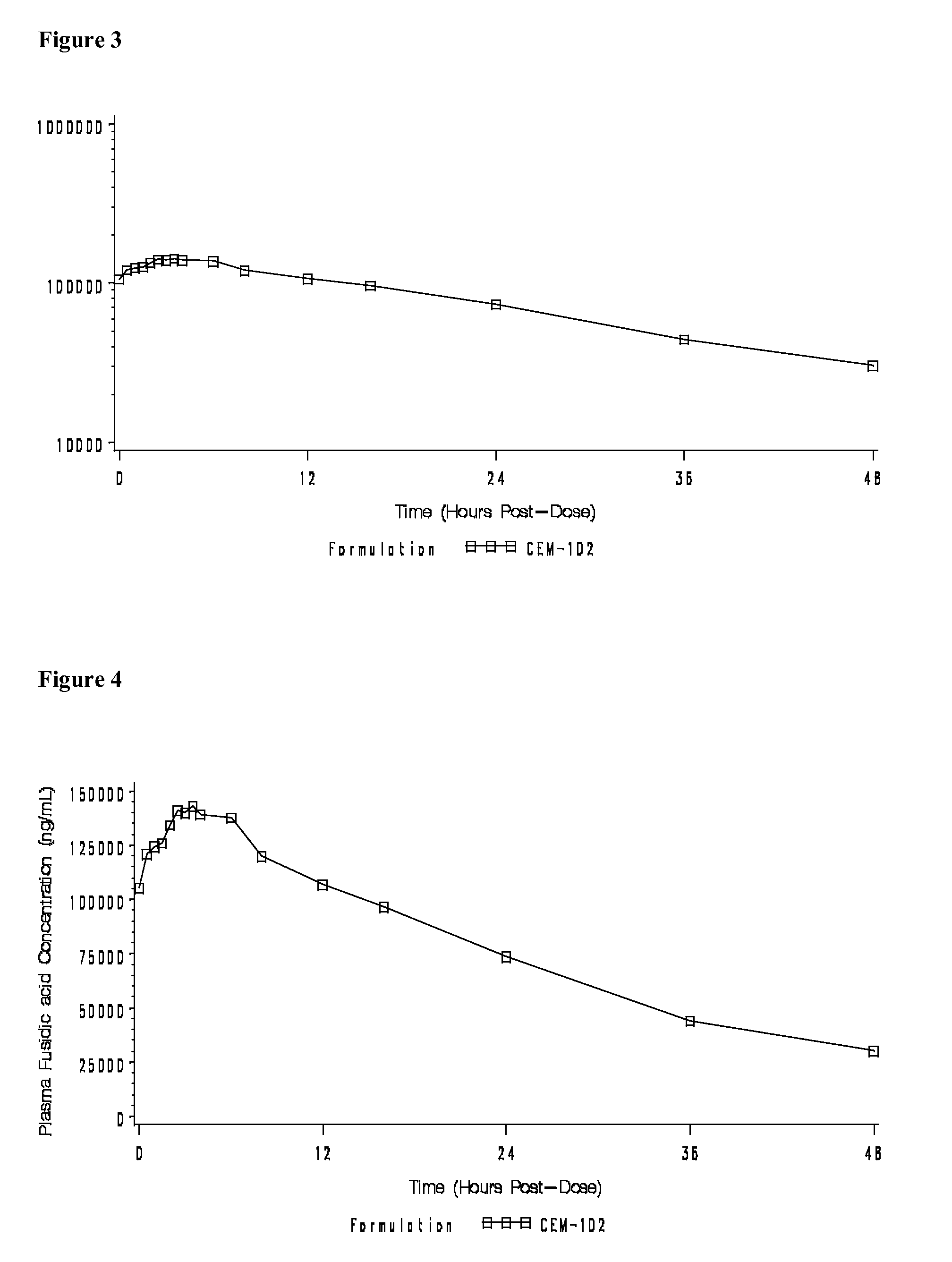Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
54 results about "Maintenance dose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In pharmacokinetics, a maintenance dose is the maintenance rate [mg/h] of drug administration equal to the rate of elimination at steady state. This is not to be confused with dose regimen, which is a type of drug therapy in which the dose [mg] of a drug is given at a regular dosing interval on a repetitive basis. Continuing the maintenance dose for about 4 to 5 half lives (t½) of the drug will approximate the steady state level. One or more doses higher than the maintenance dose can be given together at the beginning of therapy with a loading dose.
Methods and Kits For Administering Probiotics
InactiveUS20080241226A1Improve tolerability and perception of benefitBiocideNervous disorderMedicineDrug loading dose
Methods for administering probiotics comprising the steps of: administering a loading dose of a loading probiotic for a loading time period; and administering a dose of a botanical and / or additional materials for the loading time period are disclosed. The methods also include administering a maintenance dose of a maintenance probiotic, and / or a botanical and / or an additional material for a maintenance time period. Also disclosed are kits for use in administering probiotics.
Owner:THE PROCTER & GAMBLE COMPANY
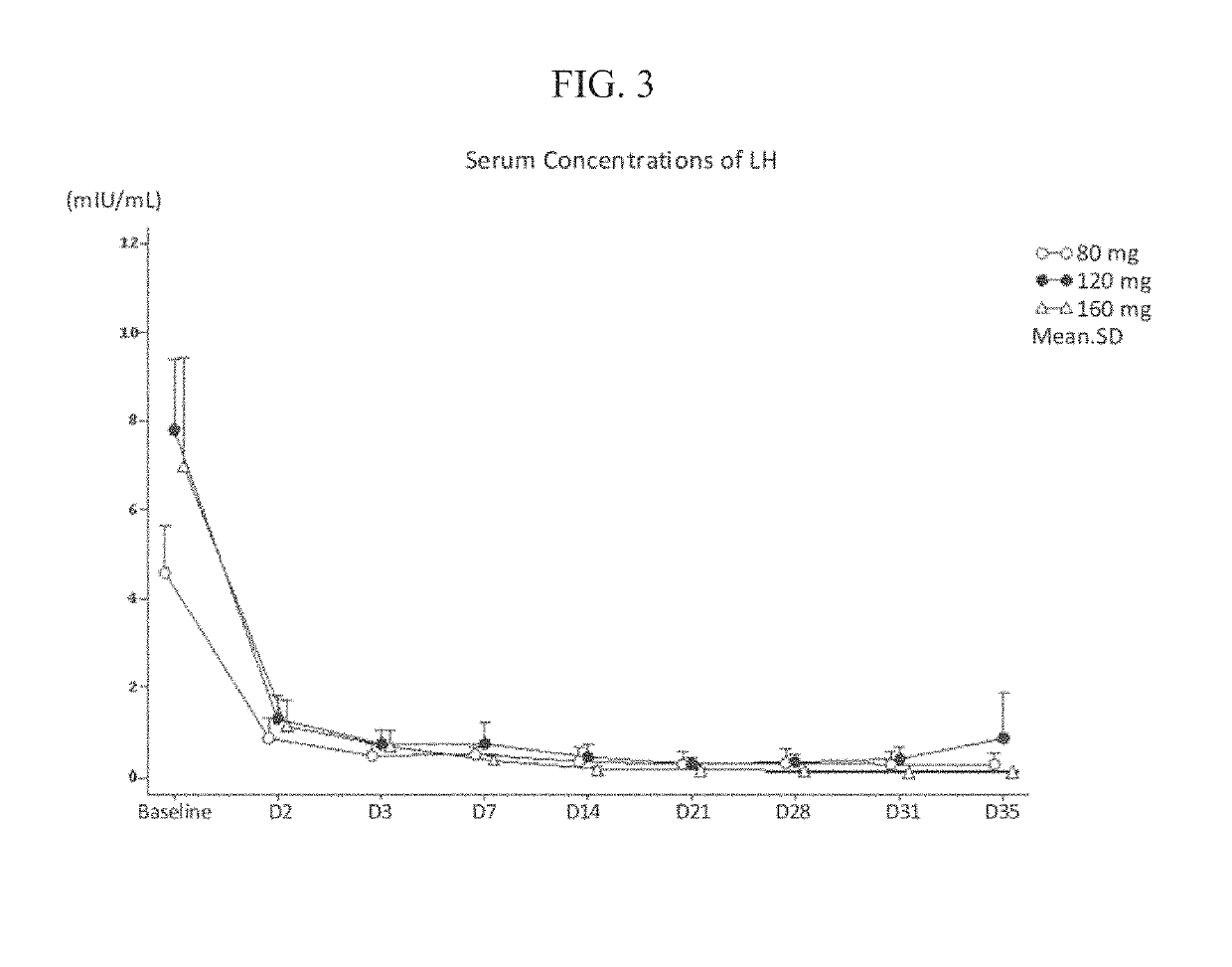
Application of initial doses of LHRH analogues and maintenance doses of LHRH antagonists for the treatment of hormone-dependent cancers and corresponding pharmaceutical kits
ActiveUS20080032935A1Negative hormone withdrawal symptomsPeptide/protein ingredientsLuteinising hormone-releasing hormoneGynecologyInitial dose
LHRH analogues and LHRH antagonists for use in the treatment or prophylaxis of hormone-dependent cancers, in particular prostate cancer, prostate carcinoma and / or advanced prostate carcinoma, by administering an initial dose of an LHRH analogue over a first period sufficient to effect hormonal castration, then administering a maintenance dose of an LHRH antagonist over a second period, the dose being insufficient to achieve and / or maintain hormonal castration.
Owner:AETERNA ZENTARIS GMBH
User score-based project recommendation method
InactiveCN105740444AGood sparsity resistanceAddressing Concept DriftSpecial data processing applicationsPersonalizationTime factor
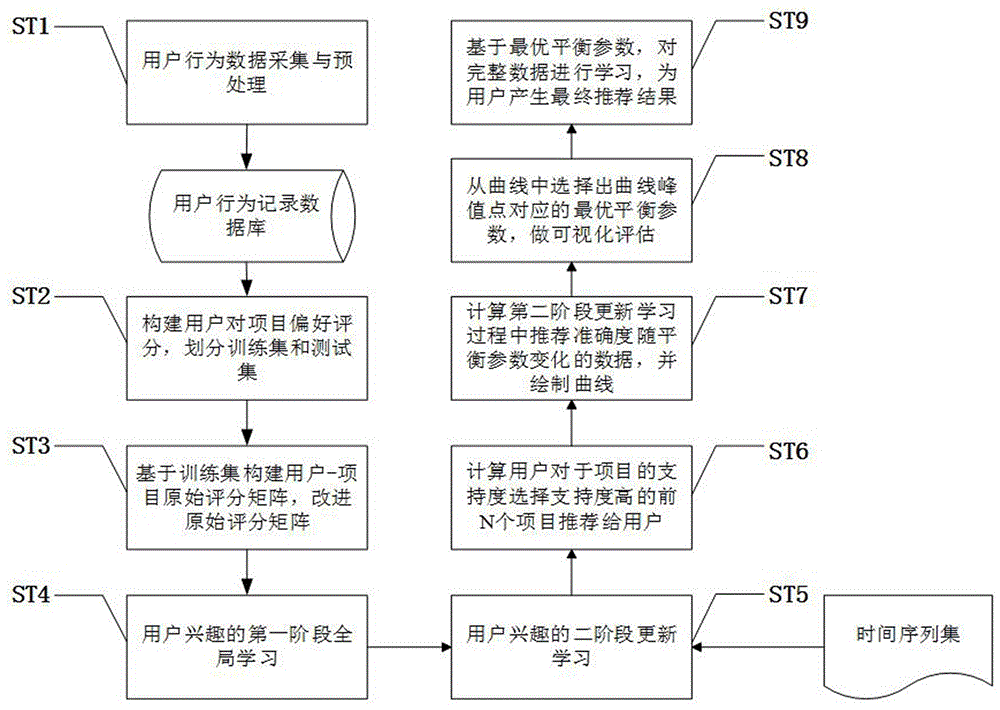
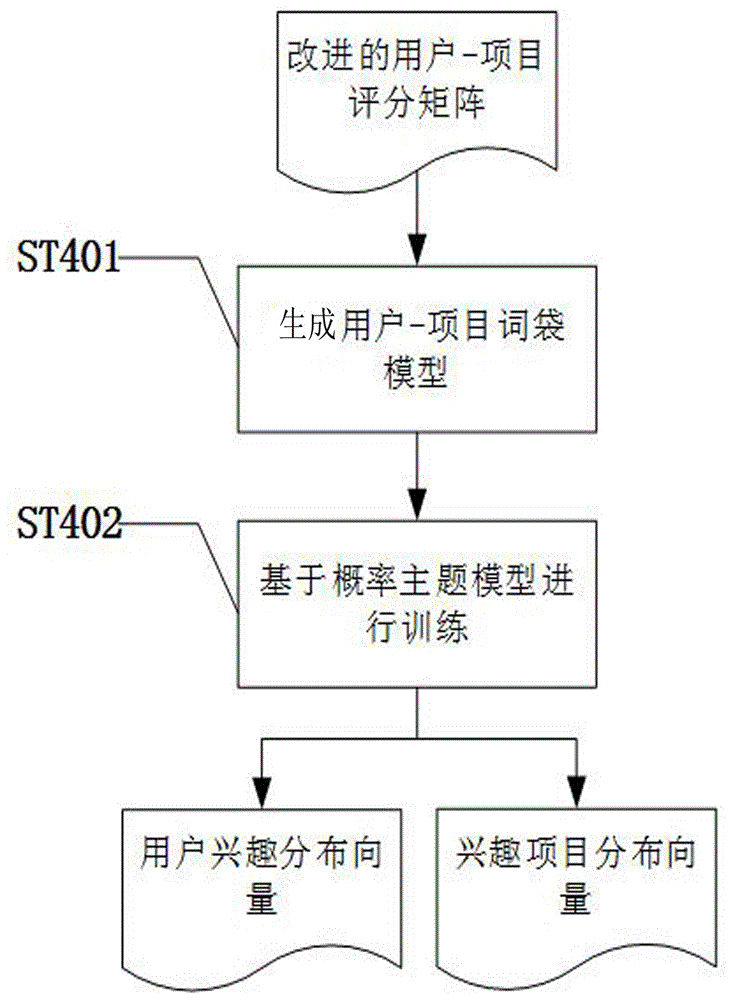
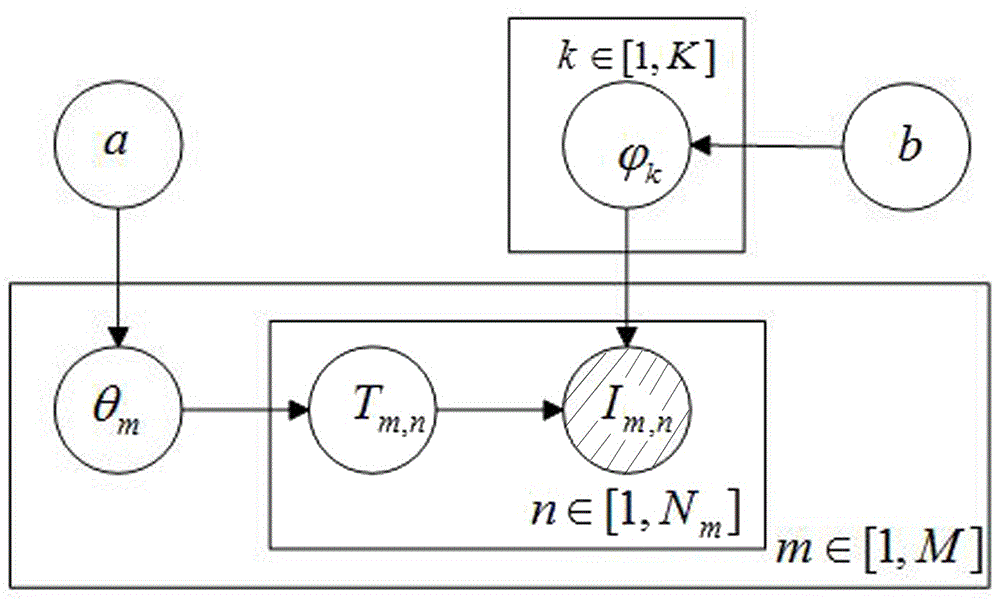
The invention discloses a user score-based project recommendation method. The method comprises the following steps: in allusion to the dynamism and diversity of interests of a user in a recommendation system, effectively fusing a maintenance dose function on the basis of the sores of user projects and completing the global learning of potential interests of the user by adopting a probability topic model according to the global influences, on the interests of the user, of the time factors; and in allusion to the sensitivity, for potential scenario change, of the learning process, user personalization-oriented secondary updating learning is carried out on the interests on the basis of a concept drift problem according to the local influences, on the potential interests of the user, of the time factors; and finally calculating the degrees of supporting the projects by the user through analyzing the interests of the user, and carrying out sorting to generate a project recommendation list. According to the method, the influences, caused by the recommendation performance, of the concept drift problem can be effectively avoided and the whole recommendation quality of the system can be improved under the condition of sufficiently mining the potential interests of the user.
Owner:GUILIN UNIV OF ELECTRONIC TECH
Bacterial Management in Animal Holding Systems
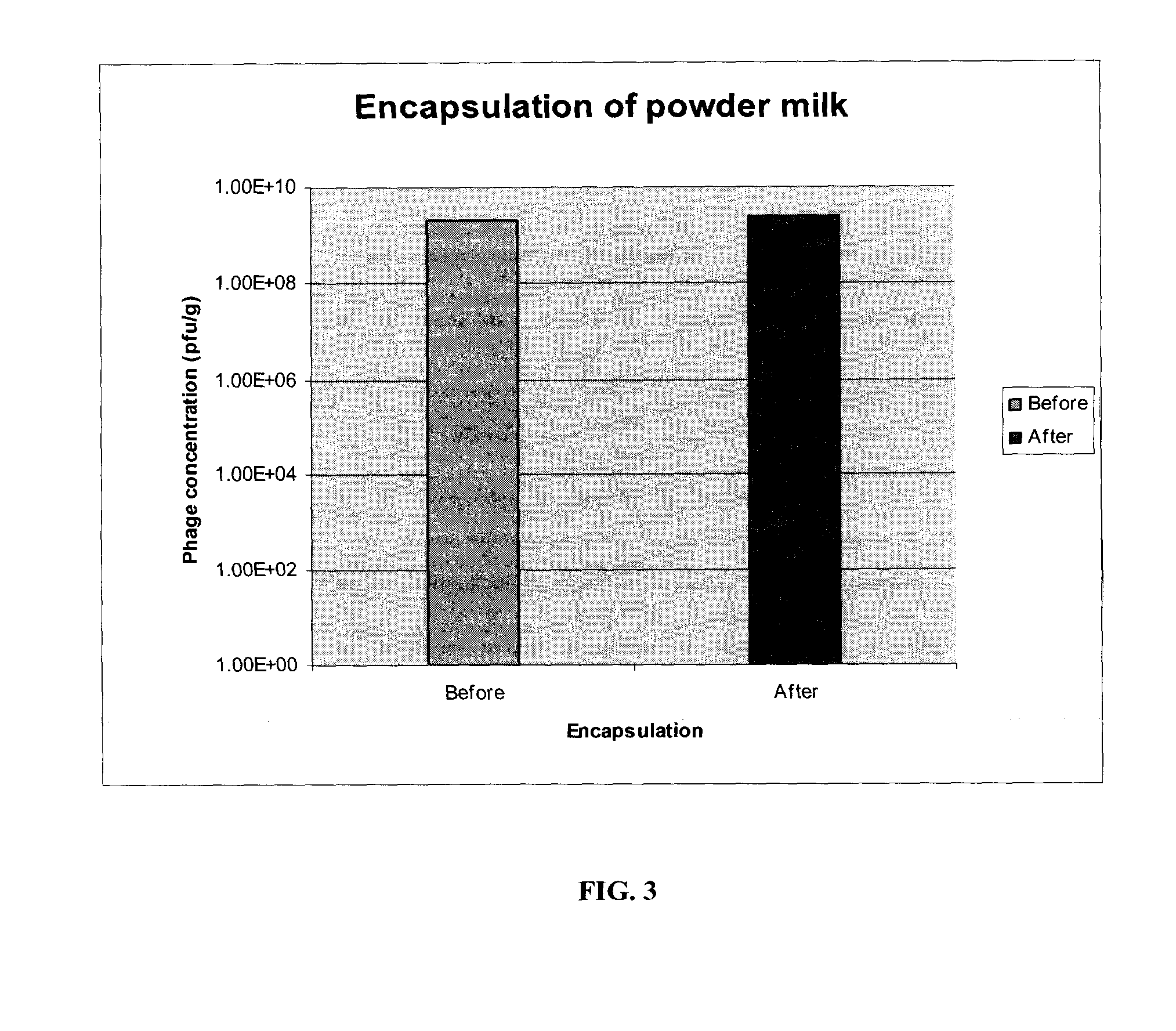
InactiveUS20080260697A1Improve securityReduce pathogen contaminationAntibacterial agentsBiocideControlled releaseBacteriophage
The present invention is directed to method for reducing a population of a target pathogen in an animal or within a feedlot. The method involves administering one or more than one controlled release bacteriophage strain or phage component, or both, to the animal, so that the one, or more than one bacteriophage strain is released in vivo and adsorbs to the one or more than one target pathogen, thereby reducing the one, or more than one pathogen from the animal. The controlled release bacteriophage strain or phage component may be administered as a treatment dose prior to further processing of the animal, a treatment dose followed by a maintenance dose, or a maintenance dose, to manage feedlot target pathogens.
Owner:CHR HANSEN AS
Method for quickly and efficiently testing liquid absorptivity of pole piece
InactiveCN106814004AHigh flash pointHydrophobicWeighing by absorbing componentObservational errorPole piece
The invention relates to a method for quickly and efficiently testing liquid absorptivity of a pole piece. The method comprises the following steps: cutting a pole piece sample, and weighing the pole piece sample to obtain the mass M0; putting the weighed pole piece sample into a container; pouring a soaking solution into the container, and immersing the pole piece sample; putting the container into a vacuum drying oven, and keeping for 15-20 minutes; taking out the pole piece, wiping the free soaking solution on the surface of the pole piece sample by using filter paper, and weighing the pole piece sample to obtain the mass M1; and calculating the liquid absorptivity epsilon=(M1-M0) / M0 and the electrolyte maintenance dose M=epsilon*M0*rho1 / rho2, wherein rho1 is the electrolyte concentration, and rho2 is the density of the soaking solution. The used octadecane has the advantages of high stability, high flash point and low volatility, is hydrophobic, and thus, is suitable to be used as a solvent for soaking the pole piece. The method overcomes the defects of long time consumption, big measurement errors, great environmental pollution caused by the electrolyte and potential hazards to the operating personnel when the electrolyte is used for soaking the pole piece and testing the liquid absorptivity in the past.
Owner:ZHEJIANG CHAOWEI CHUANGYUAN INDUSTRAIAL
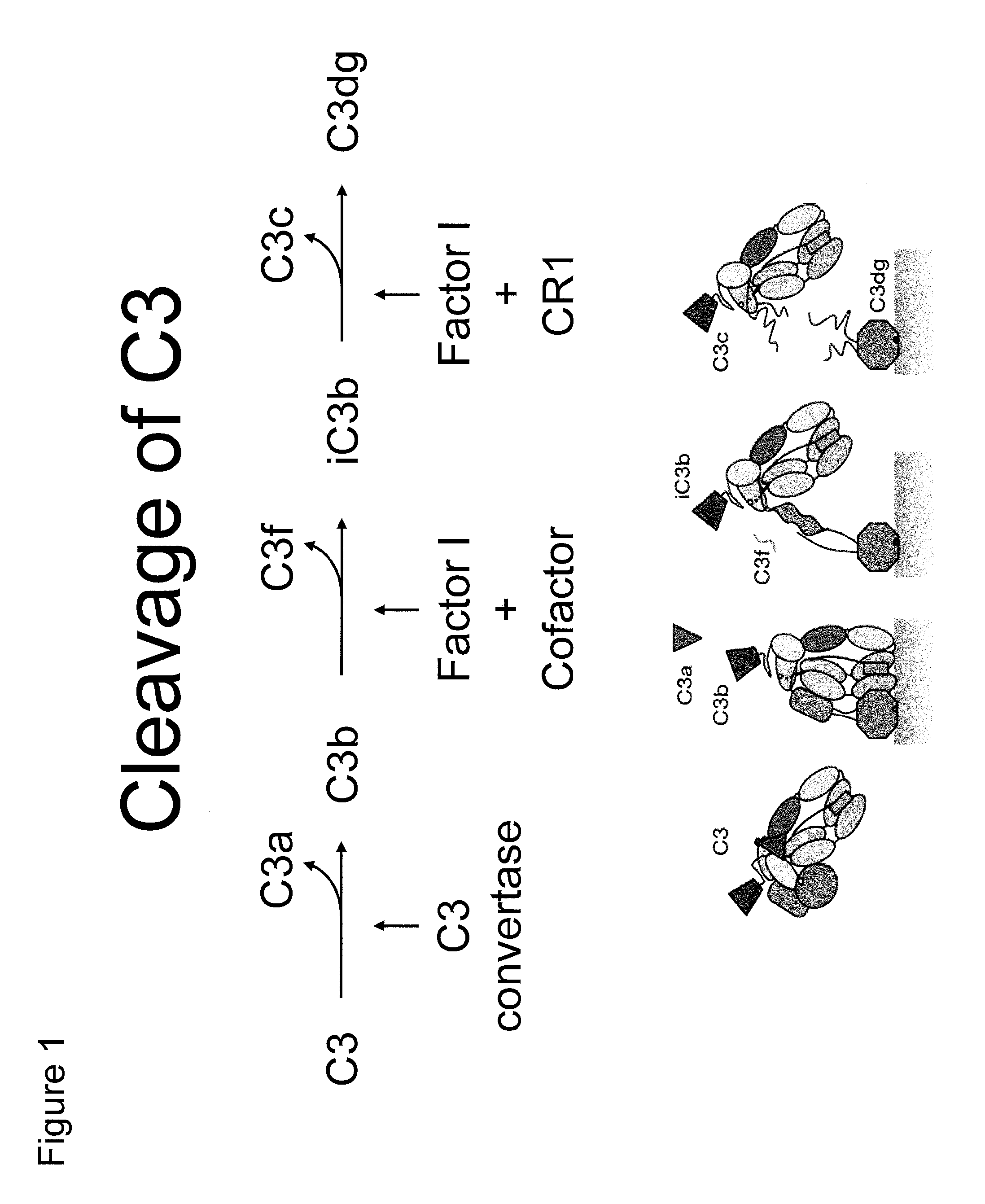
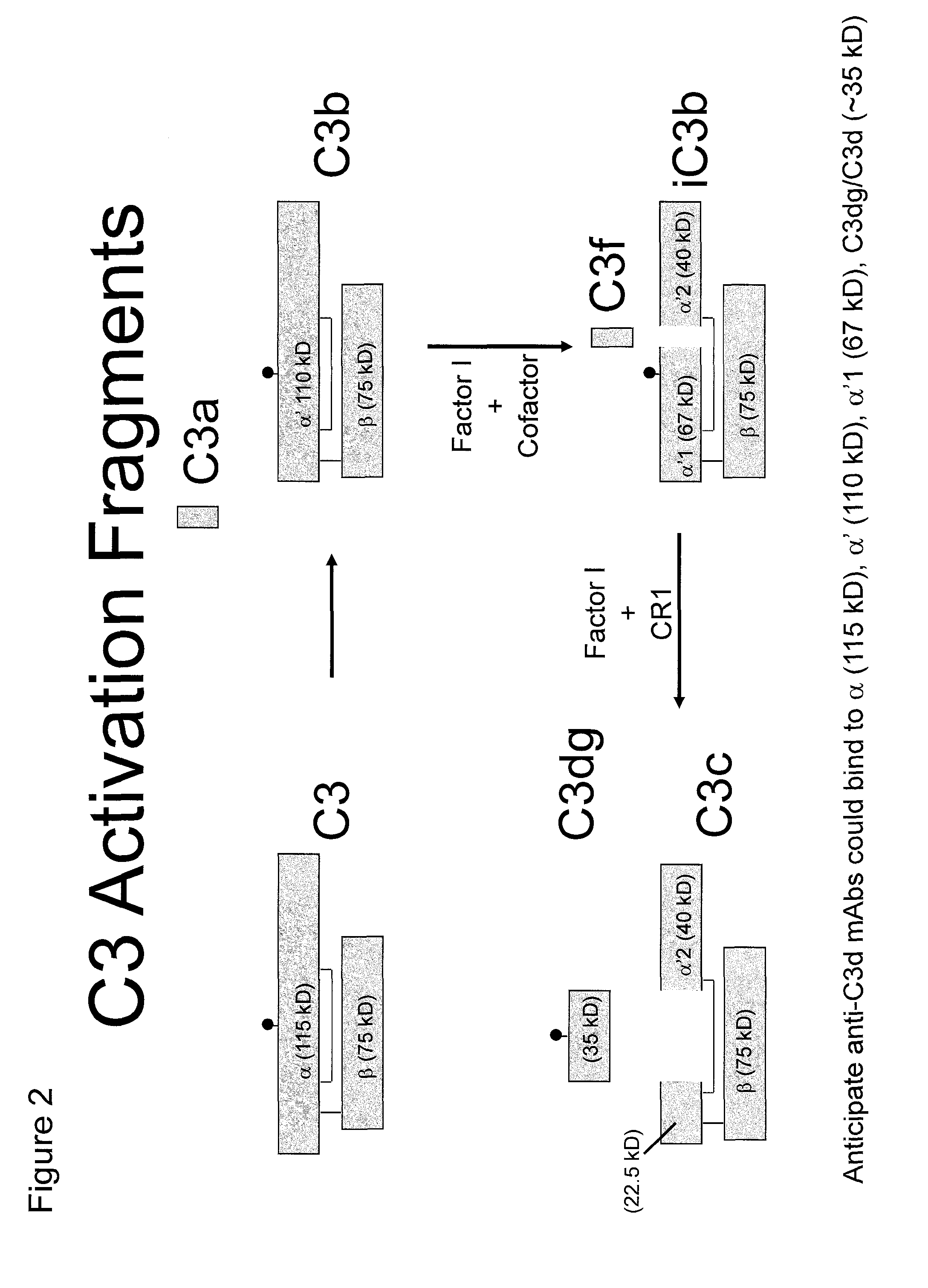
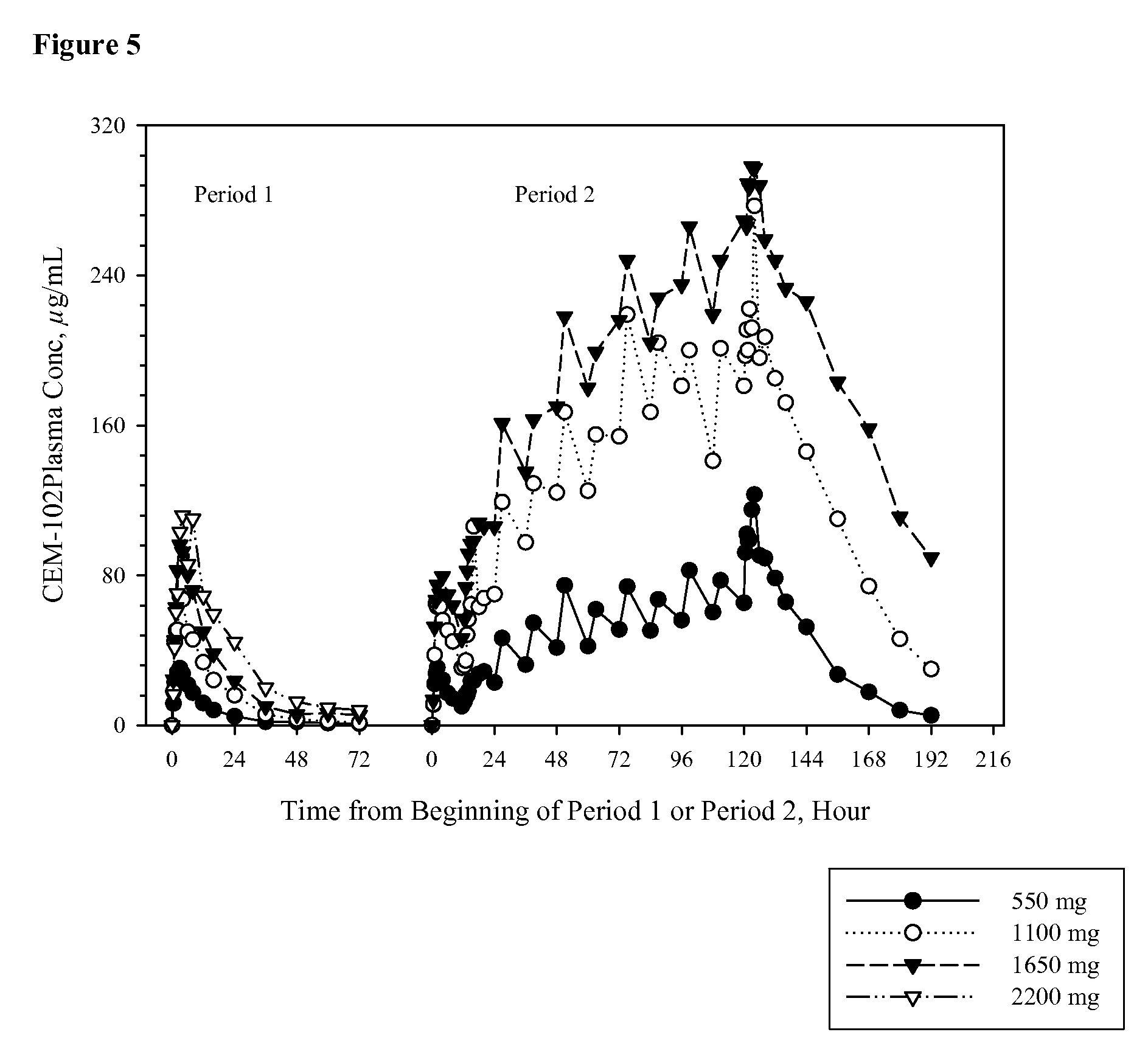
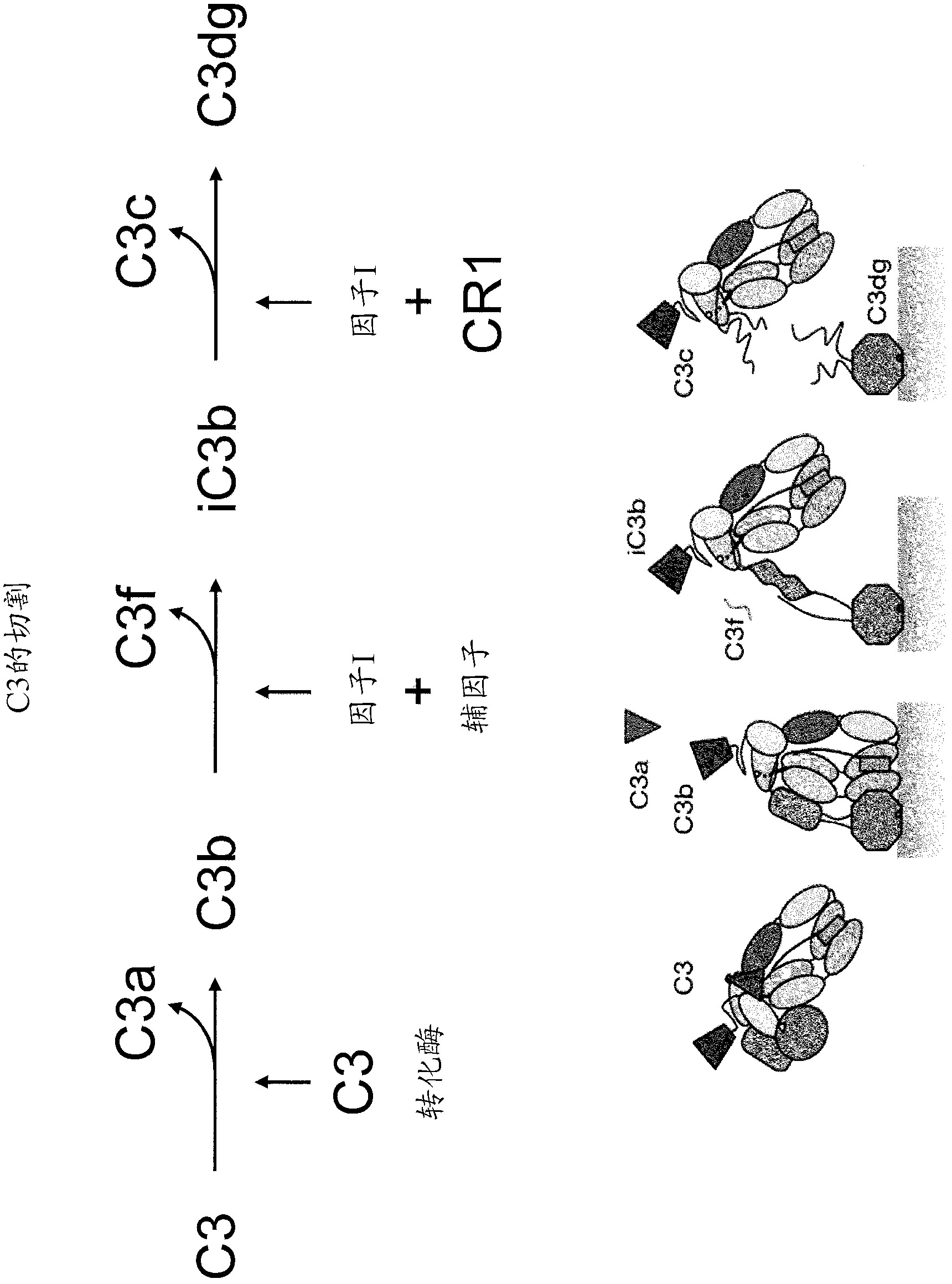
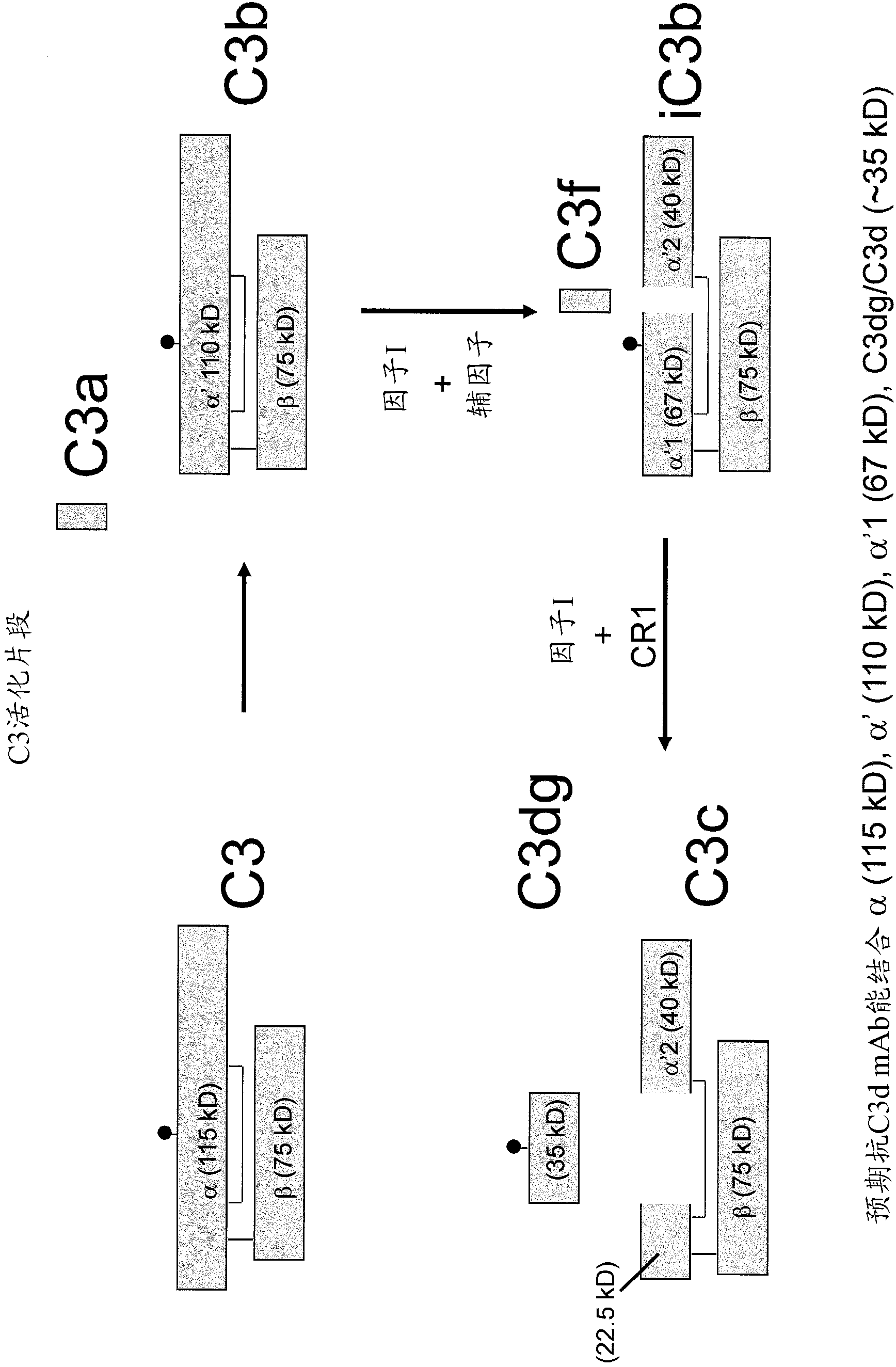
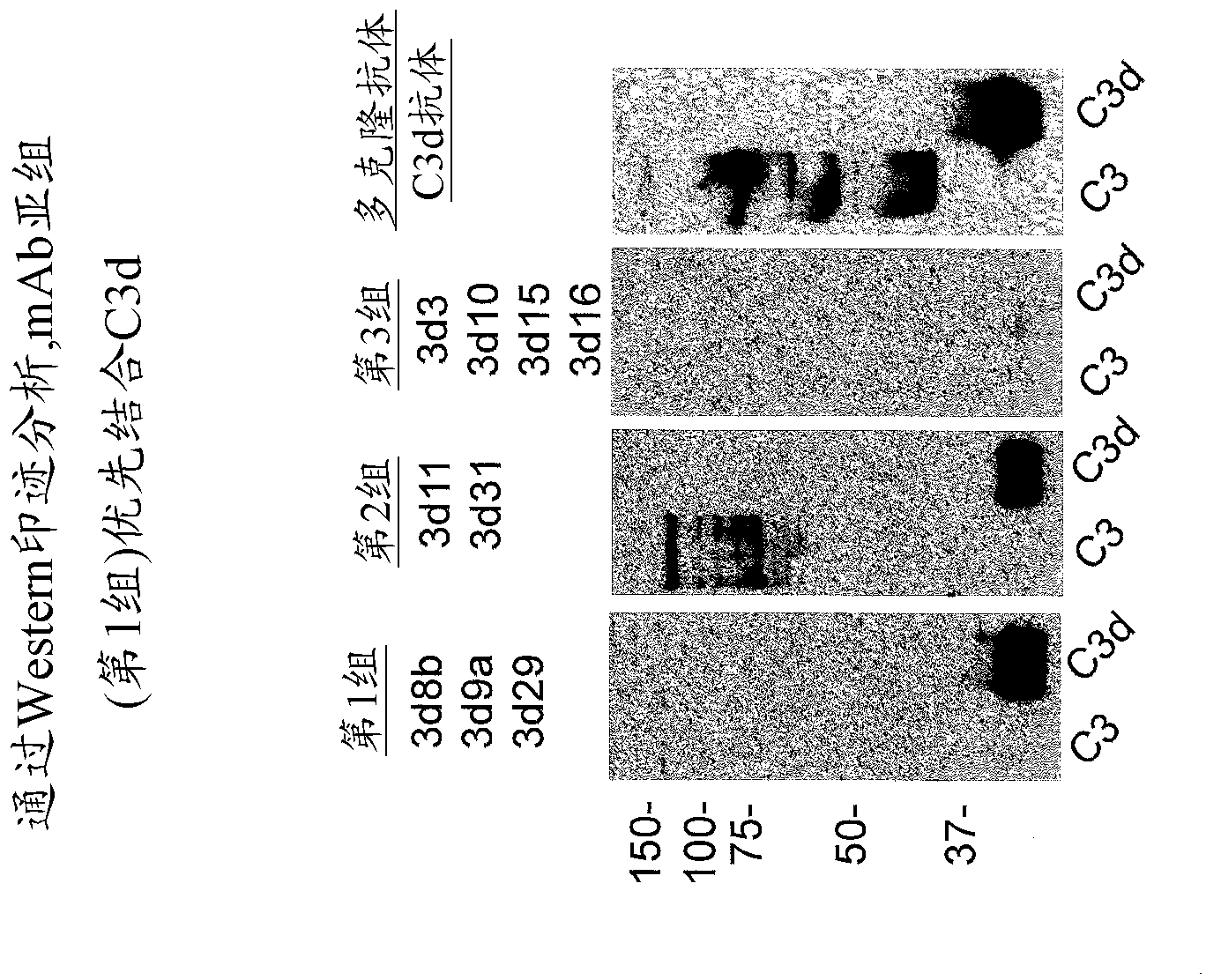
Antibodies to the c3d fragment of complement component 3
ActiveUS20130129728A1Effective treatment and preventionReduce systemic side effectsAntibacterial agentsSenses disorderWhole bodyComplement component 3
The present invention relates to methods and materials for modulating the complement alternative pathway (CAP), the complement classical pathway (CCP), the complement lectin / mannose pathway (CMP), or combinations thereof, as well as methods and materials for targeting diagnostic, prophylactic and therapeutic agents to localized areas of tissue within the body where they may more directly exert their effects upon the intended target cells or tissue, with reduced, associated systemic effects compared with administration of the same or similar agents in an untargeted, systemic manner. The methods and materials of the present invention may therefore allow for increased efficacy, lower threshold effective dosages and / or lower effective maintenance doses, and / or reduced associated undesired or adverse effects in terms of frequency or severity of occurrence, or both. The present invention also relates to methods and materials for modulating a host humoral immune response, especially reducing, inhibiting, or preventing a host humoral immune response.
Owner:MUSC FOUND FOR RES DEV +1
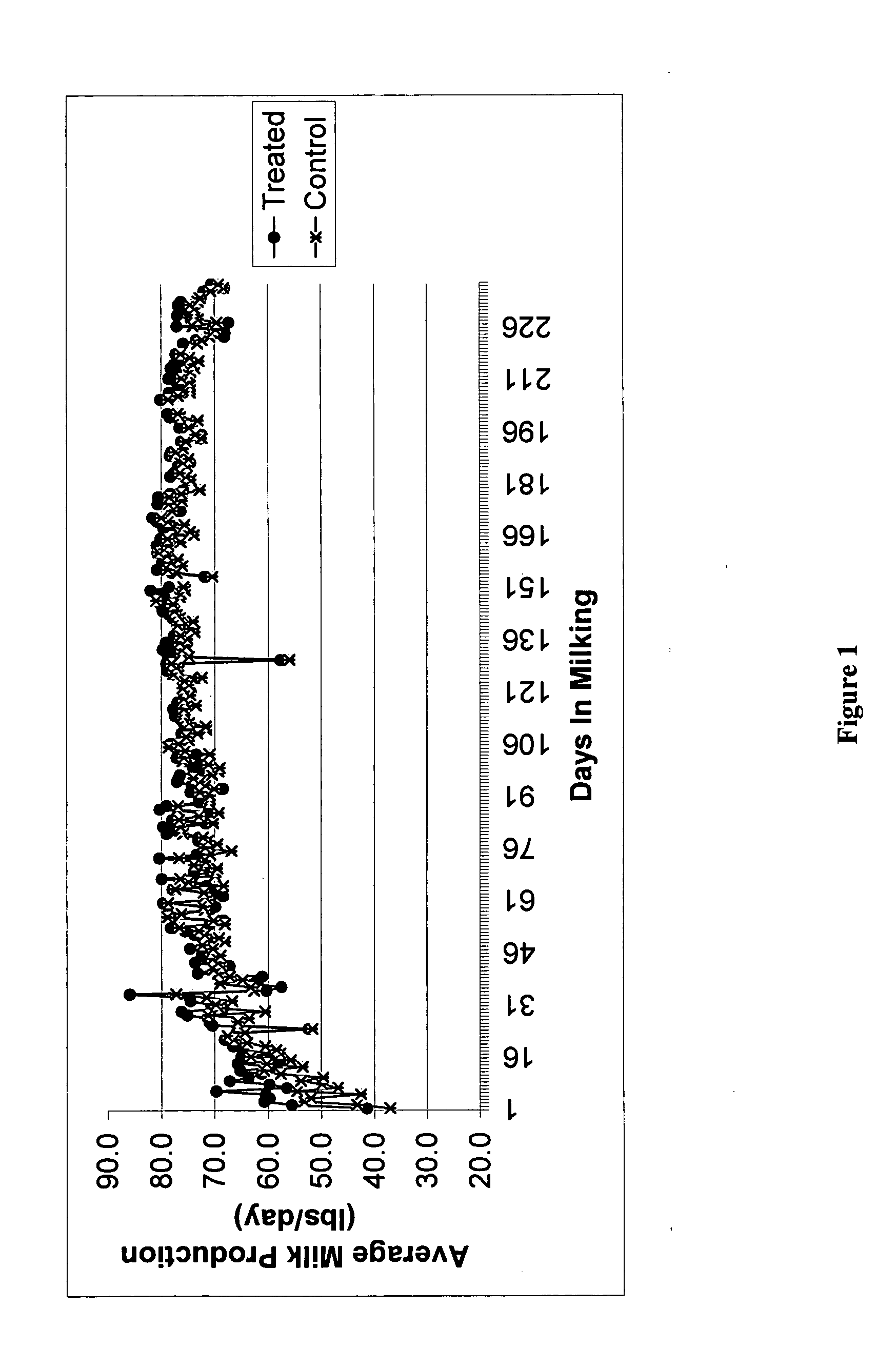
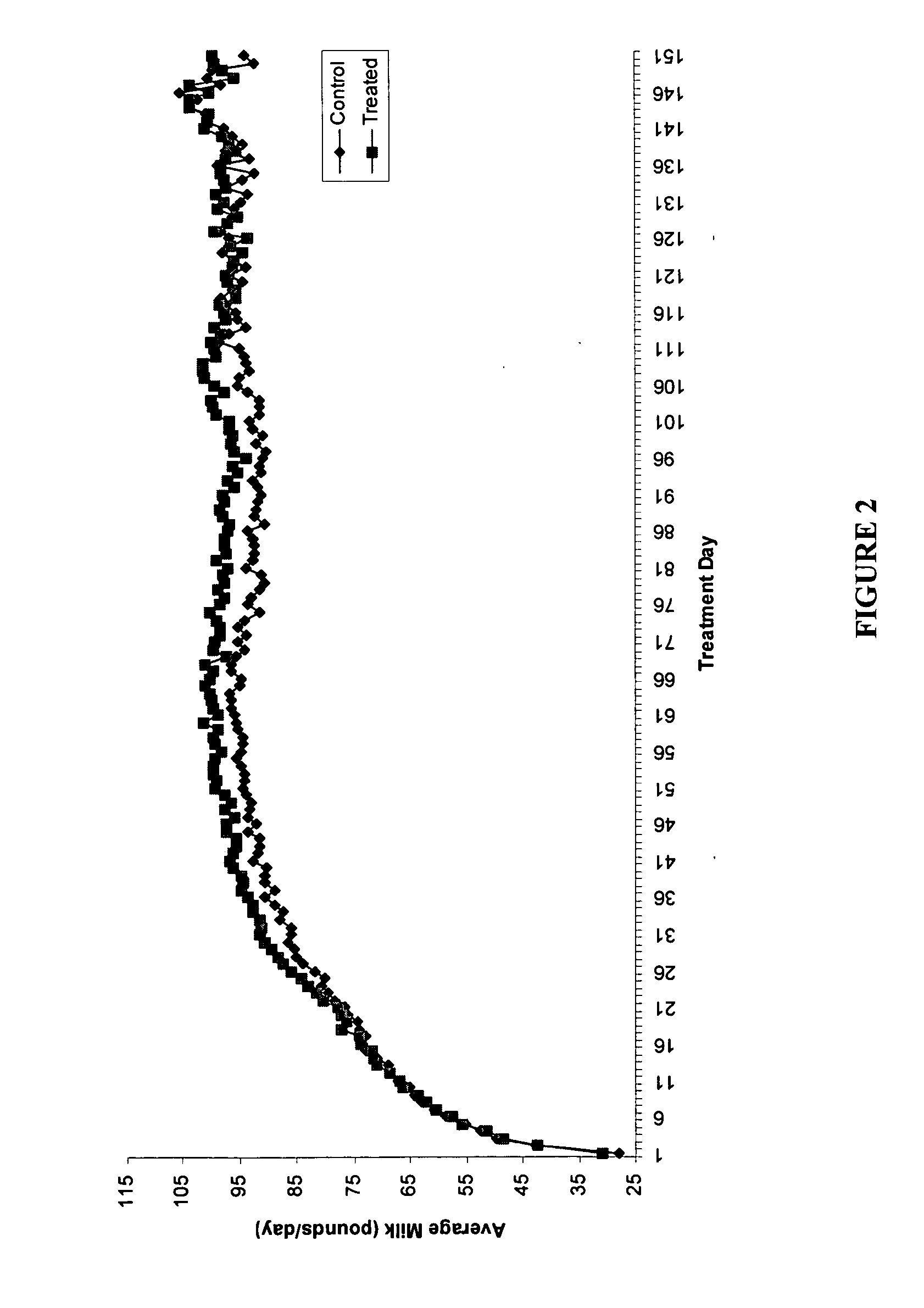
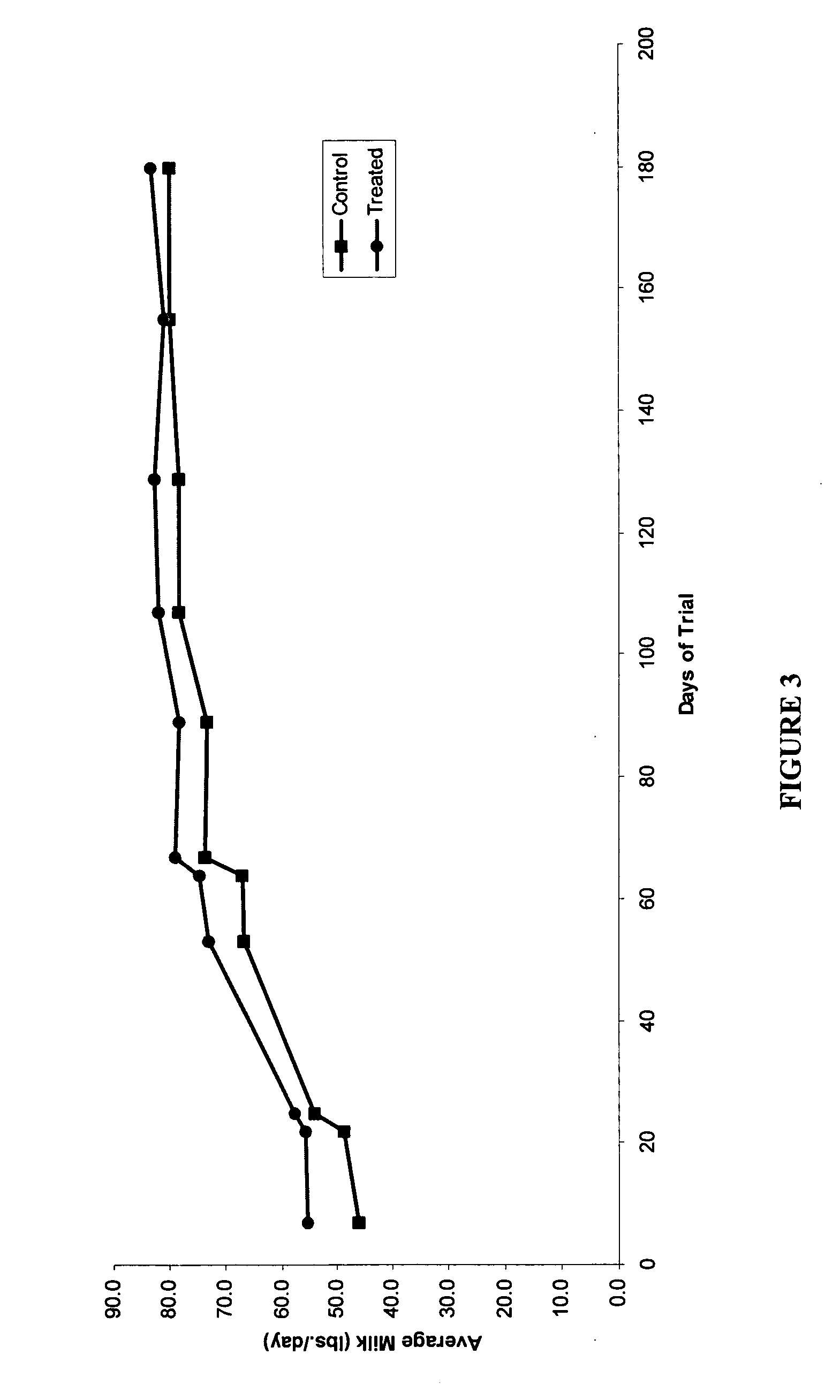
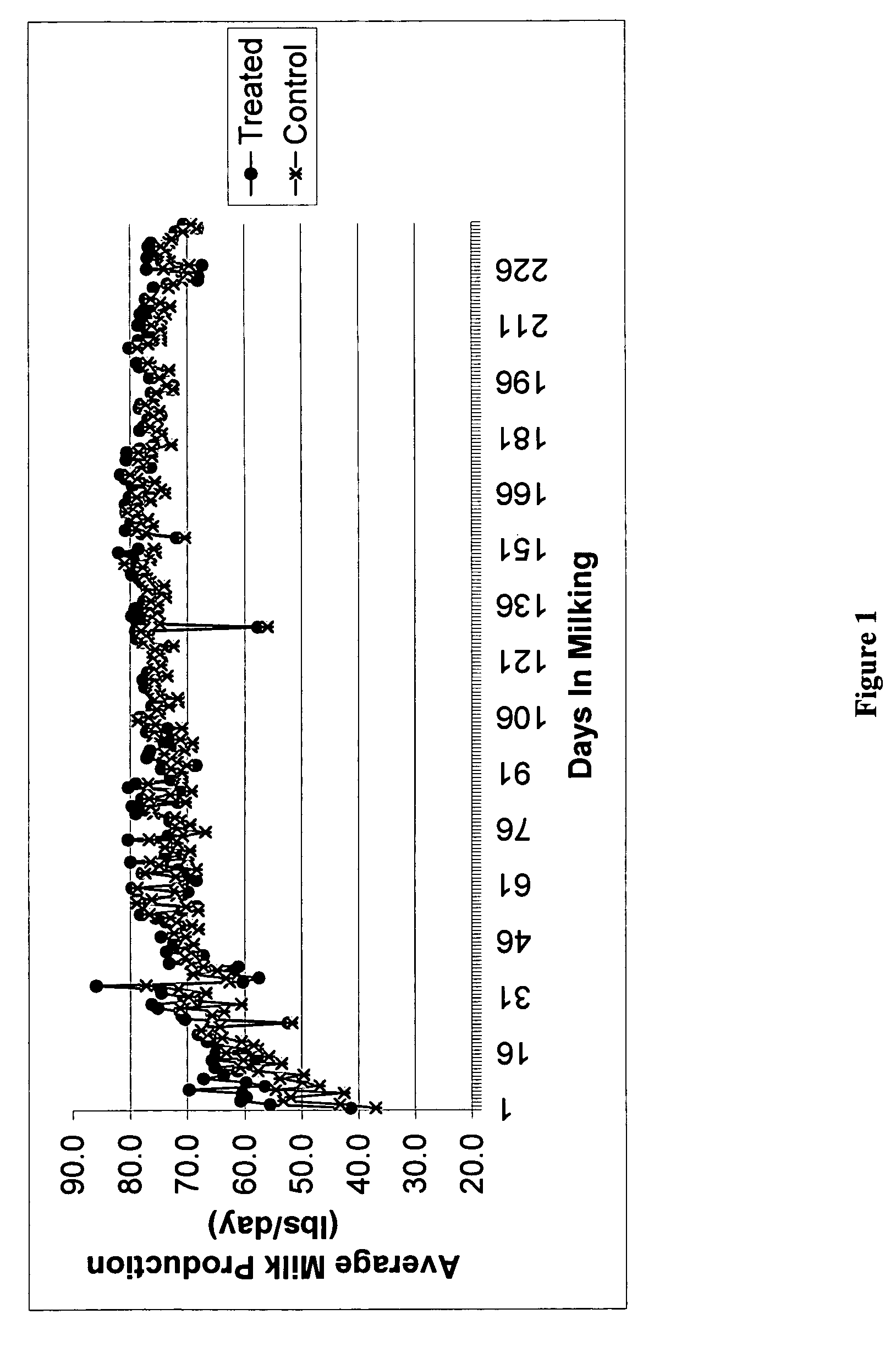
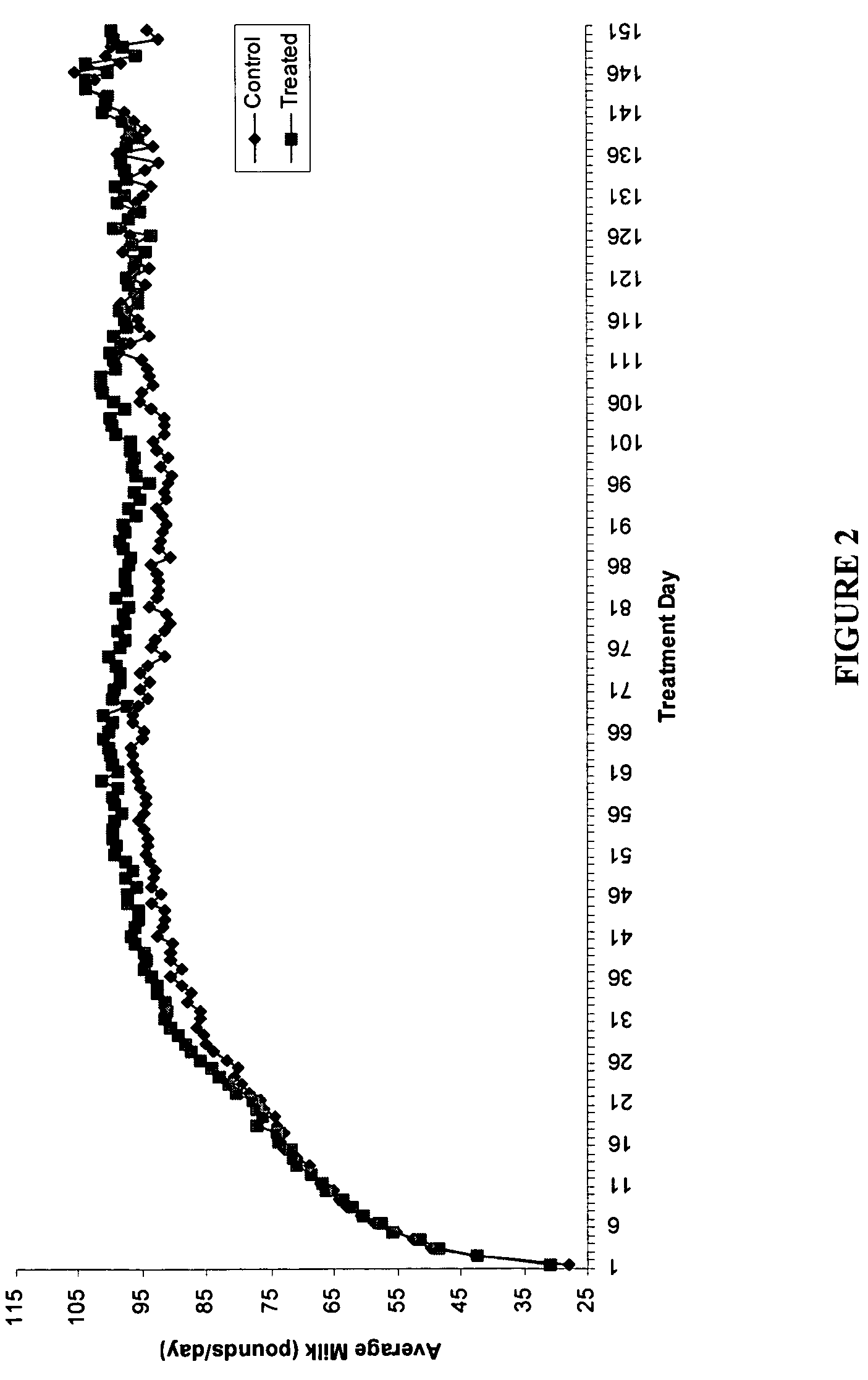
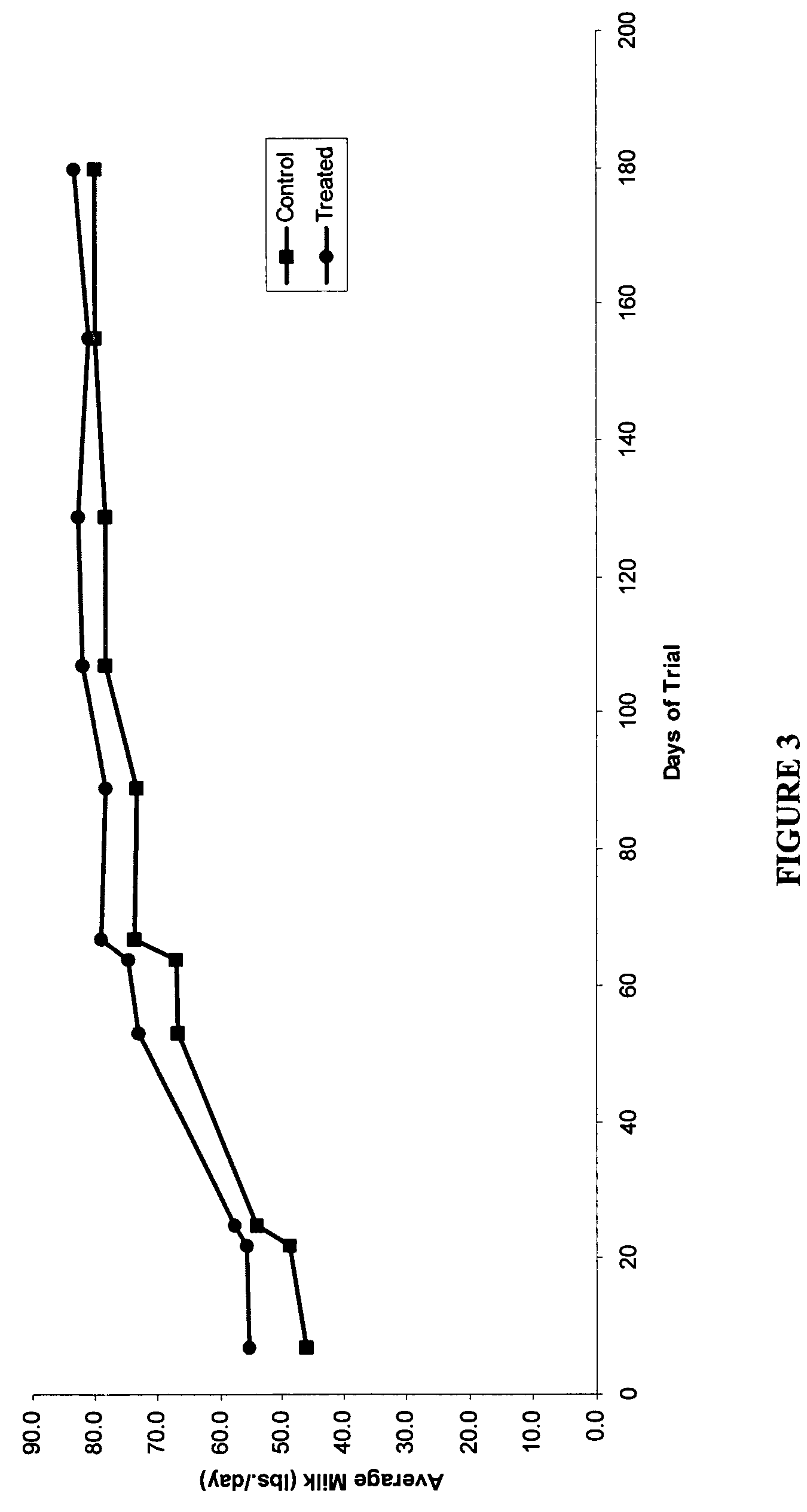
Methods and compositions for increasing milk production in animals
The invention is related to methods and compositions for increasing milk production in animals using a saponin containing composition. In an embodiment, the invention is a method for increasing milk production of an animal comprising administering an initiation dose of a saponin-containing composition to the animal within five days before or after the time of freshening of the animal, and administering a plurality of maintenance doses of the saponin-containing composition to the animal.
Owner:SARTEC
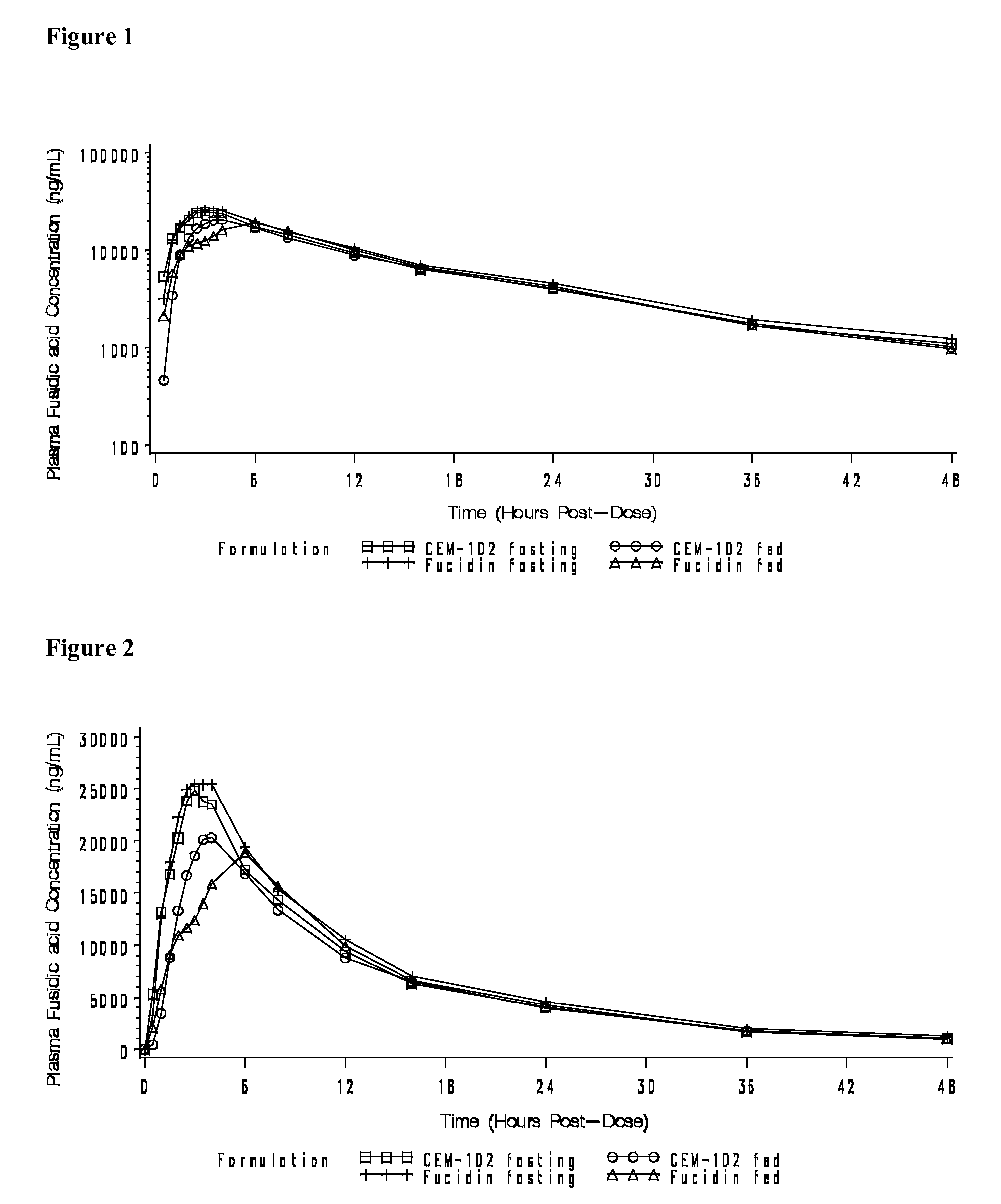
Fusidic acid regimens for treatment of bacterial infections
Novel dosing regimens for the treatment and prevention of bacterial infections using fusidic acid are described. The use of a high loading dose of fusidic acid, followed by moderate maintenance doses of the drug, have been found to prevent development of drug-resistant strains of bacteria, to increase the effective spectrum of the drug, and to avoid nausea and vomiting associated with a prolonged course of therapy of high amounts of the drug.
Owner:ARREVUS INC
Method of water treatment
InactiveUS7094353B2Low levelMinimize exposureWater treatment parameter controlSpecific water treatment objectivesMedicineTurbidity
The invention relates to a method of treatment of bodies of water such as recreational pools, spas and hot tubs with maintenance doses of water treatment chemicals to achieve consistent sanitization and aesthetically pleasing levels of properties such as turbidity. The amount of the maintenance doses is based on the volume of water to be treated in order to achieve hygienic and clear water. The method can be automated for accurate, consistent and safe treatment of water such as found in swimming pools, spas or hot tubs.
Owner:ARCH CHEM INC
Antibodies to the c3d fragment of complement component 3
Owner:UNIV OF COLORADO THE REGENTS OF
Methods and compositions for increasing milk production in animals
The invention is related to methods and compositions for increasing milk production in animals using a saponin containing composition. In an embodiment, the invention is a method for increasing milk production of an animal comprising administering an initiation dose of a saponin-containing composition to the animal within five days before or after the time of freshening of the animal, and administering a plurality of maintenance doses of the saponin-containing composition to the animal.
Owner:SARTEC
Controlled muscle relaxation
In general, embodiments of the present invention provide a method of applying controlled neuromuscular block in a surgical patient until the end of the surgical procedure comprising the steps of (1) inducement of deep neuromuscular block by intravenous administration of an initial bolus dose of a neuromuscular blocking agent, (2) if needed, maintenance of deep neuromuscular block by intravenous application of maintenance doses of the blocking agent, and, in various embodiments, (3) reversal of the neuromuscular block by intravenous application of a bolus dose of a chemical chelator of the pertinent neuromuscular blocking agent.
Owner:NV ORGANON
Dosage and administration of bispecific scfv conjugates
Disclosed are methods for the therapeutic administration of bispecific scFv conjugates as single doses at at least weekly intervals. In certain embodiments the conjugate is MM-111 and is administered at intervals of every two weeks or every three weeks. In other embodiments a single loading dose of MM-111 is administered to a human patient followed at at least weekly intervals by a least a single maintenance dose of MM-111. The loading dose is larger than the maintenance dose.
Owner:MERRIMACK PHARMACEUTICALS INC
Methods and Compositions for pH Control
InactiveUS20090081806A1Aid in sanitizationImprove buffering effectWater treatment parameter controlAnalysis using chemical indicatorsOrganic acidPh control
The present invention comprises methods and compositions for controlling the pH of a confined body of water, such as a swimming pool, spas, and hot tubs, using an organic acid specifically citric acid alone or in combination with other organic acids, borates or boric acid or other chelants or clarifiers. The amount of organic acid added to the confined body of water is determined relative to the pH of the water prior to such addition and the volume of water being treated. The amount of maintenance doses of organic acid is based upon the volume of water being treated and the magnitude of pH change required for adjustment to desired pH. The organic acid can be dispensed automatically or manually and can be dispensed as a solid, including a dispersable powder form, a capsule, or tablet, or as a liquid concentrate to be diluted in the confined body of water.
Owner:REEVES III CHARLES E +1
Method of water treatment
InactiveUS20060283811A1Low levelMinimize exposureSolid sorbent liquid separationSedimentation separationMedicineTurbidity
The invention relates to a method of treatment of bodies of water such as recreational pools, spas and hot tubs with maintenance doses of water treatment chemicals to achieve consistent sanitization and aesthetically pleasing levels of properties such as turbidity. The amount f the maintenance doses is based on the volume of water to be treated in order to achieve hygienic and clear water. The method can bt automated for accurate, consistent and safe treatment of water such as found in swimming pools, spas or hot tubs.
Owner:ARCH CHEM INC
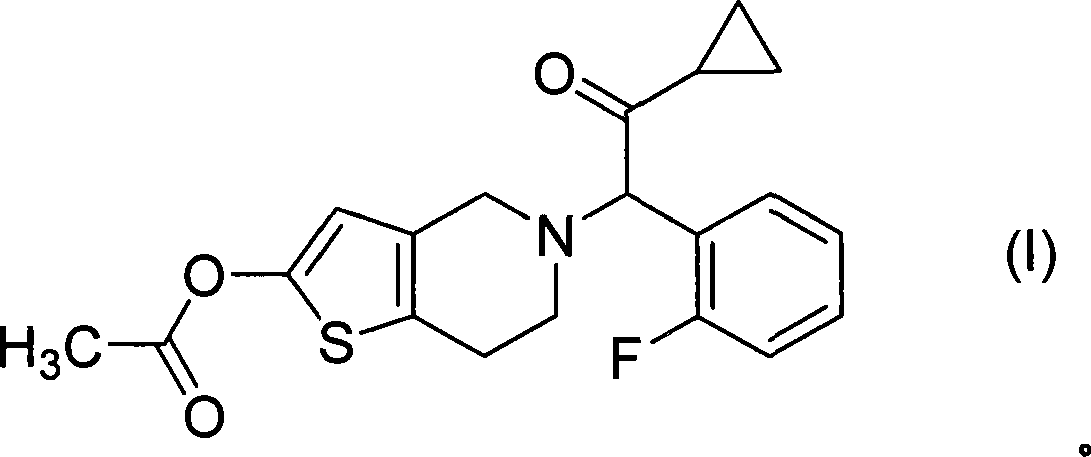
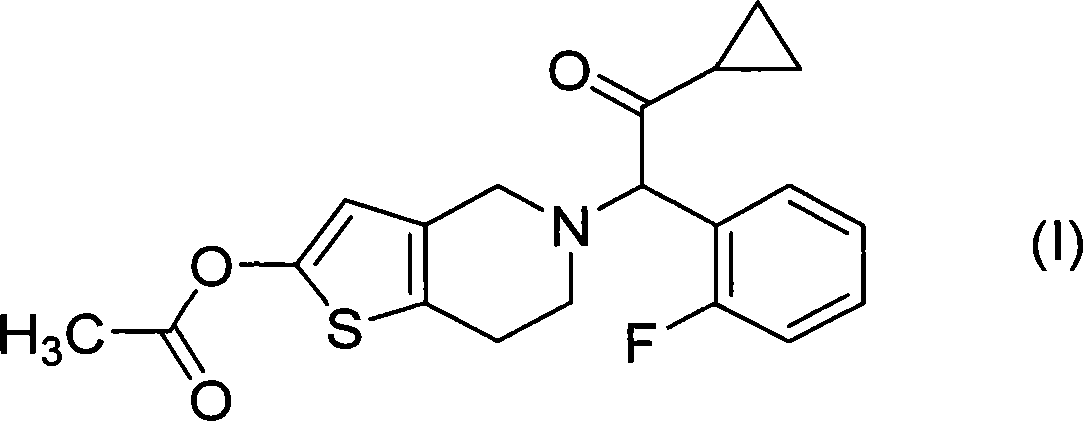
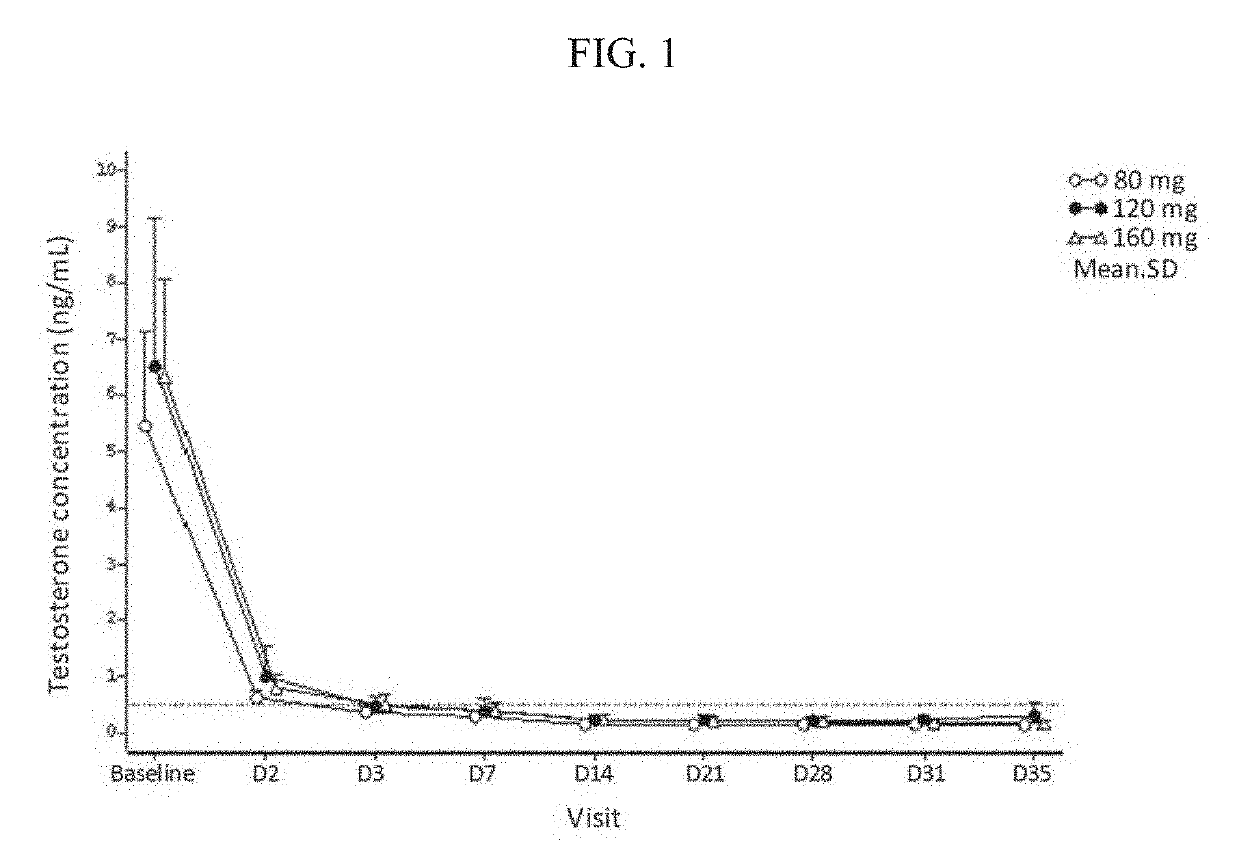
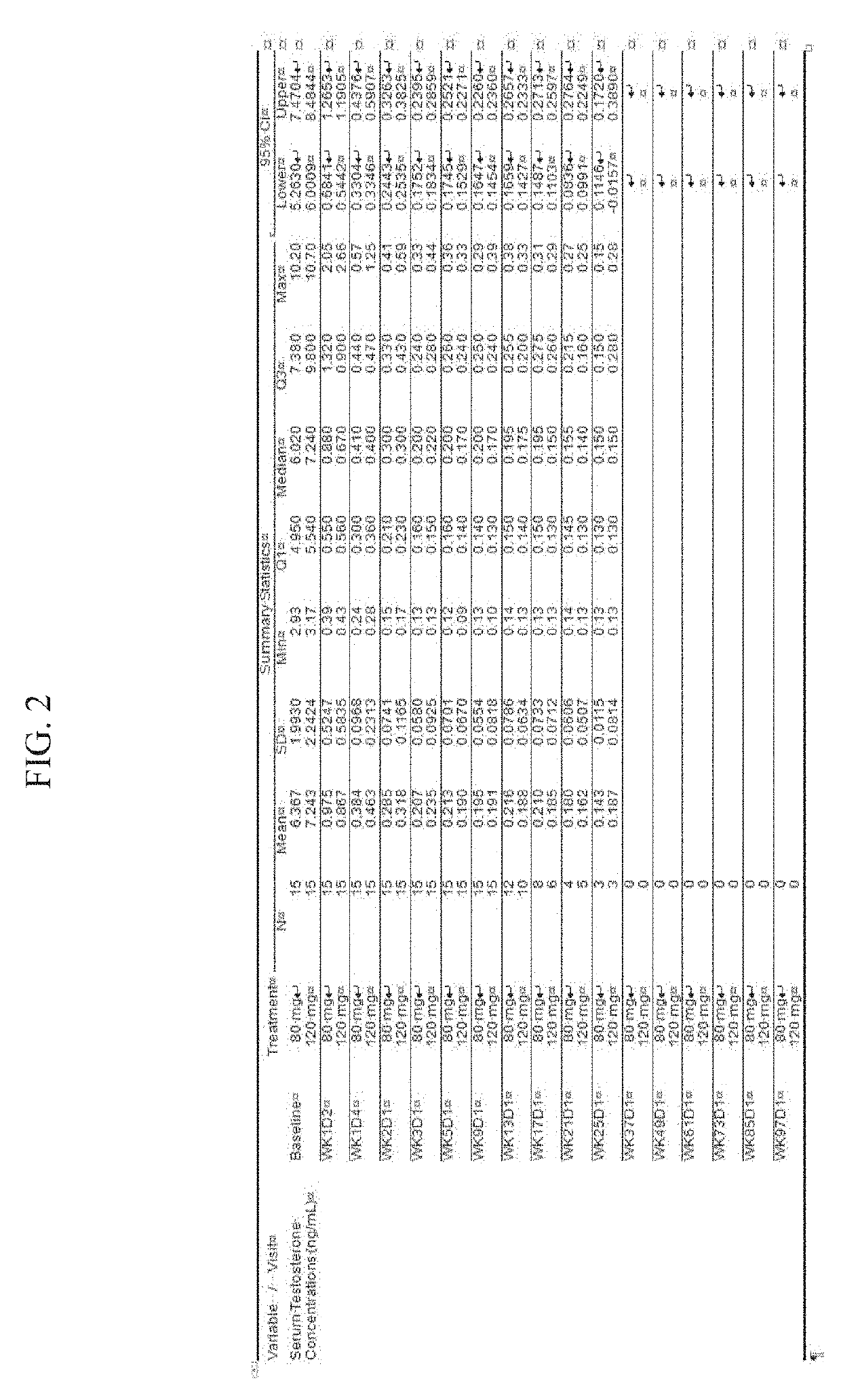
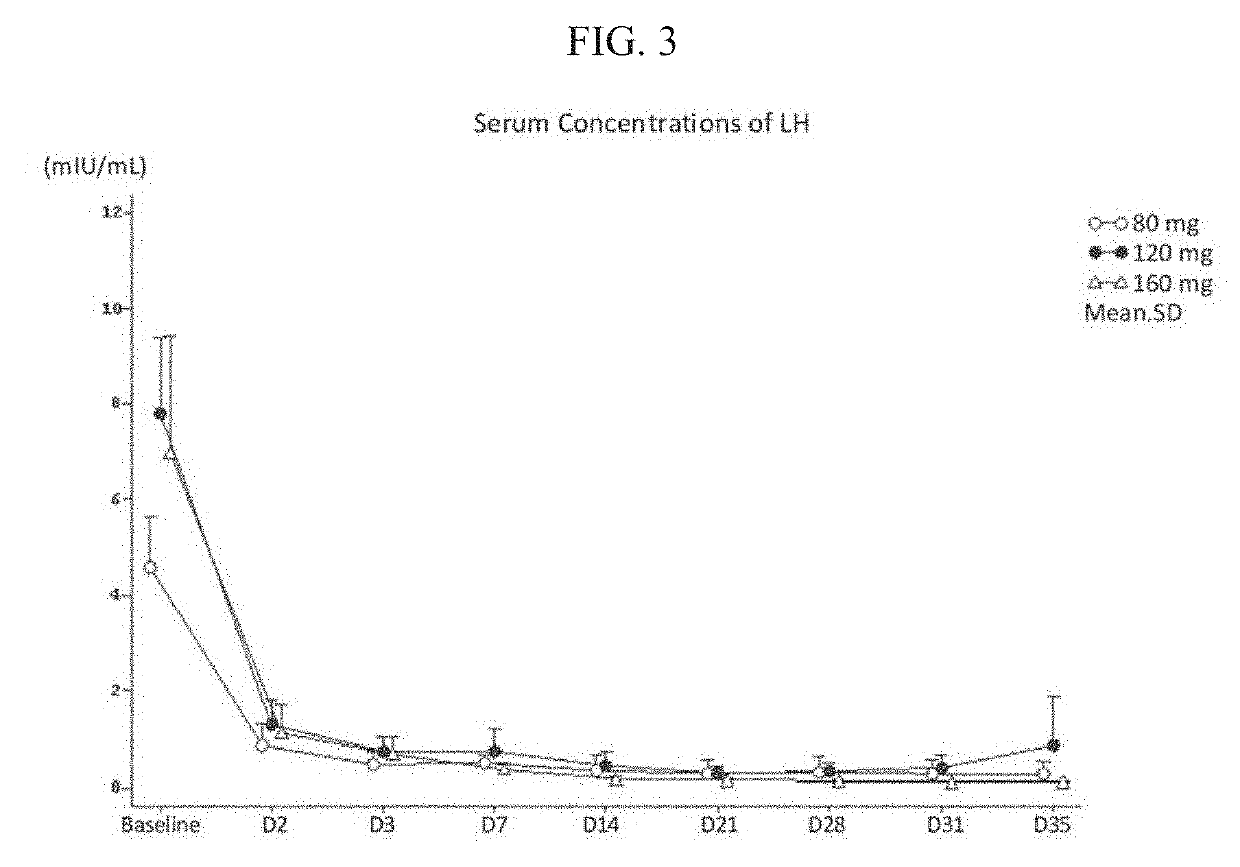
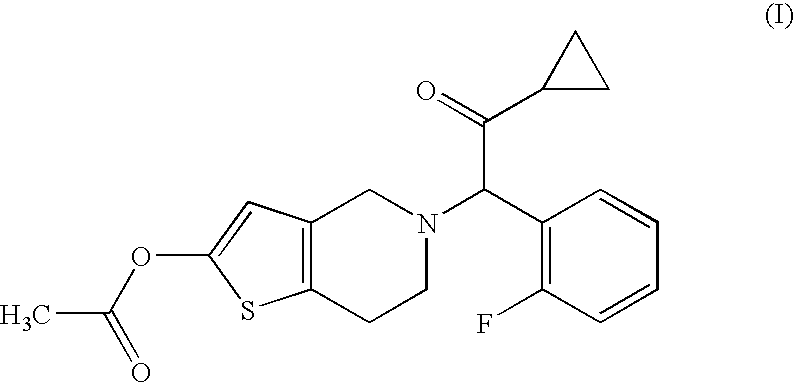
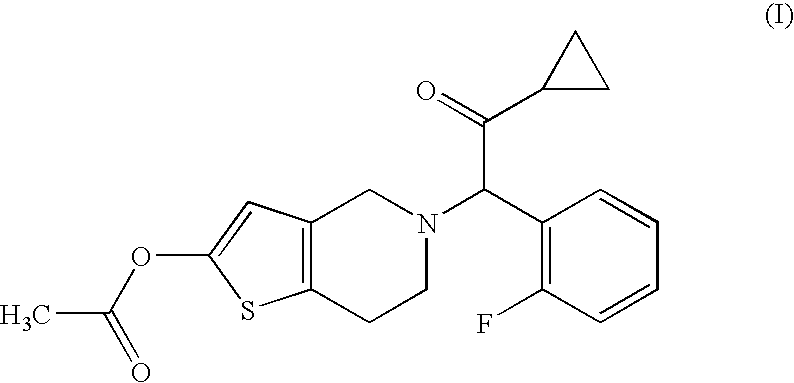
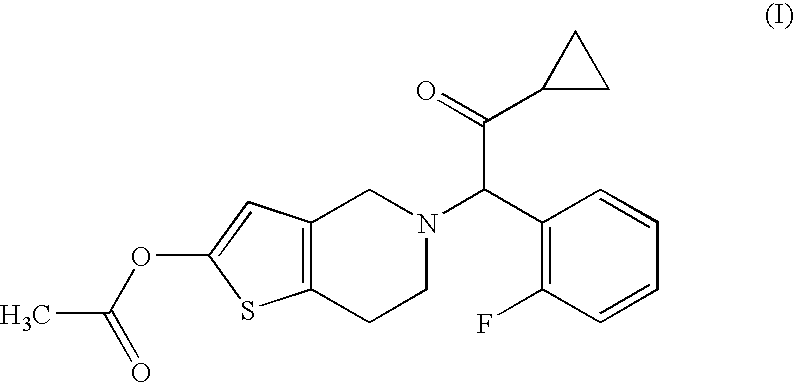
Treatment of prostate cancer
Methods for treating prostate cancer, including advanced prostate cancer, in a subject in need thereof, include administering once-daily to the subject, at least 80 mg of N-(4-(1-(2,6-difluorobenzyl)-5-((dimethylamino)methyl)-3-(6-methoxy-3-pyridazinyl)-2,4-dioxo-1,2,3,4- tetrahydrothieno[2,3-d]pyrimidin-6-yl)phenyl)-N′-methoxyurea, or a corresponding amount of a pharmaceutically acceptable salt thereof. Another method includes: administering once-daily to the subject in need thereof, an oral load dose formulation having from 240 mg to 480 mg of N-(4-(1-(2,6-difluorobenzyl)-5-((dimethylamino)methyl)-3-(6-methoxy-3-pyridazinyl)-2,4-dioxo-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidin-6-yl)phenyl)-N′-methoxyurea, or a corresponding amount of a pharmaceutically acceptable salt thereof; and thereafter administering once-daily to the subject, an oral maintenance dose formulation having 80 mg to 160 mg of N-(4-(1-(2,6-difluorobenzyl)-5-((dimethylamino)methyl)-3-(6-methoxy-3-pyridazinyl)-2,4-dioxo-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidin-6-yl)phenyl)-N′-methoxyurea, or a corresponding amount of a pharmaceutically acceptable salt thereof.
Owner:TAKEDA PHARMA CO LTD +1
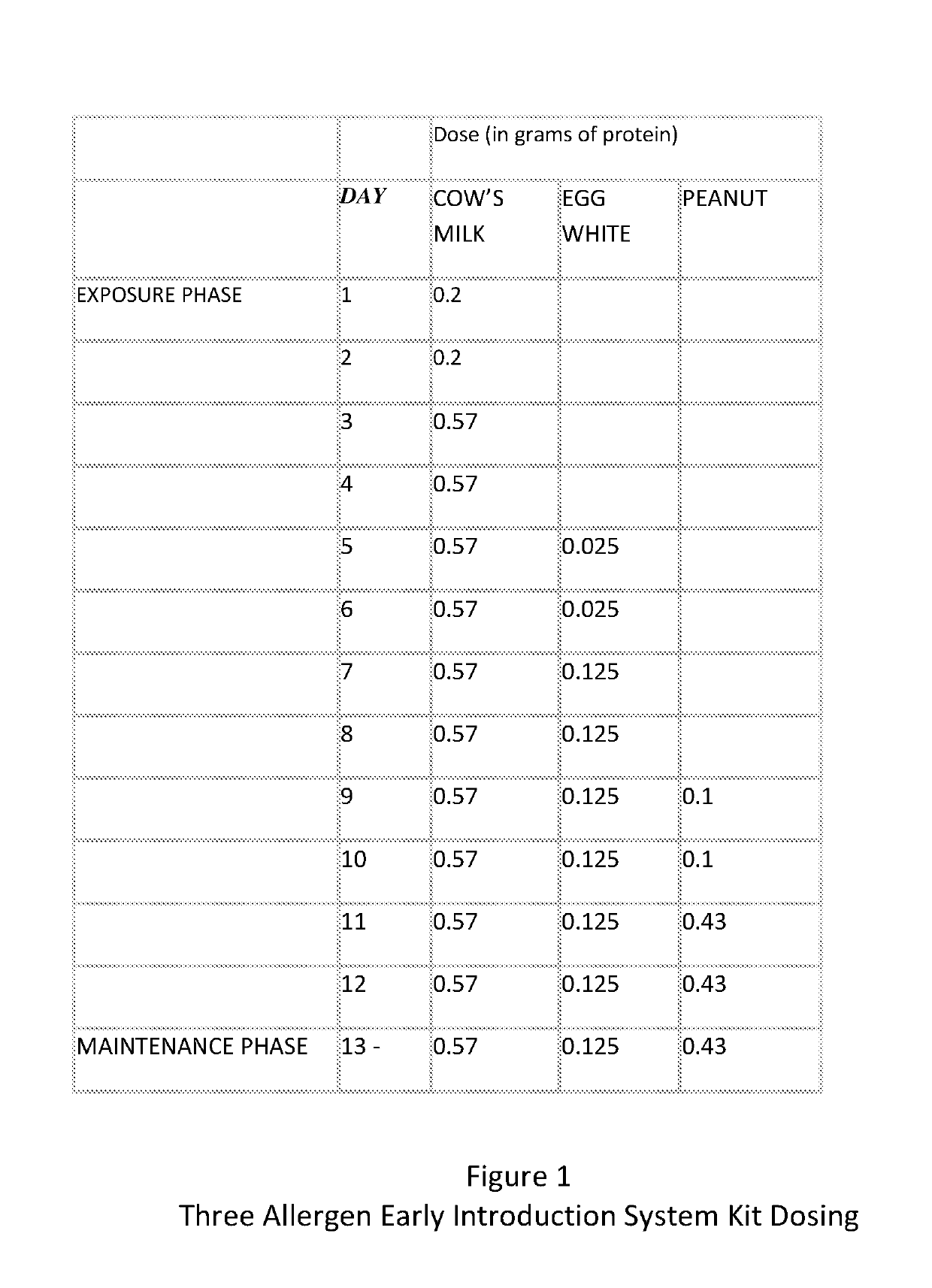
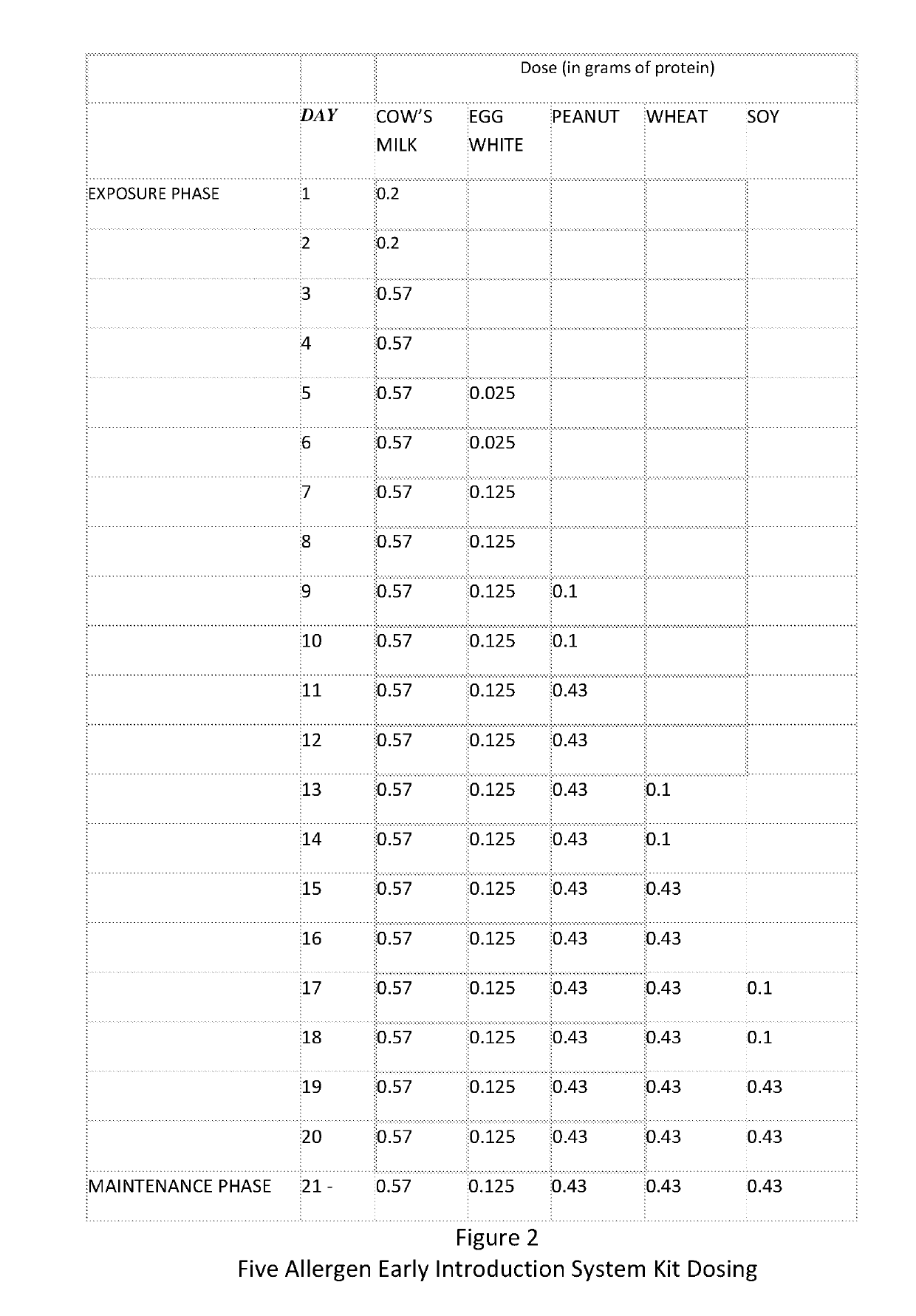
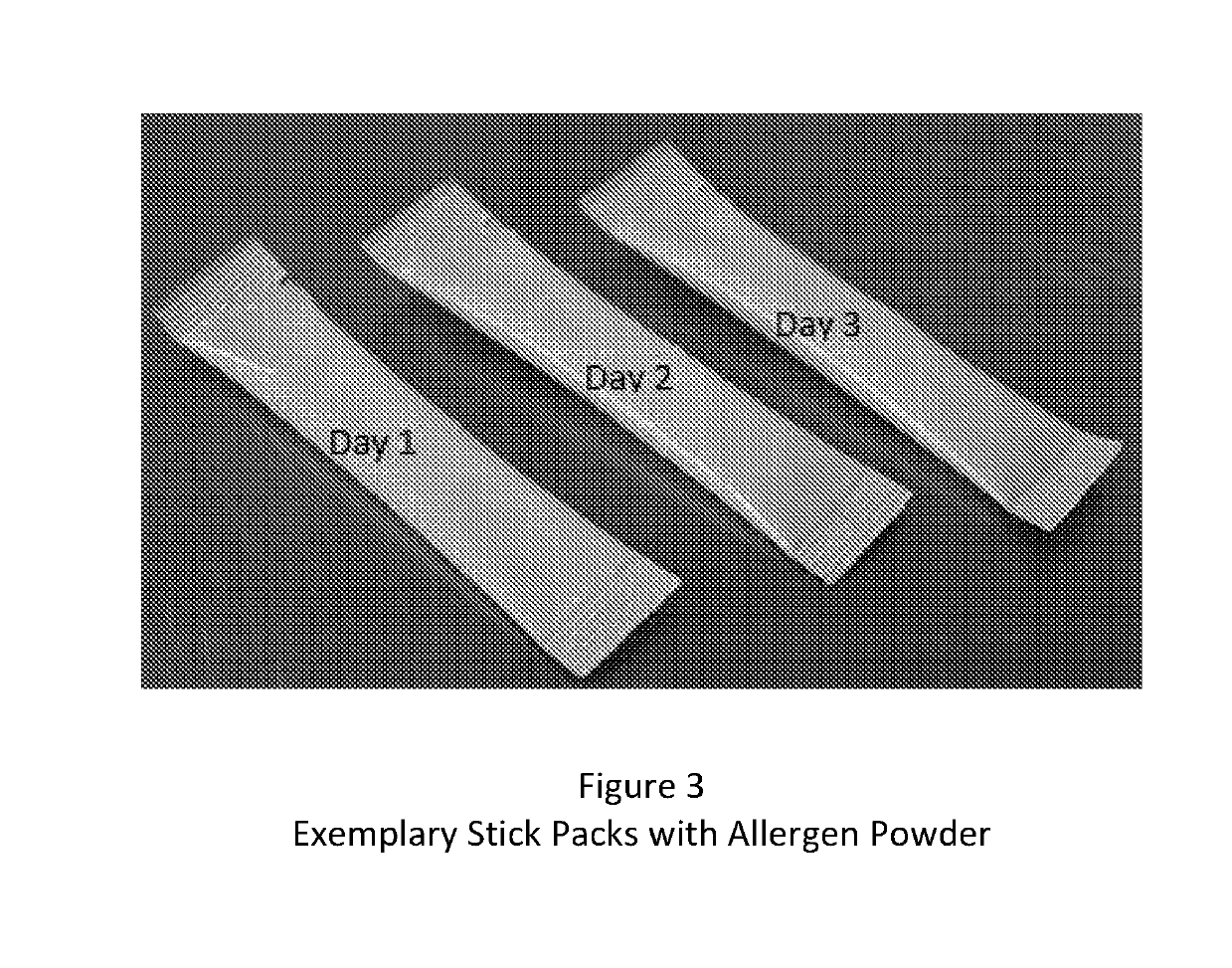
Composition and method for reducing allergic response
A method and kit for the sequential early introduction to an infant of at least two allergens to decrease the infant's risk for developing allergies, the method involving administering an initial lower, exposure dose of a first allergen for a day or two, followed by administering a higher maintenance dose of the first allergen for several days, followed by administering the maintenance dose of the first allergen and an initial lower, exposure dose of a second allergen for a day or two, followed by administering the higher maintenance dose of the first allergen and a higher maintenance does of the second allergen for several days. The allergens can be provided in powdered protein form in premeasured pouches for addition to baby formula or to mother's milk. Alternatively, the allergens can already be provided in baby formula, or in other foods such as snack bars, cookies, or gels.
Owner:PROLLERGY CORP
Iodine therapy dosing for treating medical conditions
A method for therapeutically treating medical conditions requiring chronic therapy such as fibrocystic breast disease, endometriosis, ovarian cysts, uterine fibroids and including the treatment of women considered to be at risk to breast and / or ovarian cancer without causing an overt thyroid disease by first administering to a patient a daily loading dose of molecular iodine (I2) for a time period not to exceed six months and preferably 2 to 4 months at a concentration level sufficient to remediate symptoms associated with the medical condition followed by daily dosing with a maintenance dose of I2 after treatment of the loading dose is completed with the concentration of the maintenance dose being substantially less than the concentration of the loading dose.
Owner:IOGEN LLC
Use of carbazole compounds for the treatment of congestive heart failure
A method of treatment using carvedilol is disclosed, wherein the carvedilol decreases the risk of mortality caused by congestive heart failure in patients. The patients are titrated with low amounts of carvedilol, with the initial titration dosage being only 10 to 30% of the daily maintenance dose.
Owner:SMITHKLINE BEECHAM (CORK) LTD
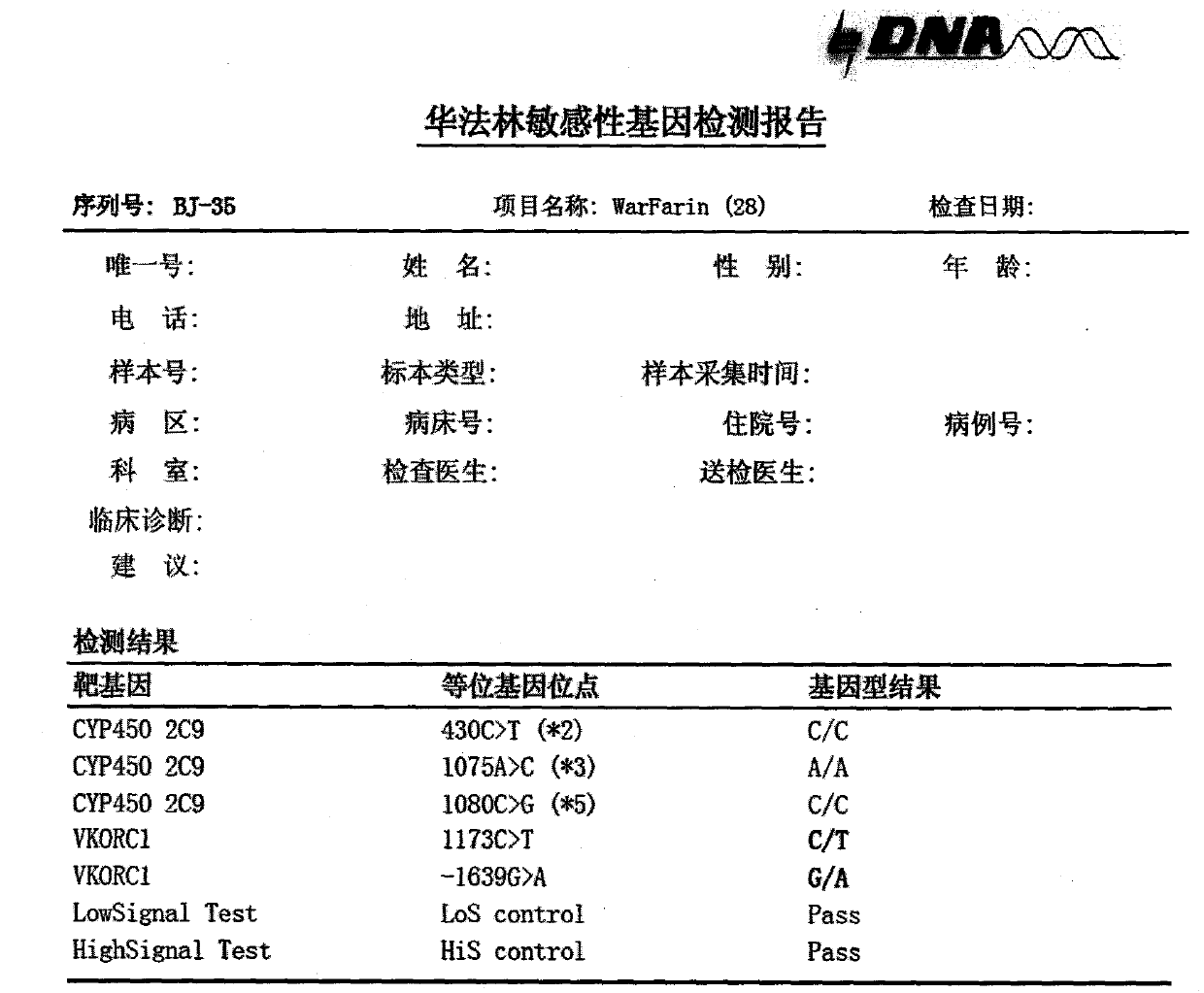
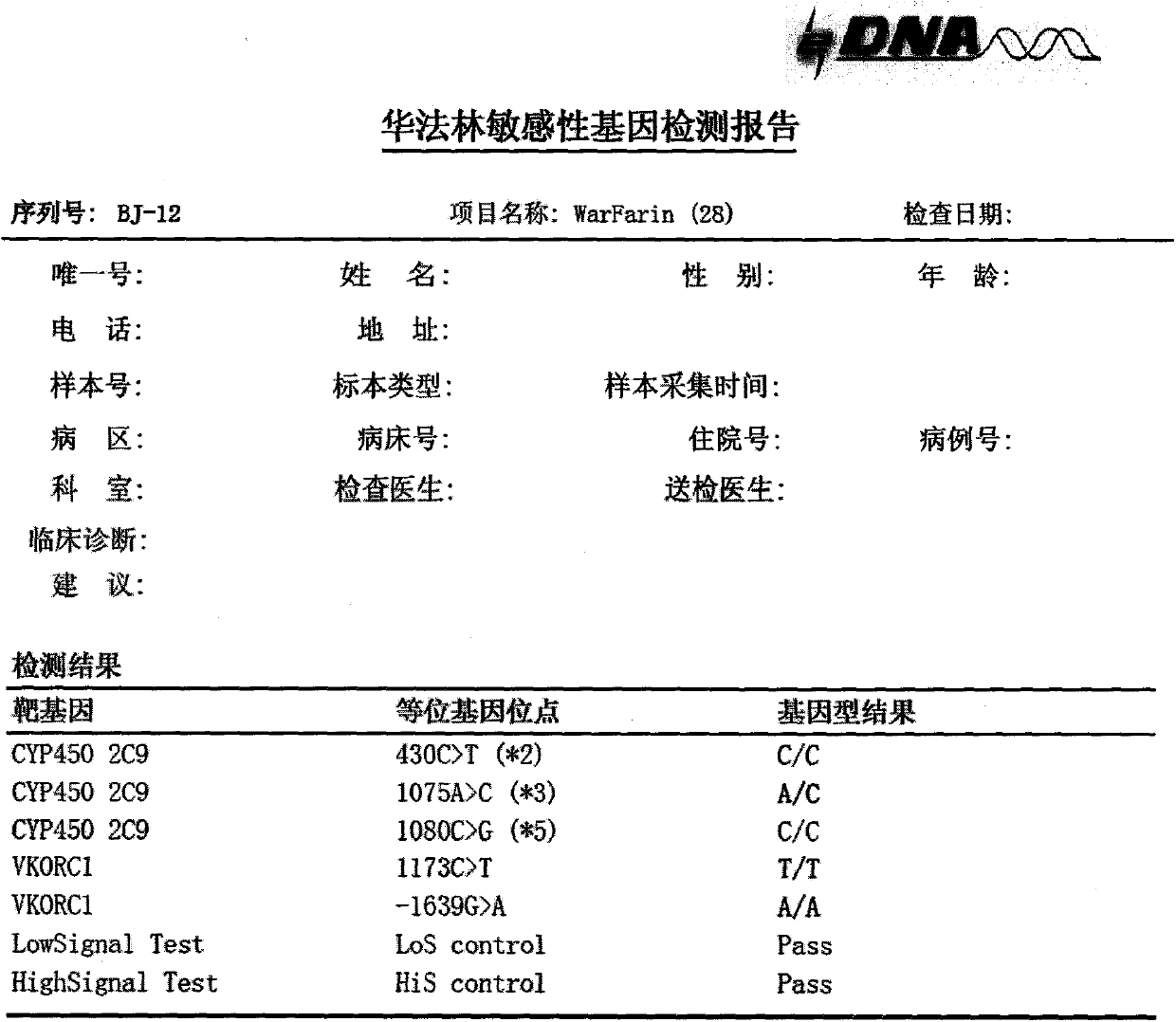
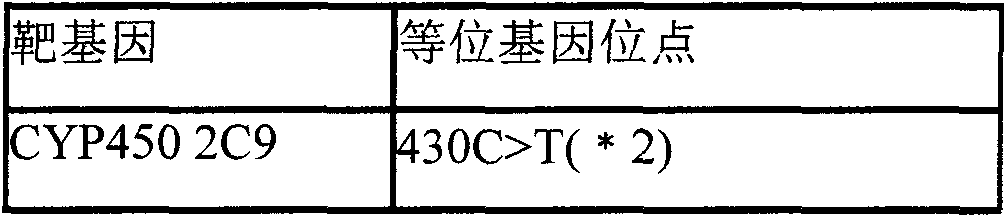
Kit for detecting warfarin sensitivity gene by using electrochemical gene sensor method
InactiveCN103627789AHigh sensitivityStrong specificityMicrobiological testing/measurementHuman DNA sequencingWarfarin
The invention provides a warfarin sensitivity gene detection kit (an electrochemical gene sensor method) which is a kit used for rapid detection of a warfarin sensitivity gene in a human genome by using electrochemical gene sensor technology. The kit is applicable to qualitative detection of *2, *3 and *5 allele types of the cytochrome CYP2C9 gene and 1639G>A and 1173C>T allele types of vitamin K epoxide reductase complex 1 (VKORC 1) in clinical specimens like human peripheral blood and a variety of tissue and cells; the above-mentioned genotypes are helpful for clinical discrimination of patients with increased drug susceptibility to warfarin and enable the dosage range of warfarin to be rapidly determined, curative effects to be guaranteed and the risk of hemorrhage to be reduced. Combination of warfarin gene detection results with INR monitoring enables the maintenance dose of warfarin to be effectively and rapidly adjusted, thereby lowering down the risk of hemorrhage while achieving curative effects.
Owner:DAAN GENE CO LTD
Method of water treatment
InactiveUS7575673B2Low levelMinimize exposureSolid sorbent liquid separationSedimentation separationMedicineTurbidity
The invention relates to a method of treatment of bodies of water such as recreational pools, spas and hot tubs with maintenance doses of water treatment chemicals to achieve consistent sanitization and aesthetically pleasing levels of properties such as turbidity. The amount of the maintenance doses is based on the volume of water to be treated in order to achieve hygienic and clear water. The method can be automated for accurate, consistent and safe treatment of water such as found in swimming pools, spas or hot tubs.
Owner:ARCH CHEM INC
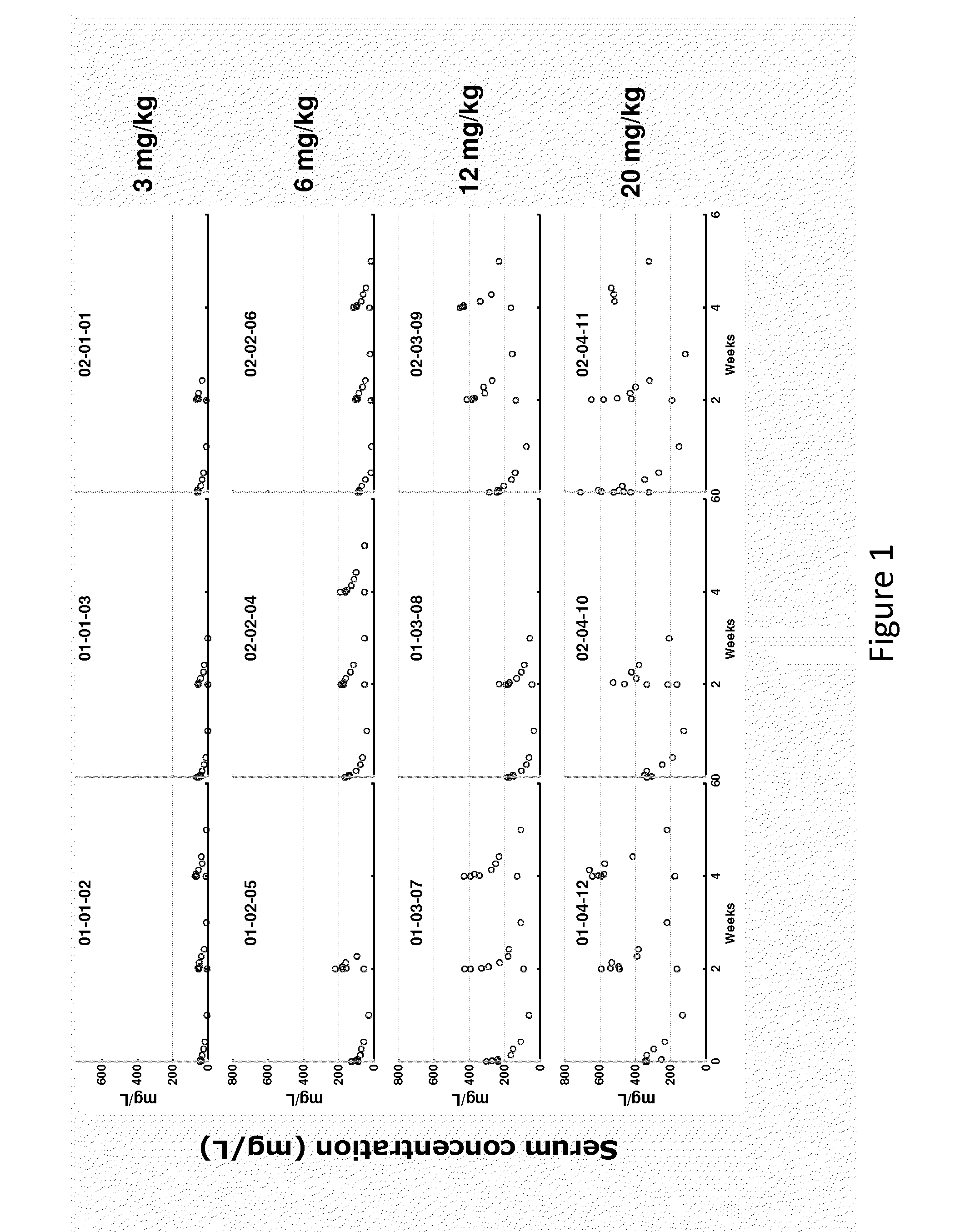
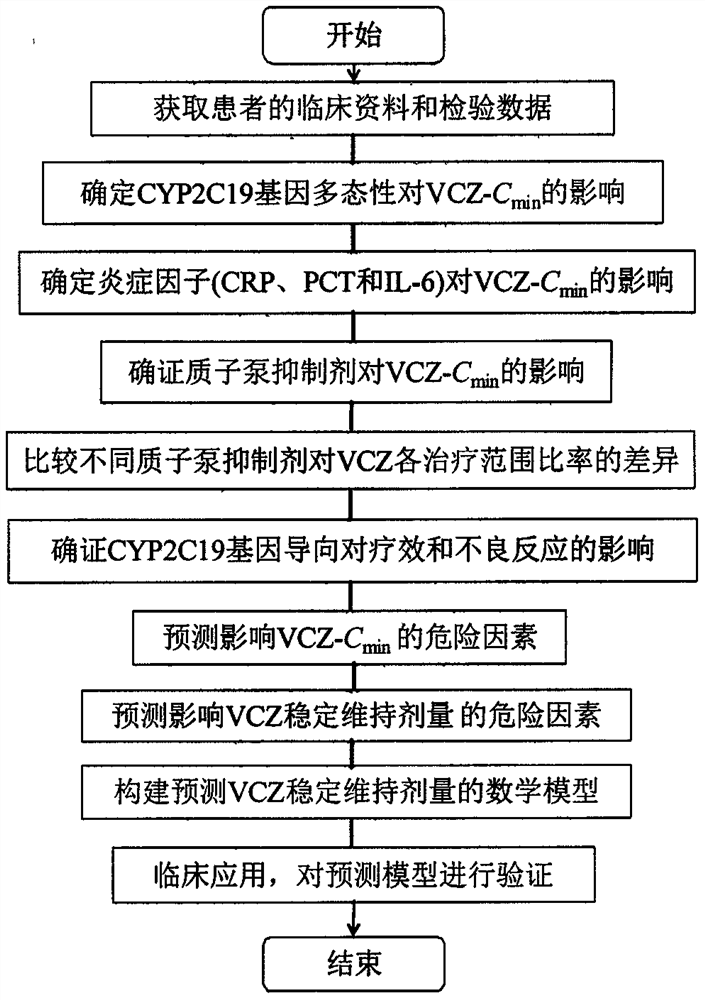
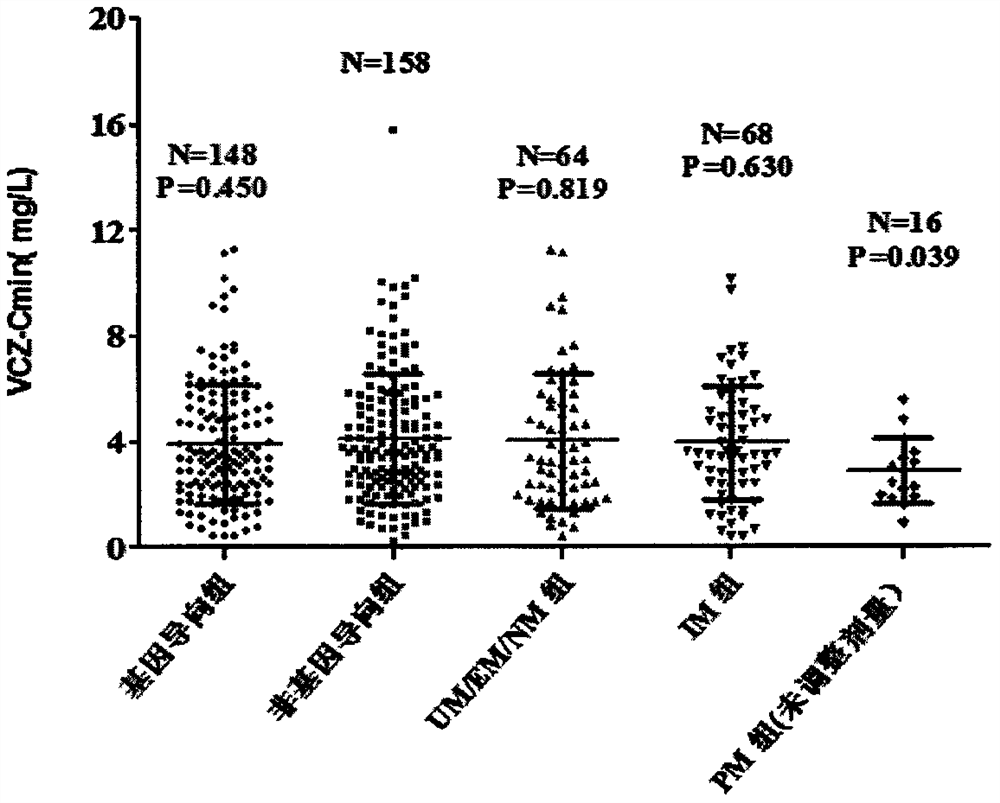
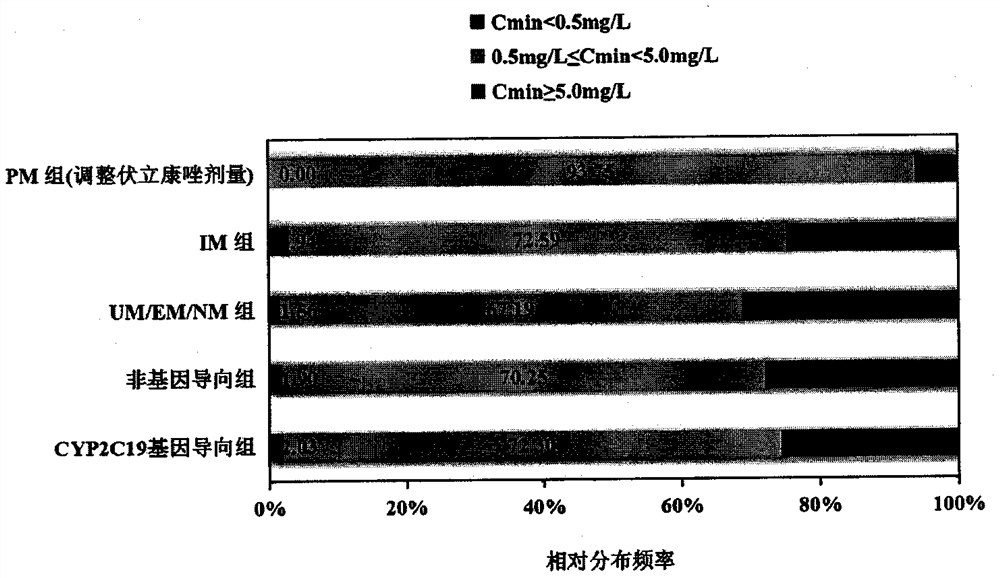
Voriconazole maintenance dose prediction mathematical model and construction method and application thereof
PendingCN114255883AEnsure safetyTo achieve the effect of individualized medicineMedical data miningDrug referencesMathematical modelCmin
The invention discloses a mathematical model for predicting voriconazole maintenance dose by integrating multiple factors and application, and relates to the technical field of medical treatment, and the model is prepared by the following steps: (1) determining the influence of CYP2C19 gene polymorphism on the use of voriconazole steady-state plasma concentration (VCZ-Cmin) by invasive fungal infection patients through variance analysis; (2) confirming the influence of CRP, PCT, IL-6 and combined use of a proton pump inhibitor on VCZ-Cmin; (3) confirming the influence of oriented use of voriconazole and non-oriented medication of different CYP2C19 genotypes on the curative effect and adverse reaction of a patient; (4) predicting risk factors influencing the VCZ-Cmin and risk factors influencing the voriconazole maintenance dosage; and (5) establishing a mathematical model for predicting the voriconazole maintenance dose and verifying the model. According to the influence factors and the mathematical prediction model disclosed by the invention, the accuracy of the sustaining dose of the clinically used voriconazole is improved, the drug concentration can be quickly stabilized in a target valley concentration range, the medication of the voriconazole is more accurate, safe and effective, and the adverse reaction is reduced.
Owner:ZHENGZHOU CENT HOSPITAL
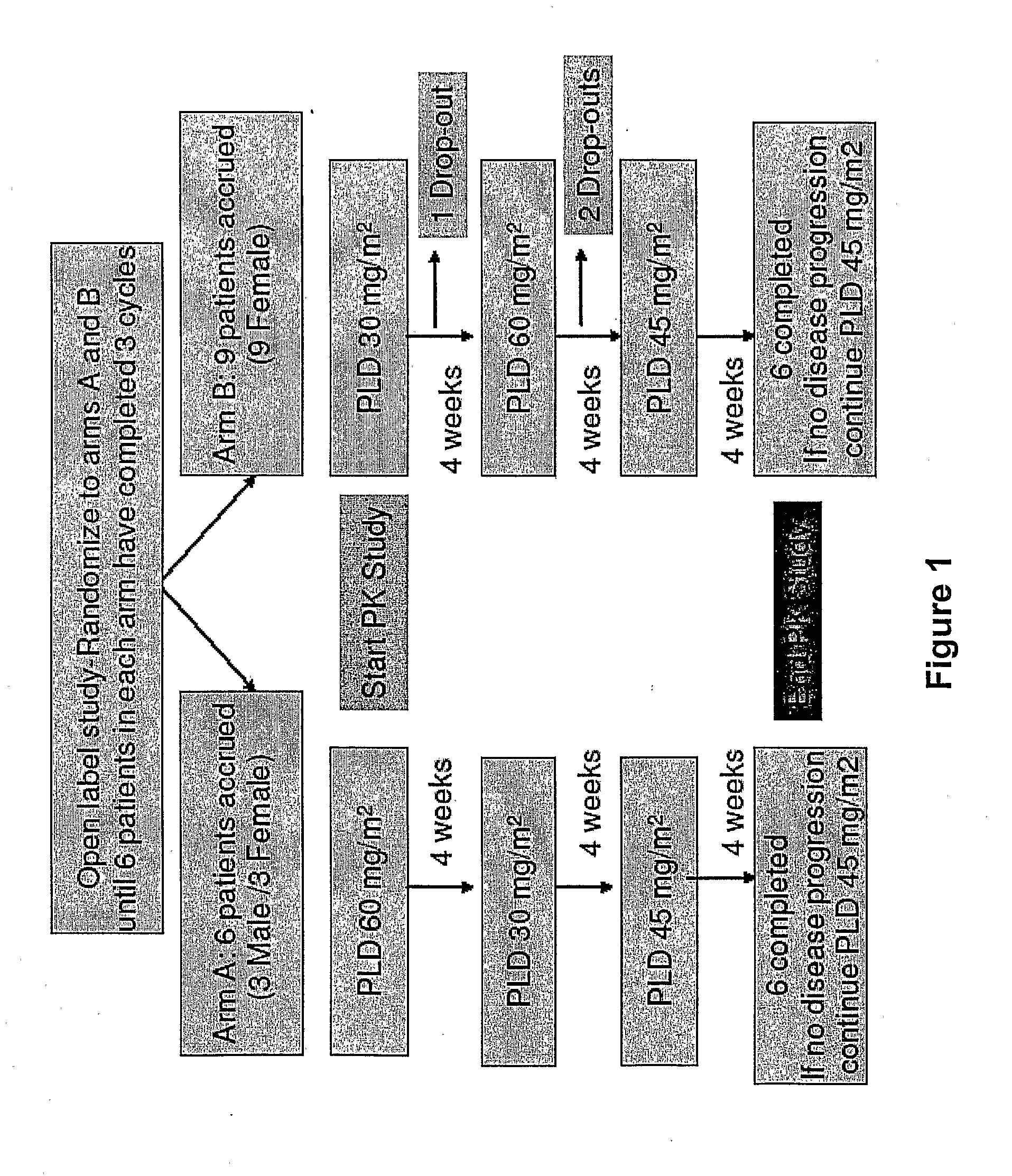
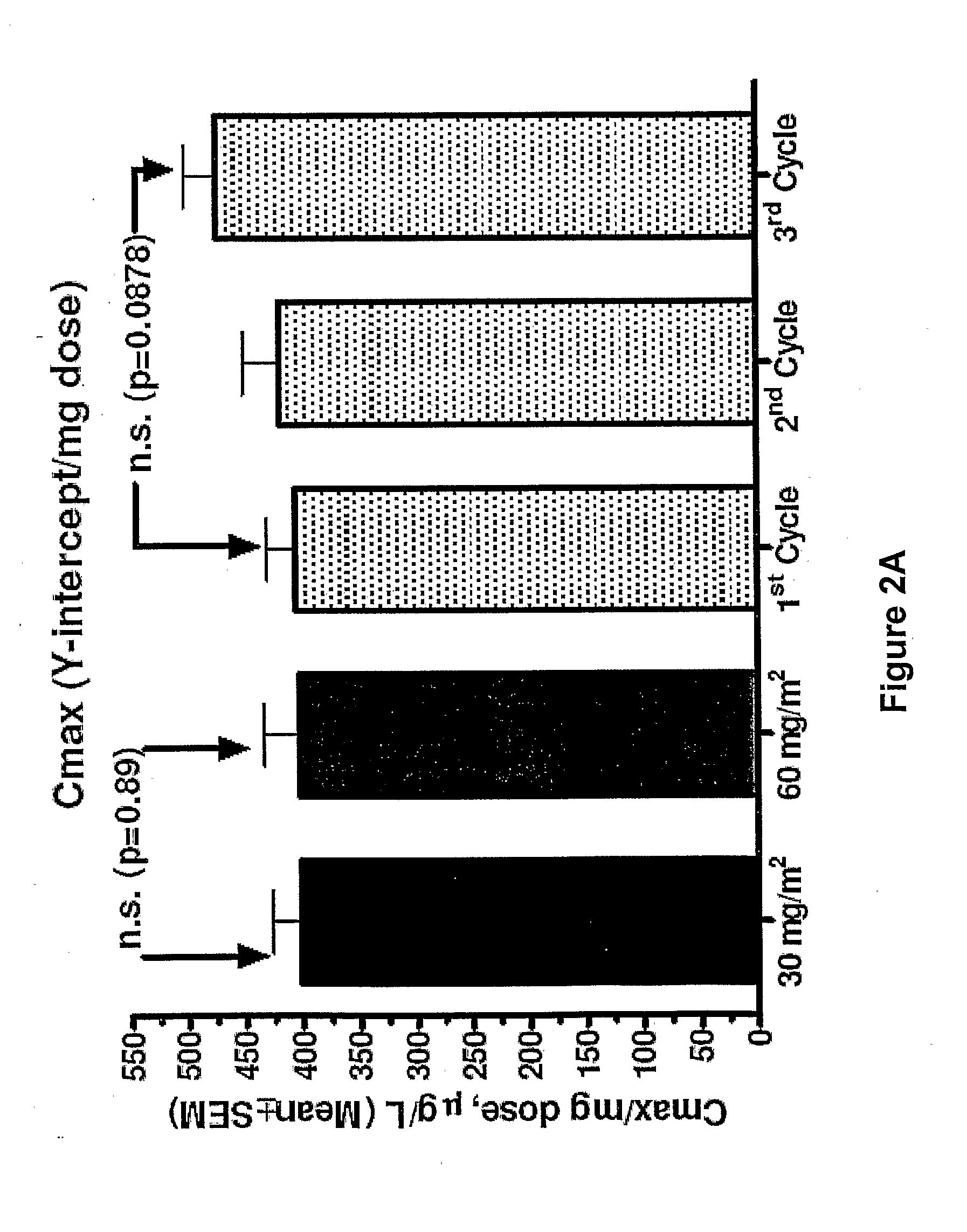
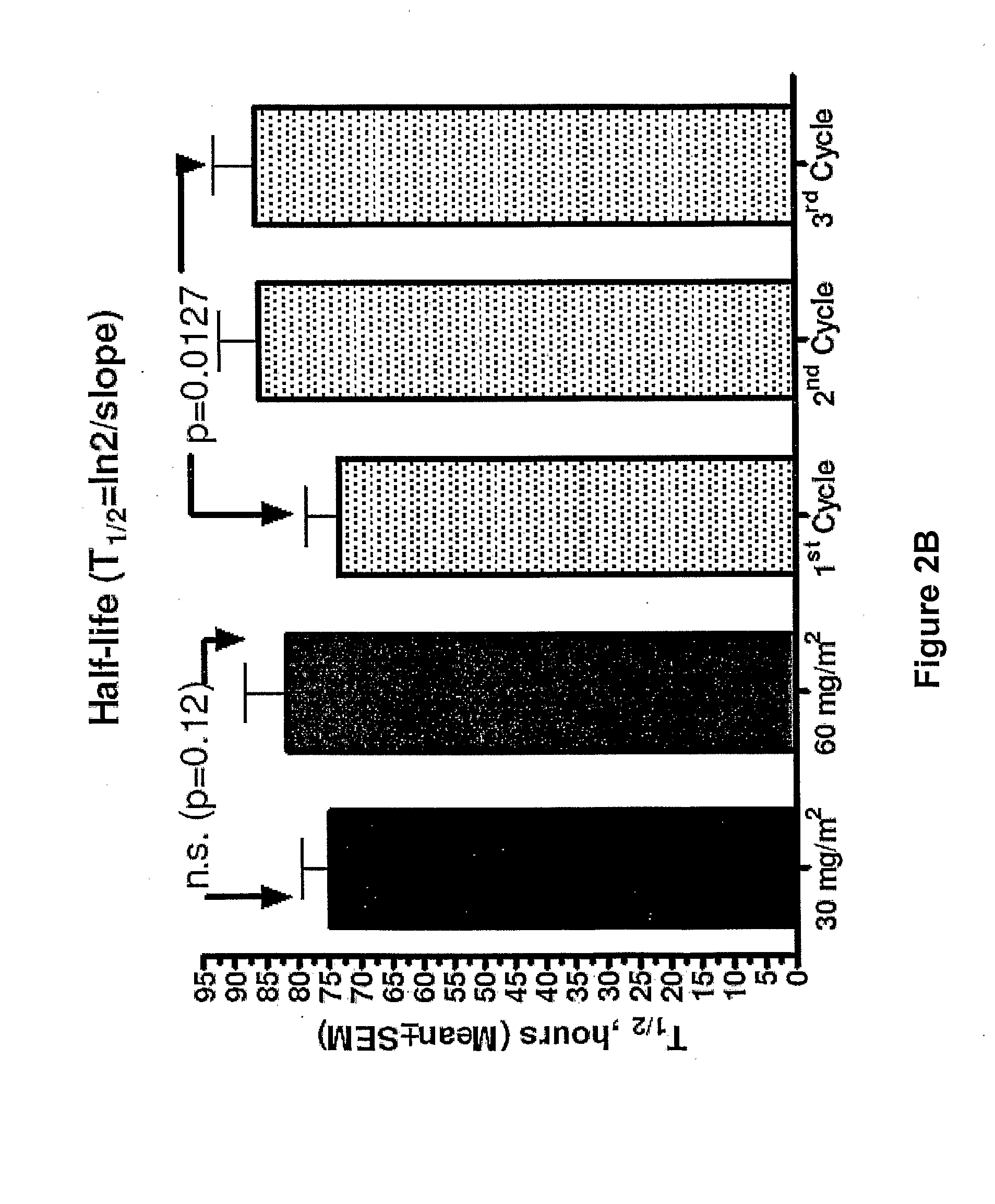
Method for administration of pegylated liposomal doxorubicin
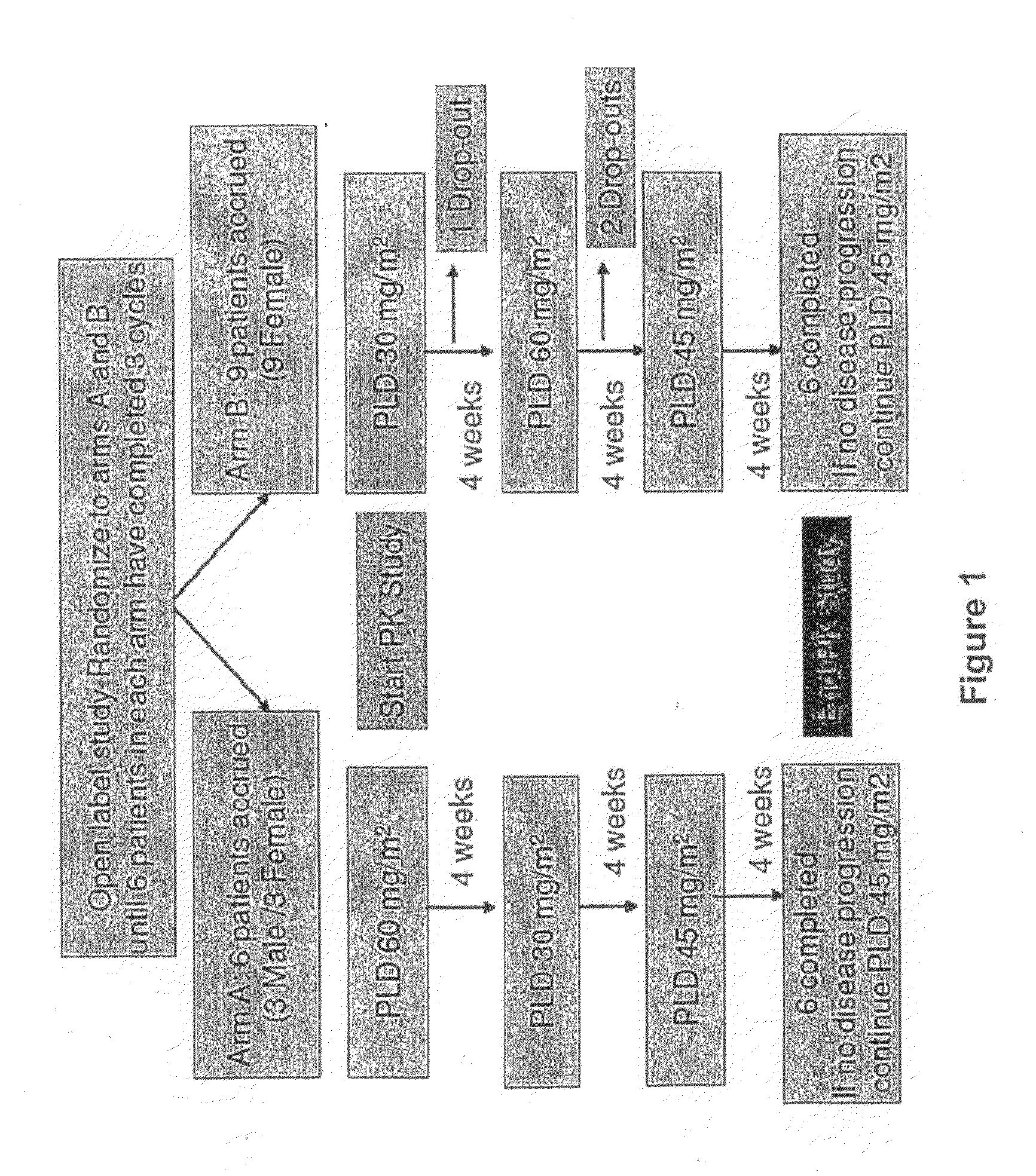
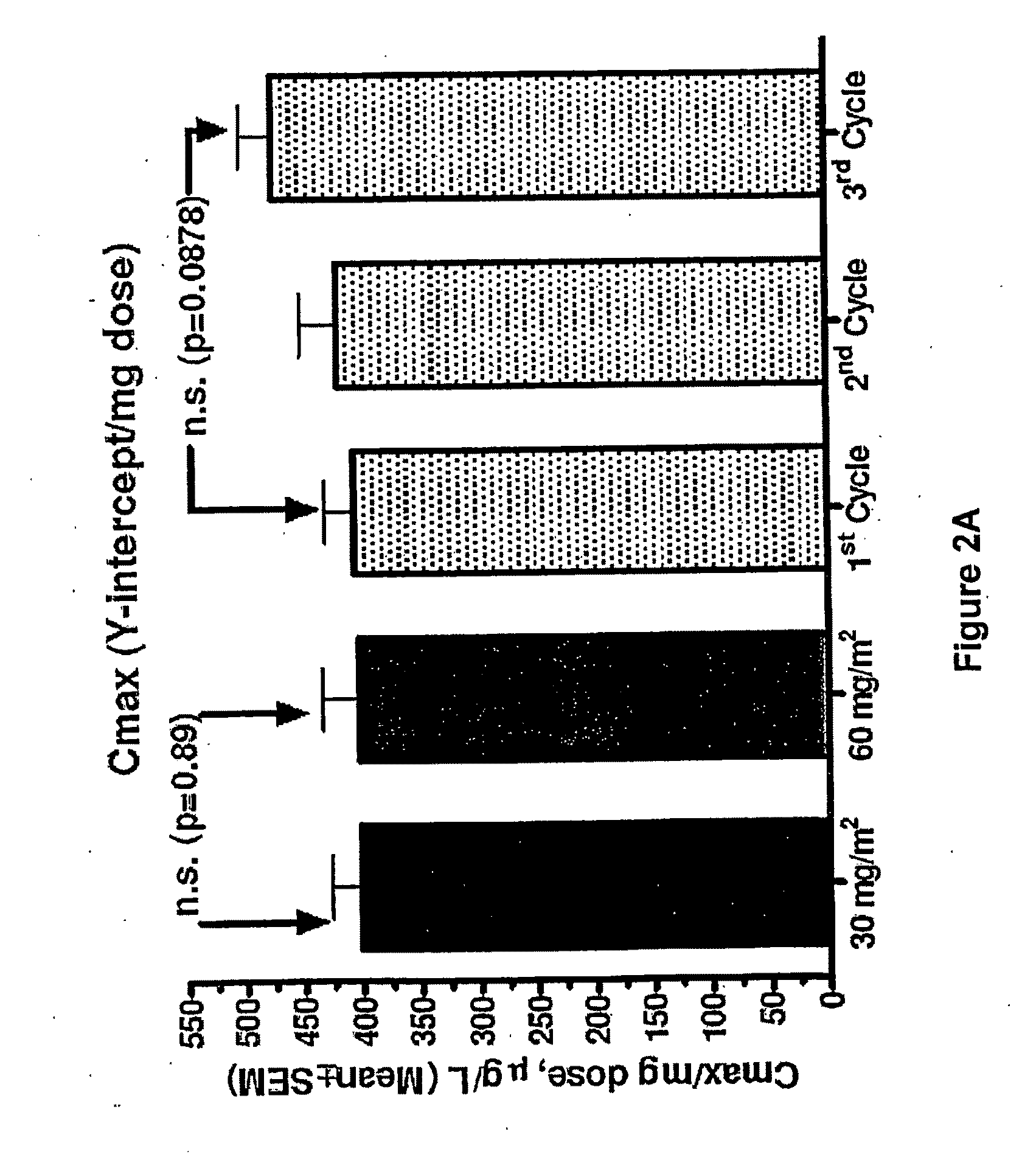
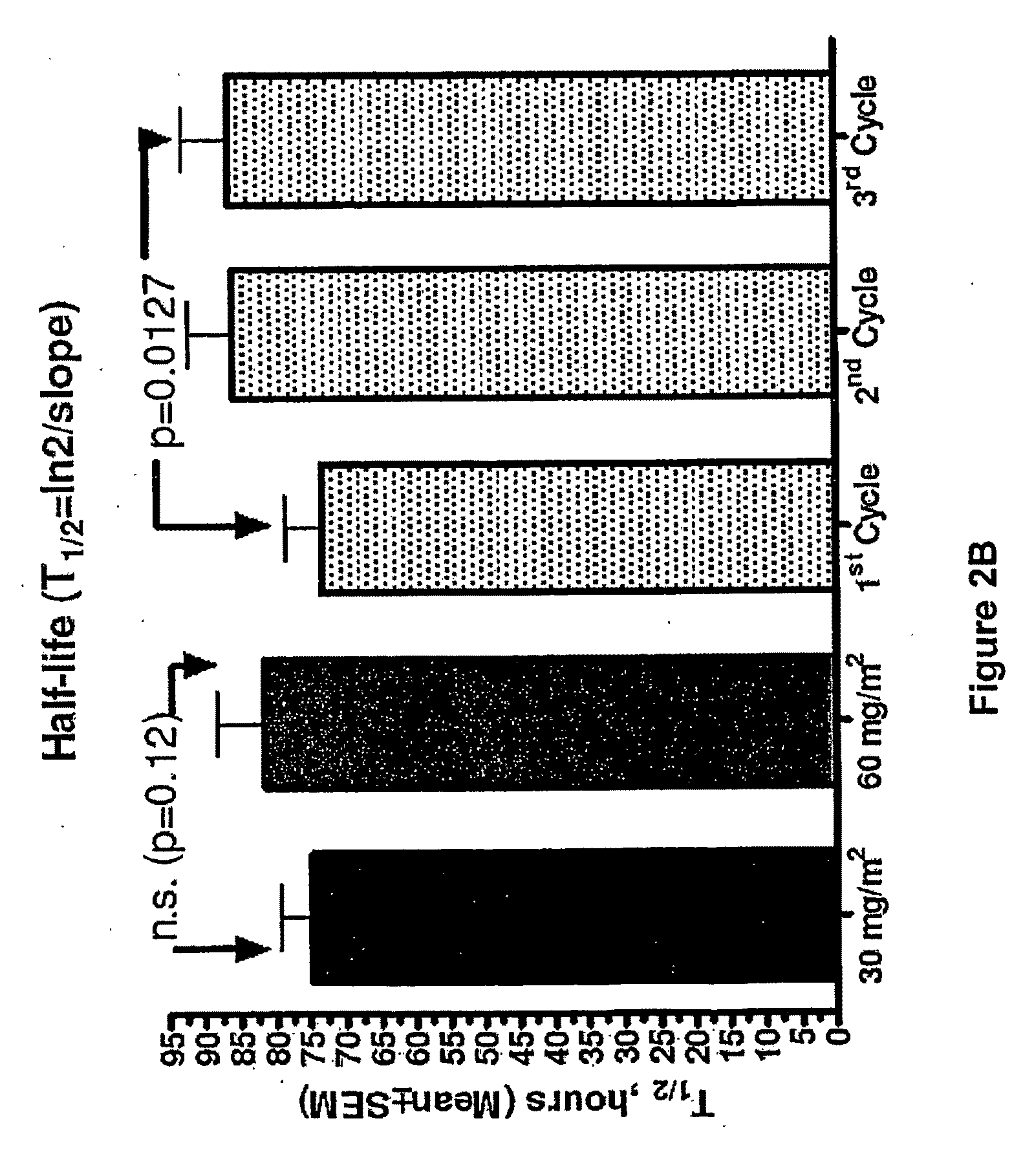
An embodiment of the present invention comprises a method of treating malignancies in a subject in need of treatment comprising administering to the subject a high loading dose of a pegylated liposomal doxorubicin (PLD) in an initial cycle, followed by a reduced dose in a second cycle, wherein the second cycle reduced dose is in the range of 20% to 50%, preferably 50%, of the initial loading dose, and thereafter one or more maintenance doses in further cycles. The interval between dose cycles is in the range of about three-to-four weeks, preferably about four weeks. The initial loading dose is in the range of between the maximum tolerated dose (MTD) and the recommended dose, preferably the MTD (for instance, in the range of about 70 mg / m2 to 50 mg / m2, preferably 60 mg / m2). The one or more maintenance doses are in the range of about 40 mg / m2 to 50 mg / m2, preferably 45 mg / m2).
Owner:GABIZON ALBERTO A
Method for administration of pegylated liposomal doxorubicin
An embodiment of the present invention comprises a method of treating malignancies in a subject in need of treatment comprising administering to the subject a high loading dose of a pegylated liposomal doxorubicin (PLD) in an initial cycle, followed by a reduced dose in a second cycle, wherein the second cycle reduced dose is in the range of 20% to 50%, preferably 50%, of the initial loading dose, and thereafter one or more maintenance doses in further cycles. The interval between dose cycles is in the range of about three-to-four weeks, preferably about four weeks. The initial loading dose is in the range of between the maximum tolerated dose (MTD) and the recommended dose, preferably the MTD (for instance, in the range of about 70 mg / m2 to 50 mg / m2, preferably 60 mg / m2). The one or more maintenance doses are in the range of about 40 mg / m2 to 50 mg / m2, preferably 45 mg / m2).
Owner:GABIZON ALBERTO A
Dosage regimen for prasugrel
InactiveCN101198329ABlood disorderHeterocyclic compound active ingredientsVascular diseaseDosing regimen
Owner:ELI LILLY & CO
Treatment of prostate cancer
ActiveUS20190224196A1Increase serum testosterone levelsOrganic active ingredientsPharmaceutical delivery mechanismProstate cancerPharmacology
Methods for treating prostate cancer, including advanced prostate cancer, in a subject in need thereof, include administering once-daily to the subject, at least 80 mg of N-(4-(1-(2,6-difluorobenzyl)-5-((dimethylamino)methyl)-3-(6-methoxy-3-pyridazinyl)-2,4-dioxo-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidin-6-yl)phenyl)-N′-methoxyurea, or a corresponding amount of a pharmaceutically acceptable salt thereof. Another method includes: administering once-daily to the subject in need thereof, an oral load dose formulation having from 240 mg to 480 mg of N-(4-(1-(2,6-difluorobenzyl)-5-((dimethylamino)methyl)-3-(6-methoxy-3-pyridazinyl)-2,4-dioxo-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidin-6-yl)phenyl)-N′-methoxyurea, or a corresponding amount of a pharmaceutically acceptable salt thereof; and thereafter administering once-daily to the subject, an oral maintenance dose formulation having 80 mg to 160 mg of N-(4-(1-(2,6-difluorobenzyl)-5-((dimethylamino)methyl)-3-(6-methoxy-3-pyridazinyl)-2,4-dioxo-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidin-6-yl)phenyl)-N′-methoxyurea, or a corresponding amount of a pharmaceutically acceptable salt thereof.
Owner:MYOVANT SCI GMBH +1
Dosage regimen for prasugrel
A dosage regimen for treating vascular disease in a human comprising the steps of administering a loading dosage of about 30 mg to 70 mg of loading dose of prasugrel or a pharmaceutically acceptable salt thereof, and thereafter administering a daily dosage regimen of about 7.5 mg to 15 mg maintenance dose of prasugrel or a pharmaceutically acceptable salt thereof.
Owner:ELI LILLY & CO
ITE for Cancer Intervention and Eradication
ActiveUS20130310429A1Good curative effectInhibit angiogenesisPhotovoltaic supportsBiocideProstate cancerCarrier system
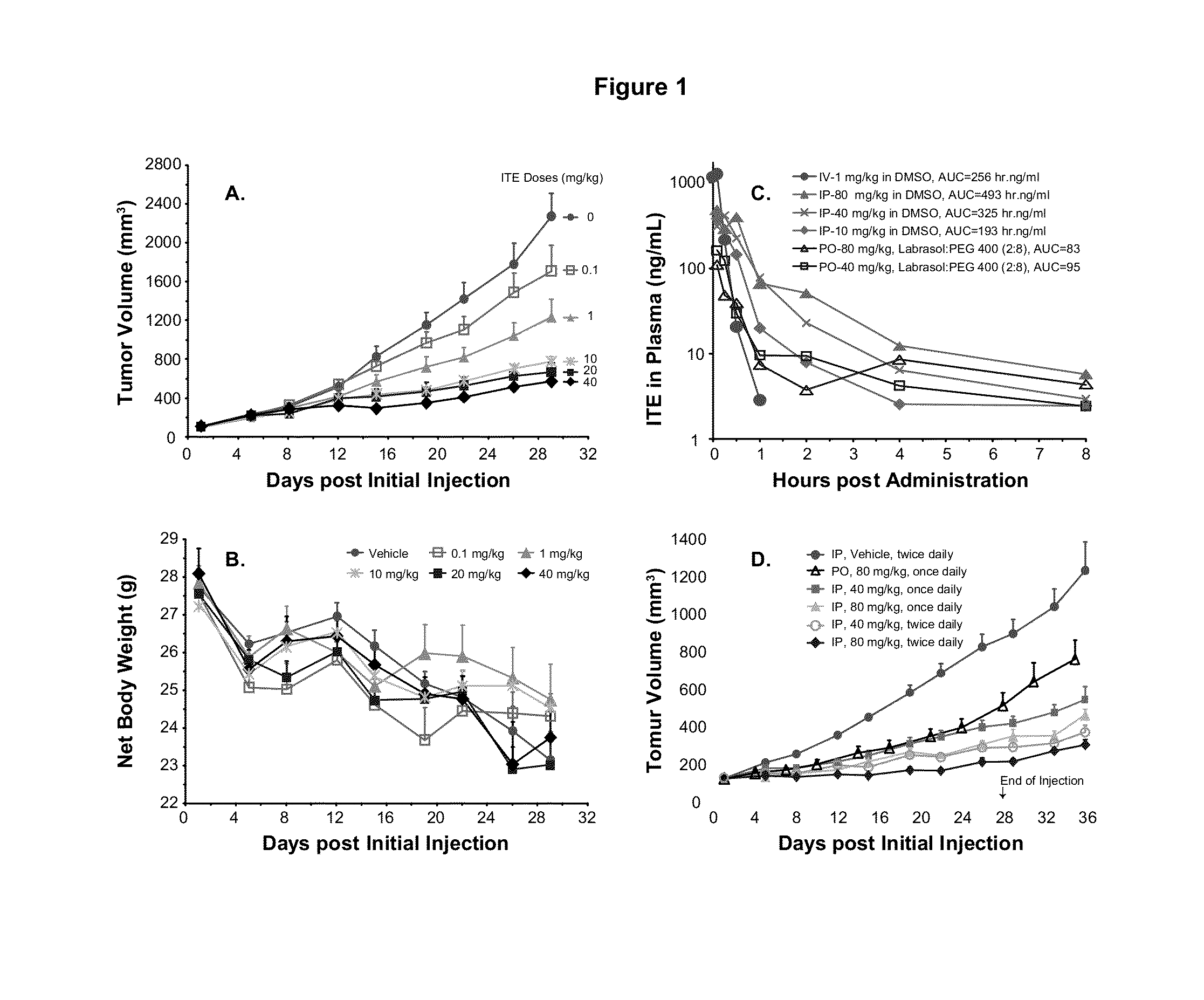
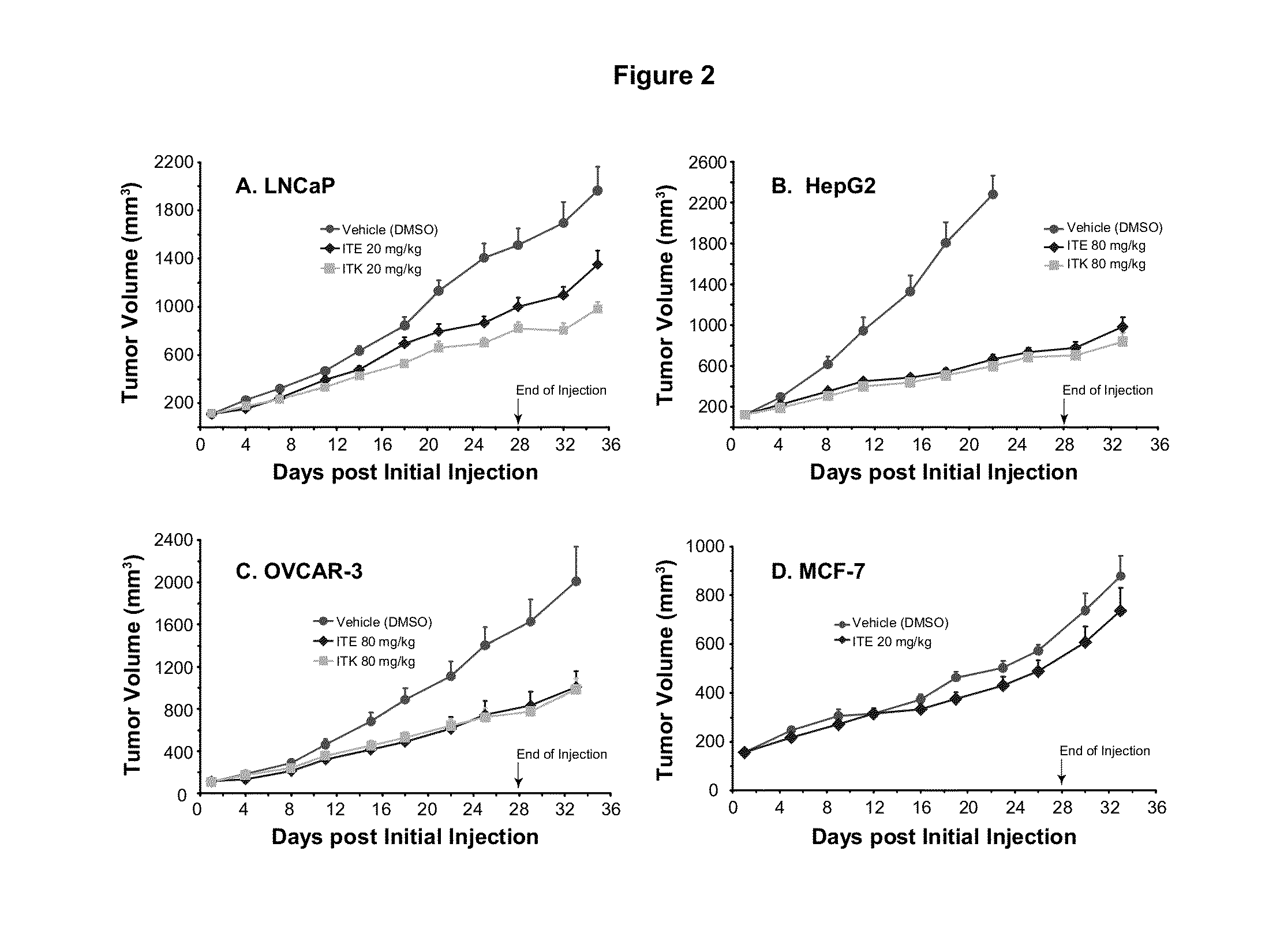
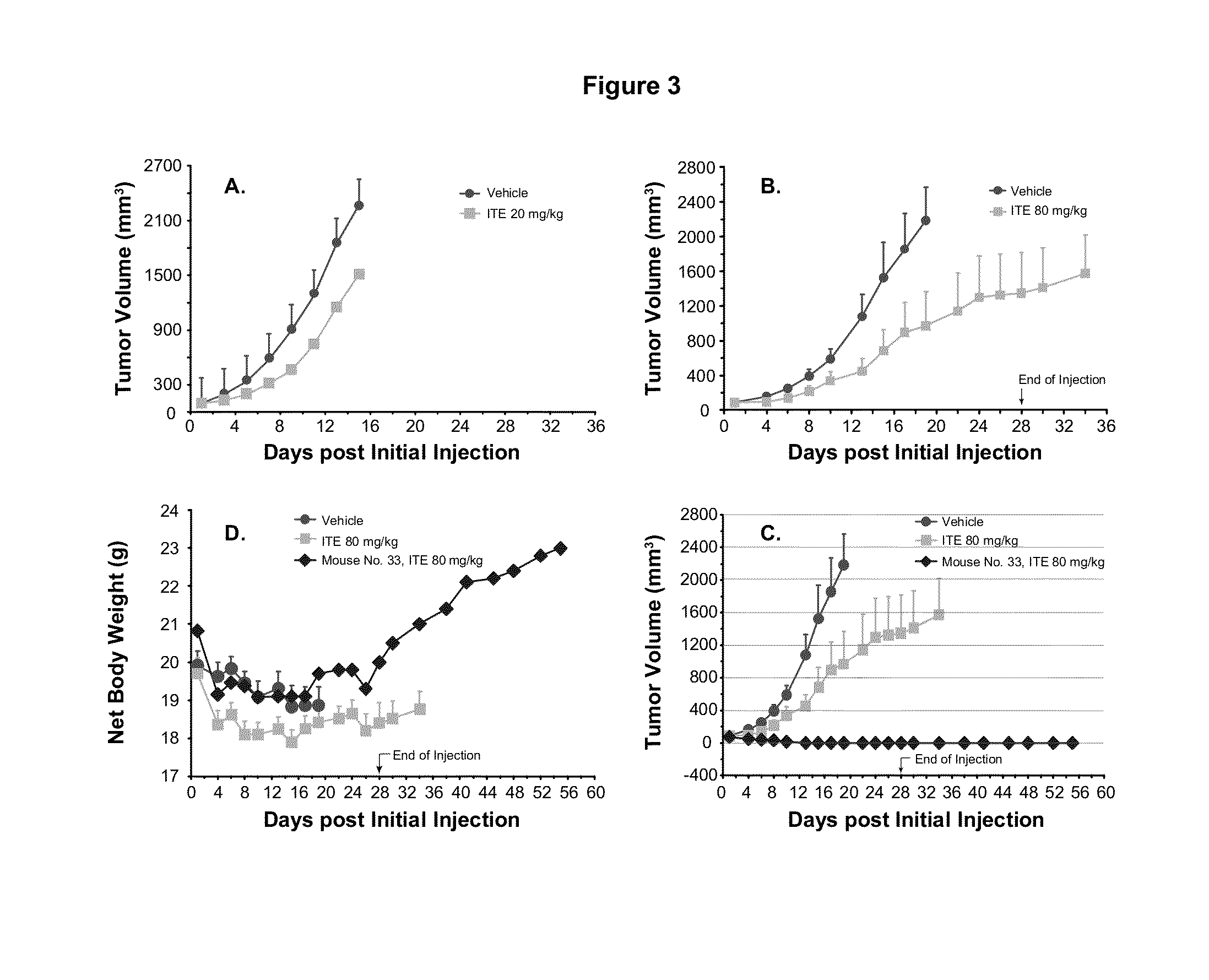
A method of cancer intervention or eradication by administering an effective amount of an endogenous ligand for the aryl hydrocarbon (Ah) receptor (AhR) named ITE or one of its analogs (the active ingredient) to a subject with cancer is disclosed. An effective dose and dosing frequency of the active ingredient are determined by measuring its blood levels of the subject after dosing. The active ingredient formulated with a carrier system is applied topically, enterally, or parenterally to the subject. The formulated drug can also be administered together with one or more of other cancer therapeutic agents. A maintenance dosing is provided after the subject is free of cancer to insure the cancer eradication. Subjects with cancers of prostate, liver, lung, ovarian, and breast are preferably accepted for treatment.
Owner:ARIAGEN INC
Vacuum pressure impregnation full-load alternate machining method for wood
InactiveCN106142252AImprove retentionStrong penetrating powerPressure impregnationTreatment effectVacuum pressure
The invention discloses a vacuum pressure impregnation full-load alternate machining method for wood. The method includes the steps that 1, multiple times of a full-load cell impregnation method are superposed; 2, after a planned time, the direction of a four-way valve is changed again, a modifier is pumped out through a circulating pump, vacuum is produced in an impregnation tank, and the step is repeated multiple times; and 3, the vacuum degree and the boosting pressure are main technological parameters affecting the treatment effect and efficiency, and selection of the parameters should be determined according to the properties of treated materials and the impregnation difficulty of the modifier. The method is actually generated by improving a vacuum pressure impregnation full-load machining method for the wood, the multiple times of the full-load cell impregnation methods are superposed to increase the maintenance dose and penetrance of the modifier in the wood, and the maximum modifier absorption amount and depth of penetration can be achieved. The method is suitable for the wood and wet wood difficult to impregnate.
Owner:潘杨基
Method of treating musculoskeletal and connective tissue inflammations
A method of treating said musculoskeletal and connective tissue inflammations including osteoarthritis and associated articular and periarticular inflammations, and non-articular Rheumatism including capsulitis, tendonitis, fibrositis, and perarticular inflammations is disclosed. The method includes administering to a patient of a therapeutically effective amount of a composition comprising 13-cis-retinoic acid. Preferably, the treatment method includes administering to a patient of an initial dosage of a composition comprising 13-cis-retinoic acid for an initial treatment period, and thereafter administering a maintenance dosage of the composition.
Owner:PARKS L DEAN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com