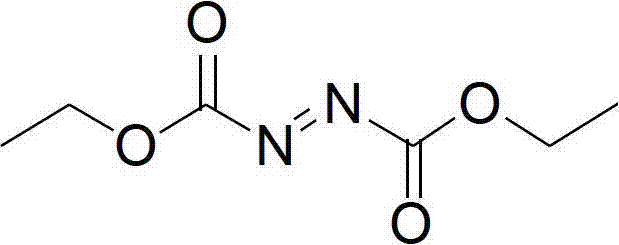
Synthesis method of diethyl azodicarboxylate and intermediate of diethyl azodicarboxylate
A technology of diethyl azodicarboxylate and diethyl hydrogen azodicarboxylate is applied in the preparation of hydrazide, organic chemistry, etc., can solve problems such as environmental pollution, and achieves good economy, wide reaction temperature range, and operation. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] In a 500ml three-necked flask equipped with mechanical stirring, 250ml of diethyl carbonate was added, and 125g of hydrazine hydrate with a mass percentage of 80% was added. Heated to reflux in an oil bath for 4 hours, distilled off excess diethyl carbonate, and the remaining mixed solution was spin-dried under reduced pressure to obtain crude ethyl carbazate. Recrystallize with acetone to obtain 198 g (95.2%) of pure ethyl carbazate.
[0043] II, the preparation of diethyl hydroazodicarboxylate
Embodiment 2
[0045] In a 250ml three-neck flask equipped with mechanical stirring, add 120ml of absolute ethanol, and add 4.8g of metallic sodium under nitrogen protection. After the metallic sodium is completely dissolved, add 31.2g of ethyl carbazate. After 0.5h, the solution turns orange red. Slowly add 40 g of diethyl carbonate dropwise. After the dropwise addition, react at room temperature for 6 hours to obtain an orange-red turbid liquid, and recover ethanol and diethyl carbonate by atmospheric distillation. Use 10% hydrochloric acid solution to adjust the pH to 6.0. White crystals are precipitated and washed with cold water to obtain the crude product diethyl hydroazodicarboxylate. Recrystallize the above crude product with acetone and petroleum ether to obtain a white solid hydrogenation Diethyl azodicarboxylate 6.6g (12.5%) mp=130-132℃.
[0046] 1 HNMR (CDCl 3 ,300MHz)δ(ppm):8.97(2H,s,NH);3.99-4.06(4H,m,CH 2 ); 1.16 (6H,t,CH 3 ).
Embodiment 3
[0048]In a 250ml three-neck flask equipped with mechanical stirring, add 120ml of absolute ethanol, and add 7.1g of sodium metal under nitrogen protection. After the sodium metal is completely dissolved, add 31.2g of ethyl carbazate. After 0.5h, the solution turns orange. red. Slowly add 40 g of diethyl carbonate dropwise. After the dropwise addition, react at 75° C. for 6 h to obtain an orange-red turbid liquid. Ethanol and diethyl carbonate are recovered by atmospheric distillation. Use a hydrochloric acid solution with a mass concentration of 20% to adjust the pH ≈ 5.0, white crystals precipitate, wash with cold water to obtain the crude product diethyl hydroazodicarboxylate, and recrystallize the above crude product with acetone and petroleum ether in turn to obtain a white solid hydrogenation Diethyl azodicarboxylate 26.6g (50.7%) mp=130-132°C.
[0049] 1 HNMR (CDCl 3 ,300MHz)δ(ppm):8.97(2H,s,NH);3.99-4.06(4H,m,CH 2 ); 1.16 (6H,t,CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com