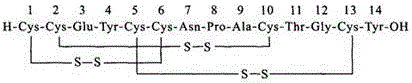
Method for synthesizing linaclotide
A technology of linaclotide and crude peptide, applied in the field of drug synthesis, can solve the problems of short cyclization time and expensive raw materials, and achieve the effects of simple cyclization system, short cyclization time and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Weigh 1g of linaclotide linear crude peptide without protecting group, and conduct purity test, the obtained linear crude peptide has a purity of 45%. Prepared in advance according to the ratio of 100mg ammonium carbonate / 1mlDMSO / 10ml water. Then, 0.8 g of hydroquinone was added to the solution to start cyclization, and the cyclization was completed for 2 hours to complete the oxidation of the three pairs of disulfide bonds, followed by filtration. The filtered crude linaclotide solution was injected into a preparative high performance liquid chromatograph. The preparation conditions are as follows: phase A is 0.1% TFA aqueous solution, phase B is 0.1% TFA acetonitrile solution, choose a C18 diameter 50MMDAC preparation column, the flow rate is 40ml / min, and the column temperature is 25 degrees. The mobile phase was collected and freeze-dried to obtain pure linaclotide, which weighed 317 mg. The pure product was detected, the purity was 99.3%, and the recovery rate wa...
Embodiment 2
[0015] Weigh 1g of linaclotide linear crude peptide without protecting group, and conduct purity test, the obtained linear crude peptide has a purity of 65%. Prepared in advance according to the ratio of 100mg ammonium carbonate / 1mlDMSO / 10ml water. Then add 0.5g TCEP to the solution to start cyclization, cyclization for 1 hour, the oxidation of the three pairs of disulfide bonds can be completed, and then filtered. The filtered crude linaclotide solution was injected into a preparative high performance liquid chromatograph. The preparation conditions are as follows: phase A is 0.1% TFA aqueous solution, phase B is 0.1% TFA acetonitrile solution, choose a C18 diameter 50MMDAC preparation column, the flow rate is 40ml / min, and the column temperature is 25 degrees. The mobile phase was collected and freeze-dried to obtain pure linaclotide, which weighed 579 mg. The pure product was detected, the purity was above 99.1%, and the recovery rate was 89%.
Embodiment 3
[0017] Weigh 1g of linaclotide linear crude peptide without protecting group, and conduct purity test, the obtained linear crude peptide has a purity of 45%. Prepared in advance according to the ratio of 100mg ammonium carbonate / 1mlDMSO / 10ml water. Then 0.8 g of hydroquinone was added to the solution to start the cyclization, and the cyclization was carried out for 6 hours and filtered. The filtered crude linaclotide solution was injected into a preparative high performance liquid chromatograph. The preparation conditions are as follows: phase A is 0.1% TFA aqueous solution, phase B is 0.1% TFA acetonitrile solution, choose a C18 diameter 50MMDAC preparation column, the flow rate is 40ml / min, and the column temperature is 25 degrees. The mobile phase was collected and freeze-dried to obtain pure linaclotide, weighing 321 mg. The pure product was detected, the purity was 99%, and the recovery rate was 71.3%. Compared with Example 1, by prolonging the cyclization time in this...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com