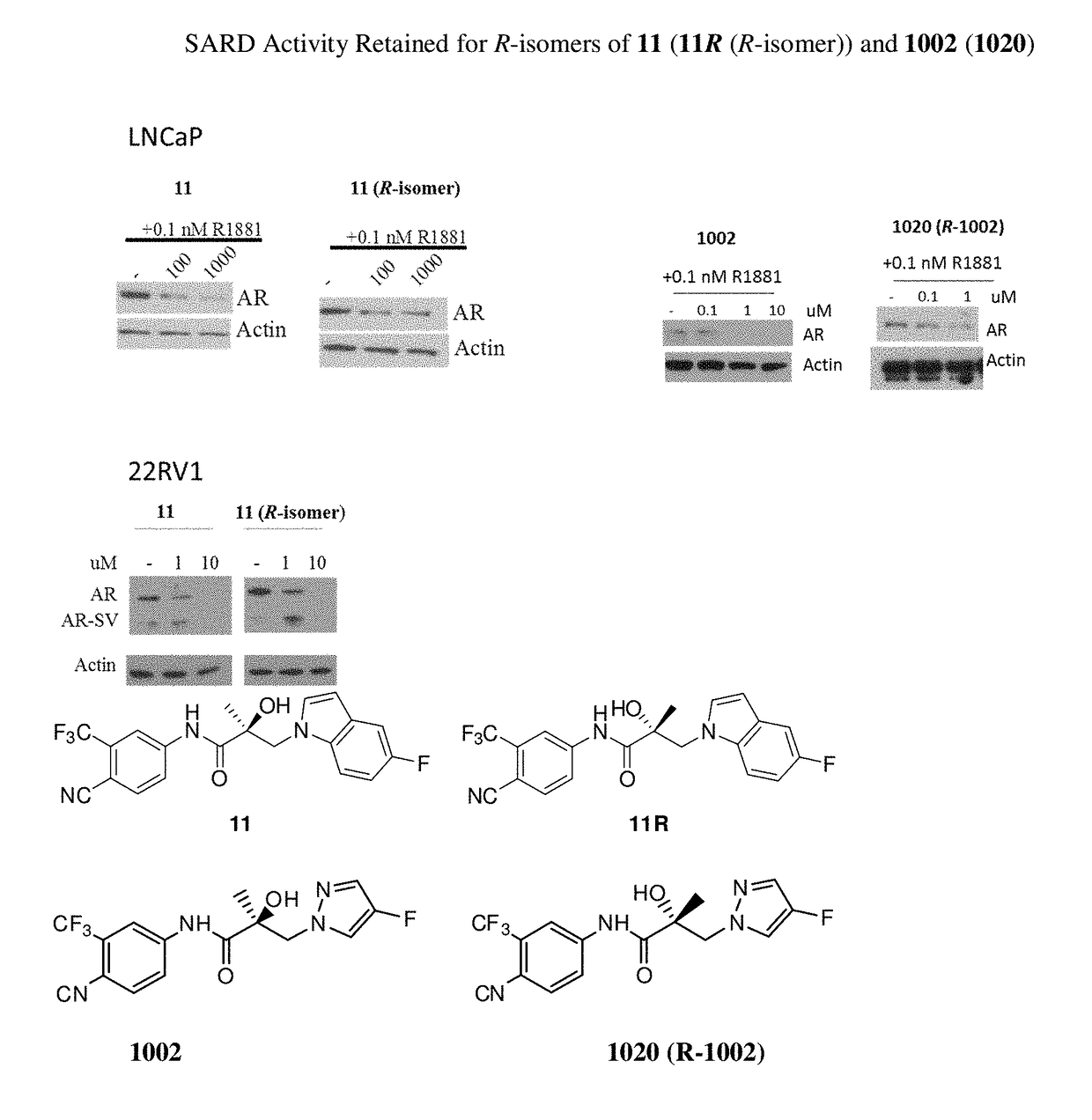
This invention is directed to pyrrole, pyrazole, imidazole, triazole, and morpholine based selective androgen receptor degrader (SARD) compounds including heterocyclic anilide rings and their synthetic precursors, R-isomers, and non-hydroxylated and / or non-chiral propanamides, and pharmaceutical compositions and uses thereof in treating prostate cancer, advanced prostate cancer, castration resistant prostate cancer, triple negative breast cancer, other cancers expressing the androgen receptor, androgenic alopecia or other hyperandrogenic dermal diseases, Kennedy's disease, amyotrophic lateral sclerosis (ALS), abdominal aortic aneurysm (AAA), and uterine fibroids, and to methods for reducing the levels of androgen receptor-full length (AR-FL) including pathogenic or resistance mutations, AR-splice variants (AR-SV), and pathogenic polyglutamine (polyQ) polymorphisms of AR in a subject.
Owner:UNIV OF TENNESSEE RES FOUND
Method of Treating Cancer
ActiveUS20150133494A1Improve survivalTreat and ameliorate and reduce severityBiocideSkeletal disorderDual inhibitorOncology
This invention is directed to the treatment of cancer, particularly castration-resistant prostate cancer and osteolytic bone metastases, with a dual inhibitor of MET and VEGF.
Owner:EXELIXIS INC
Combination Therapies and Methods of Use Thereof for Treating Cancer
ActiveUS20150209359A1Reduced viabilityReduce proliferationBiocideOrganic active ingredientsCombined Modality TherapyActive agent
Pharmaceutical compositions including an effective amount of an antiandrogen or androgen antagonist in combination with a Plk inhibitor and methods of use thereof for treating cancer are disclosed. Administration of the combination of the active agents can be effective to reduce cancer cell proliferation or viability in a subject with cancer to the same degree, or a greater degree than administering to the subject the same amount of either active agent alone. The active agents can be administered together or separately. Methods of selecting and treating subjects with cancers, particular prostate cancers including castration resistant prostate cancer, breast cancers, particularly androgen receptor positive breast cancers, and pancreatic cancers are also provided.
Owner:MASSACHUSETTS INST OF TECH
Selective androgen receptor degrader (SARD) ligands and methods of use thereof
This invention provides novel indole, indazole, benzimidazole, indoline, quinolone, isoquinoline, and carbazole selective androgen receptor degrader (SARD) compounds, pharmaceutical compositions and uses thereof in treating prostate cancer, advanced prostate cancer, castration resistant prostate cancer, other AR-expressing cancers, androgenic alopecia or other hyper androgenic dermal diseases, Kennedy's disease, amyotrophic lateral sclerosis (ALS), abdominal aortic aneurysm (AAA), and uterine fibroids, and to methods for reducing the levels (through degradation) and / or activity (through inhibition) of any androgen receptor including androgen receptor-full length (AR-FL) including pathogenic and / or resistance mutations, AR-splice variants (AR-SV), and pathogenic polyglutamine (polyQ) polymorphisms of AR in a subject.
Owner:UNIV OF TENNESSEE RES FOUND +1
Selective androgen receptor degrader (SARD) ligands and methods of use thereof
ActiveUS20170368003A1Lower Level RequirementsShorten the lengthHalogenated hydrocarbon active ingredientsNervous disorderMorpholineResistance mutation
This invention is directed to pyrrole, pyrazole, imidazole, triazole, and morpholine based selective androgen receptor degrader (SARD) compounds including heterocyclic anilide rings and their synthetic precursors, R-isomers, and non-hydroxylated and / or non-chiral propanamides, and pharmaceutical compositions and uses thereof in treating prostate cancer, advanced prostate cancer, castration resistant prostate cancer, triple negative breast cancer, other cancers expressing the androgen receptor, androgenic alopecia or other hyperandrogenic dermal diseases, Kennedy's disease, amyotrophic lateral sclerosis (ALS), abdominal aortic aneurysm (AAA), and uterine fibroids, and to methods for reducing the levels of androgen receptor-full length (AR-FL) including pathogenic or resistance mutations, AR-splice variants (AR-SV), and pathogenic polyglutamine (polyQ) polymorphisms of AR in a subject.
Owner:UNIV OF TENNESSEE RES FOUND
Selective androgen receptor degrader (SARD) ligands and methods of use thereof
ActiveUS9814698B2Improve survivalReduce morbidityOrganic chemistryPharmaceutical delivery mechanismResistance mutationGlutamine
This invention provides novel indole, indazole, benzimidazole, indoline, quinolone, isoquinoline, and carbazole selective androgen receptor degrader (SARD) compounds, pharmaceutical compositions and uses thereof in treating prostate cancer, advanced prostate cancer, castration resistant prostate cancer, androgenic alopecia or other hyper androgenic dermal diseases, Kennedy's disease, amyotrophic lateral sclerosis (ALS), and uterine fibroids, and to methods for reducing the levels of androgen receptor-full length (AR-FL) including pathogenic and / or resistance mutations, AR-splice variants (AR-SV), and pathogenic polyglutamine (polyQ) polymorphisms of AR in a subject.
Owner:UNIV OF TENNESSEE RES FOUND +1
Methods for diagnosing or treating prostate cancer
InactiveUS20110294123A1Relieve symptomsReduced expression levelCompound screeningApoptosis detectionCancer cellNormal cell
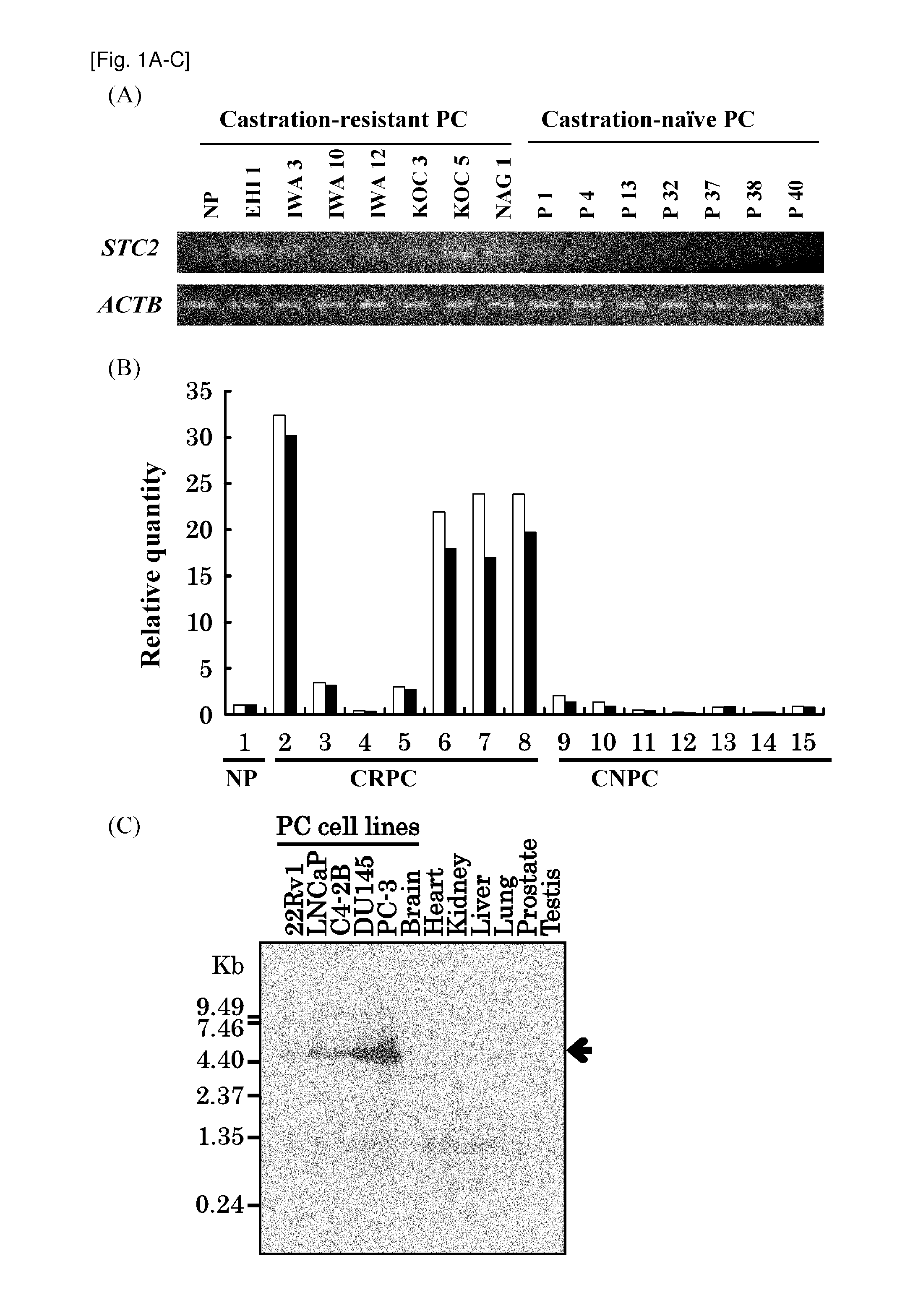
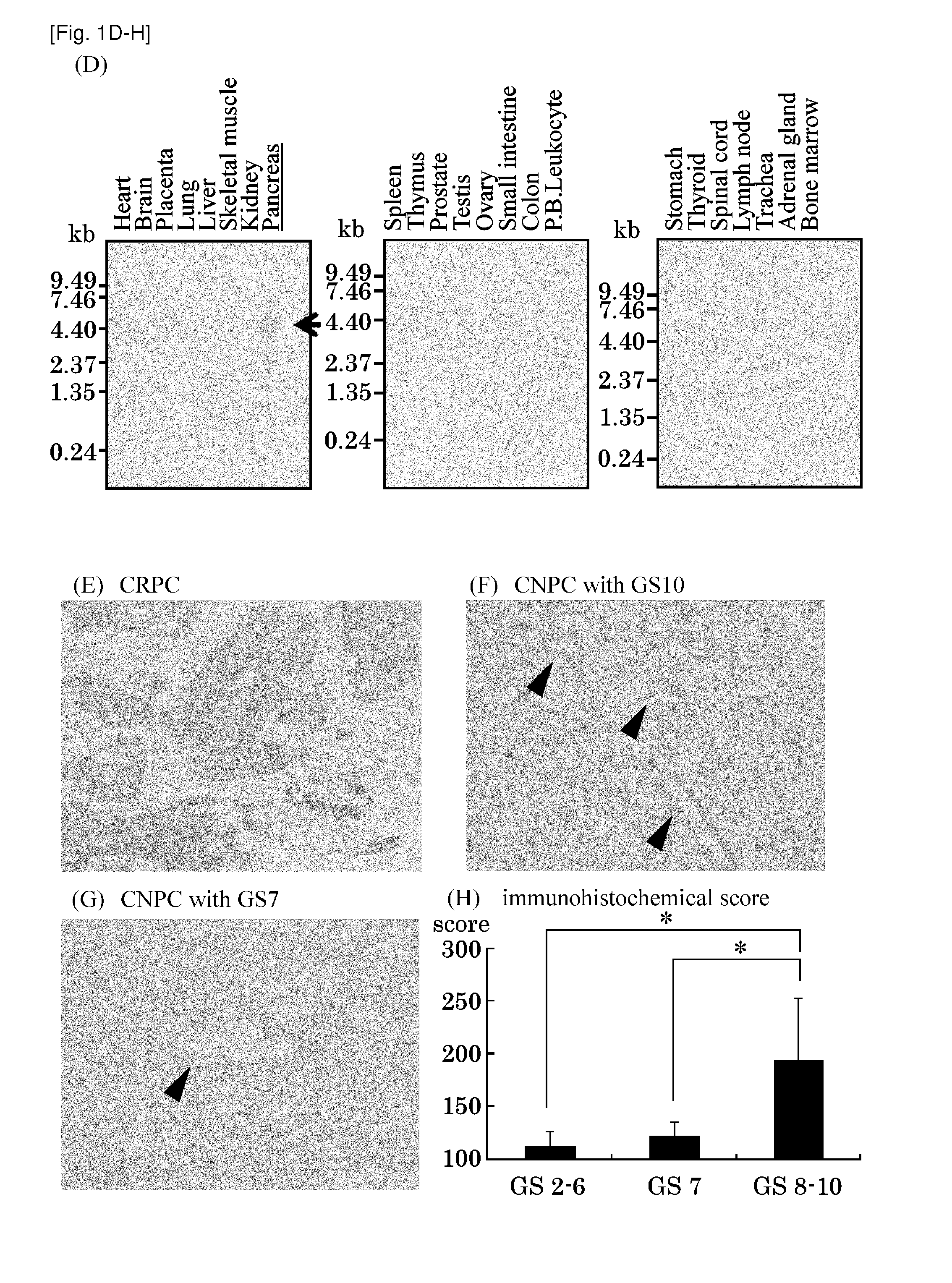
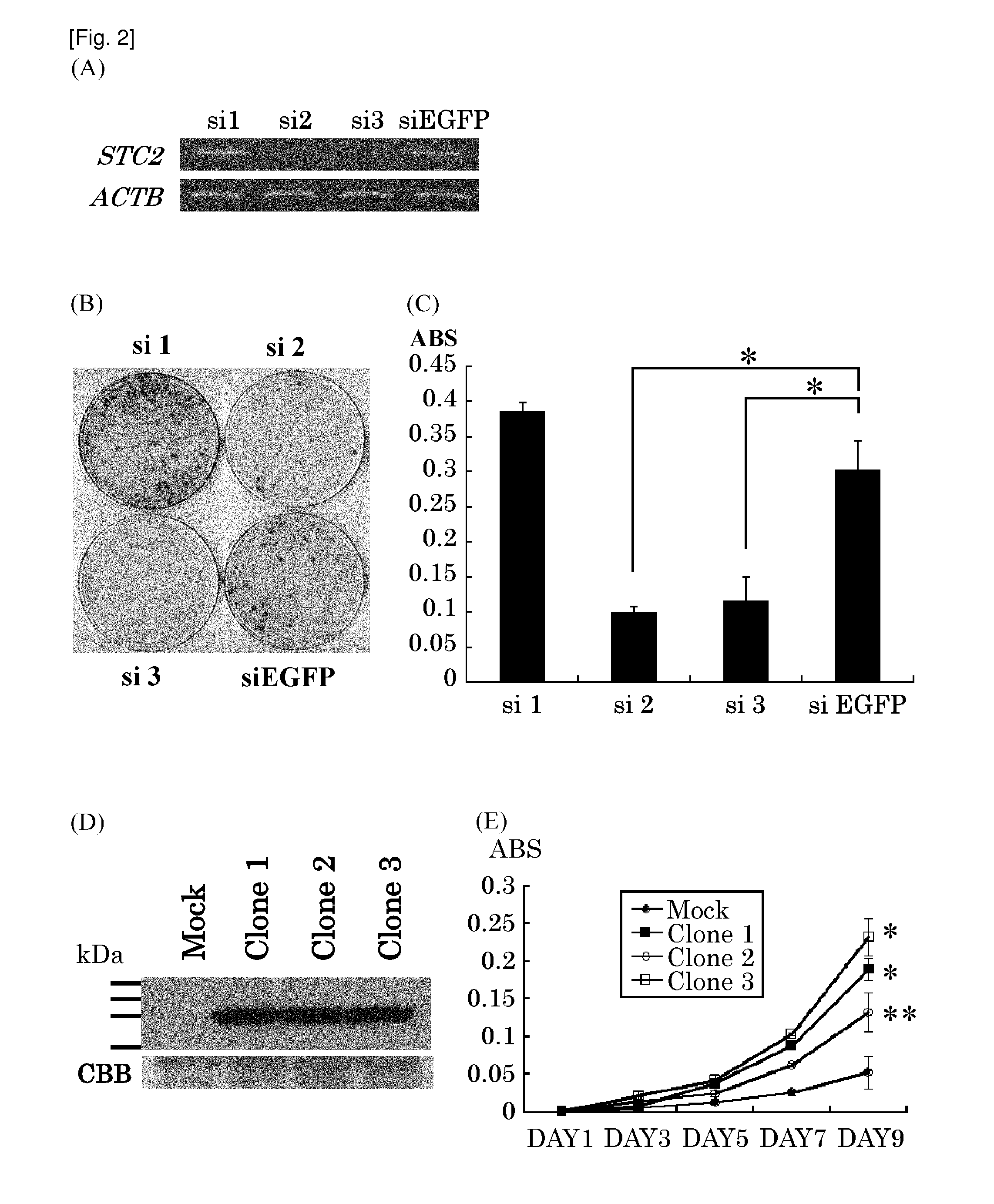
The present invention provides methods for detecting and / or diagnosing cancer through the determination of the expression level of the STC2 gene. The gene was discovered to discriminate cancer cells from normal cells. Furthermore, the present invention provides methods of screening for therapeutic agents useful in the treatment of cancer, methods for treating cancer. Moreover, the present invention provides double-stranded molecules targeting the STC2 gene, which are suggested to be useful in the treatment of cancer. The compositions and methods of the present invention find particular applicability to prostate cancer, more specifically, castration-resistant prostate cancer and aggressive prostate cancer.
Owner:ONCOTHERAPY SCI INC
Selective androgen receptor degrader (SARD) ligands and methods of use thereof
ActiveUS10035763B2Improve survivalReduce morbidityOrganic chemistryPharmaceutical delivery mechanismResistance mutationGlutamine
This invention provides novel indole, indazole, benzimidazole, indoline, quinolone, isoquinoline, and carbazole selective androgen receptor degrader (SARD) compounds, pharmaceutical compositions and uses thereof in treating prostate cancer, advanced prostate cancer, castration resistant prostate cancer, other AR-expressing cancers, androgenic alopecia or other hyper androgenic dermal diseases, Kennedy's disease, amyotrophic lateral sclerosis (ALS), abdominal aortic aneurysm (AAA), and uterine fibroids, and to methods for reducing the levels (through degradation) and / or activity (through inhibition) of any androgen receptor including androgen receptor-full length (AR-FL) including pathogenic and / or resistance mutations, AR-splice variants (AR-SV), and pathogenic polyglutamine (polyQ) polymorphisms of AR in a subject.
Owner:UNIV OF TENNESSEE RES FOUND +1
Selective androgen receptor degrader (SARD) ligands and methods of use thereof
This invention provides novel indole, indazole, benzimidazole, indoline, quinolone, isoquinoline, and carbazole selective androgen receptor degrader (SARD) compounds, pharmaceutical compositions and uses thereof in treating prostate cancer, advanced prostate cancer, castration resistant prostate cancer, other AR-expressing cancers, androgenic alopecia or other hyper androgenic dermal diseases, Kennedy's disease, amyotrophic lateral sclerosis (ALS), abdominal aortic aneurysm (AAA), and uterine fibroids, and to methods for reducing the levels (through degradation) and / or activity (through inhibition) of any androgen receptor including androgen receptor-full length (AR-FL) including pathogenic and / or resistance mutations, AR-splice variants (AR-SV), and pathogenic polyglutamine (polyQ) polymorphisms of AR in a subject.
Owner:UNIV OF TENNESSEE RES FOUND +1
Processes for the synthesis of diarylthiohydantoin and diarylhydantoin compounds
ActiveUS20130190507A1Organic active ingredientsOrganic compound preparationPharmacy medicineMedicine
Owner:MEDIVATION PROSTATE THERAPEUTICS LLC
Selective androgen receptor degrader (SARD) ligands and methods of use thereof
ActiveUS10314797B2Halogenated hydrocarbon active ingredientsNervous disorderMorpholineResistance mutation
This invention is directed to pyrrole, pyrazole, imidazole, triazole, and morpholine based selective androgen receptor degrader (SARD) compounds including heterocyclic anilide rings and their synthetic precursors, R-isomers, and non-hydroxylated and / or non-chiral propanamides, and pharmaceutical compositions and uses thereof in treating prostate cancer, advanced prostate cancer, castration resistant prostate cancer, triple negative breast cancer, other cancers expressing the androgen receptor, androgenic alopecia or other hyperandrogenic dermal diseases, Kennedy's disease, amyotrophic lateral sclerosis (ALS), abdominal aortic aneurysm (AAA), and uterine fibroids, and to methods for reducing the levels of androgen receptor-full length (AR-FL) including pathogenic or resistance mutations, AR-splice variants (AR-SV), and pathogenic polyglutamine (polyQ) polymorphisms of AR in a subject.
Owner:UNIV OF TENNESSEE RES FOUND
Selective androgen receptor degrader (SARD) ligands and methods of use thereof
ActiveUS9834507B2Improve survivalReduce morbidityOrganic chemistry methodsPharmaceutical delivery mechanismAmyotrophic lateral sclerosisAndrogen
This invention provides novel 3-amino propanamide selective androgen receptor degrader (SARD) compounds, pharmaceutical compositions and uses thereof in treating prostate cancer, advanced prostate cancer, castration resistant prostate cancer, androgenic alopecia or other hyperandrogenic dermal diseases, Kennedy's disease, amyotrophic lateral sclerosis (ALS), and uterine fibroids, and to methods for reducing the levels of androgen receptor-full length (AR-FL) including pathogenic or resistance mutations, AR-splice variants (AR-SV), and pathogenic polyglutamine (polyQ) polymorphisms of AR in a subject.
Owner:ONCTERNAL THERAPEUTICS INC +1
Co-targeting androgen receptor splice variants and mtor signaling pathway for the treatment of castration-resistant prostate cancer
InactiveUS20170056336A1Reducing and preventing tumor growthPrevent and reduce tumor growthEther/acetal active ingredientsAntineoplastic agentsMedicineAndrogen Receptor Gene
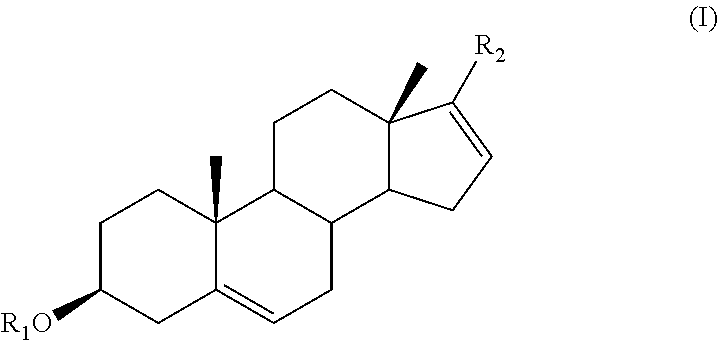
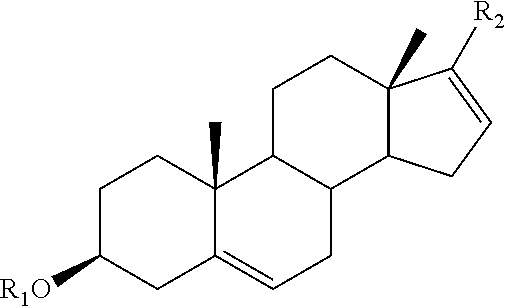
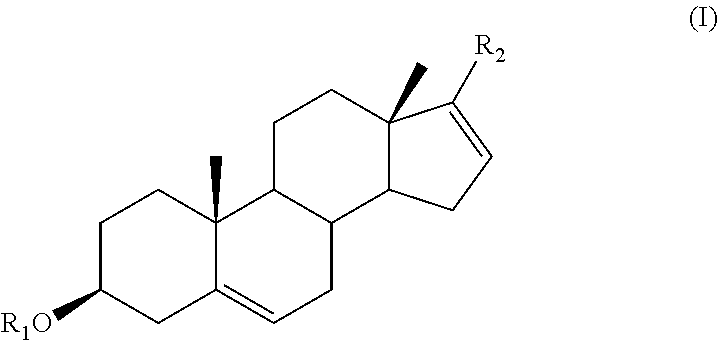
The present invention provides methods, compositions, and combinations for treating cancer via combined use of a compound of formula (I), and / or their subgenra, or a pharmaceutically acceptable salt, tautomer or stereoisomer thereof, wherein R1, R2, R3, R8, R9, R11a, R11b, R11c, and R11d are as defined herein, and at least one therapeutically active agents selected from inhibitors of PI3K / AKT / mTOR pathway, active agents associated with the treatment of prostate cancer, and anticancer agents.
Owner:BRITISH COLUMBIA CANCER AGENCY BRANCH
Selective androgen receptor degrader (SARD) ligands and methods of use thereof
ActiveUS9815776B2Improve survivalReduce morbidityNervous disorderOrganic chemistry methodsResistance mutationGlutamine
Owner:ONCTERNAL THERAPEUTICS INC +1
Novel therapy for prostate carcinoma
InactiveUS20160022606A1Growth inhibitionInhibits and delays onsetBiocideAnimal repellantsLyase activityHormone dependence
Disclosed herein are methods of inhibiting or delaying the growth of androgen-dependent prostate cancer, and / or inhibiting or delaying the onset of castration-resistant prostate cancer (CRPC) by administering naphthoquinone analogs, such as plumbagin, and specified hormone therapy agents, including selective inhibitors of 17,20-lyase activity of CYP 17.
Owner:PELLFICURE PHARMA
Selective androgen receptor degrader (SARD) ligands and methods of use thereof
ActiveUS20180360805A1Antineoplastic agentsHeterocyclic compound active ingredientsMalignancyResistance mutation
This invention provides novel indole, indazole, benzimidazole, benzotriazole, indoline, quinolone, isoquinoline, and carbazole selective androgen receptor degrader (SARD) compounds, pharmaceutical compositions and uses thereof in treating hyperproliferations of the prostate including pre-malignancies and benign prostatic hyperplasia, prostate cancer, advanced prostate cancer, castration resistant prostate cancer, other AR-expressing cancers, androgenic alopecia or other hyper androgenic dermal diseases, Kennedy's disease, amyotrophic lateral sclerosis (ALS), abdominal aortic aneurysm (AAA), and uterine fibroids, and to methods for reducing the levels (through degradation) and / or activity (through inhibition) of any androgen receptor including androgen receptor-full length (AR-FL) including pathogenic and / or resistance mutations, AR-splice variants (AR-SV), and pathogenic polyglutamine (polyQ) polymorphisms of AR in a subject.
Owner:UNIV OF TENNESSEE RES FOUND +1
Kit for detecting metastatic castration resistant prostate cancer (mCRPC) drug resistance
ActiveCN105779599AIt has the function of guiding medicineGuaranteed PCR amplification requirementsMicrobiological testing/measurementAntigenFluorescence
The invention provides a kit for detecting metastatic castration resistant prostate cancer (mCRPC) drug resistance. The kit is characterized by comprising an antibody-oligonucleotide probe obtained by independently coupling PSA (prostate specific antigen) and PSMA (prostate specific membrane antigen) antibodies with different oligonucleotides; an amplification primer and a buffer solution for performing PCR (polymerase chain reaction) amplification on the antibody-oligonucleotide probe; and a fluorescent probe. By detecting the expression quantity of PSA and PSMA antigens, the kit can analyze the relative activity of AR signals, thereby detecting the mCRPC drug resistance of a patient.
Owner:SHANGHAI MAJORBIO BIO PHARM TECH
Estrogen receptor ligands and methods of use thereof
InactiveUS20120077845A1Decreased bone mineral densityIncreased riskBiocideAmine active ingredientsIncreased riskEstrogen receptor
The present invention relates to methods for reducing testosterone levels by reduction of luteinizing hormone (LH) or independent of LH levels in a male subject and methods of treating, suppressing, reducing the incidence, reducing the severity, or inhibiting prostate cancer, advanced prostate cancer, and castration-resistant prostate cancer (CRPC) and palliative treatment of prostate cancer, advanced prostate cancer and castration-resistant prostate cancer (CRPC). The compounds of this invention suppress free or total testosterone levels to castrate levels which may be used to treat prostate cancer, advanced prostate cancer, and CRPC without causing bone loss, decreased bone mineral density, increased risk of bone fractures, increased body fat, hot flashes and / or gynecomastia.
Owner:GTX INCORPORATED
Selective androgen receptor degrader (SARD) Ligands and methods of use thereof
ActiveUS10441570B2Antineoplastic agentsHeterocyclic compound active ingredientsResistance mutationMalignancy
Owner:UNIV OF TENNESSEE RES FOUND +1
Biomarkers for treatment of neoplastic disorders using androgen-targeted therapies
InactiveUS20150374721A1Organic active ingredientsMicrobiological testing/measurementLyase inhibitorMolecular Targeted Therapies
Described herein are methods and compositions for the treatment of prostate cancer in a subject in need thereof. The prostate cancer may be a castration resistant and an androgen receptor antagonist-resistant prostate cancer. The methods may comprise administering to the subject a CYP17-lyase inhibitor of Formula II.
Owner:UNIV OF MARYLAND +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com