Synthesis method based on amination reaction of aryl siloxane
A technology of aryl siloxane amine and synthesis method, which is applied in the direction of organic chemistry, can solve problems such as poor applicability, dangerous environment, and polluted environment, and achieve the effects of mild reaction conditions, environmental friendliness, and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
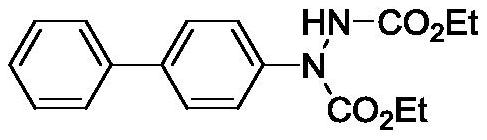
[0017] Embodiment 1, Preparation of 1-([1,1'-biphenyl]-4-yl)hydrazine-1,2-dicarboxylate diethyl ester:
[0018]
[0019] Add 0.3mmol of AgF, 0.2mmol of 4-triethoxysilylbiphenyl, 0.3mmol of diethyl azodicarboxylate, 2mL of methyl ethyl ketone solution and one No. 5 magnet in sequence, and pass the condenser tube from bottom to top After the water was condensed, the reactor was placed in a 60°C oil bath and heated to react for 12 hours. The spherical container at the bottom of the reactor is immersed in the silicone oil, and the immersion depth is such that the height of the silicone oil is higher than the height of the reaction solution in the spherical container of the micro reaction tube. The rotating speed of the magnetic stirrer was adjusted to 700 rpm. After the reaction, the reaction solution was poured into a separatory funnel, 15 mL of distilled water was added, and extracted three times with 10 mL of ethyl acetate. The obtained organic phases were combined, spin-dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com