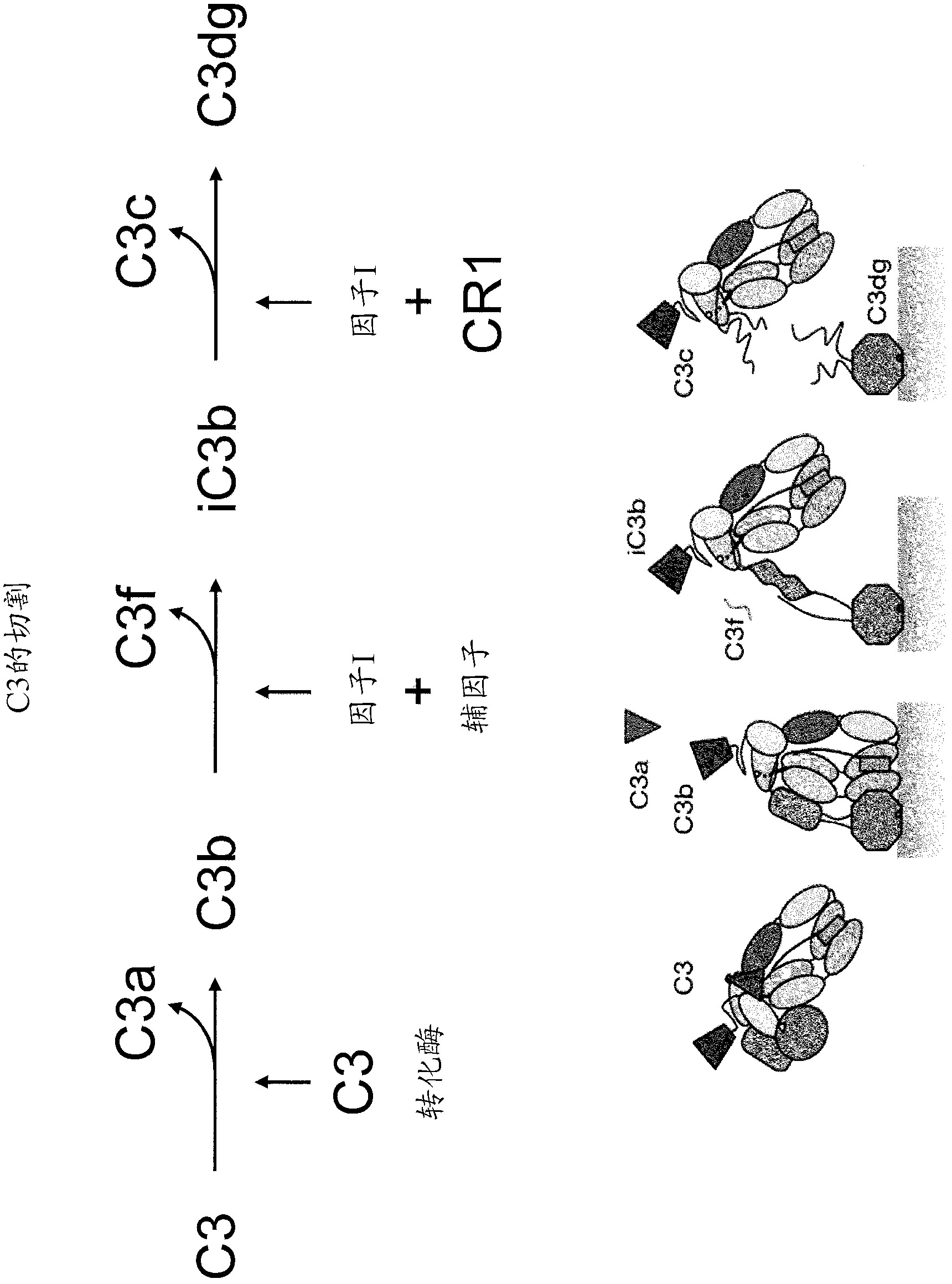
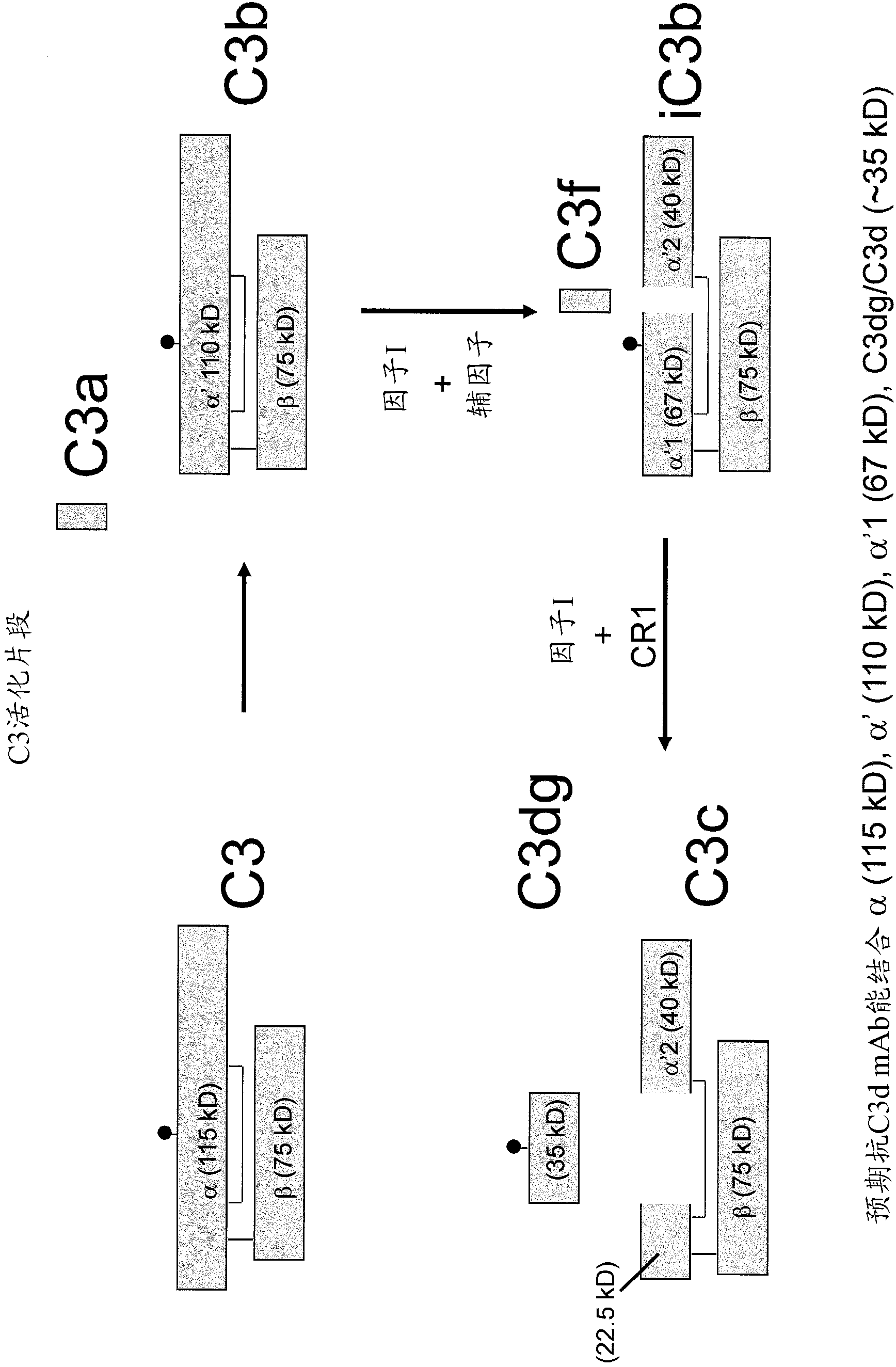
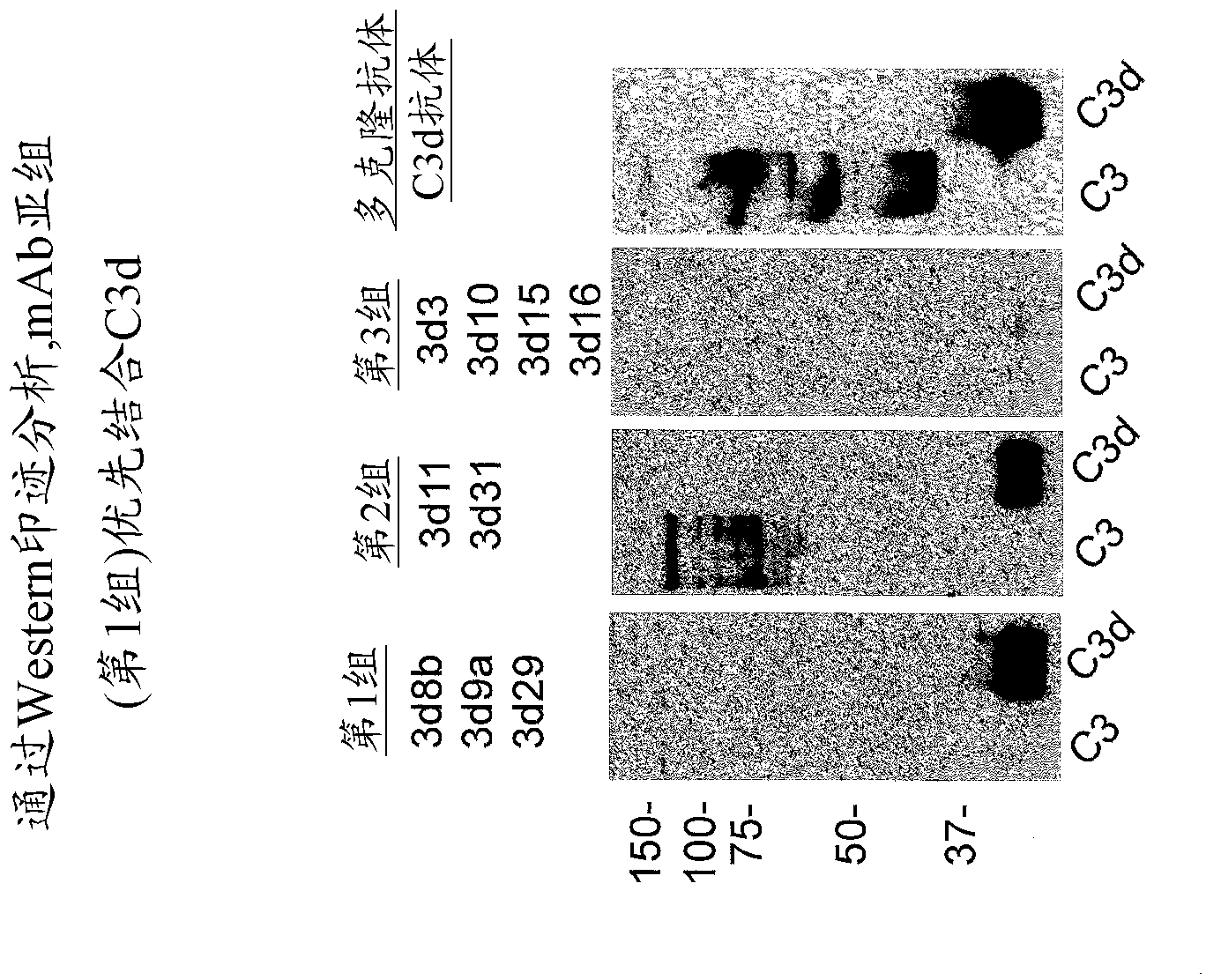
Antibodies to the c3d fragment of complement component 3
一种抗体、补体的技术,应用在抗体、补体蛋白、抗体模拟物/支架等方向
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0350] Anti-C3d antibody:
[0351] Recombinant human C3d was produced in E. coli using the pGEX expression system as described in Hannan, J.P., et al. (2005) J. Mol. Biol. 346, 845-858. Briefly, ampicillin-resistant clones were used to initiate overnight cultures, and cultures were expanded to 1 L and grown at 37°C until reaching A 600 =0.3. Cultures were induced with 0.3 mM isopropyl-β-D-thiogalactoside overnight at 30°C and then harvested by centrifugation. The harvested bacterial pellet was resuspended in glutathione-s-transferase column buffer (50 mM Tris-HCl, pH 8.0, 250 mM NaCl, 1 mM EDTA) and lysed by sonication. Lysates were clarified by centrifugation and applied to GStrap columns (GE Biosciences). C3d was cleaved from the column by digestion with 50 units of thrombin overnight at 4°C, followed by purification by size exclusion chromatography. The purity of C3d was monitored by SDS-PAGE.
[0352] C3 was immunized with 60 μg of recombinant C3d emulsified in Freund...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com