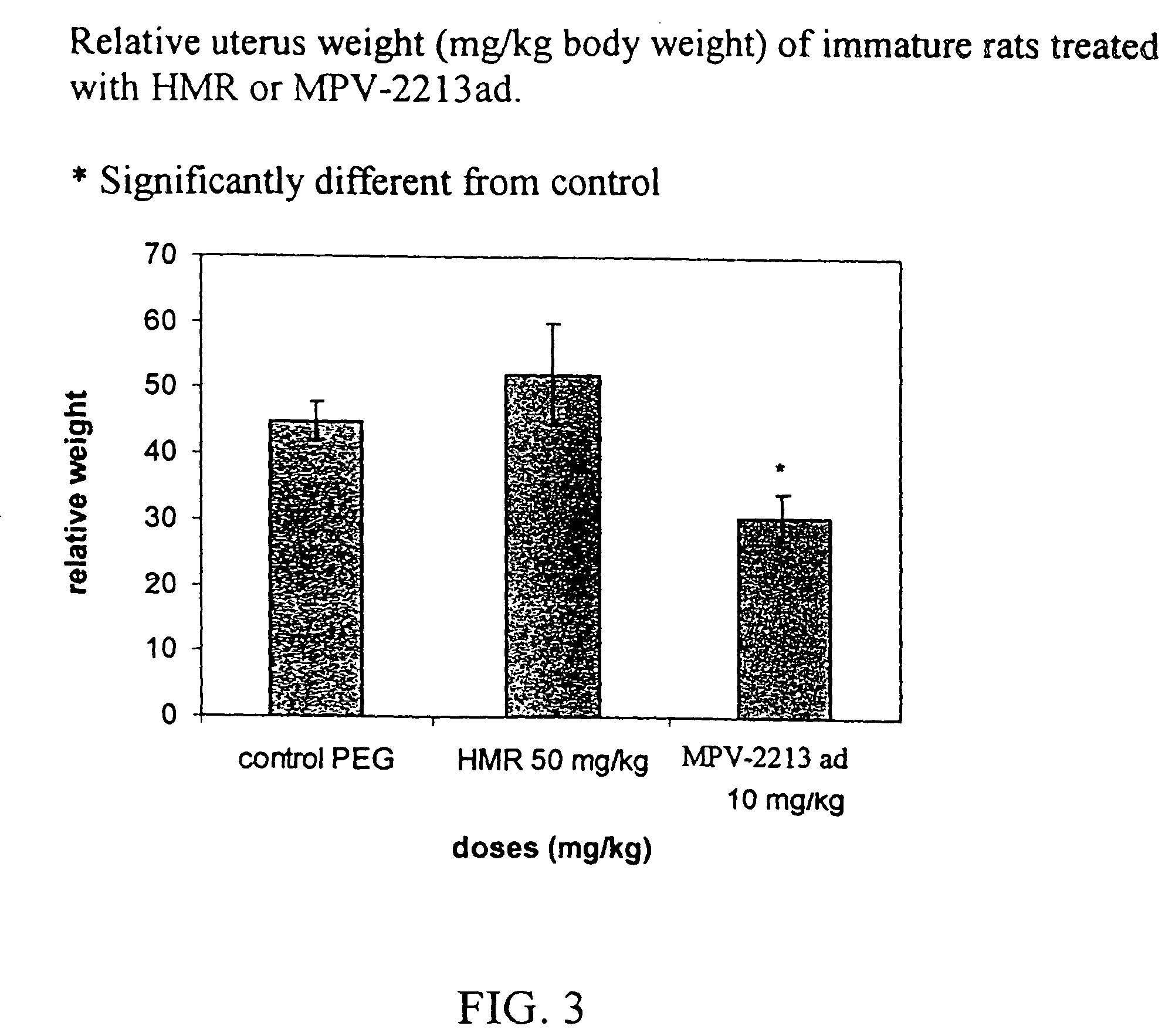
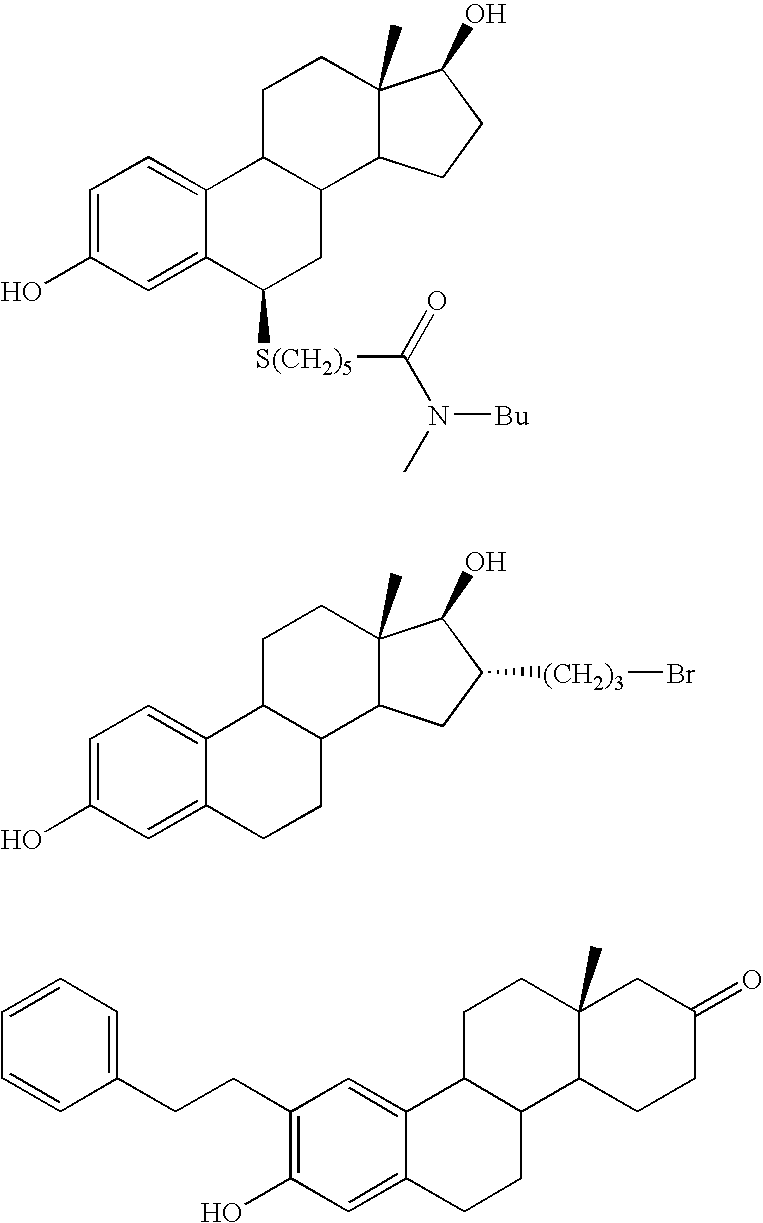
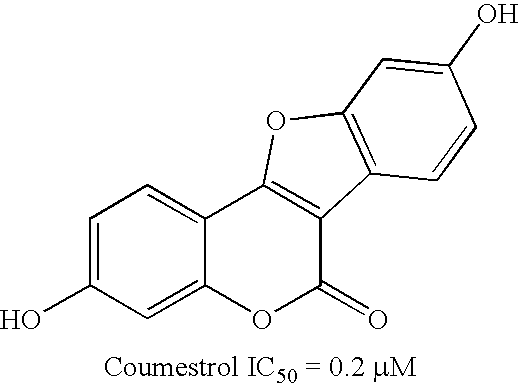
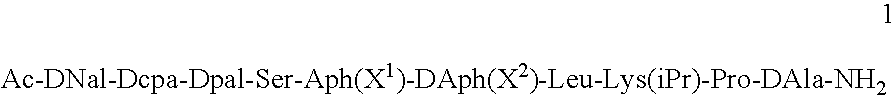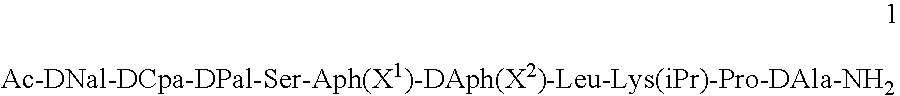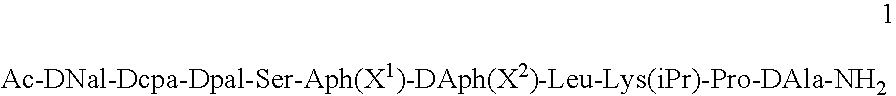Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
219 results about "Hormone dependence" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Types of hormone signaling. Hormonal effects are dependent on where they are released, as they can be released in different manners. Not all hormones are released from a cell and into the blood until it binds to a receptor on a target.
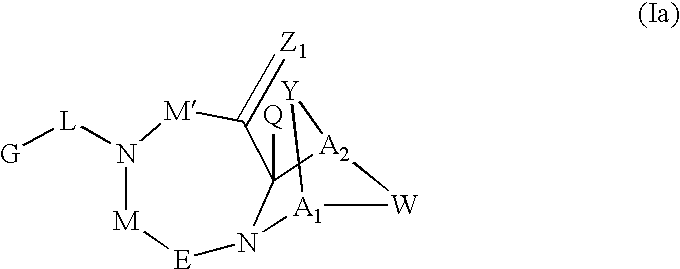
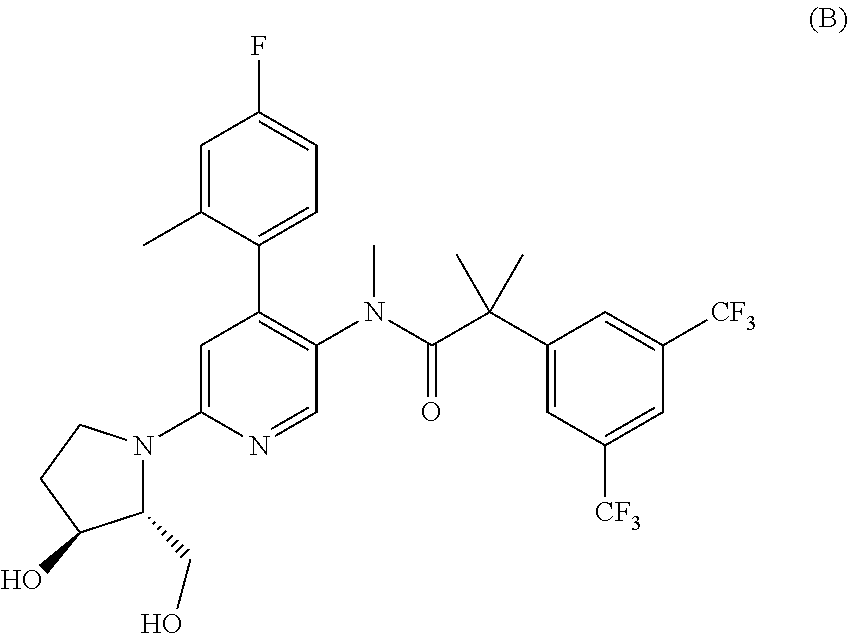
Method for the treatment of a condition remediable by administration of a selective androgen receptor modulator
InactiveUS6960474B2BiocidePeptide/protein ingredientsSelective androgen receptor modulatorHormone dependence
Owner:BRISTOL MYERS SQUIBB CO
17Beta-Hydroxysteroid Dehydrogenase Type 1 Inhibitors for the Treatment of Hormone-Related Diseases
The invention relates to the use of non-steroidal 17beta-hydroxysteroid dehydrogenase type 1 inhibitors for the treatment and prophylaxis of hormone-dependent, particularly estrogen-dependent, diseases. The invention further relates to suitable inhibitors and to a method for the production thereof.
Owner:UNIV DES SAARLANDES
Use of progesterone receptor modulators
A progesterone receptor modulator of the structure is provided. Use of compositions containing this compound for contraception, hormone replacement therapy, treating hormone-dependent disease, synchronizing estrus, treating dysmenorrhea, treating dysfunctional uterine bleeding, inducing amenorrhea, or treating symptoms of premenstrual syndrome and premenstrual dysphoric disorder in a mammal are described.
Owner:WYETH LLC
Compositions and methods for treating estrogen-dependent diseases and conditions
A pharmaceutical composition for the treatment of an estrogen-dependent disease or condition comprises: (1) at least one polysaccharide selected from the group consisting of an alginate and a fucoidan in a quantity effective to treat an estrogen-dependent disease or condition; and (2) a pharmaceutically acceptable carrier. The composition can include both an alginate and a fucoidan. The composition can include other ingredients such as at least one compound selected from the group consisting of diindolylmethane and indole-3-carbinol in a quantity sufficient to inhibit the activity of estrogen. Methods for use of the composition for the treatment of an estrogen-dependent disease or condition, especially endometriosis, are described.
Owner:CONCOURSE HEALTH SCI
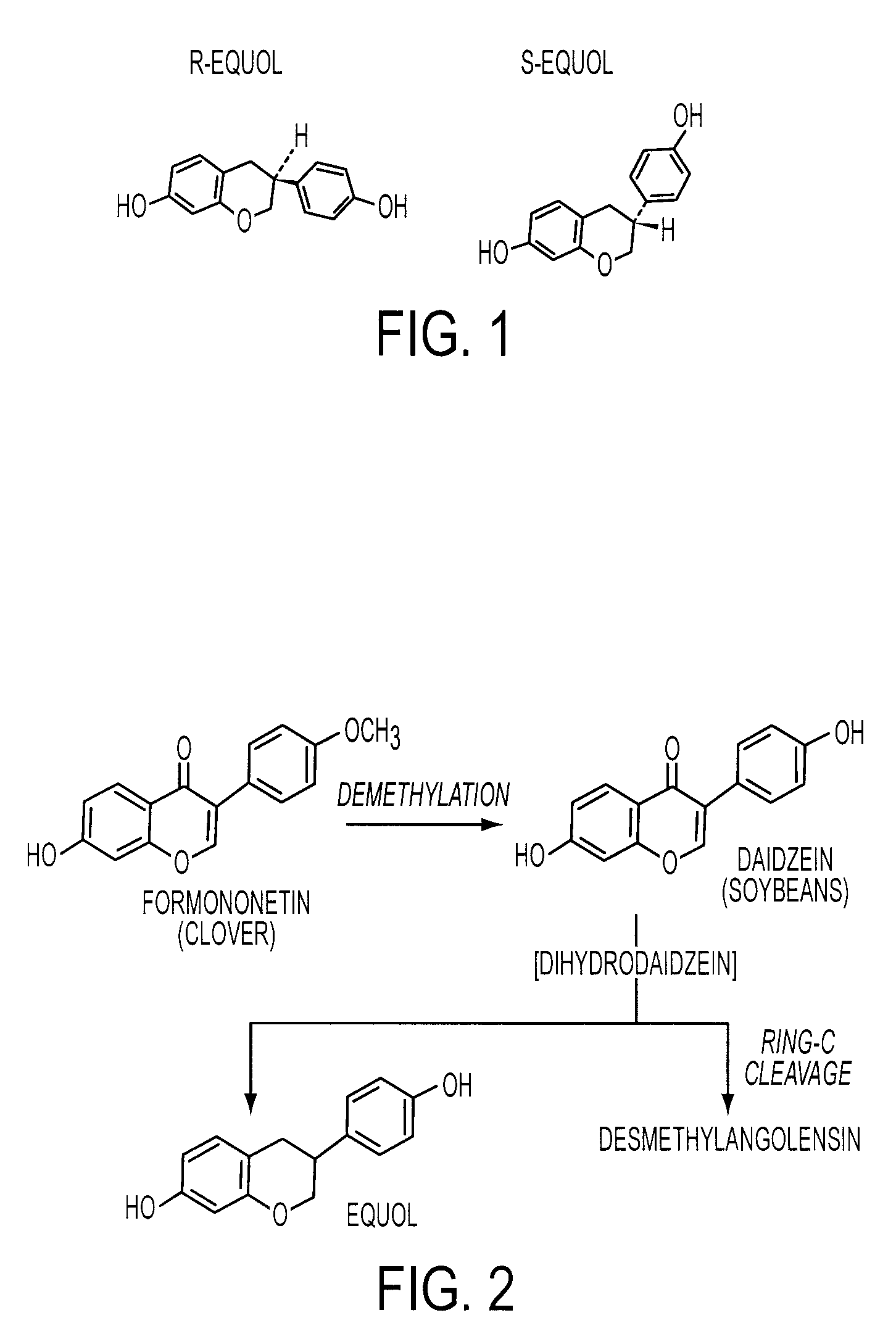
Compositions and products containing S-equol, and methods for their making
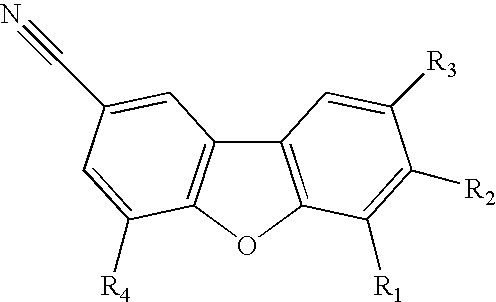
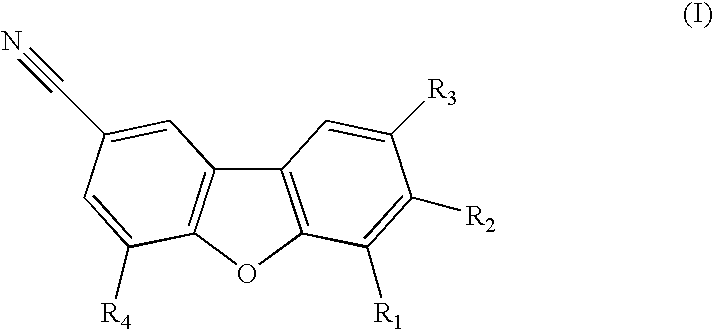
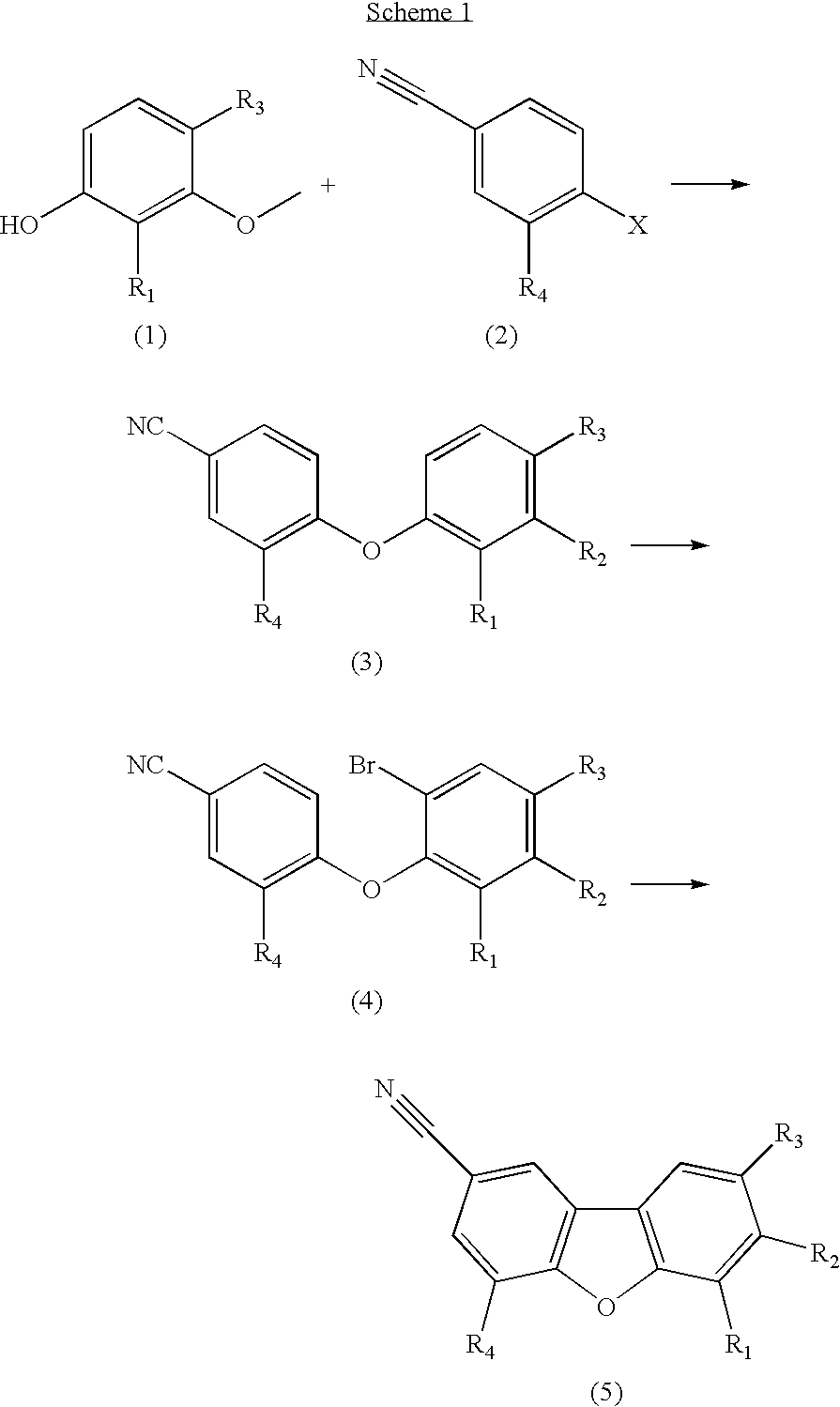
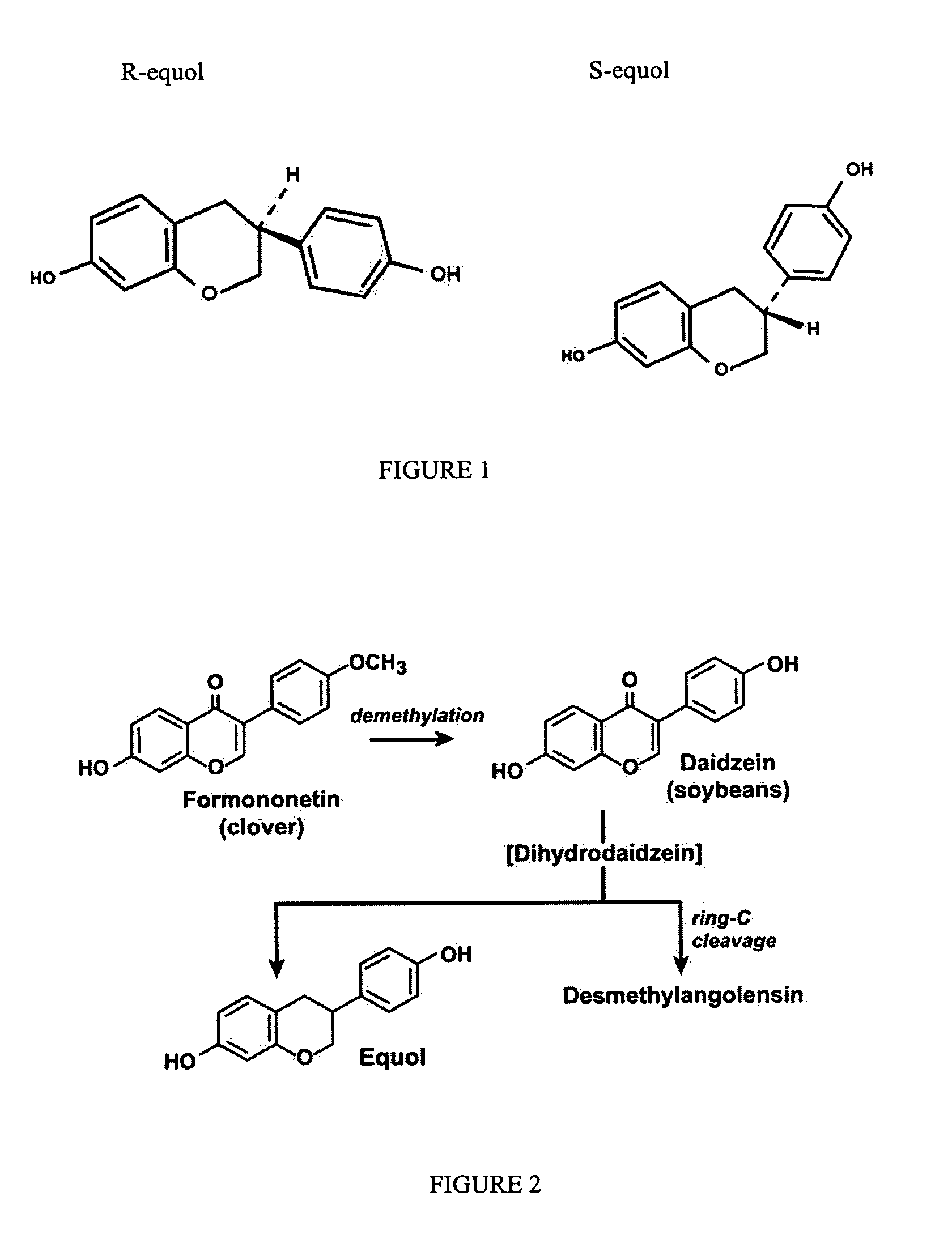
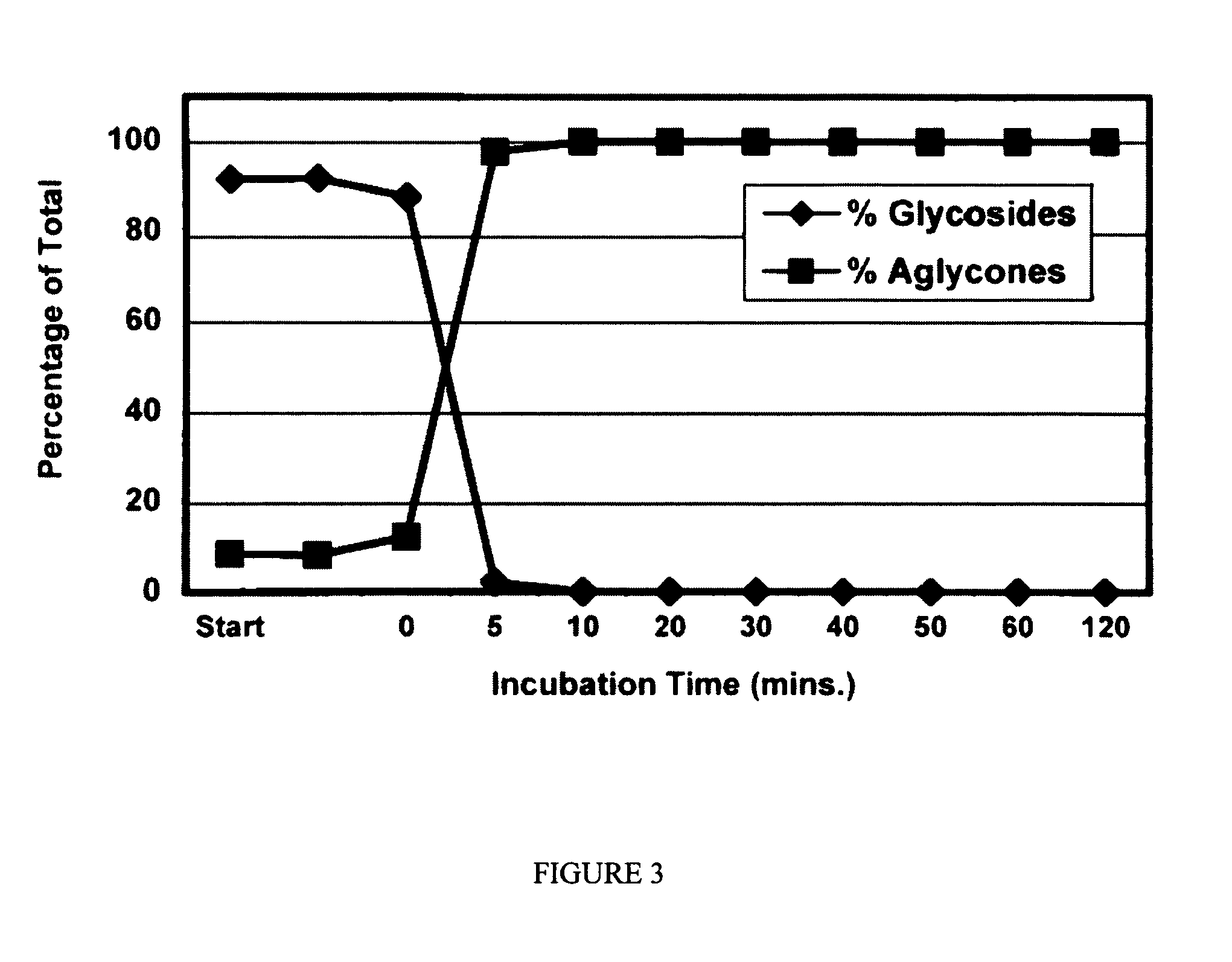
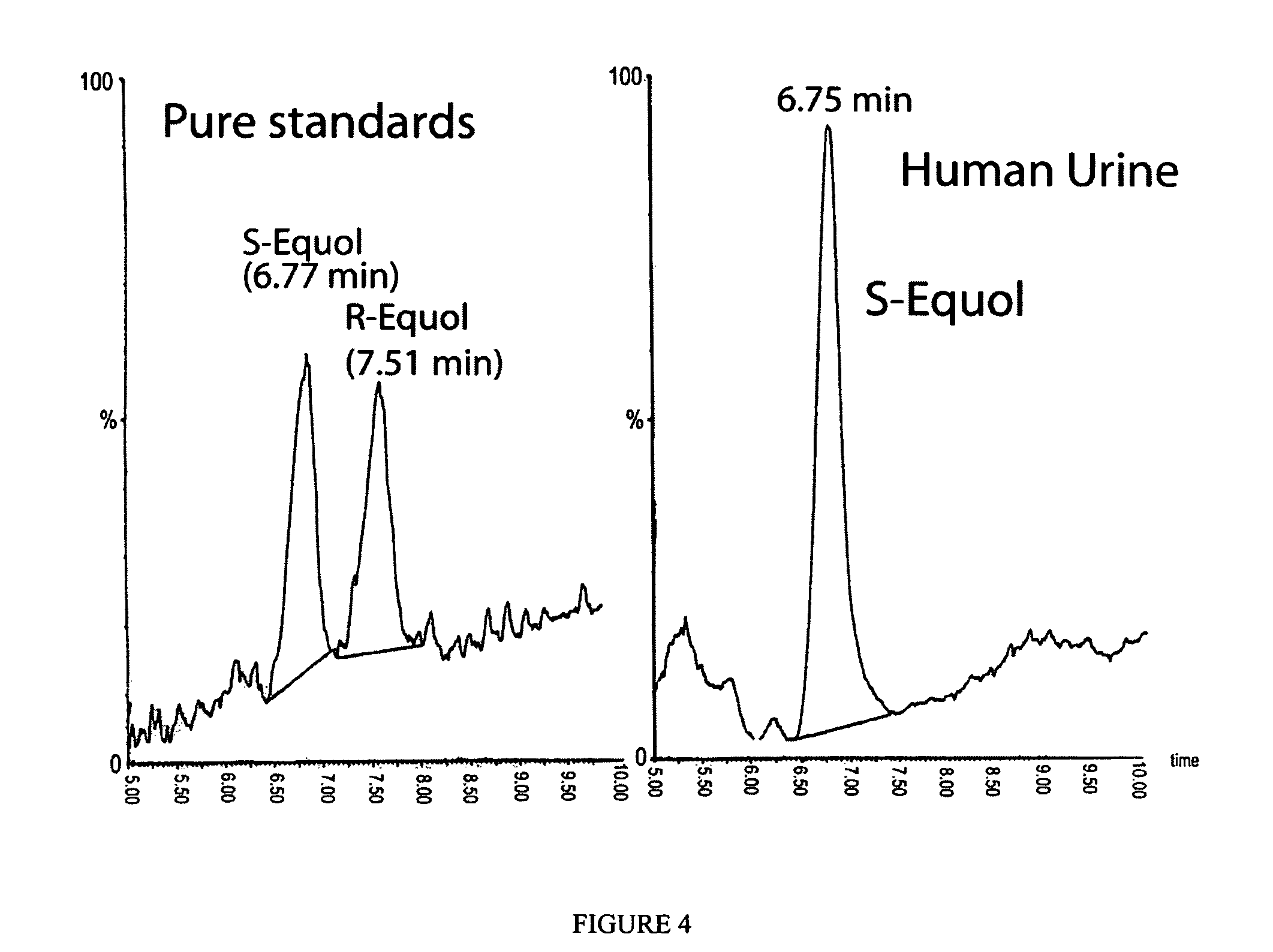
A composition for use in making commercial food and skin products comprising S-equol or mixtures, including both a non-racemic mixture and a racemic mixture, of S-equol and R-equol. The composition can be used to make articles of commerce such as food supplements, pharmaceuticals, and medicaments. The compositions are useful in a method of delivering S-equol to a mammal to prevent or treat a disease or associated condition, including hormone-dependent diseases or conditions such as cardiovascular disease, lipid disorder, osteopenia, osteoporosis, liver disease, and acute ovarian estrogen deficiency. The S-equol enantiomer can be produced in a biological synthesis from the metabolism of an isoflavone by an organism.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI +1
Food product comprising hydroxymatairesinol
InactiveUS7005447B2Improve stabilityBiocideCarbohydrate active ingredientsHydroxymatairesinolNon cancer
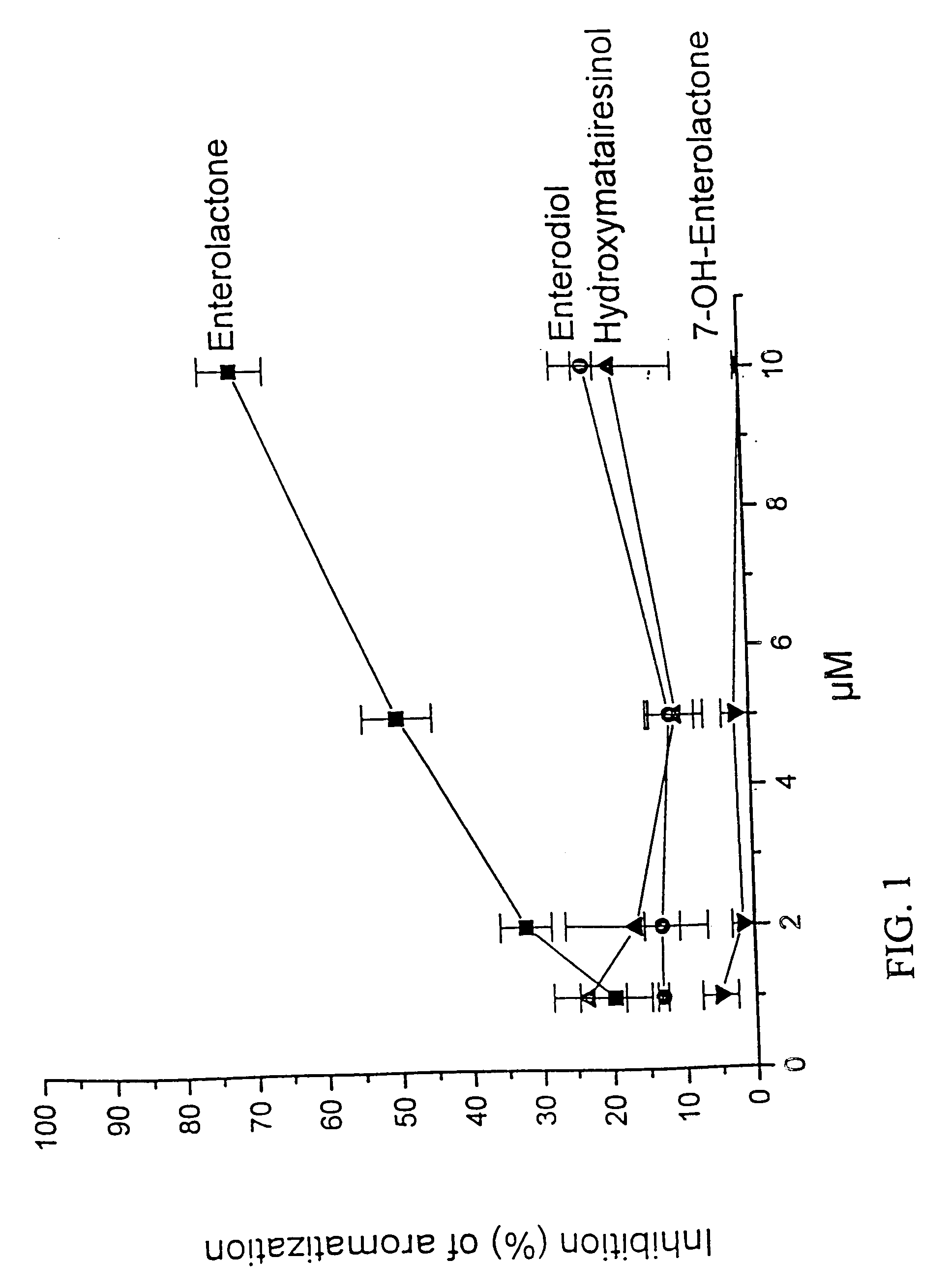
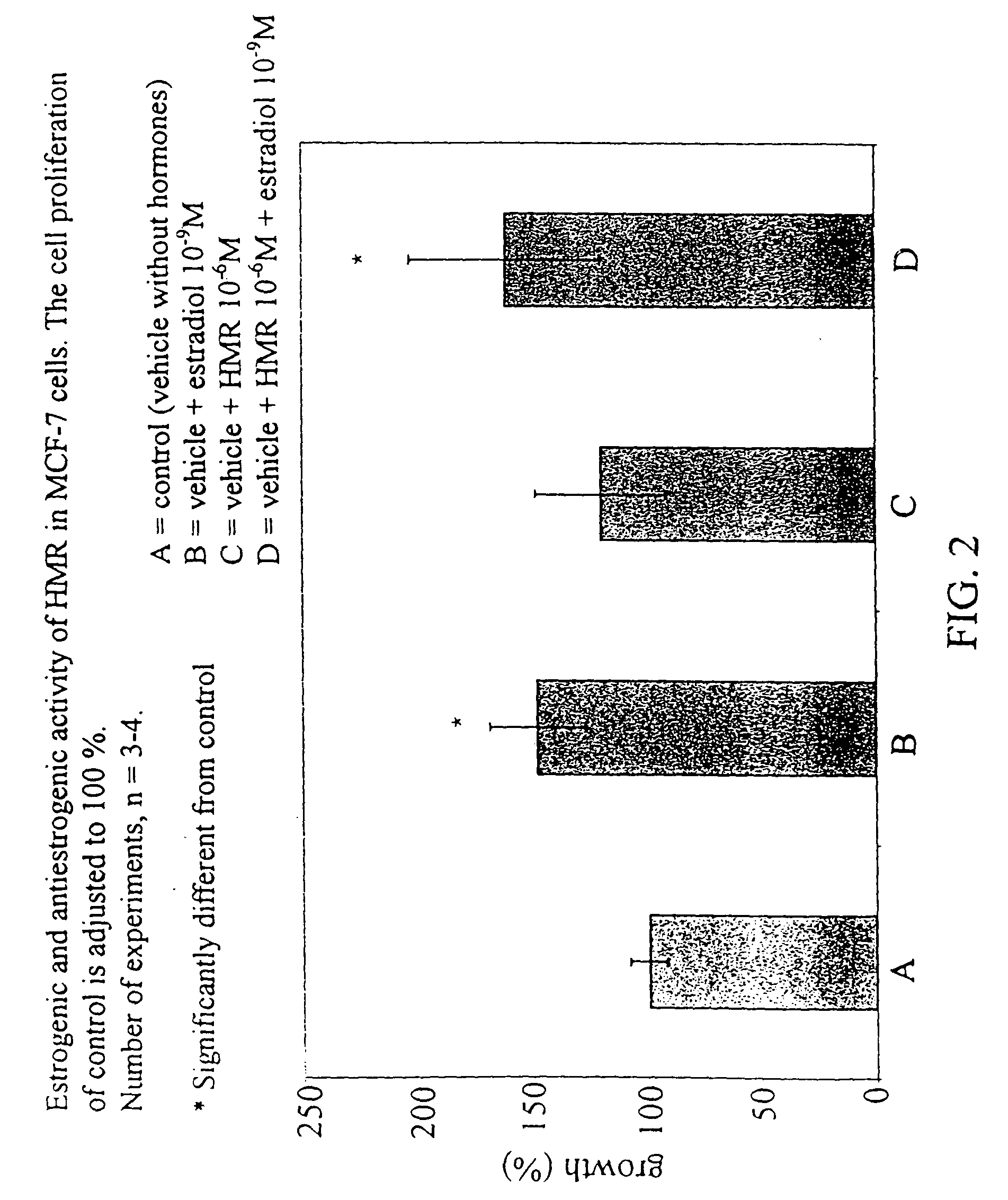
A food product containing an effective amount of an active agent which is hydroxymatairesinol, a geometric isomer or stereoisomer thereof, or an acceptable salt thereof, or a mixture thereof, where the food product is selected from the group consisting of a nutritional supplement and a nutrient. The food product can increase the level of enterolactone or another metabolite of hydroxymatairesinol in a person's serum thereby causing prevention of a cancer or a certain non-cancer, hormone dependent disease in a person, based on the administration of hydroxymatairesinol to the person.
Owner:HORMOS NUTRACEUTICAL
17Beta-hydroxysteroid dehydrogenase type 1 inhibitors for the treatment of hormone-related diseases
The invention relates to the use of non-steroidal 17beta-hydroxysteroid dehydrogenase type 1 inhibitors for the treatment and prophylaxis of hormone-dependent, particularly estrogen-dependent, diseases. The invention further relates to suitable inhibitors and to a method for the production thereof.
Owner:UNIV DES SAARLANDES
Gel composition containing tacrolimu and its preparation method and medicinal application
InactiveCN101288643AEasy to cleanNo bad smellOrganic active ingredientsPharmaceutical delivery mechanismDiseaseAdditive ingredient
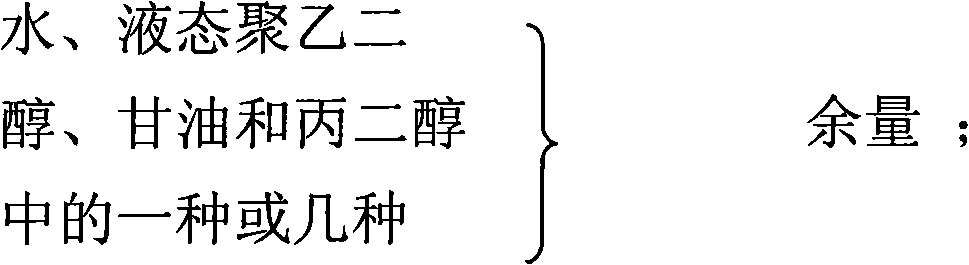
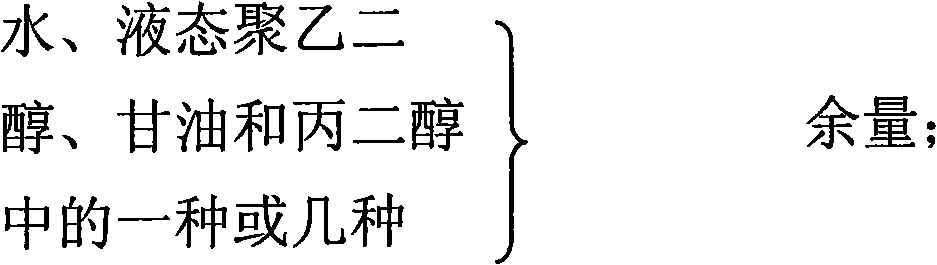
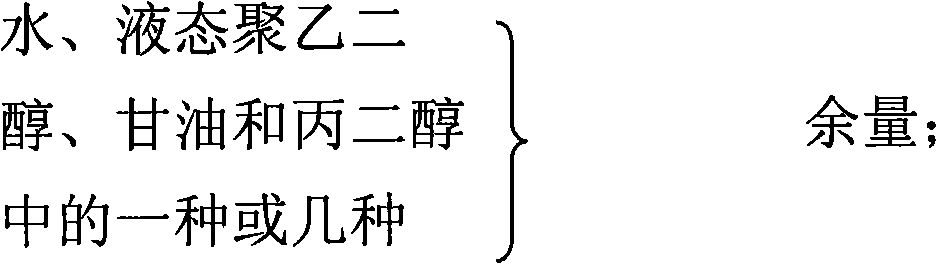
The invention relates to a gel composition containing tacrolimus, which contains the tacrolimus and ingredients of a matrix, wherein, the ingredients of the matrix contain one or more of liquid polyethylene glycol, glycerin and propylene glycol, the content of the tacrolimus in the gel is 0.01 percent to 0.5 percent, and the weight ratio of the tacrolimus to one or more of the liquid polyethylene glycol, the glycerin and the propylene glycol is 1: (50 to 3000). The invention further relates to a preparation method of the gel composition of the tacrolimus and the application of the gel composition in the preparation of drugs for the treatment of atopic dermatitis, vitiligo, psoriasis, hormone-dependent dermatitis, intractable neurodermatitis, lupus erythematosus, alopecia areata and other diseases.
Owner:杨喜鸿
Application of initial doses of LHRH analogues and maintenance doses of LHRH antagonists for the treatment of hormone-dependent cancers and corresponding pharmaceutical kits
ActiveUS20080032935A1Negative hormone withdrawal symptomsPeptide/protein ingredientsLuteinising hormone-releasing hormoneGynecologyInitial dose
LHRH analogues and LHRH antagonists for use in the treatment or prophylaxis of hormone-dependent cancers, in particular prostate cancer, prostate carcinoma and / or advanced prostate carcinoma, by administering an initial dose of an LHRH analogue over a first period sufficient to effect hormonal castration, then administering a maintenance dose of an LHRH antagonist over a second period, the dose being insufficient to achieve and / or maintain hormonal castration.
Owner:AETERNA ZENTARIS GMBH
Testosterone compounds and use for the protection of neurons
Androgens and their derivates and analogs, such as anabolic agents, are characterized by at least one substituent or substituent grouping with radical trapping properties. These compounds are used as androgen or anabolic agent substitutes or therapeutical agents for treating androgen defficiency; for treating benign prostate hypertrophy and prostate carcinome, in particular with a testosterone-based compound; for treating osteoporosis, in particular post-menopausal osteoporosis in women, preferably associated with estrogen and / or gestagens; for treating brain oedema induced by vasculary or ischemic troubles, subarachnoidal bleeding, ischemic shock and cerebral insult; for treating asthma in its various forms, for treating Alzheimer's disease, Parkinson's disease; for organ transplants; and for treating androgen-dependent and non androgen-dependent malign neoplasia
Owner:MITHOKO +1
Quinoline derivatives, their production and use
The present compounds are intermediates for the preparation of quinoline derivatives and compositions having gonadotropin-releasing hormone antagonistic activity useful as propylactics or therapeutic agent for the prevention or treatment of several hormone dependent diseases, for example, a sex hormone dependent cancer (e.g. prostatic cancer, uterine or cervical cancer, breast cancer, pituitary adenoma), benign prostatic hypertrophy, myoma of the uterus, endometriosis, precocious puberty, amenorrhea, premenstrual syndrome, polycystic ovary syndrome and acne vulgaris; are effective as a fertility controlling agent in both sexes (e.g. a pregnancy controlling agent and a menstrual cycle controlling agent); can be used as a male or female contraceptive, as an ovulation-inducing agent; can be used as an infertility treating agent by using a rebound effect owing to a stoppage of administration thereof; and are useful for modulating estrous cycles in animals in the field of animal husbandry, as agents for improving the quality of edible meat or promoting the growth of animals, and as agents for promoting spawning in fish.
Owner:TAKEDA PHARMA CO LTD
Use of a GnRH antagonist peptide in the treatment of sex hormone-dependent diseases
InactiveUS20090018085A1Peptide/protein ingredientsLuteinising hormone-releasing hormonePrecocious pubertyPhysiology
Owner:FERRING BV
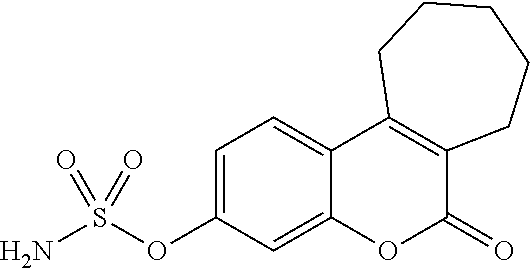
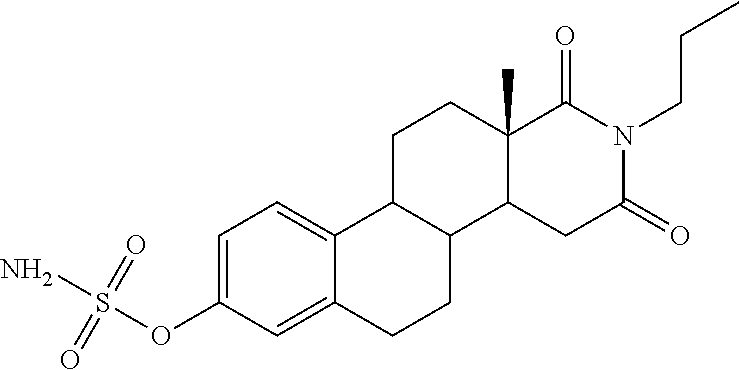
Steroid sulfamates, method for the production and use thereof
InactiveUS6339079B1Improve effectivenessReduced effectivenessBiocideOrganic active ingredientsSterolPharmaceutical industry
A novel class of gonane type and D-homo-gonane type steroids having sulfatase-inhibiting and / or estrogenic activity is presented for application in the pharmaceutical industry. The number and location of sulfamoyloxy groups on the steroids provides for sulfatase inhibiting and estrogenic activities that independently vary over a wide range, allowing the customization of the pharmaceutical for specific purposes, including treatment and diagnosis of estrogen-dependent tumors.
Owner:DR HELMUT KASCH +1
Compositions containing micronized tanaproget prepared by wet granulation
Compositions, preferably pharmaceutical compositions, containing micronized tanaproget, or pharmaceutically acceptable salt thereof, microcrystalline cellulose, croscarmellose sodium, sodium lauryl sulfate, butylated hydroxyanisole, povidone, and magnesium stearate, are provided. The compositions are useful in contraception and hormone replacement therapy and in the treatment and / or prevention of uterine myometrial fibroids, benign prostatic hypertrophy, benign and malignant neoplastic disease, dysfunctional bleeding, uterine leiomyomata, endometriosis, polycystic ovary syndrome, and carcinomas and adenocarcinomas of the pituitary, endometrium, kidney, ovary, breast, colon, and prostate and other hormone-dependent tumors, and in the preparation of medicaments useful therefor. Additional uses include stimulation of food intake.
Owner:WYETH LLC
Medicine composite containing icaritin and demethylicaritin and its application
InactiveCN1194701CClear compositionOrganic active ingredientsPharmaceutical delivery mechanismHepatic fibrosisSenile dementia
The present invention provides a medicine composition including icaritin and / or demethylicaritin as active component, medicinal carrier and / or excipient and having plant female hormone action, it can be used in the preparation of medicines for curing various diseases of hormone-dependend type cancers, cardiovascular diseases, after-menopausal osteoporosis, climacteric indisposition, senile dementia, hepatitis, diabetes and others, also can be used as precursor compound of the medicine.
Owner:ZHEJIANG UNIV
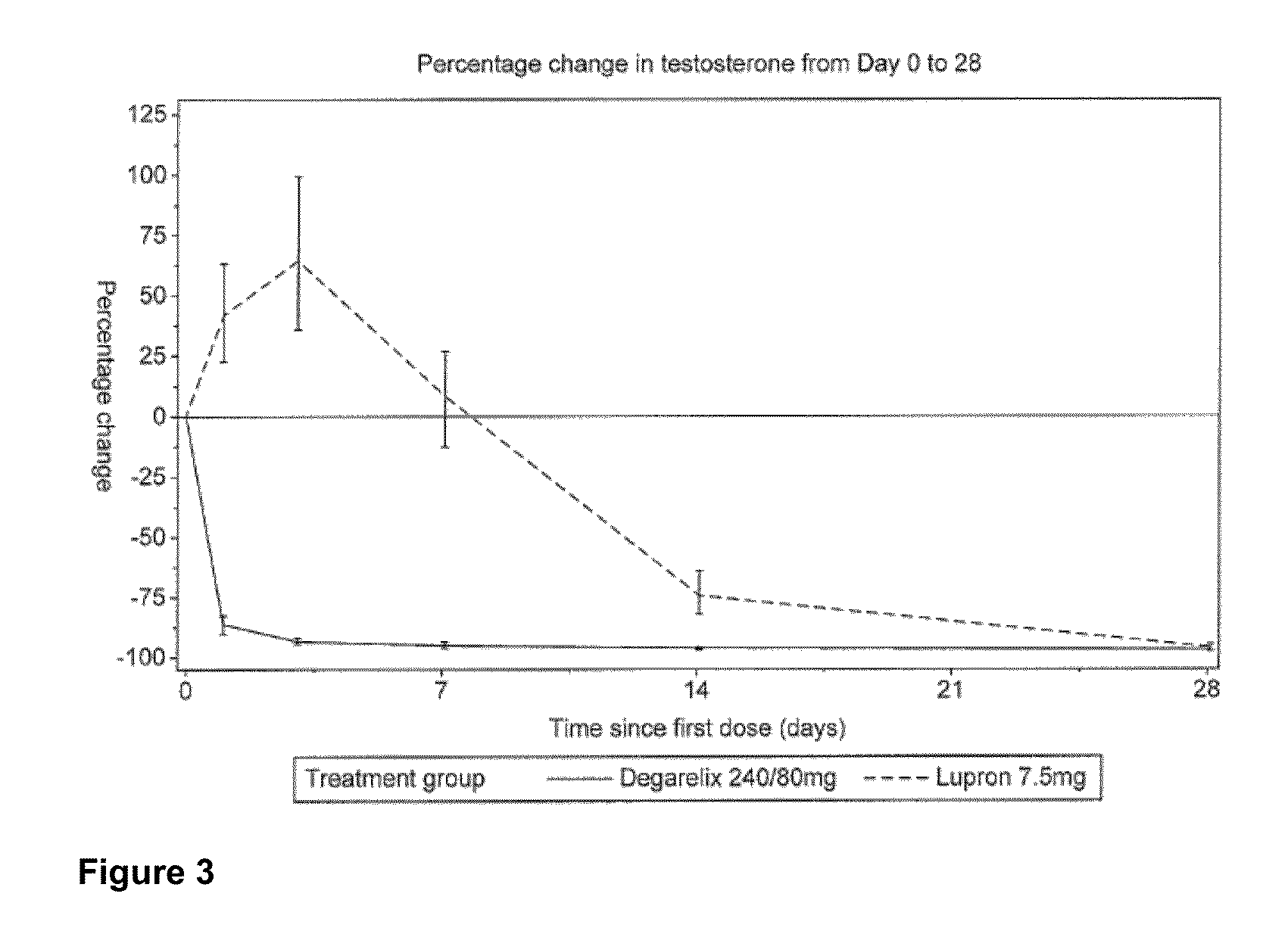
METHOD OF TREATING PROSTATE CANCER WITH GnRH ANTAGONIST
The invention provides methods and dosing regimens for safely and effectively treating androgen-dependent prostate cancer with a gonadotrophin releasing hormone (GnRH) antagonist without causing a testosterone spike and / or other side effect of GnRH agonist therapy such as a urinary tract infection, or an arthralgia-related or cardiovascular side effect. The present disclosure also provides for methods for treating prostate cancer in a patient with a history of at least one cardiovascular event, wherein administration of degarelix to the subject decreases the likelihood of developing or experiencing an additional cardiovascular event compared to treatment with a gonadotrophin releasing hormone (GnRH) agonist.
Owner:FERRING BV
Prinsepia utilis Royle oil-containing functional skin care product and preparation method thereof
ActiveCN102406576AMild textureImprove securityCosmetic preparationsToilet preparationsAllergic dermatitisDisease
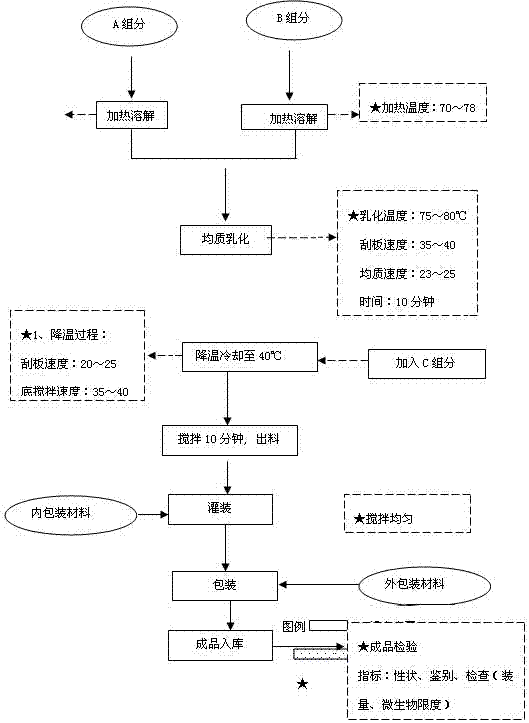
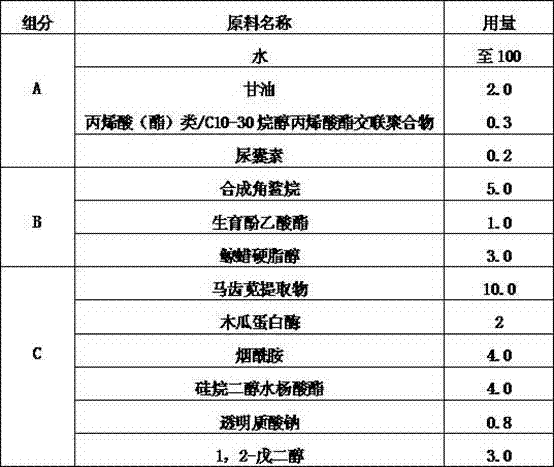
The invention discloses a Prinsepia utilis Royle oil-containing functional skin care product and a preparation method thereof. The Prinsepia utilis Royle oil-containing functional skin care product is prepared from the following raw materials in part by weight: 1 to 2 parts of Prinsepia utilis Royle oil, 10 to 25 parts of purslane extracting solution, 5 parts of glycerol, 2 to 3 parts of 1,2-pentanediol, 5 to 7 parts of caprylic / caprictriglycerides, 2 parts of synthetic squalane, 1 to 5 parts of tocopheryl acetate, 1 to 3 parts of cetearyl alcohol, 0.1 to 0.2 part of allantoin, 0.6 to 0.9 part of coconut oil-base glucoside, 0.5 to 1 part of coconut oil alcohol, 0.5 to 2 parts of glyceryl stearate, 0.1 to 1 part of sodium hyaluronate and the balance of water. The functional skin care product has mild property and high safety without toxic or side reaction and anaphylactic reaction, can repair the destructed skin barrier, resists inflammation and skin ageing, and has a good effect and high safety of assisting in treating skin diseases related with skin barrier destruction, such as facial dermatitis (hormone dependence dermatitis, allergic dermatitis and acne), atopic dermatitis and the like.
Owner:YUNNAN BOTANEE BIO TECH GRP CO LTD
Functional skin care product containing purslane extractive and preparation method thereof
ActiveCN102764217AMild textureImprove securityCosmetic preparationsAntipyreticGlycerolPurslane extract
The invention is a functional skin care product containing purslane extractive. Raw materials of the functional skin care product include, by weight, 10 parts of purslane extractive, 1-2 parts of Prinsepia utilis rogle oil, 2-5 parts of glycerol, 0.3-0.5 parts of crylic acid (ester) / C10-30 alkanol acrylate cross-linked polymer, 2-3 parts of 1,2-pentanediol, 1-5 parts of synthesized squalane, 1-2 parts of tocopheryl acetate, 2-3 parts of cetostearyl alcohol, 0.1-0.2 part of allantoin, 0.3-0.8 part of sodium hyaluronate, 3-5 parts of Butyrospermum parkii butter, and 100 parts of deionized water. Or the Prinsepia utilis rogle oil and the Butyrospermum parkii butter are not added. By the aid of animal experiments, namely, mouse auricle swelling models due to dimethlbenzene, the functional skin care product containing purslane extractive is fine in anti-inflammation and anti-allergy effects, mild in nature and fine in safety, and is free of toxic reaction and anaphylaxis. The functional skin care product has an adjuvant therapy function to skin barrier recovery of common discosmetic dermatosis such as hormone dependent dermatitis and acne, and is fine in curative effect and safety.
Owner:YUNNAN BOTANEE BIO TECH GRP CO LTD
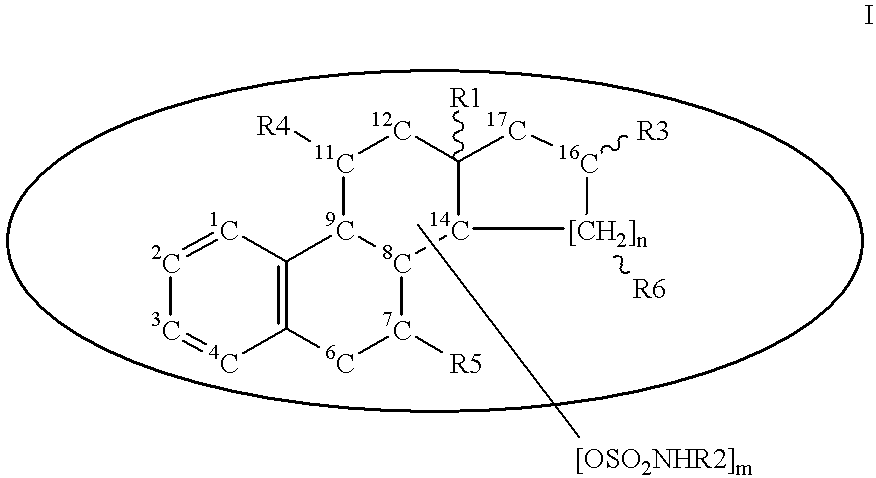
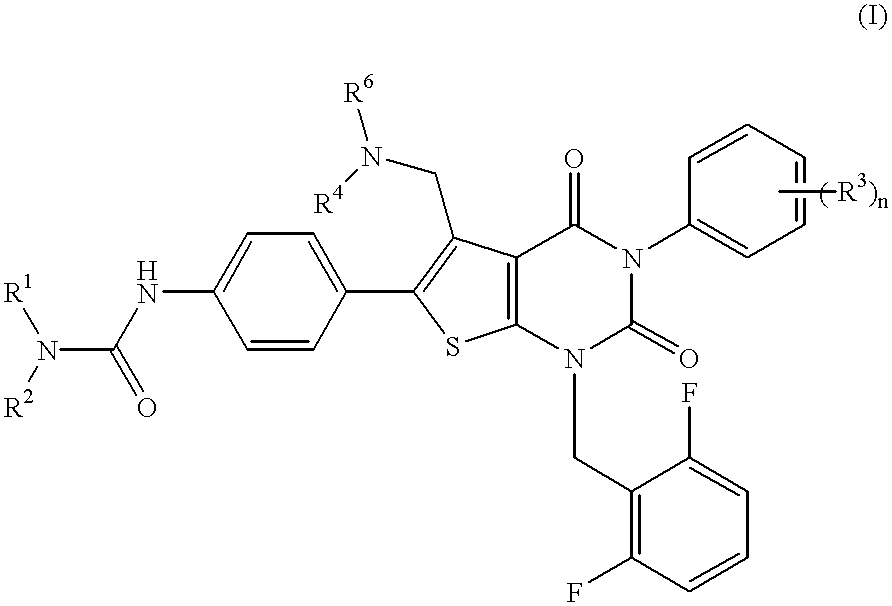
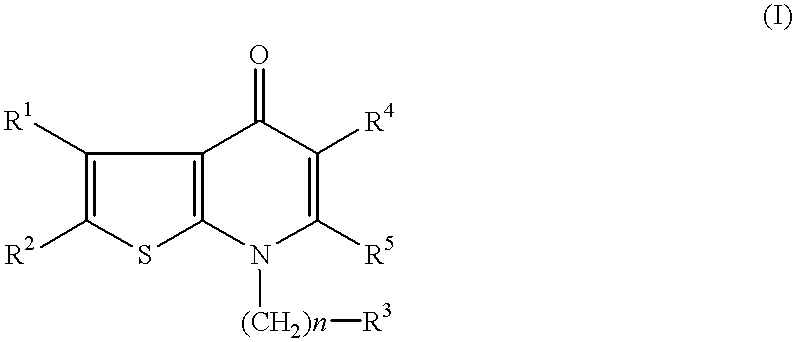
Thienopyrimidine compounds, their production and use
InactiveUS6297379B1Easy to useHigh activityOrganic active ingredientsOrganic chemistryHalogenHydrogen
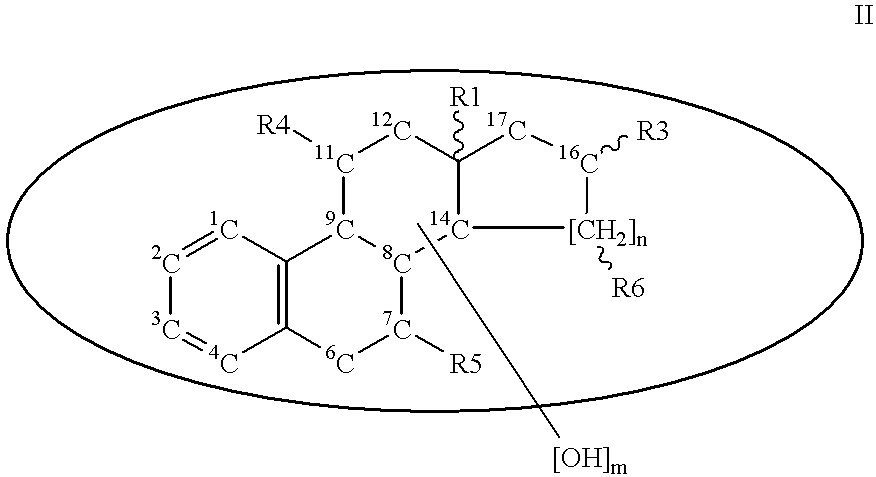
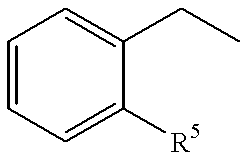
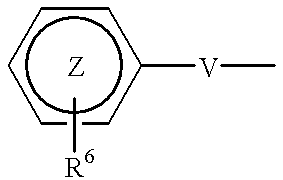
A compound of formula (I) wherein R1 and R2 each is hydrogen, hydroxy, C1-4 alkoxy, C1-4 alkoxy-carbonyl or C1-4 alkyl which may be substituted; R3 is hydrogen, halogen, hydroxy or C1-4 alkoxy which may be substituted; or adjacent two R3 may form C1-4 alkylenedioxy; R4 is hydrogen or C1-4 alkyl; R6 is C1-4 alkyl which may be substituted or a group of the formula (A) wherein R5 is hydrogen of R4 and R5 may form heterocycle; and n is 0-5, or a salt thereof, has an excellent GnRH-antagonizing activity, and is useful for preventing or treating sex hormone-dependent diseases.
Owner:TAKEDA PHARMA CO LTD
Micronized tanaproget, compositions, and methods of preparing the same
The present invention provides compositions, desirably pharmaceutical compositions, containing micronized tanaproget. The compositions can also contain microcrystalline cellulose, croscarmellose sodium, anhydrous lactose, magnesium stearate, micronized edetate calcium disodium hydrous, and micronized sodium thiosulfate pentahydrate. The compositions are useful in contraception and hormone replacement therapy and in the treatment and / or prevention of uterine myometrial fibroids, benign prostatic hypertrophy, benign and malignant neoplastic disease, dysfunctional bleeding, uterine leiomyomata, endometriosis, polycystic ovary syndrome, and carcinomas and adenocarcinomas of the pituitary, endometrium, kidney, ovary, breast, colon, and prostate and other hormone-dependent tumors, and in the preparation of medicaments useful therefor. Additional uses include stimulation of food intake.
Owner:WYETH LLC
Micronized tanaproget and compositions containing same
The present invention provides compositions, desirably pharmaceutical compositions, containing micronized tanaproget. The compositions can also contain microcrystalline cellulose, croscarmellose sodium, anhydrous lactose, and magnesium stearate; or can contain microcrystalline cellulose, croscarmellose sodium, sodium lauryl sulfate, povidone, and magnesium stearate. The compositions are useful in contraception and hormone replacement therapy and in the treatment and / or prevention of uterine myometrial fibroids, benign prostatic hypertrophy, benign and malignant neoplastic disease, dysfunctional bleeding, uterine leiomyomata, endometriosis, polycystic ovary syndrome, and carcinomas and adenocarcinomas of the pituitary, endometrium, kidney, ovary, breast, colon, and prostate and other hormone-dependent tumors, and in the preparation of medicaments useful therefore Additional uses include stimulation of food intake.
Owner:WYETH LLC
IGF-IR antagonists as adjuvants for treatment of prostate cancer
InactiveUS20090175868A1Inhibits and prevents transitionBiocideOrganic active ingredientsCancer cellAdjuvant
The present invention relates to a method of treating prostate cancer with androgen deprivation therapy and an insulin-like growth factor receptor (IGF-IR) antagonist. Although the response rate of prostate cancer to androgen deprivation therapy (ADT) is high, surviving cancer cells invariably become androgen independent (AI) and tumor growth follows. The invention inhibits or delays transition of androgen dependent cancer to androgen independent cancer, significantly decreases risk of recurrence, and improves treatment outcome.
Owner:UNIV OF WASHINGTON +1
Condensed-ring thiophene derivatives, their production and use
A gonadotropin-releasing hormone antagonistic composition, which comprises an optionally substituted condensed-bicyclic compound consisting of a homo or hetero 5 to 7 membered ring and a homo or hetero 5 to 7 membered ring is effective as a propylactic or therapeutic agent for the prevention or treatment of several hormone dependent diseases, for example, a sex hormone dependent cancer (e.g. prostatic cancer, cancer of uterine cervix, breast cancer, pituitary adenoma), benign prostatic hypertrophy, myoma of the uterus, endometriosis, precocious puberty, amenorrhea, premenstrual syndrome, polycystic ovary syndrome and acne vulgaris; is effective as a fertility controlling agent in both sexes (e.g. a pregnancy controlling agent and a menstrual cycle controlling agent); can be used as a contraceptive of male or female, as an ovulation-inducing agent of female; can be used as an infertility treating agent by using a rebound effect owing to a stoppage of administration thereof; is useful as modulating estrous cycles in animals in the field of animal husbandry, as an agent fro improving the quality of edible meat or promoting the growth of animals; is useful as an agent of spawning promotion in fish.
Owner:TAKEDA PHARMA CO LTD
Methods and compositions for the treatment of estrogen-dependent hyperproliferative uterine disorders
InactiveUS20100087402A1Minimization of actionReducing local biosynthesisSalicyclic acid active ingredientsBiocideUterine DisorderSide effect
The present invention relates to the treatment of estrogen-dependent hyperproliferative uterine disorders including endometriosis, uterine fibroids, endometrial hyperplasia, uterine cancer, and their related symptoms by intravaginally administering at least two active agents selected from an aromatase inhibitor, an antiinflammatory agent, and a uterine-selective estrogen receptor antagonist. This combination therapy reduces local estrogen production, blocks local estrogen action, and suppresses inflammation locally, resulting in starvation of the estrogen-dependent diseased tissues, relief of related symptoms, and retardation of disease progression. Intravaginal delivery maximizes local inhibition of estrogen production without significantly affecting systemic circulating estrogen levels. This results in enhanced clinical efficacy and reduced side effects.
Owner:VIVUS
Method of treatment of prostate cancer
InactiveUS7241753B2Heavy metal active ingredientsPhosphorous compound active ingredientsCancer preventionHormone dependence
The present invention relates to the field of cancer, and in particular hormone dependent cancers including, but not limited to prostate, breast, endometrial, ovarian, thyroid, bone, and testis. The present invention also relates to the use of steroid analogues, and in particular analogues of Δ5-androstene-3-β, 17α-diol, and its epimer Δ5-androstene-3-β, 17β-diol for the treatment and prevention of cancer.
Owner:HARBOR DIVERSIFIED
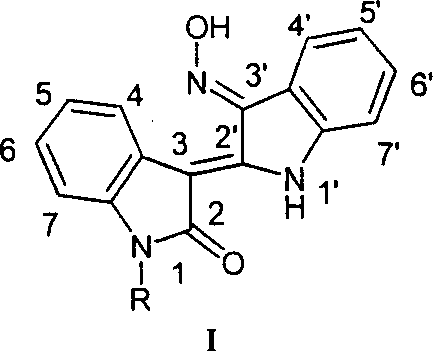
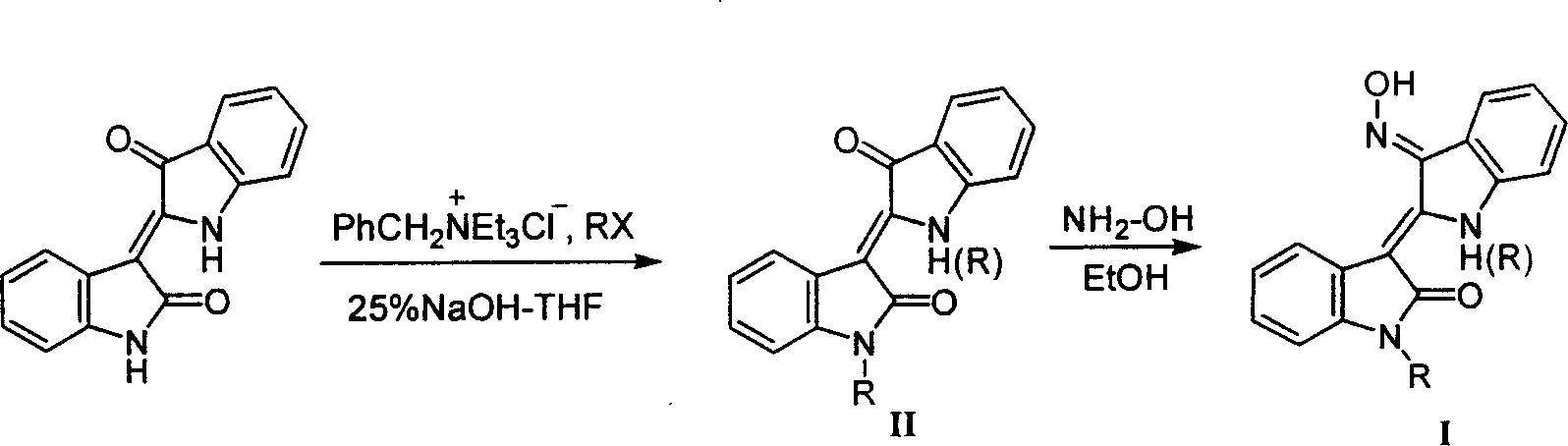
Preparation method and medical uses of Nú¿1ú®-hydrocarbyl-3íõ-nitrotylindirubin derivative 1
InactiveCN1763005ACheap methodThe method is simple and fastOrganic active ingredientsPowder deliveryHalohydrocarbonHydroxylamine
The present invention is preparation process and antitumor effect of N(1)-alkyl-3'-oximino indirubin derivative (I, JN-2528). The new synthesis process uses indirubin as initial material, and under the action of alkaline compound as promoter, indirubin is reacted directly with halohydrocarbon or other halide to obtain N(1)-alkyl indirubin derivative, which is then reacted with hydroxylamine to obtain N(1)-alkyl-3'-oximino indirubin derivative. The MTT and SRB process and transplanting C57 mouse Lewis lung cancer mold test proves the antitumor activity of N(1)-alkyl-3'-oximino indirubin. The compound shows extracorporeal and intracorporeal cancer inhibiting effect.
Owner:JC (WUXI) CO INC
Method of treatment of EGFR inhibitor toxicity
InactiveUS20110190244A1Reduce severityGood curative effectBiocideSenses disorderErlotinibTyrosine-kinase inhibitor
The invention provides a method of treating and / or preventing a toxicity associated with epidermal growth factor receptor (EGFR) inhibitor therapy in a subject, the method comprising administering to the subject an effective amount of a steroid sulfatase (STS) inhibitor. The toxicity may be ocular toxicity; or dermatologic toxicity, such as papulopustular rash. The EGFR inhibitor may be selected from the group consisting of: a small molecule; an antibody or derivative or fragment thereof; another agent that targets the extracellular or intracellular domain of the EGFR, such as a tyrosine kinase inhibitor selected from the group consisting of: erlotinib; gefitinib; lapatinib; and any combination thereof. The EGFR inhibitor may also be antibody selected from the group consisting of: cetuximab; panitumumab; and any combination thereof.Preferably the STS inhibitor is selected from the group consisting of: alternative STS substrates; reversible STS inhibitors; and irreversible STS inhibitors; and any combination thereof. A preferred STS inhibitor is the irreversible nonsteroidal STS inhibitor STX64.In some embodiments, the subject receiving EGFRI therapy has a cancer comprising cells that express wildtype k-ras and / or wildtype b-raf. In other embodiments, the cancer may be hormone-dependent. Cancers that may be treated with EGFRI therapy include colorectal cancer and non-small cell lung cancer.
Owner:PETER MACCALLUM CANCER INST
Compositions and products containing s-equol, and methods for their making
A composition for use in making commercial food and skin products comprising S-equol or mixtures, including both a non-racemic mixture and a racemic mixture, of S-equol and R-equol. The composition can be used to make articles of commerce such as food supplements, pharmaceuticals, and medicaments. The compositions are useful in a method of delivering S-equol to a mammal to prevent or treat a disease or associated condition, including hormone-dependent diseases or conditions such as cardiovascular disease, lipid disorder, osteopenia, osteoporosis, liver disease, and acute ovarian estrogen deficiency. The S-equol enantiomer can be produced in a biological synthesis from the metabolism of an isoflavone by an organism.
Owner:AUSTRALIAN HEALTH & NUTRITION ASSOC +1
Single-chain antibodies against human insulin-like growth factor I receptor: expression, purification, and effect on tumor growth
A method of inhibiting the growth of hormone dependent tumor cells in a mammal comprises administering to said mammal an insulin-like growth factor receptor (IGF-IR) recombinant antibody, wherein said antibody can be a single-chain recombinant antibody, which can be humanized, capable of blocking agonist interaction with the IGF-IR.
Owner:CITY OF HOPE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com