Novel method for preparing ferrocenyl polymer from controllable type polyacrylonitrile resin
A polyacrylonitrile resin and ferrocene-based technology, applied in the field of functional polymer materials, can solve problems such as side reactions, increase reaction costs, limit the use of alkyne compounds, etc., and achieve the effect of convenient design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1: Controllable polyacrylonitrile resin synthesized by Cu(0) regulated CRP method
[0057] According to the ratio [AN] 0 : [EBiB] 0 : [Cu(0)] 0 : [PMDETA] 0 = 500:1:1:1, sequentially add Cu(0), AN (2.0mL), PMDETA, DMSO (2.0 mL) and EBiB to a 5 mL ampoule, add a stirring bar, and go through 6 standard freezing- After the pump-thaw-fill cycle, seal the tube in an oxygen-free atmosphere. Place the sealed ampoule at a constant temperature (25 o C) Under the magnetic water bath, the reaction is carried out according to the predetermined time. After the reaction, take out the ampoule, open the sealed tube, dissolve it with 2-5 mL of DMF, pour it into 250 mL of methanol, leave it overnight, filter it with suction, and dry it to obtain polyacrylonitrile.
[0058] figure 1 It is the kinetic diagram of the controllable radical polymerization of acrylonitrile (AN) monomer regulated by Cu(0) in Example 1.
[0059] Polymerization conditions: AN = 2.0 mL; [AN] 0 / [E...
Embodiment 2
[0065] Example 2: Preparation of polyvinyl tetrazole (PVT) by click chemical reaction of nitrile group.
[0066] According to the mass ratio, polyacrylonitrile: sodium azide: ammonium chloride = 100:120:50, in the ampoule bottle of 5 mL, add the PAN ( M n,GPC = 16.1 × 10 3 g / mol, M w / M n = 1.29) 0.4188 g, sodium azide 0.5025 g, ammonium chloride 0.2094 g, add N , N - Dimethylformamide (DMF) 3 mL, after fully dissolved, add a stirring bar, place the ampoule at 120 o Carry out click chemical reaction of nitrile groups in a heat-collecting constant temperature heating magnetic stirrer of C. After 5 hours of reaction, take out the ampoule bottle, add an appropriate amount of deionized water to dissolve, and add 1.0 mol / L hydrochloric acid solution to acidify, and stir to obtain a flocculent Solid precipitated, washed 3-5 times with deionized water, placed in 40 oC Vacuum constant temperature drying oven, after the sample is completely dried, the intermediate product...
Embodiment 3
[0070]Example 3: Preparation of ferrocenyl polymer (PVT-FeC) by Mitsunobu reaction.
[0071] By mass ratio, PVT: ferrocenemethanol: triphenylphosphine: diethyl azodicarboxylate=1:2:2:2, in a 50 mL round bottom flask, add the intermediate product PVT 0.3075 obtained in Example 2 g, ferrocenemethanol 0.6150g, triphenylphosphine 0.6150g, diethyl azodicarboxylate 0.6150g, add 10 mL dichloromethane (CH 2 Cl 2 ), at a constant temperature (15 o C) The reaction time in the magnetic water bath is 22 hours. After the reaction, the reaction solution was poured into 200 mL deionized water, and after being fully dispersed, the solid precipitate was separated by a high-speed centrifuge, and placed in a freeze-vacuum drying oven for freeze-drying for 24 h to obtain the final product, ferrocene-based polymerization material (PVT-FeC).
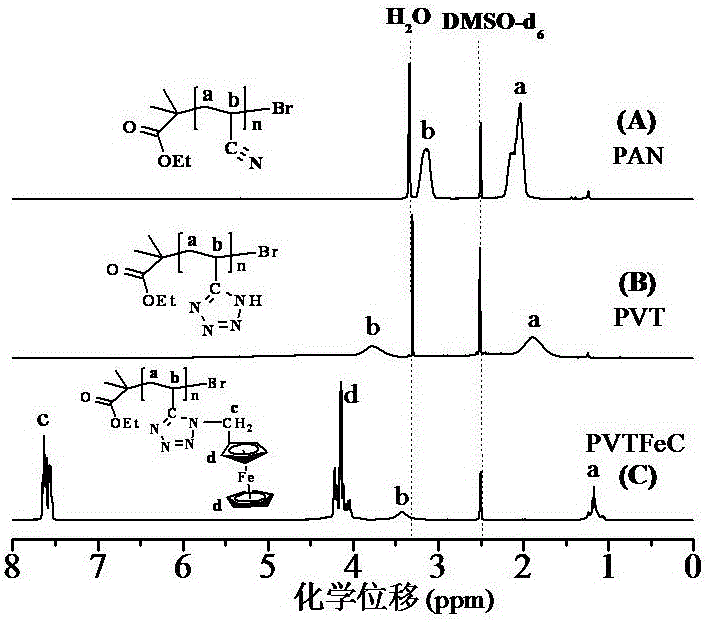
[0072] image 3 (C) is the ferrocene-based polymer (PVT-FeC) obtained in Example 3 1 H NMR chart.
[0073] Measuring conditions: INOVA 400 MHz nuclear...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com