Application of lobaplatin in the preparation of drugs for treating prostate cancer
A technology of prostate cancer and lobaplatin, which is applied in the field of new uses of chemical drugs, can solve the problems of patent literature and research reports that have no treatment effect on prostate cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
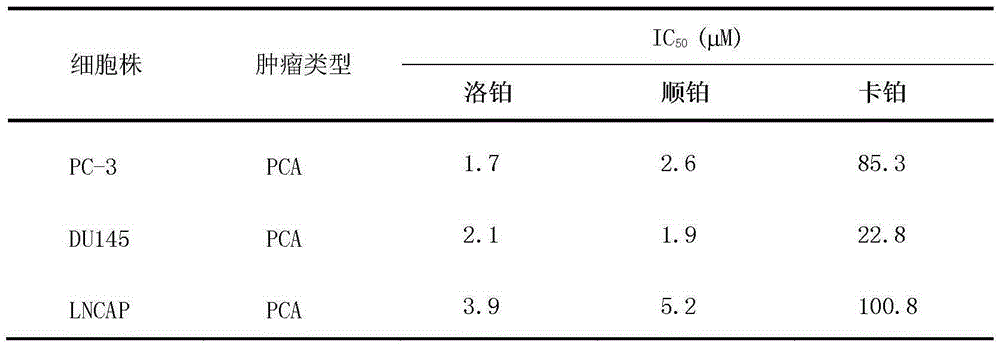
[0013] Example 1, the in vitro anti-prostate cancer cell activity of lobaplatin
[0014] 1.1 Materials and reagents:
[0015] 1.1.1 Drug name and batch number: lobaplatin is white freeze-dried powder, batch number 20070501; carboplatin is white powder, batch number 20060703; cisplatin is yellow powder, batch number 36-014050704. Docetaxel, white powder, lot number 268070506; paclitaxel, white powder, lot number 030902; vinorelbine, lot number 080504.
[0016] Provider: Lobaplatin is provided by Hainan Changan International Pharmaceutical Co., Ltd.; carboplatin is the product of Kunming Guiyan Pharmaceutical Co., Ltd.; cisplatin and vinorelbine are products of Jiangsu Hansoh Pharmaceutical Co., Ltd.; docetaxel and paclitaxel are the products of Products of Jiangsu Hengrui Pharmaceutical Co., Ltd.
[0017] Preparation method: the above drugs are formulated with corresponding concentrations in serum-free medium.
[0018] 1.1.2 Cell lines
[0019] PC-3, DU145, LNCAP: human pro...
experiment example 2
[0035] Experimental example 2. Study on the curative effect of lobaplatin on human prostate cancer in vivo
[0036] 2.1 Materials and reagents:
[0037] 2.1.1 Drug name and batch number:
[0038] Lobaplatin: white powder, produced by Hainan Changan International Pharmaceutical Co., Ltd., batch number 20070501;
[0039] Carboplatin: white powder, purchased from Kunming Guiyan Pharmaceutical Co., Ltd., batch number 20060703;
[0040] Cisplatin: yellow powder, purchased from Jiangsu Hansoh Pharmaceutical Co., Ltd., batch number 36-014050704.
[0041] All of the above are raw materials.
[0042] 2.1.2 Preparation method: Lobaplatin, carboplatin, and cisplatin were prepared with 5% GS to the corresponding concentration.
[0043] 2.1.3 Experimental animals
[0044] Male BALB / cA-nude nude mice, 6-7 weeks old, were purchased from Shanghai Slack Experimental Animal Co., Ltd. Certificate number: SCXK (Shanghai) 2003-0003. Breeding environment: SPF grade.
[0045] 2.2 Test method...
experiment example 3
[0063] Experimental Example 3. Clinical trial of lobaplatin and cisplatin in the treatment of non-hormone-dependent prostate cancer
[0064] 3.1 Clinical data: A total of 320 cases were observed, aged 40-75 years. All cases were diagnosed by imaging and confirmed as prostate cancer by prostate cancer puncture pathology; after anti-androgen treatment, it decreased significantly, and then gradually increased; The hematopoietic function of the bone marrow is not significantly damaged, and the liver and kidney functions are basically normal; he has not received radiation therapy, paclitaxel chemotherapy or other treatments within 6 months, and the expected survival time is more than 3 months. There were 242 cases in the lobaplatin group and 78 cases in the cisplatin group.
[0065] 3.2 Experimental drugs:
[0066] Lobaplatin 1: Lobaplatin API, produced by Hainan Changan International Pharmaceutical Co., Ltd., batch number 20080503;
[0067] Lobaplatin 2: prepared according to th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com