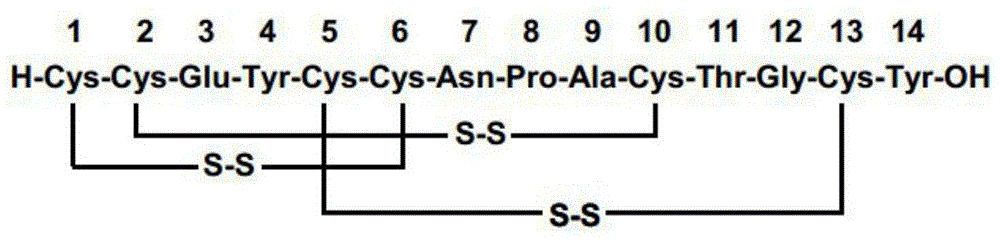
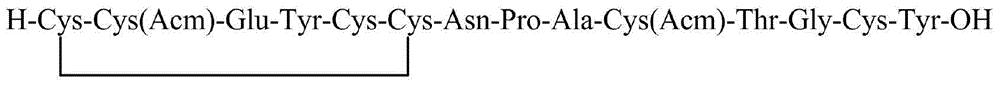Method for preparing linaclotide
A linaclotide and a pair technology, applied in the field of preparation of linaclotide, can solve the problems of complex processing process, few raw material sources and high cost, achieve simple process operation, avoid disulfide bond mismatch isomer impurities , the effect of reducing the difficulty of purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: Preparation of Fmoc-Cys(Mmt)-CTC resin
[0043] Accurately weigh 320 g of CTC resin (1.0 mmol / g) and place it in a peptide resin synthesis reactor, add 3 L of DCM to swell for 1 h. After swelling, wash 3 times with DMF, 3 L each time. Weigh 394g of Fmoc-Cys(Mmt)-OH and 87g of HOBt and dissolve them in 2L of DMF, add 110mL of DIC to activate, add the solution into the reactor, and react for 4h. After the reaction was completed, the resin was washed three times with DMF, and then a pre-prepared capping reagent (2.5L DCM, 0.3L methanol, 0.2L DIEA) was added for capping reaction for 1h. After the capping is completed, wash 4 times with DMF, 2 times with DCM, and 3 times with methanol, 3 L each time, and then vacuum-dry the resin to obtain 507 g of Fmoc-Cys(Mmt)-CTC resin. Take a small amount of resin and measure Fmoc-Cys The substitution degree of (Mmt)-CTC resin is 0.61mmol / g. The calculated synthesis scale was 309 mmol.
Embodiment 2
[0044] Embodiment 2: the preparation of fragment I peptide resin
[0045] Weigh 493g (300mmol) of the Fmoc-Cys(Mmt)-CTC resin with a substitution degree of 0.61mmol / g in Example 1 and place it in a peptide resin synthesis reactor, add 4L DCM to swell for 2h. After the swelling is completed, wash with DMF three times, 4 L each time, and then add 4 L of 20% piperidine / DMF solution for deprotection twice, for 10 min and 10 min respectively. After the deprotection was completed, the resin was washed 6 times with DMF, 4 L each time. Weigh 352g of Fmoc-Cys(Trt)-OH and 81g of HOBt and dissolve them in 2L of DMF, add 103mL of DIC to activate, add the solution into the reactor, react for 2h, and monitor the reaction end point by Kaiser test. After the reaction, the resin was washed 5 times with DMF, and then the next protected amino acid was deprotected and coupled. Repeat above-mentioned steps, carry out the coupling of Fmoc-Tyr(tBu)-OH, Fmoc-Glu(OtBu)-OH, Fmoc-Cys(Acm)-OH, Fmoc-Cys...
Embodiment 3
[0046] Example 3: Preparation of Fragment I Linear Peptide
[0047] Weigh 700 g (200 mmol) of the Fragment I peptide resin obtained in Example 2 and place it in a 10 L reaction bottle, add 7 L of cleavage reagent TFA-TIS-DCM (5-5-90), and react for 5 minutes at room temperature. After the reaction was completed, the lysate was filtered into a suction filter flask. The above cleavage reaction was repeated 3 times, and the combined filtrates were mixed with the same volume of 0.1mol / L NaHCO 3 Wash the filtrate with aqueous solution until the pH of the solution is 7-8, concentrate by rotary evaporation at 25°C, collect the separated precipitate by filtration, and dry in vacuo to obtain 261 g of Fragment I linear peptide, with a purity of 82.3% and a yield of 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com