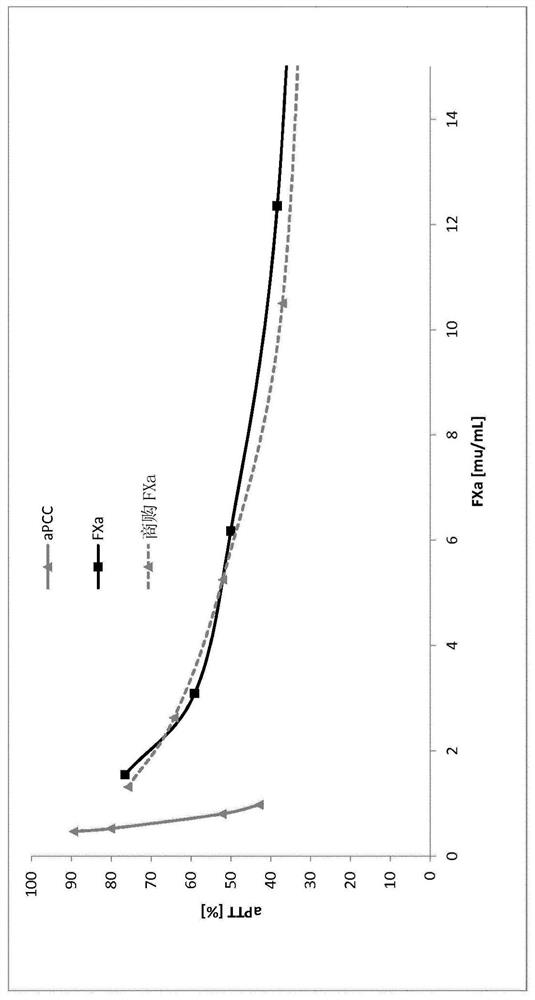
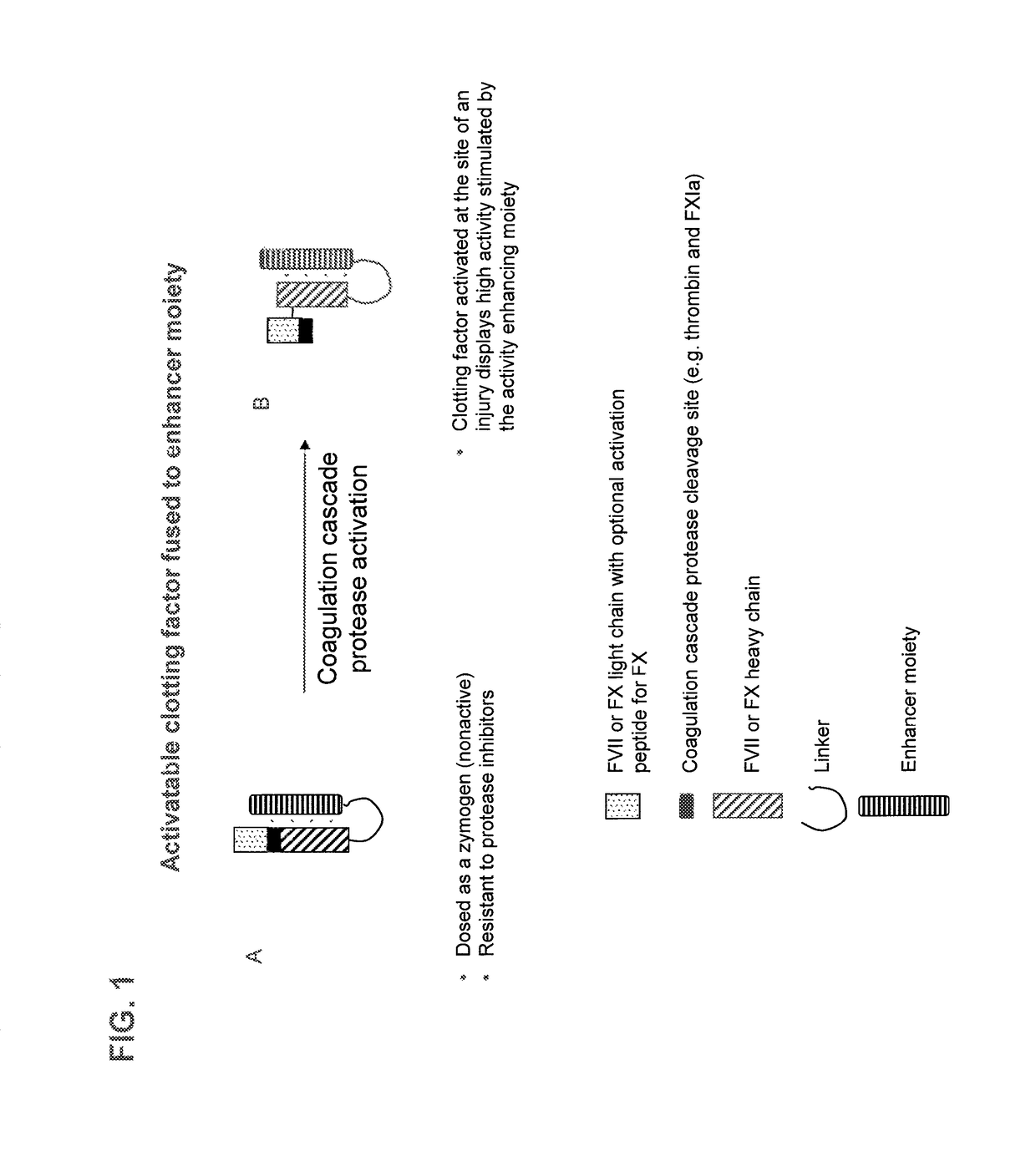
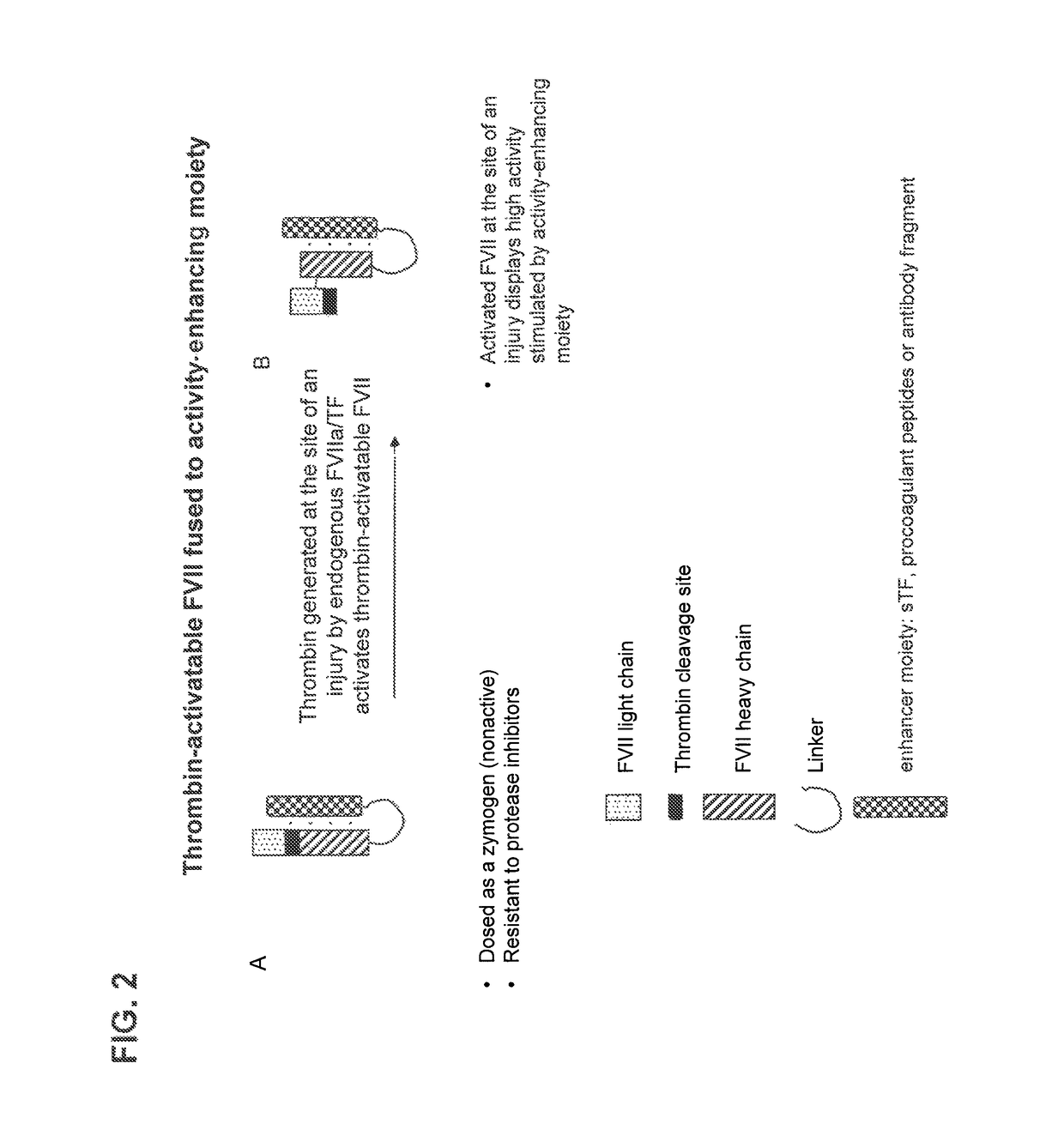
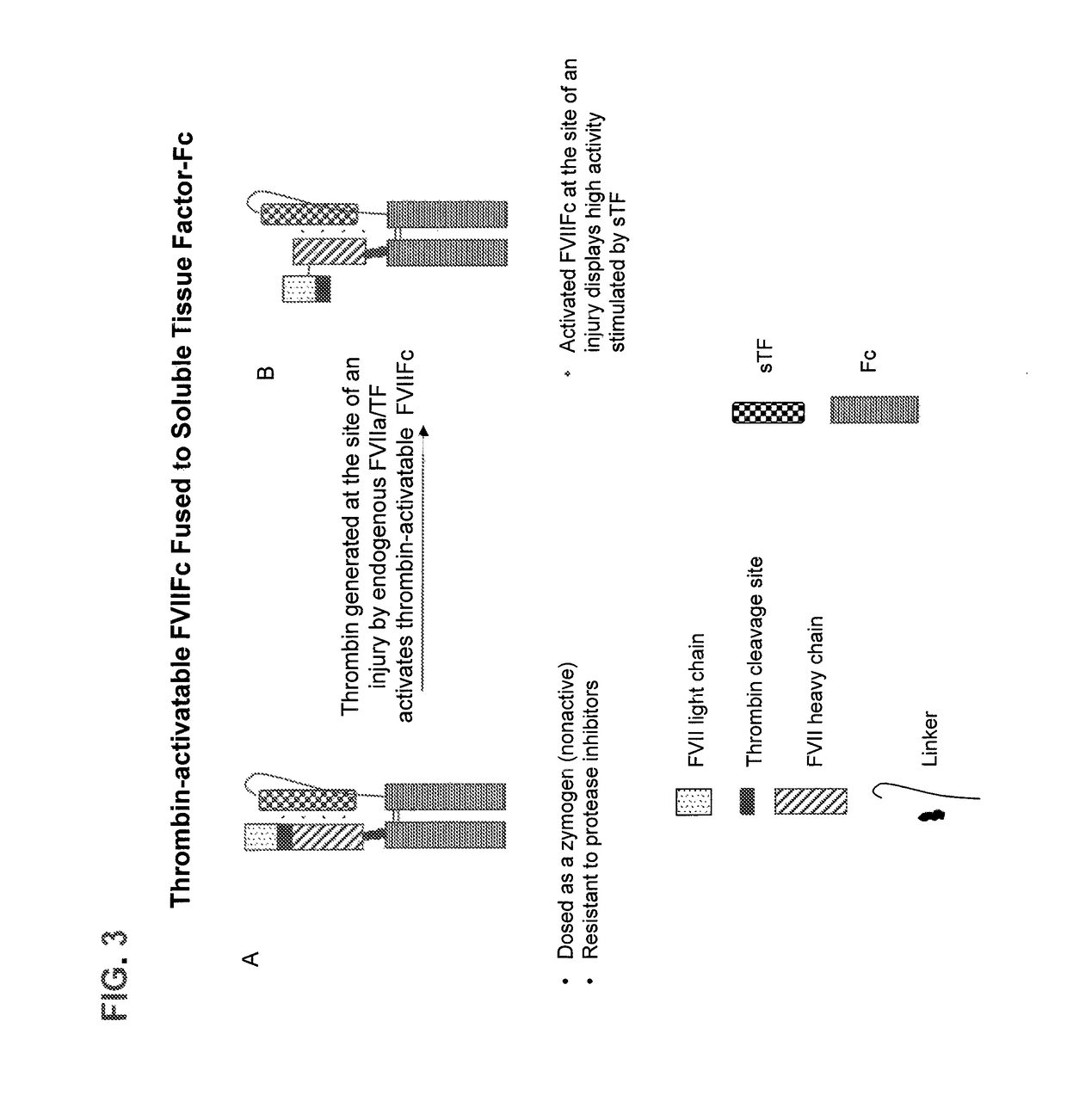
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
38 results about "Activated coagulation factor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
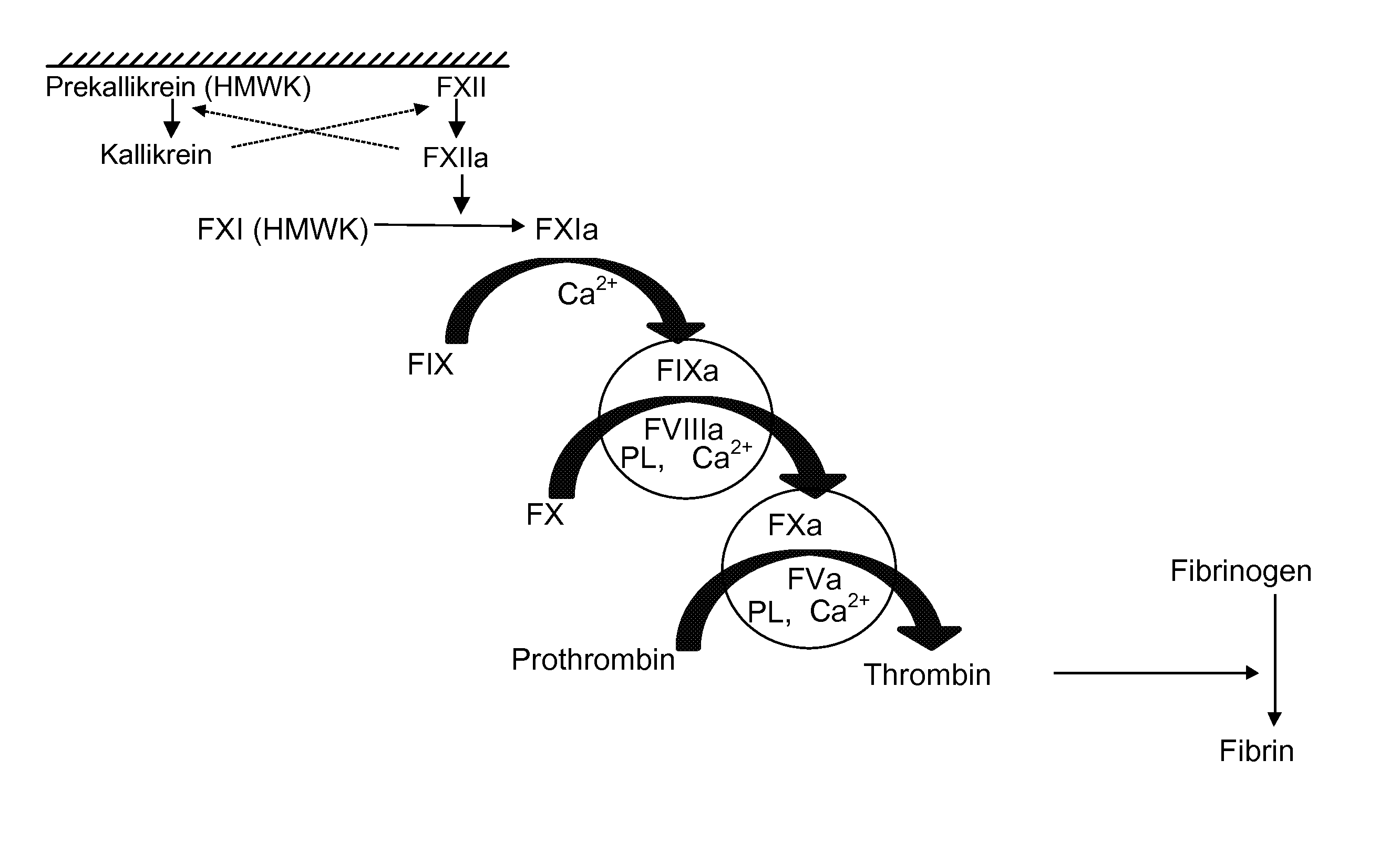
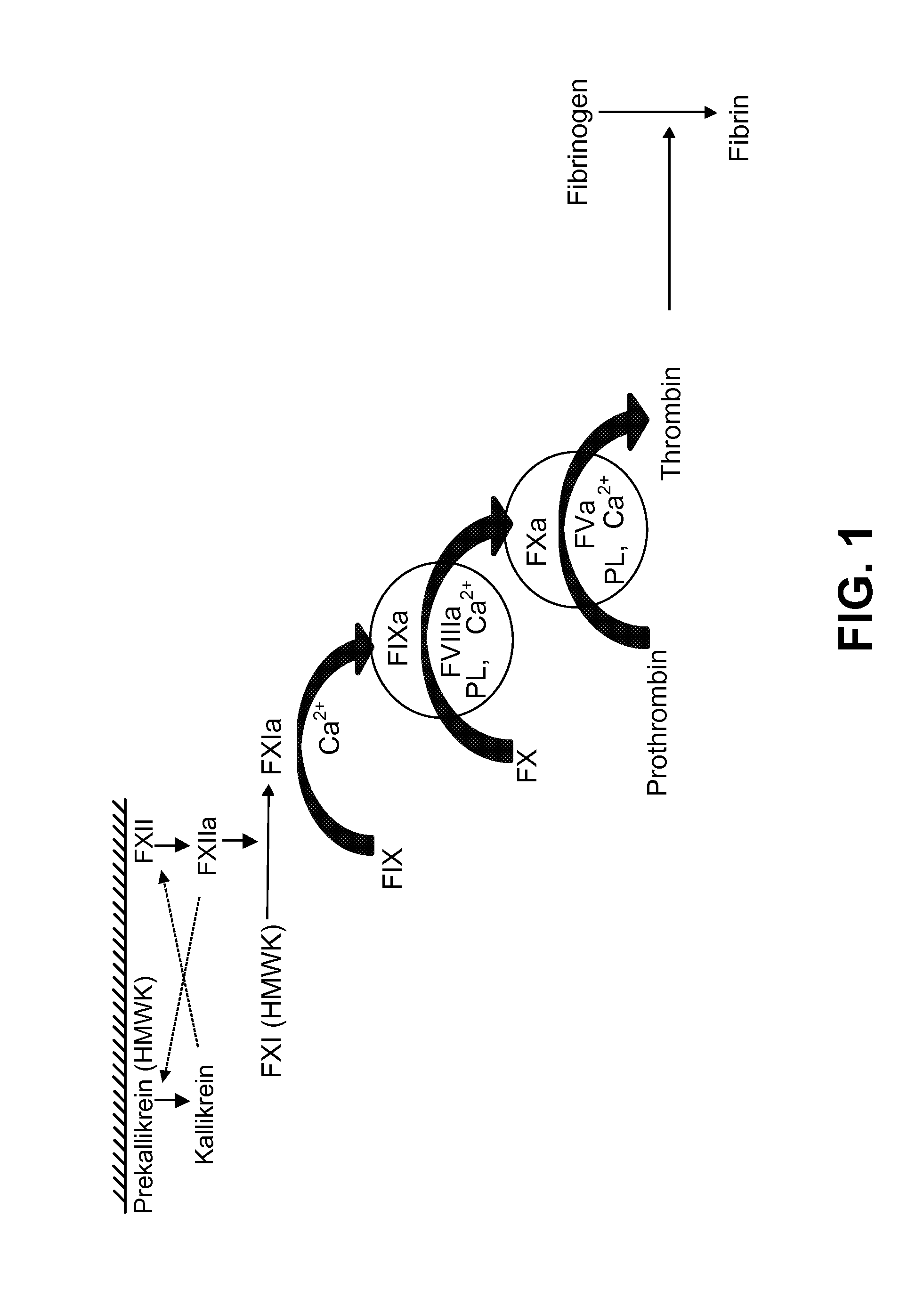
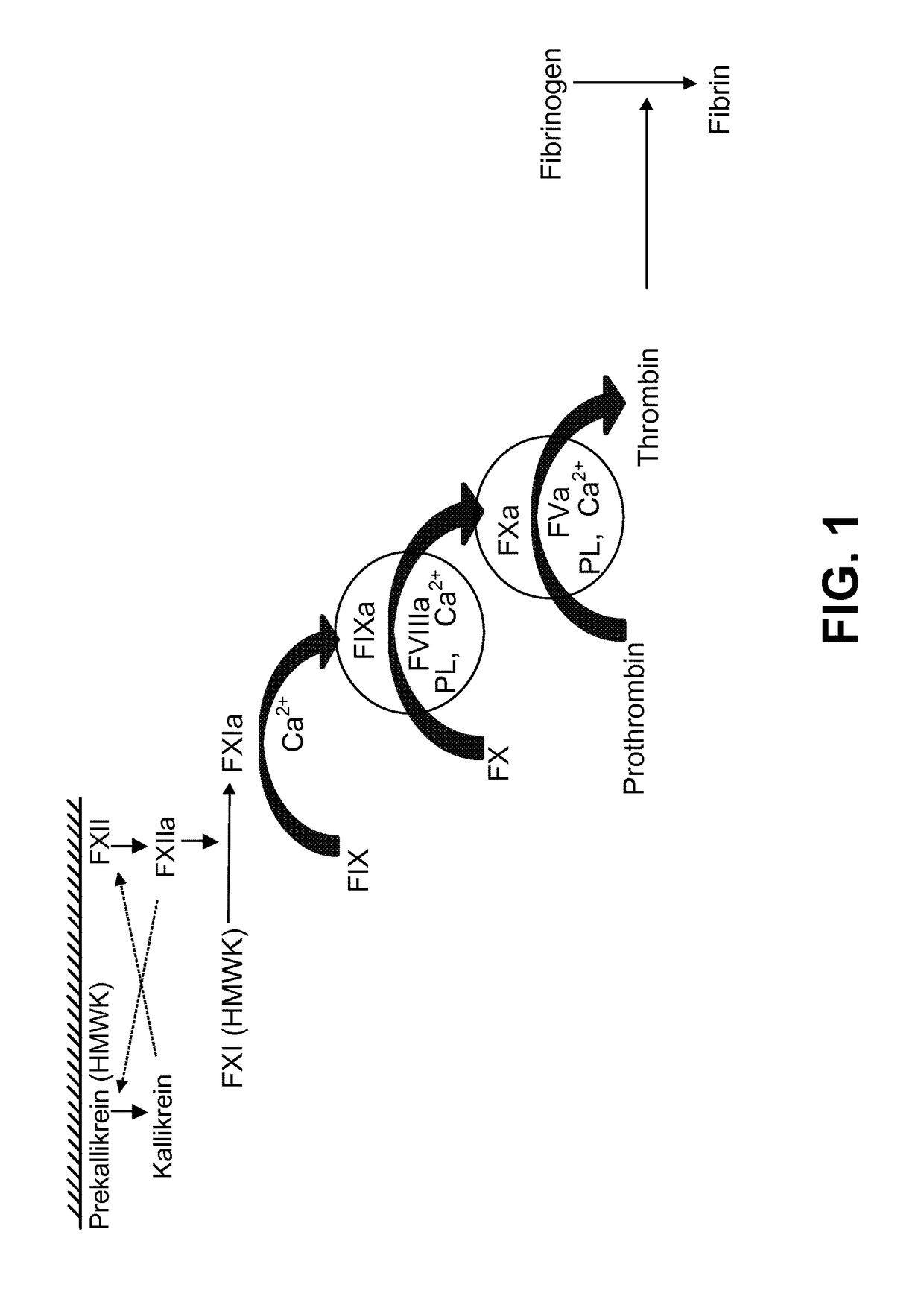
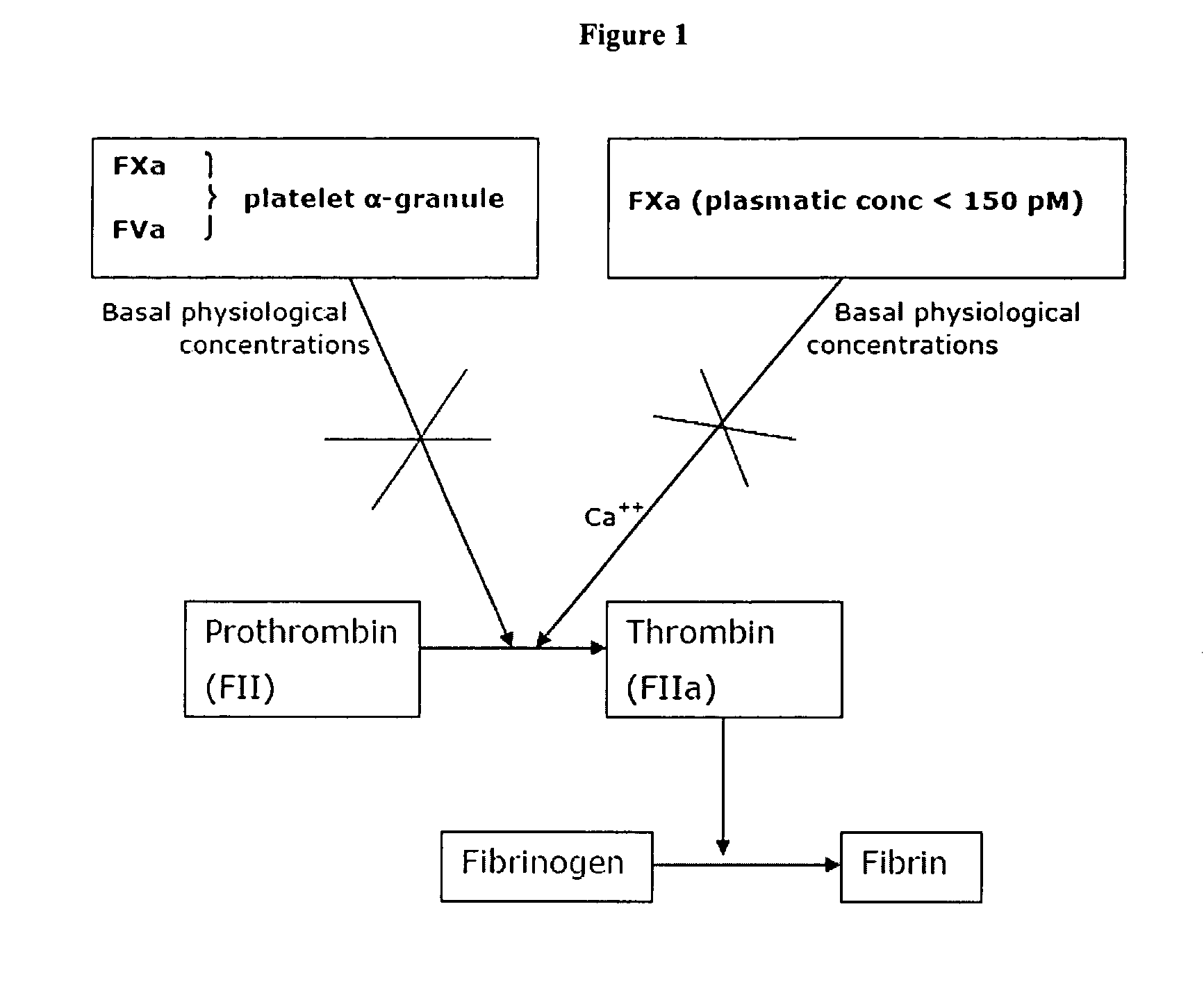
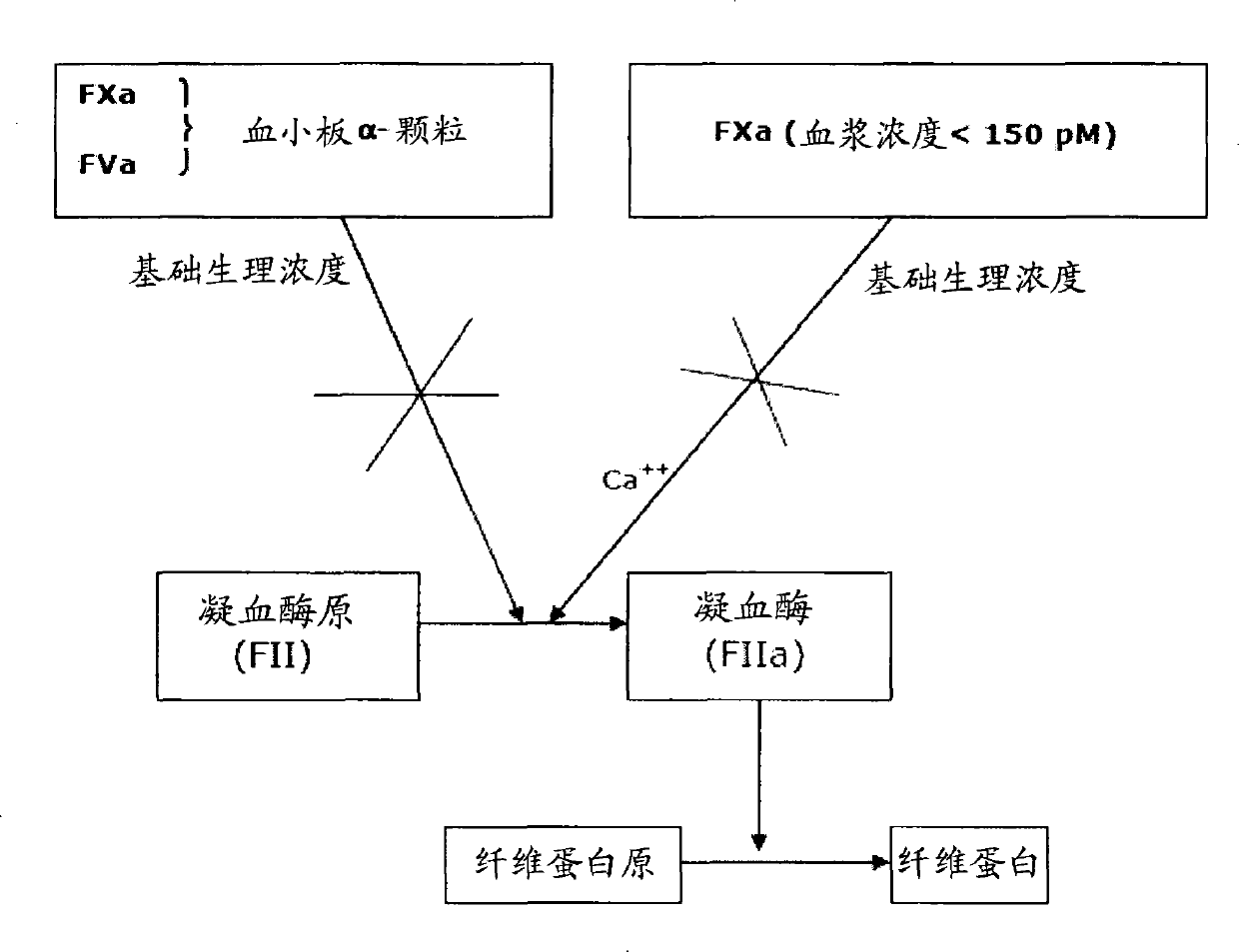
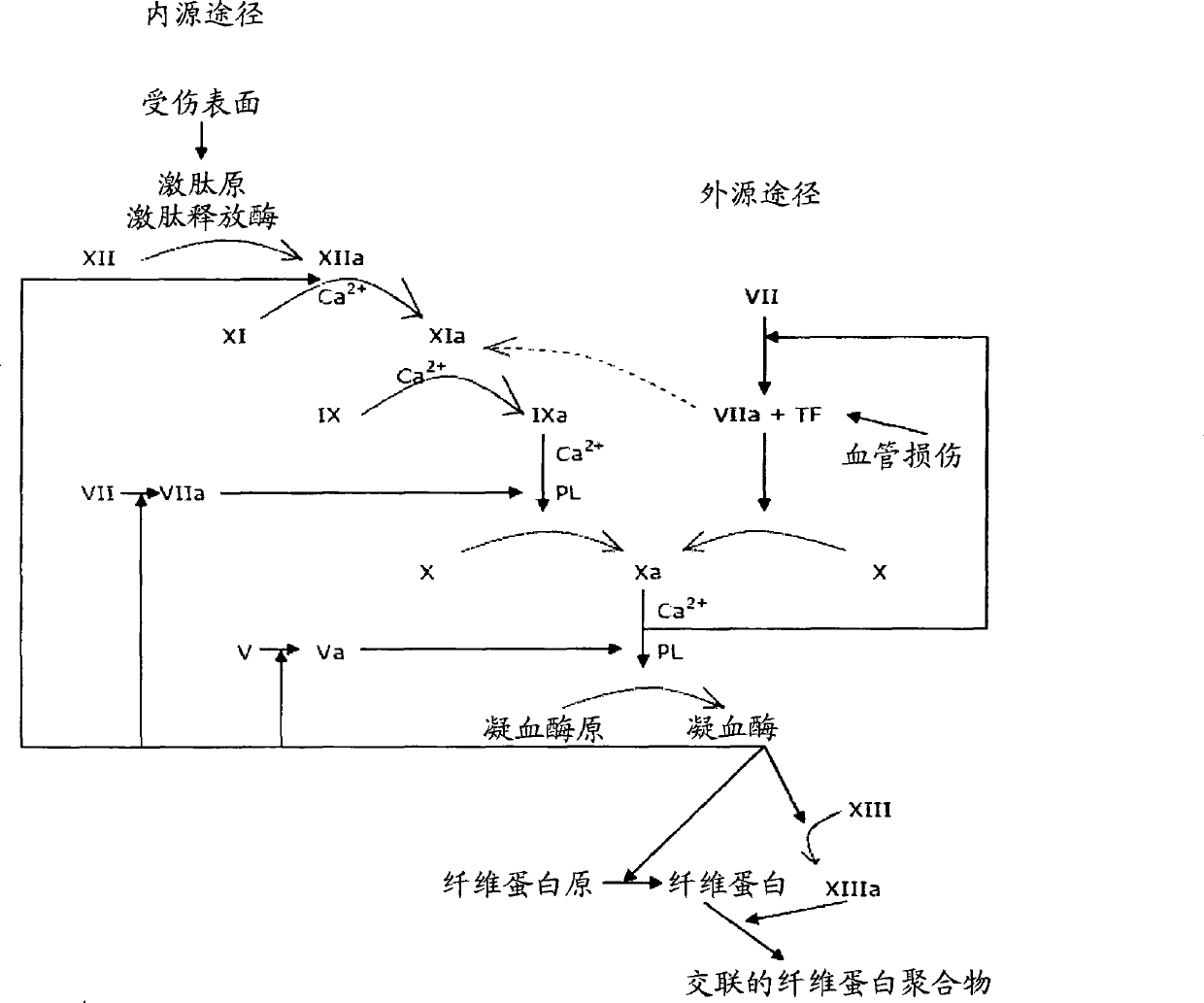
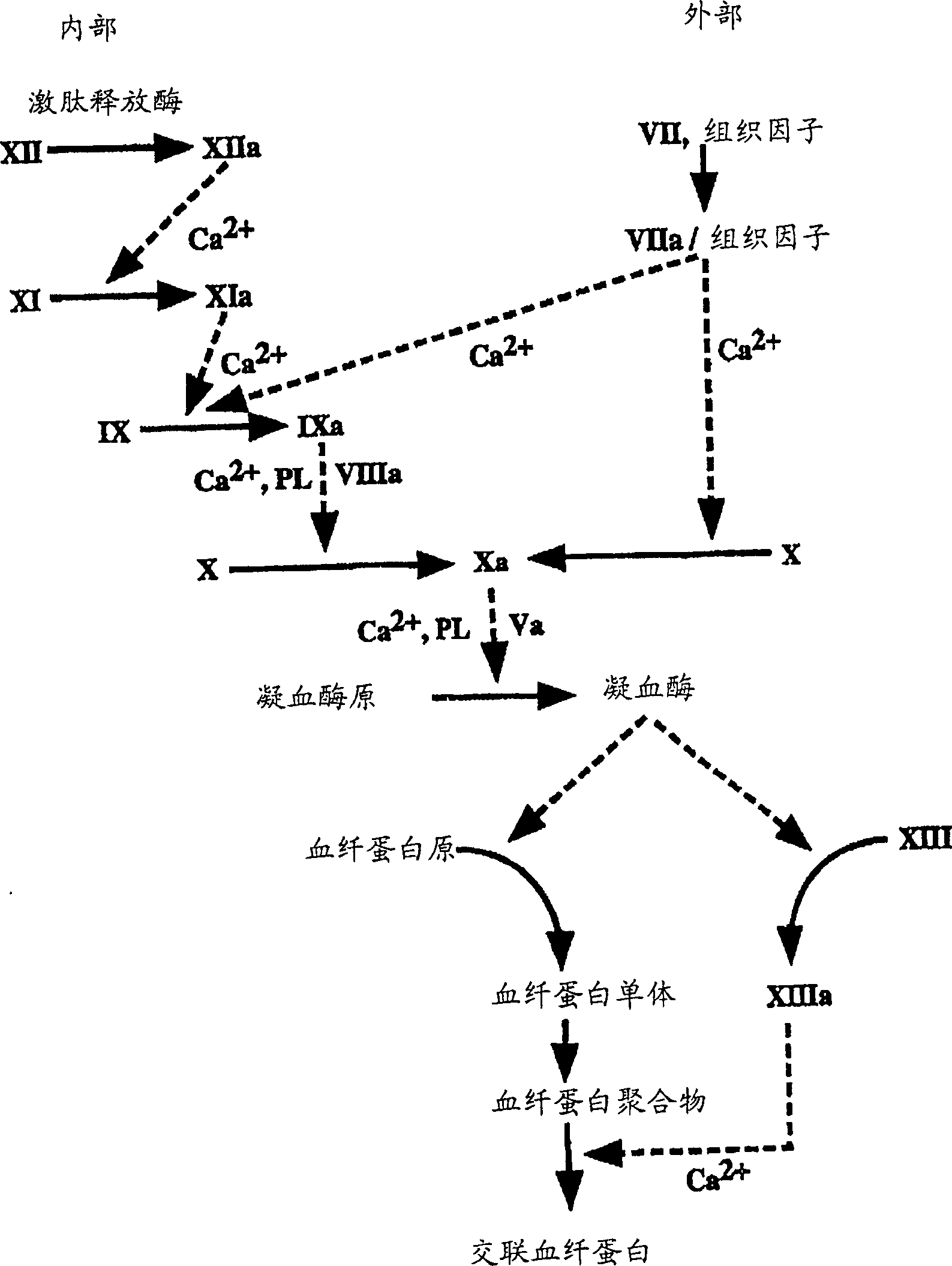
Coagulation factors are proteins that are essential for blood clot formation. Produced by the liver or blood vessels, the coagulation factors are continuously released into the bloodstream. When an injury occurs these factors are activated in a step by step process called the coagulation cascade.
Platelet aggregation function detection kit and detection method
InactiveCN102980993AStrong specificityImprove replicate assay variabilityBiological testingTesting medicinal preparationsRepeat testingBatroxobin
The present invention relates to a platelet aggregation function detection kit, and a method using the kit to detect platelet aggregation function. The detection kit mainly includes the following detection reagents: a sodium citrate whole blood activator, a fibrin activator and a platelet activator. The fibrin activator comprises: recombinant batroxobin and activated clotting factor. By using the kit of the invention to detect, blood sample processing requirements are low, repeat testing variability is small, accuracy and stability are high, use and storage are convenient, detection cost is low, and promotion is easy.
Owner:北京乐普诊断科技股份有限公司
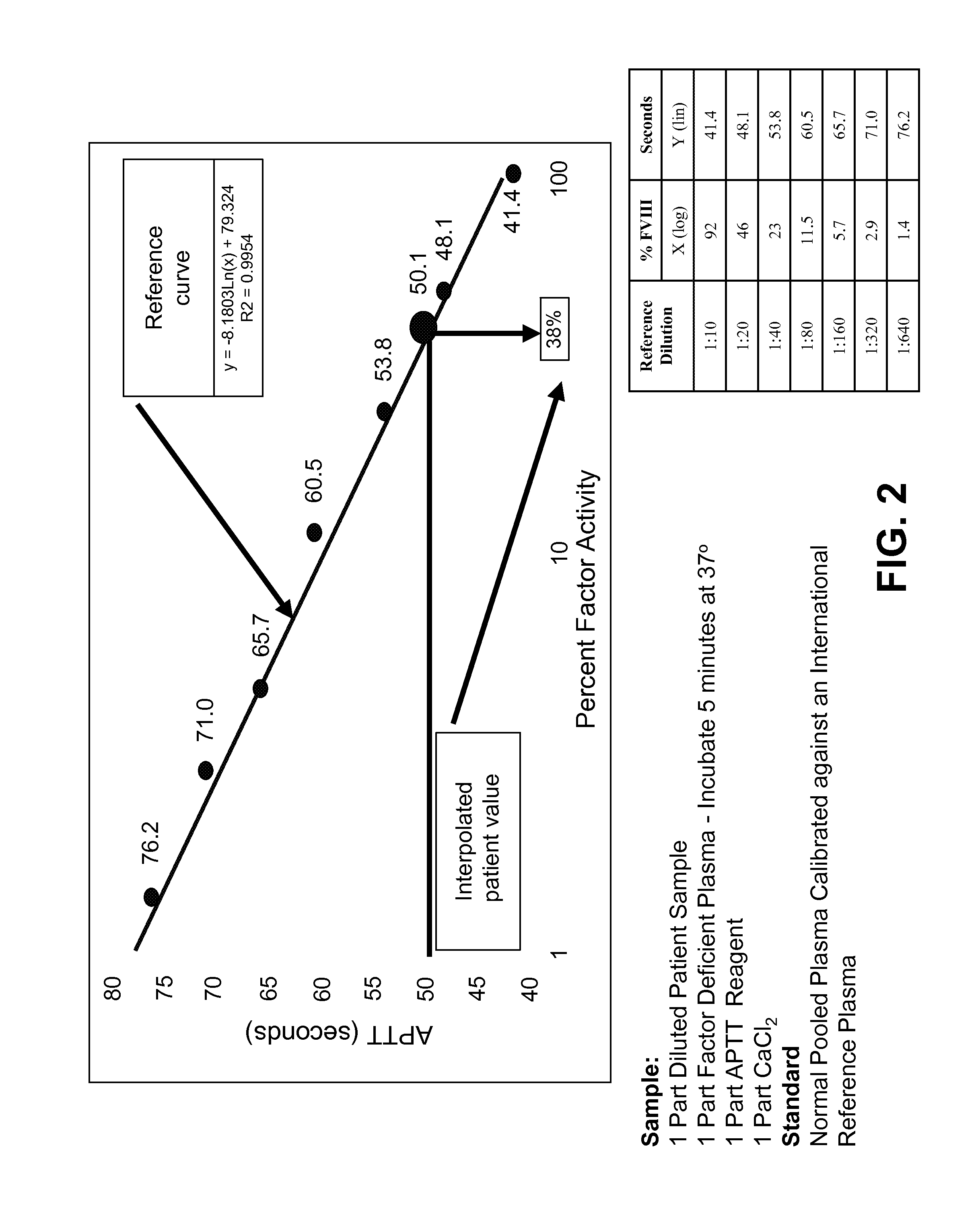
Blood Factor Monitoring Assay and Uses Thereof
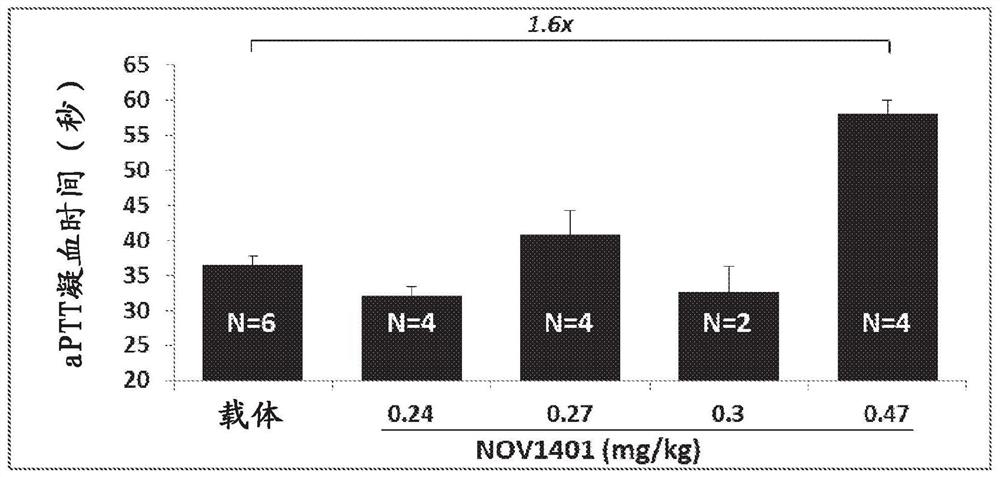
The present disclosure provides methods and compositions for diagnosing and treating subject having a bleeding disorder. The disclosed methods comprise contacting a sample, e.g., a blood or plasma sample obtained from the patient, with an activation mixture comprising an activated coagulation factor and a phospholipid mixture, wherein the activation mixture is dried onto a solid substrate. Also provided is a global hemostasis test based on the integration of clotting time (Ct) and pharmacokinetics data. The methods and compositions presented can be applied to point-of-care diagnostic systems.
Owner:BIOVERATIV THERAPEUTICS INC
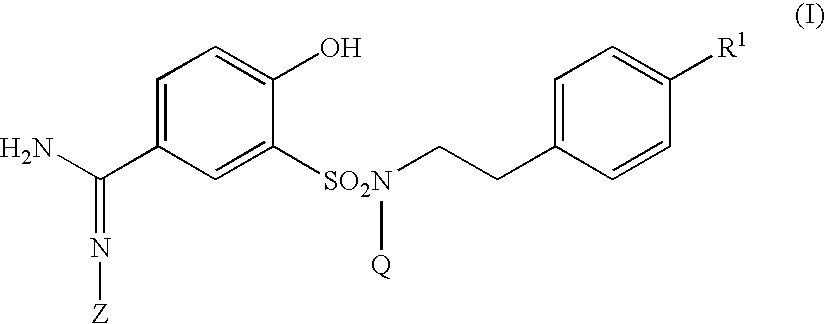
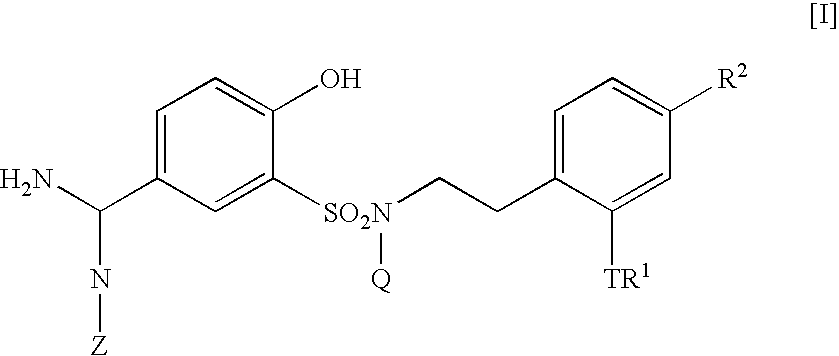
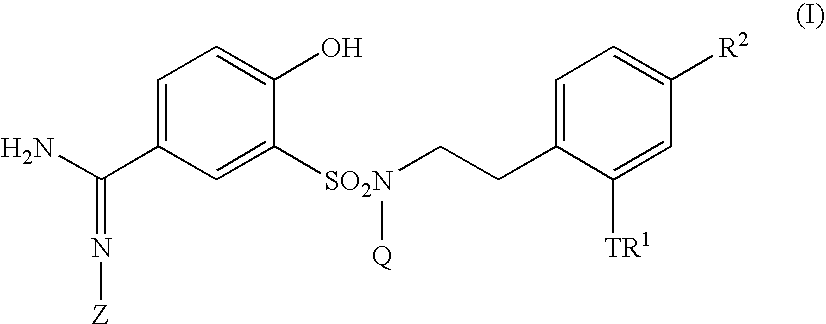
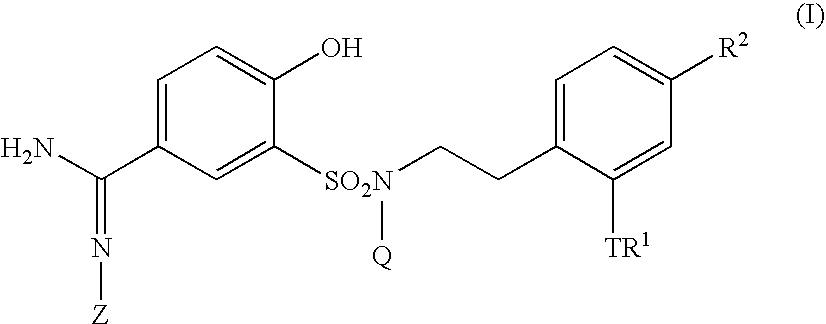
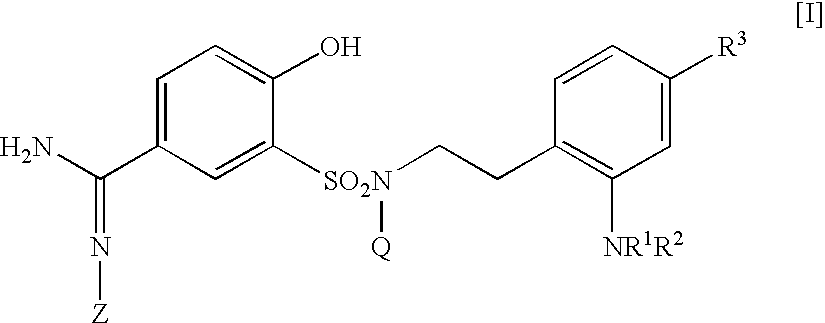
5-amidino-2-hydroxybenzenesulfonamide derivatives medicinal compoistions containing the same medicinal use thereof and intermediates in the production thereof
InactiveUS20050014787A1Reduce doseAvoided and declined adverse effectBiocideSenses disorderPulmonary artery embolismBlood Coagulation Factor X
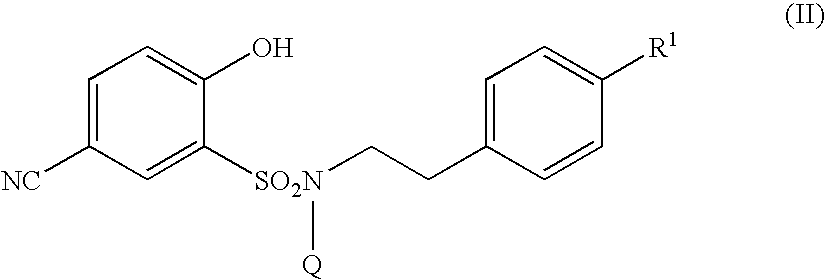
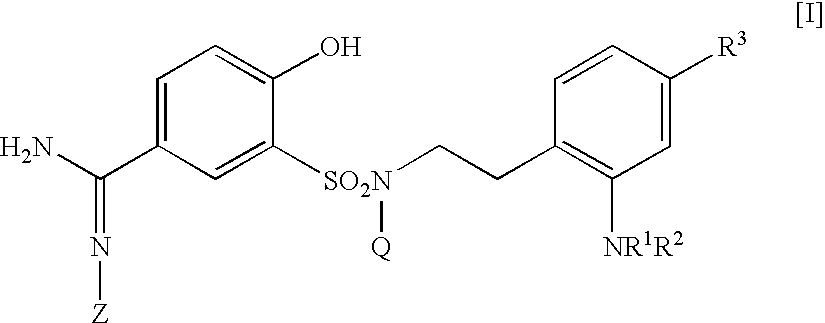
The present invention relates to a 5-amidino-2-hydroxybenzenesulfonamide derivative represented by the general formula: wherein R1 is an optionally substituted lower alkyl group, an optionally substituted lower alkoxy group, an optionally substituted lower alkenyl group, a cycloalkyl group or a lower acyl group etc.; Q is a hydrogen atom or an optionally substituted lower alkyl group; and Z is a hydrogen atom or a hydroxy group etc., or a pharmaceutically acceptable salt thereof, which exert a potent and selective activated blood coagulation factor X inhibitory activity and is useful as an agent for the prevention or treatment of a disease occurred associating an activated blood coagulation factor X, a pharmaceutical composition comprising the same and an intermediate thereof. These compounds are useful as preventives or remedies for various diseases such as brain infarction, cerebral thrombosis, cerebral embolism, TIA, cerebral vascular jerk, Alzheimer's diseases, myocardial infarction, heart attack, heart failure, thrombosis, pulmonary infarction and pulmonary embolism.
Owner:KISSEI PHARMA
Method for detecting activated blood coagulation factor XI in human intravenous immunoglobulin
ActiveCN103185710AAvoid preactivationIndirectly reflect changes in production contentFluorescence/phosphorescenceInitiation factorFactor ii
The invention discloses a method for detecting an activated blood coagulation factor XI in human intravenous immunoglobulin. The method comprises the steps as follows: (1) mixing an IVIG (intravenous immunoglobulin) sample with specially-preprocessed platelet-poor plasma (PPP); (2) adding a thrombin specific fluorogenic substrate with a phospholipid agent into a reaction system; (3) adding an initiation factor for starting a TGT (thrombin generation test), wherein the initiation factor comprises a calcium ion source and the phospholipid agent; (4) obtaining a thrombin generation curve providing parameters by the optimized reaction system; and (5) negatively correlating the peak time of thrombin (TTP) of the detected sample with the FXIa (activated blood coagulation factor XI) level in the sample. The FXIa level in the detected sample can be indirectly obtained by comparing the TTP of the detected sample with that of an FXIa standard sample. The method is suitable for detecting residual micro FXIa in human intravenous immunoglobulin (IVIG) products and is used for extracorporal evaluation of thrombosis risk of related products.
Owner:BLOOD TRASFUSION INST CHINESE ACAD OF MEDICAL SCI +1
Activated blood coagulation factor X (FXa) inhibitor
InactiveCN102325528AOrganic active ingredientsOrganic chemistryOral anticoagulationBlood Coagulation Factor X
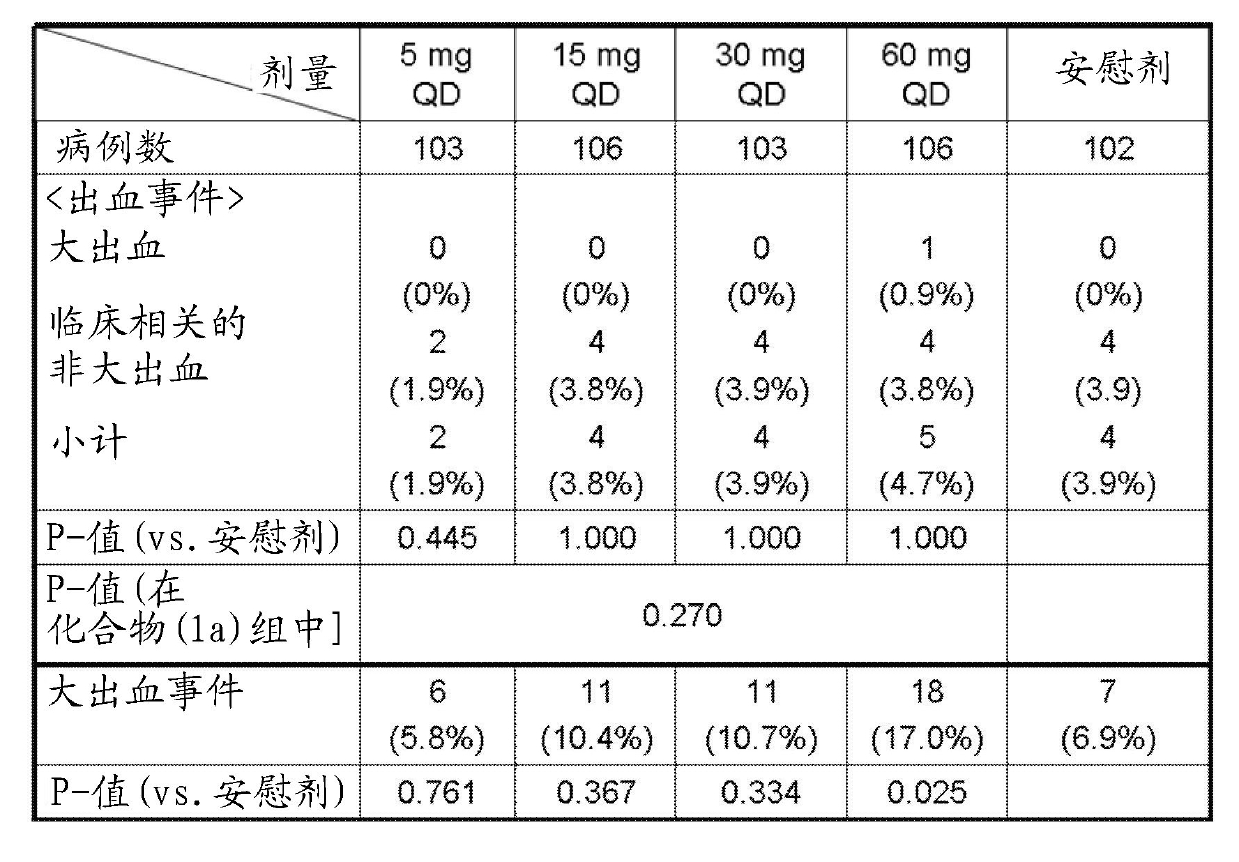
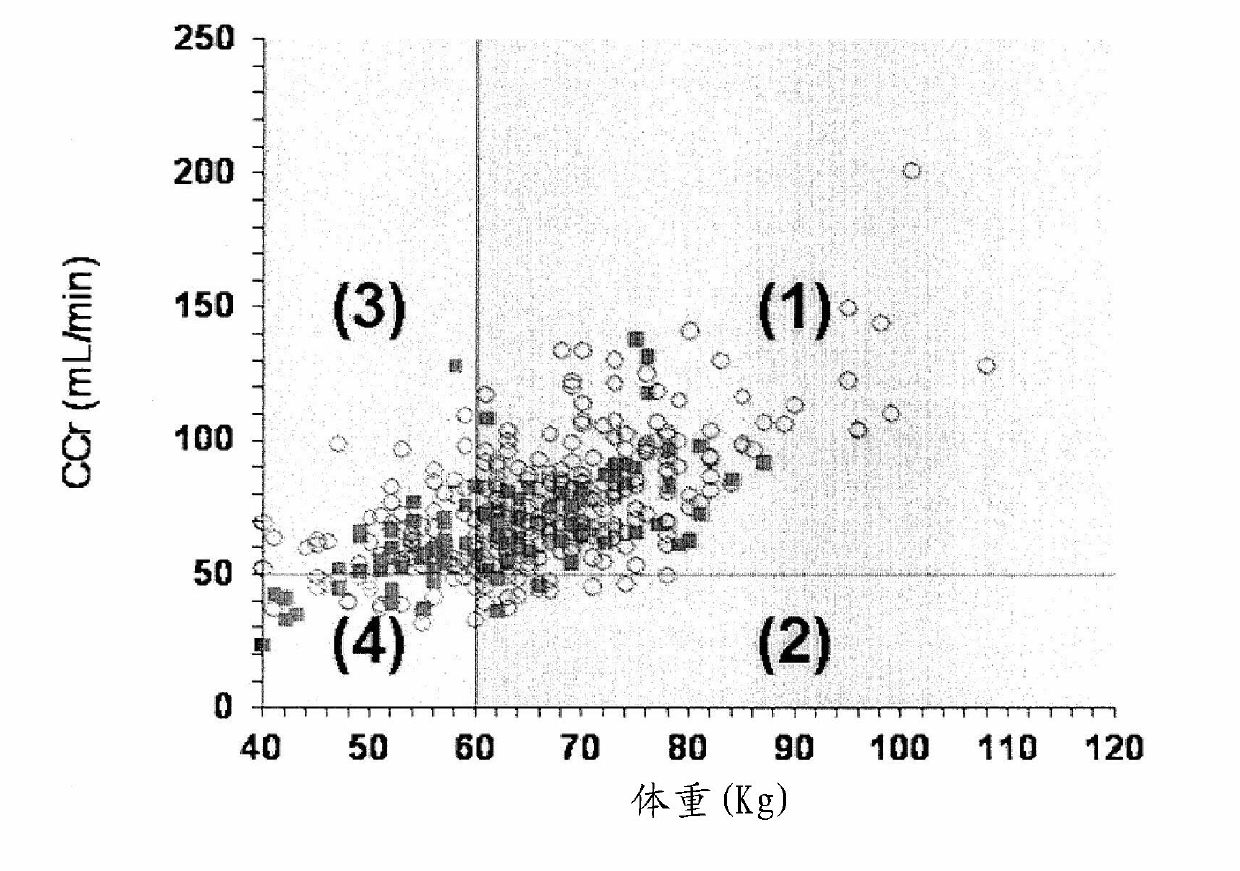
Disclosed is an activated blood coagulation factor X (FXa) inhibitor which is reduced in the risk of bleeding in the treatment of thromboembolism. Specifically disclosed is a blood coagulation inhibitor for oral administration, which comprises a compound represented by formula (1), a pharmacologically acceptable salt thereof, or a hydrate of the compound or the pharmacologically acceptable salt as an active ingredient. The blood coagulation inhibitor is characterized in that the dosage of the blood coagulation inhibitor can be determined by the following steps (A) to (D): (A) a factor associated with the risk of bleeding of the blood coagulation inhibitor is selected as a dosage determination factor; (B) a standard value for the dosage determination factor is set; (C) the level of the dosage determination factor is measured in a patient who needs the administration of the blood coagulation inhibitor; and (D) the dosage for the patient is determined by employing the standard value as a measure.
Owner:DAIICHI SANKYO CO LTD
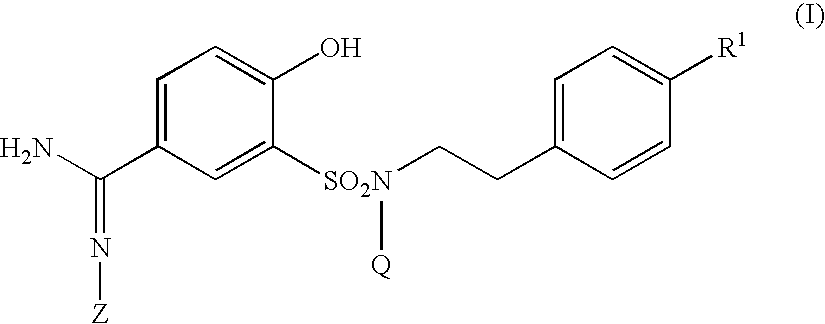
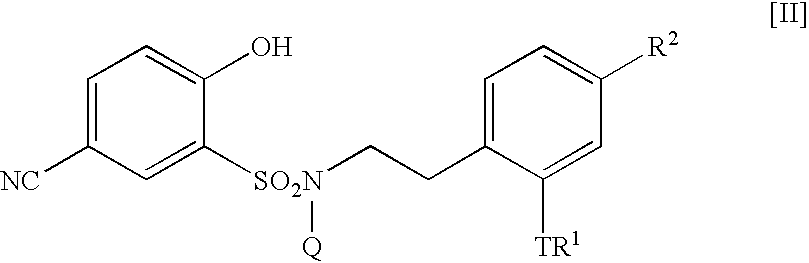
5-Amidino-2-hydroxybenzenesulfonamide derivatives, pharmaceutical compositions containing the same and intermediates for their preparation
InactiveUS20060116361A1Improve isolationEasy to purifyBiocideNervous disorderDiseaseBlood Coagulation Factor X
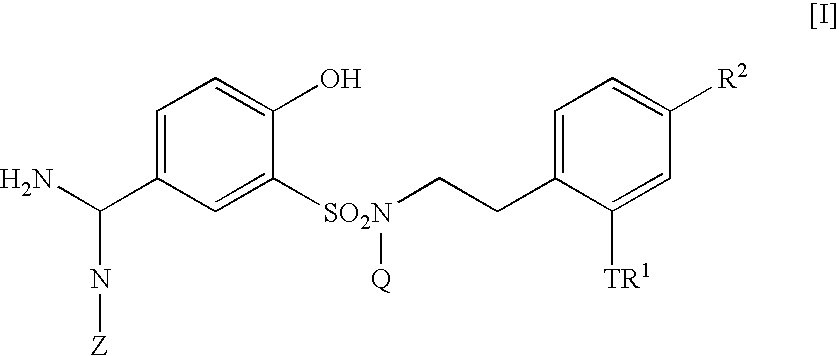
The present invention relates to a 5-amidino-2-hydroxybenzenesulfonamide derivative represented by the general formula: wherein R1 is a hydrogen atom or an optionally substituted lower alkyl group; R2 is a di(lower alkyl)amino group, a lower alkyl group, a cycloalkyl group, an optionally substituted aryl group, an optionally substituted heterocycloalkyl group, or an optionally substituted aromatic heterocyclic group; T is an oxygen atom, a sulfur atom, a sulfonyl group etc.; Q is a hydrogen atom or an optionally substituted lower alkyl group; and Z is a hydrogen atom, a hydroxy group etc., or a pharmaceutically acceptable salt thereof, which exert a potent and selective activated blood coagulation factor X inhibitory activity and is useful as an agent for the prevention or treatment of a disease occurred associating an activated blood coagulation factor X, a pharmaceutical composition comprising the same, a pharmaceutical use thereof and an intermediate thereof.
Owner:KISSEI PHARMA
Blood factor monitoring assay and uses thereof
The present disclosure provides methods and compositions for diagnosing and treating subject having a bleeding disorder. The disclosed methods comprise contacting a sample, e.g., a blood or plasma sample obtained from the patient, with an activation mixture comprising an activated coagulation factor and a phospholipid mixture, wherein the activation mixture is dried onto a solid substrate. Also provided is a global hemostasis test based on the integration of clotting time (Ct) and pharmacokinetics data. The methods and compositions presented can be applied to point-of-care diagnostic systems.
Owner:BIOVERATIV THERAPEUTICS INC
Agent neutralizint tissue factor inhibitor and agent neutralizing activated blood coagulation factor viii preparation
InactiveUS20060233786A1Suppressing bleeding tendencyPeptide/protein ingredientsAntibody ingredientsTissue factorBlood Coagulation Factor VII
Formulations for neutralizing the anticoagulant action of a human tissue factor inhibitor, comprising a blood coagulation factor complex product or an activated blood coagulation factor VII product as an active ingredient; and formulations for neutralizing the blood coagulation-promoting action of an activated blood coagulation factor VII product, comprising a human tissue factor inhibitor as an active ingredient.
Owner:CHUGAI PHARMA CO LTD
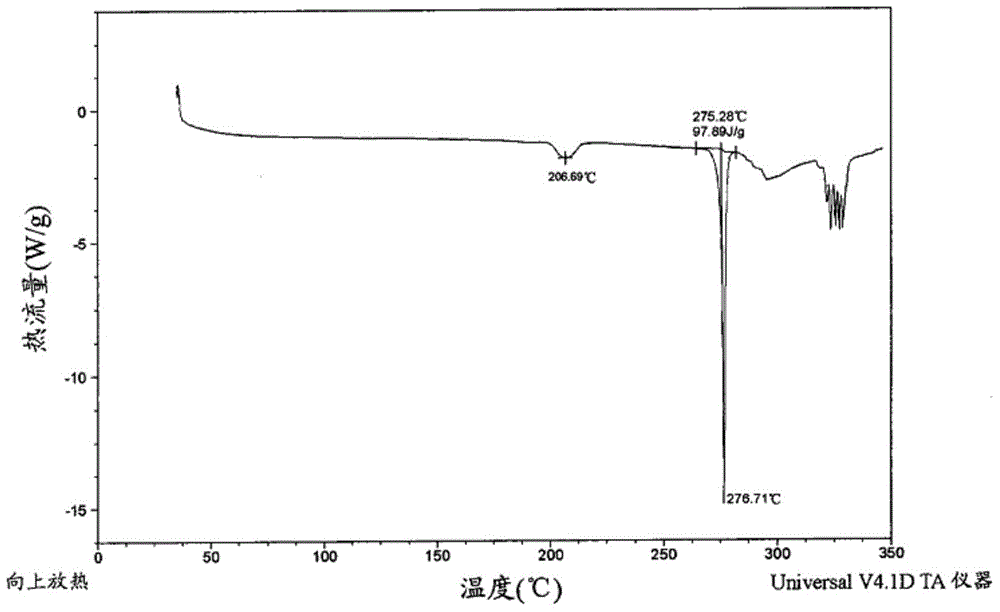
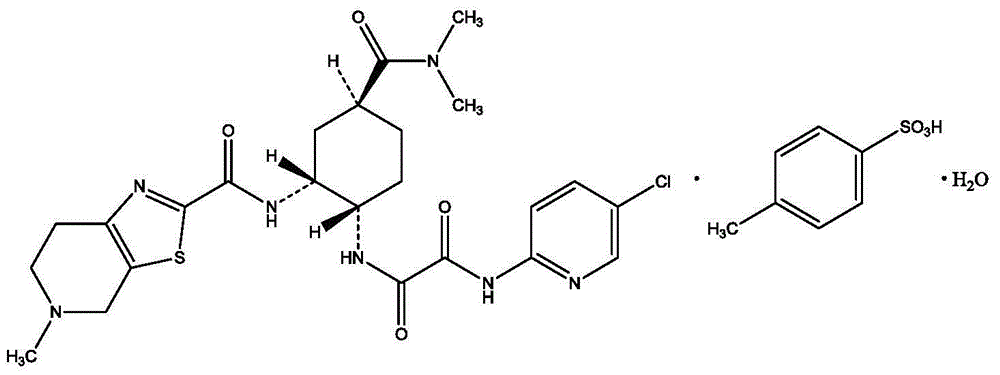
Edoxaban tosilate hydrate
ActiveCN105777779ALow impurity contentPrinciples of safe and controllable drug useOrganic active ingredientsOrganic chemistryBlood Coagulation Factor XEdoxaban Tosilate
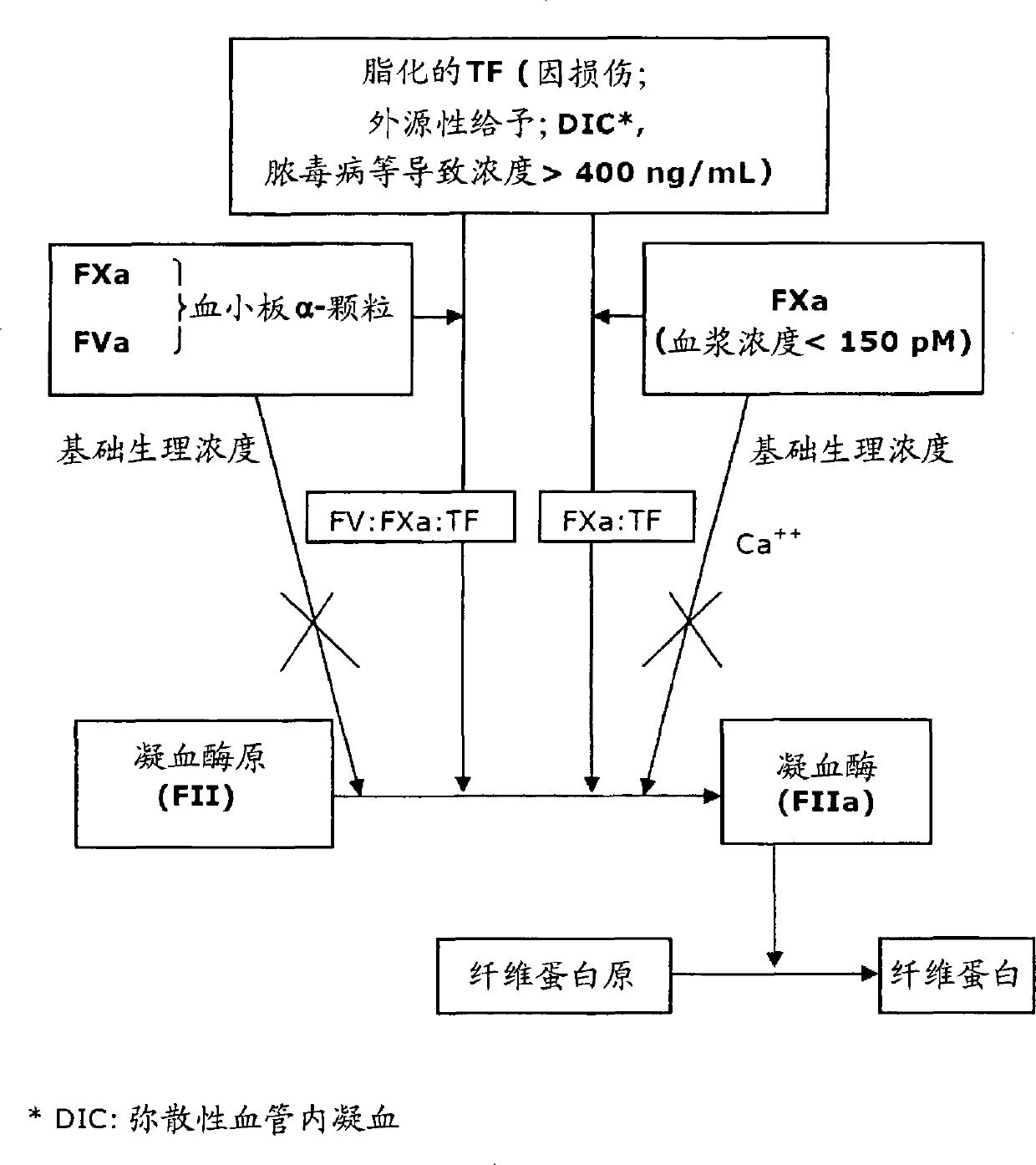
The invention provides edoxaban tosilate hydrate. Edoxaban is a micromolecule oral anticoagulant drug, and is a blood coagulation factor X(FXa) retarding agent. In the process of blood coagulation, the activated coagulation factor X(FXa) activates prothrombin (FII) into thrombin (FIIa), formation of fibrin is promoted, and thrombus is formed, so that FXa is developed as a main target of a new generation anticoagulant drug. Edoxaban is the oral anticoagulant drug by selectively, reversibly and directly inhibiting FXa in order to inhibit formation of thrombus.
Owner:海思科制药(眉山)有限公司
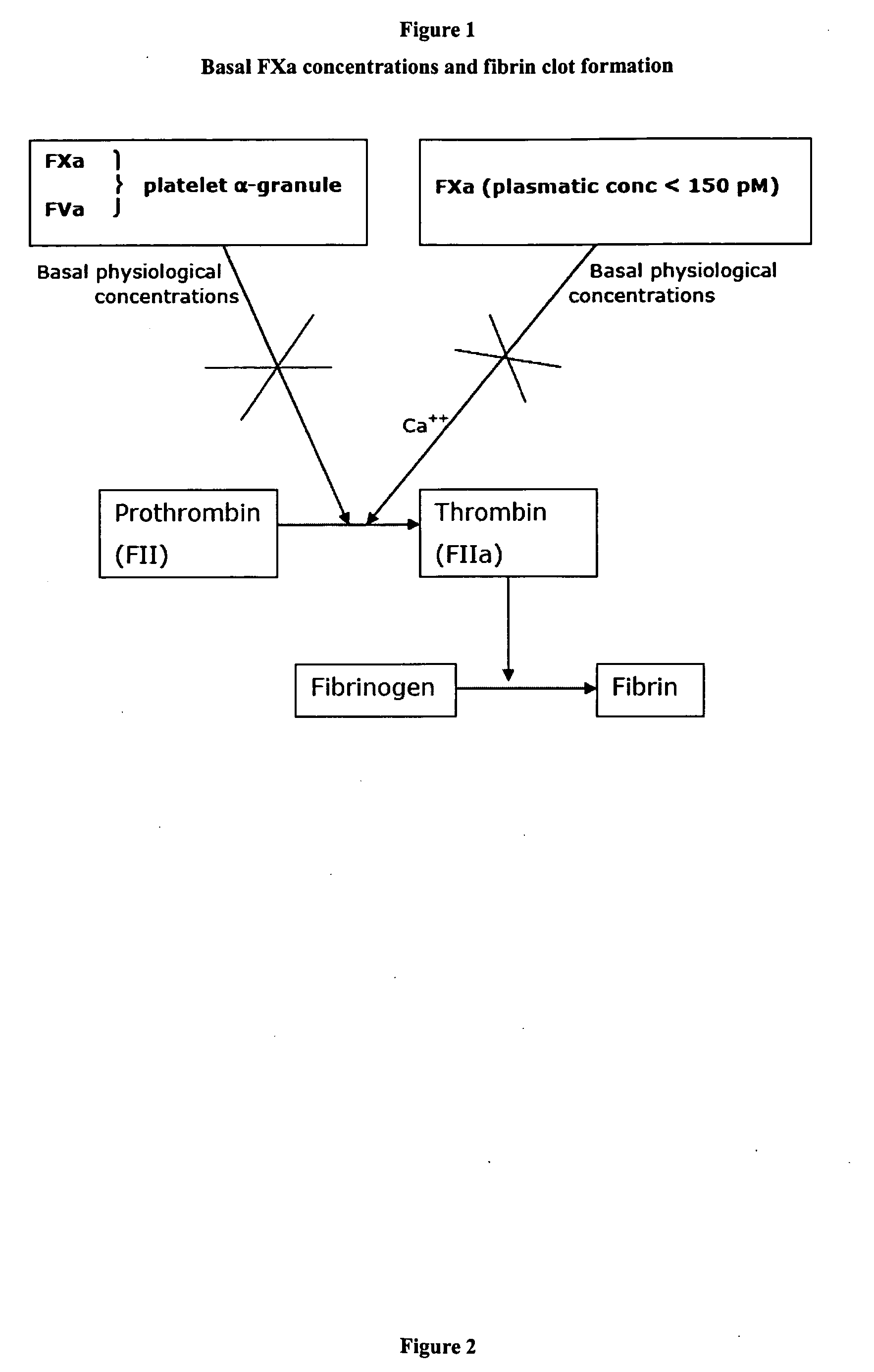
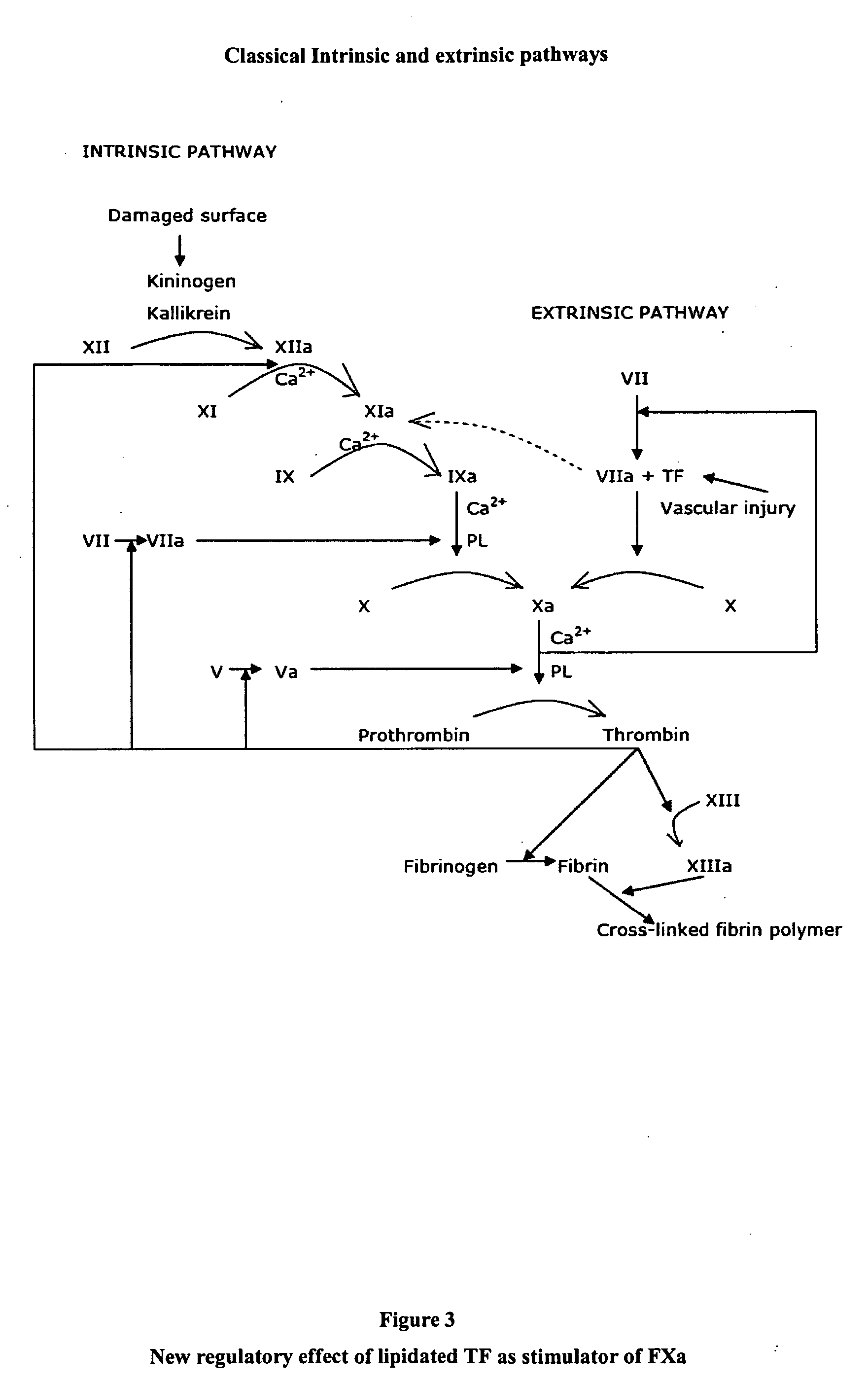
Stimulators of Factor X activated (FXa) as new topical antihemorrhagic agents
InactiveUS20070032424A1Quickly initiate thrombin formationPotent hemostasicOrganic active ingredientsFactor VIIFactor XCompound (substance)
The activated coagulation Factor X (FXa) stimulating agents may be used in the treatment of hemorrhages in a subject. Compounds and combinations are described which are particularly useful for the topical treatment of hemorrhaging in healthy subjects or in patients with hemorrhagic diathesis.
Owner:THROMBOTARGETAB CORP
5-Amidino-2-hydroxybenzenesulfonamide derivatives, pharmaceutical compositions containing the same and intermediates for their preparation
InactiveUS20060205812A1Improve isolationEasy to purifyBiocideNervous disorderDiseaseBlood Coagulation Factor X
Owner:KISSEI PHARMA
Stimulators of Factor X activated (FXa) as new topical antihemorrhagic agents
InactiveUS7772371B2Promote rapid formationPotent hemostasicFactor VIIPeptide/protein ingredientsFactor XFactor ii
The activated coagulation Factor X (FXa) stimulating agents may be used in the treatment of hemorrhages in a subject. Compounds and combinations are described which are particularly useful for the topical treatment of hemorrhaging in healthy subjects or in patients with hemorrhagic diathesis.
Owner:THROMBOTARGETAB CORP
Activated factor X(FXa) stimulants as new antihemorrhagic agents for topical use
The activated coagulation Factor X (FXa) stimulating agents may be used in the treatment of hemorrhages in a subject. Compounds and combinations are described which are particularly useful for the topical treatment of hemorrhaging in healthy subjects or in patients with hemorrhagic diathesis.
Owner:THROMBOTARGETS EURO SL
Pharmaceutical composition of hemocoagulase and application of pharmaceutical composition
PendingCN108785665APromote aggregationAggregate recoveryPeptide/protein ingredientsPharmaceutical delivery mechanismFIBRINOGEN/THROMBINHaemophilia B
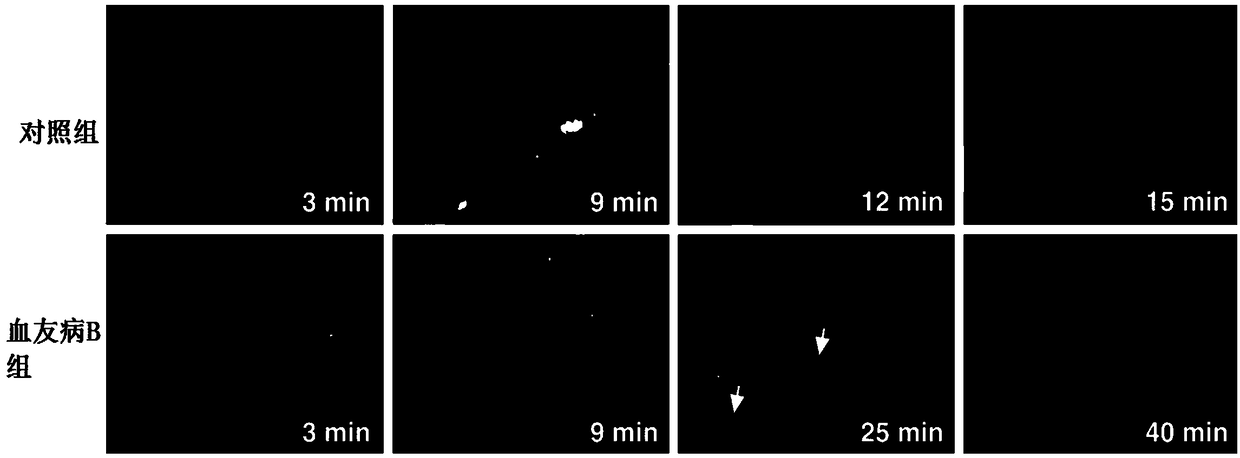
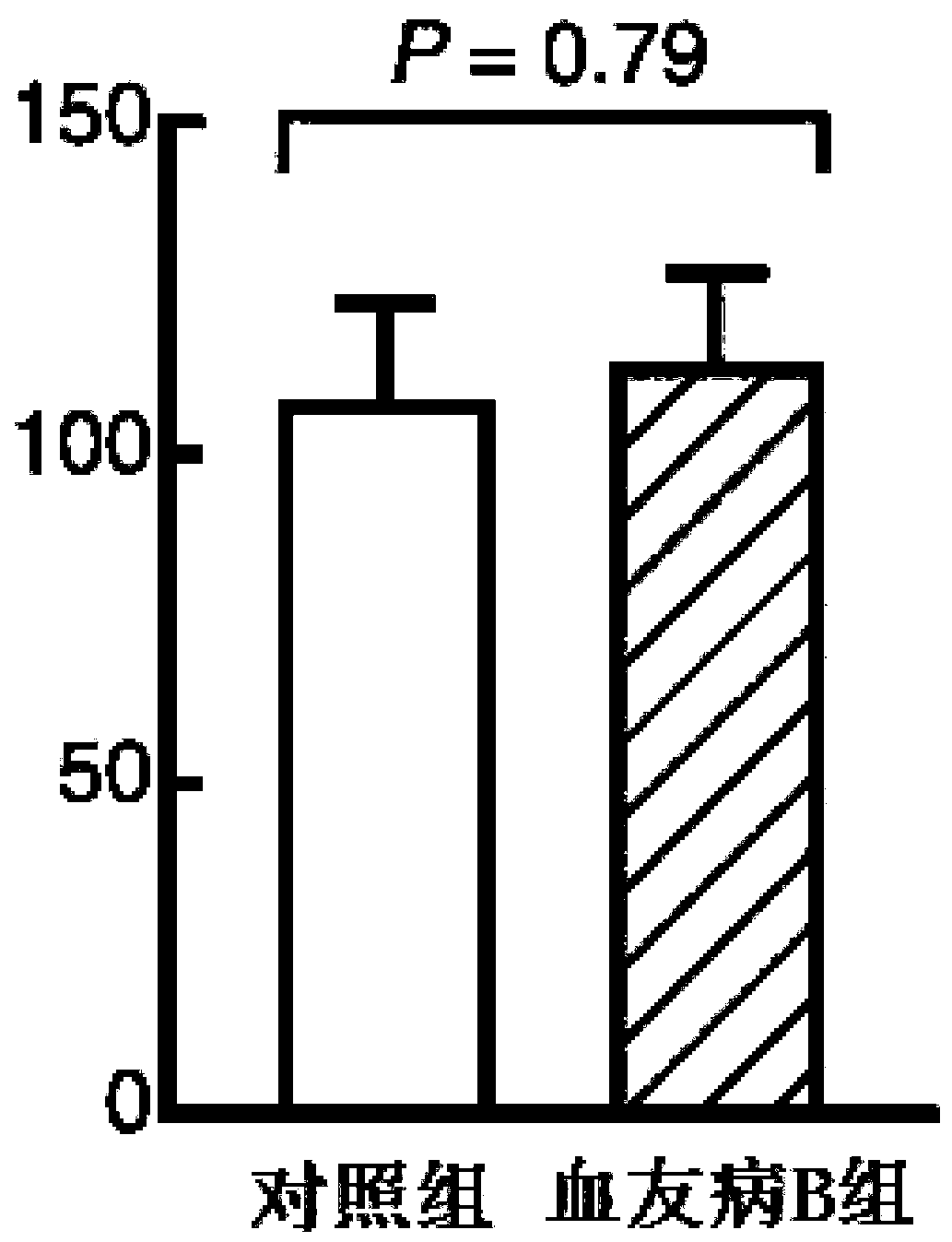
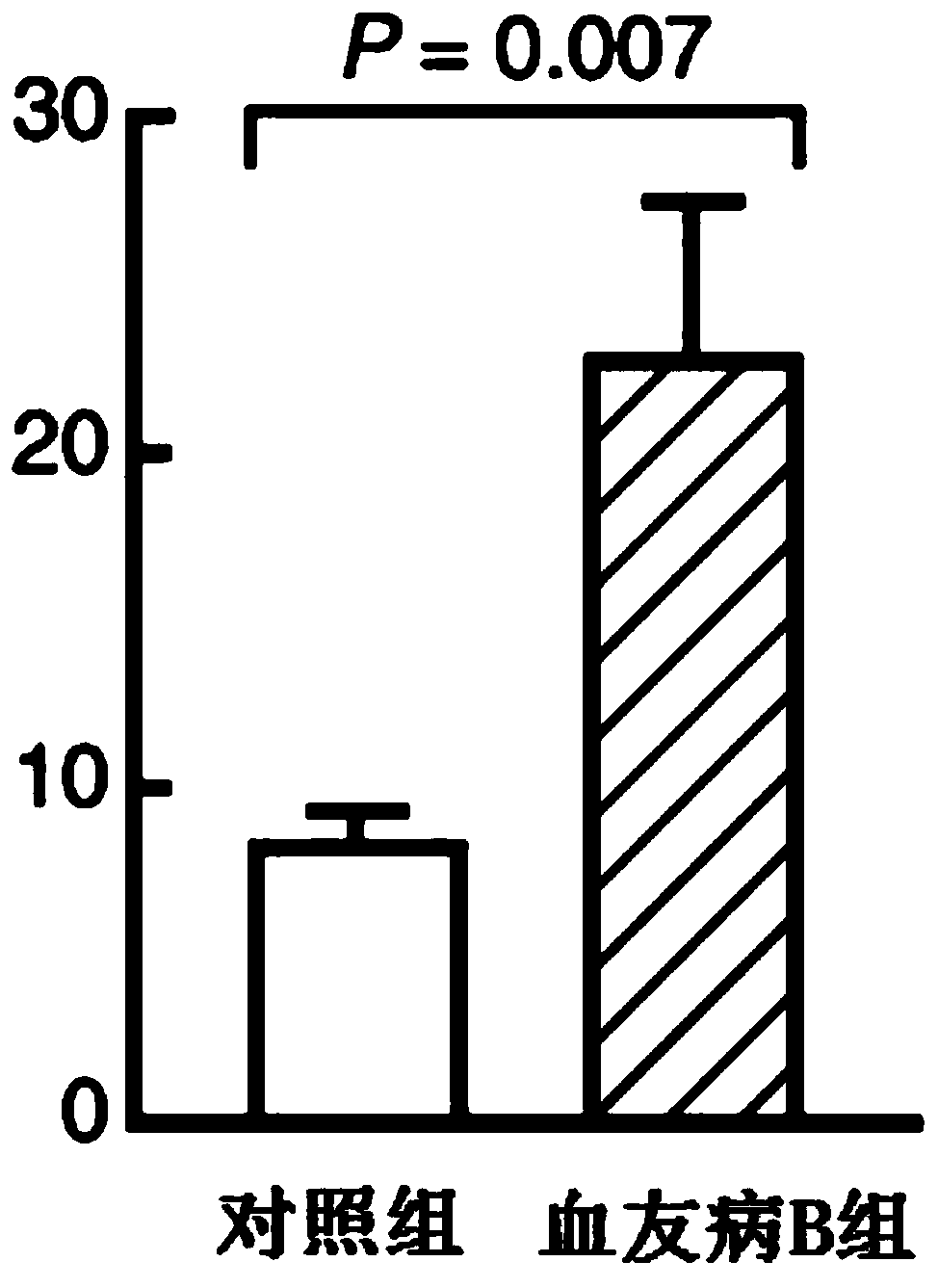
The invention discloses a pharmaceutical composition of hemocoagulase. The pharmaceutical composition is characterized in that the pharmaceutical composition is prepared from 0.00005 to 0.0005 percentof a hemocoagulase composition and the balance of a pharmaceutically acceptable carrier. The composition is applied to preparation of a medicine for treating Haemophilia B (coagulation factor IX deficiency). Active components in the hemocoagulase comprise hemocoagulase, i.e., a thrombin-like enzyme (batroxobin), and a phospholipid dependent coagulation factor X activating agent (FXA). A bleedingstopping process of the hemocoagulase does not depend on a coagulation factor IX and is mainly used for fibrin and an activated coagulation factor X, and has the effect of treating bleeding of the haemophilia B. After the hemocoagulase is used for treating, platelet aggregation can be remarkably enhanced, and the formation of blood clots or fibrin clots in a patient with the haemophilia B is recovered.
Owner:ZHAOKE PHARMA HEFEI
Method for evaluating blood coagulation reaction
ActiveCN104937423AThe effect is accurateAccurate evaluationMicrobiological testing/measurementDisease diagnosisHematological testPhospholipid
Owner:CHUGAI PHARMA CO LTD +1
Application of heparin sodium to preparation of external drug for treating haemorrhoids
PendingCN108606976AOrganic active ingredientsPharmaceutical delivery mechanismAnti coagulationIn vivo
The invention provides the application of heparin sodium to the preparation of an external drug for treating haemorrhoids. The heparin sodium is acid mucopolysaccharide-heparin (The molecular weight is 4000-15000), can interfere with many links in the hemagglutination process and has an anti-coagulation effect both in vivo and in vitro; in combination with antithrombin III (AT-III), the heparin sodium mainly enhances an inhibitory effect of the latter on activated coagulation factors II, IX, X, XI and XII and has the effects of preventing platelet aggregation and damage, hindering the formation of thromboplastin, preventing prothrombin from becoming thrombin and inhibiting thrombin, thereby preventing fibrinogen from becoming fibrinogen and playing an anti-coagulation role. The invention utilizes heparin for dermatology (heparin sodium cream) to treat internal and external hemorrhoids, mixed hemorrhoids and acute thrombotic hemorrhoid and applies the anticoagulation, blood stasis and thrombolysis effects of the heparin sodium to relieve swelling and pain; a blood vessel is dredged, retracted and restored, so that the haemorrhoids is treated. The heparin sodium is applied to an anus(Pay attention to discontinuation when bleeding occurs) and is safe, convenient and effective.
Owner:周恩志
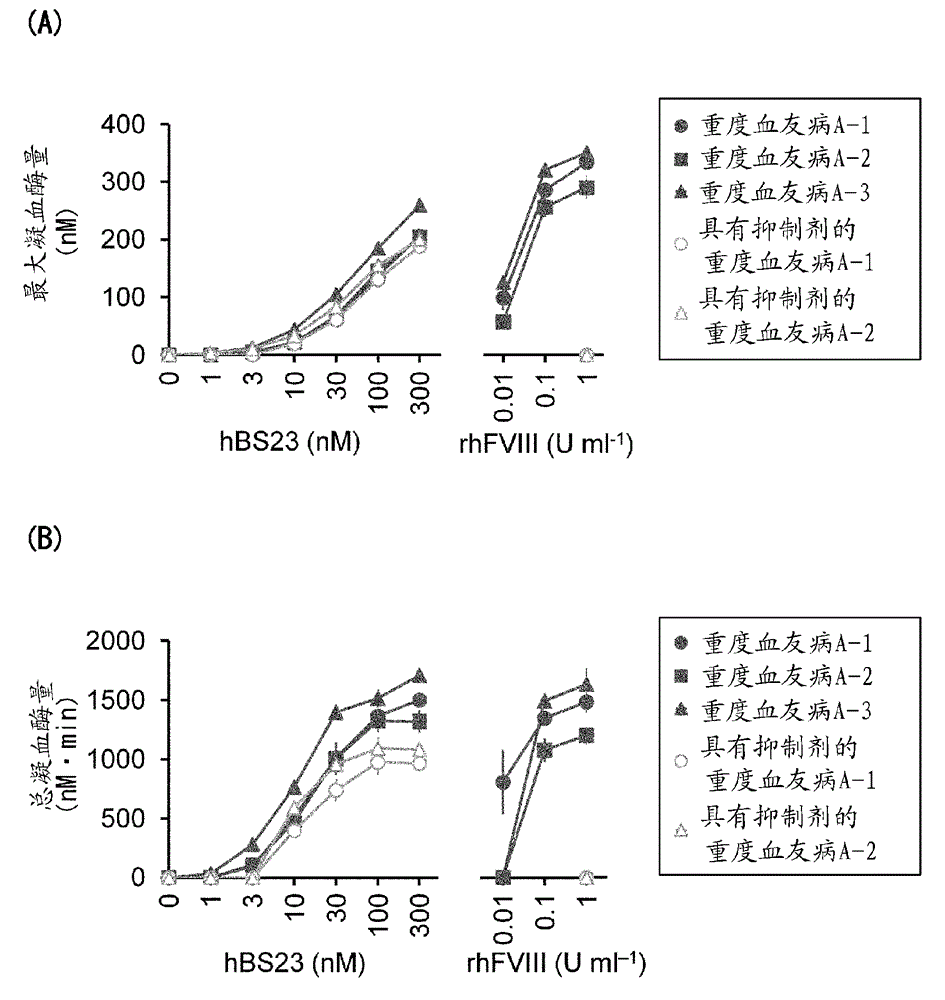
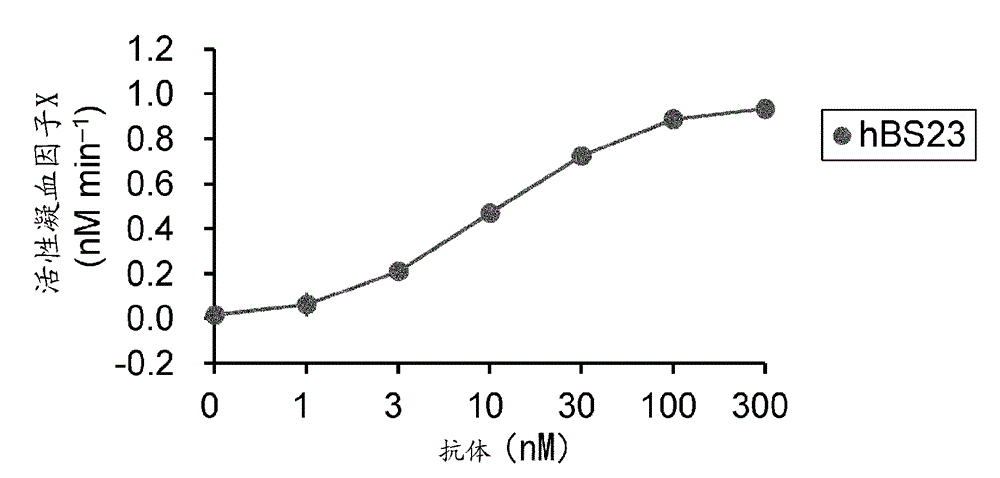
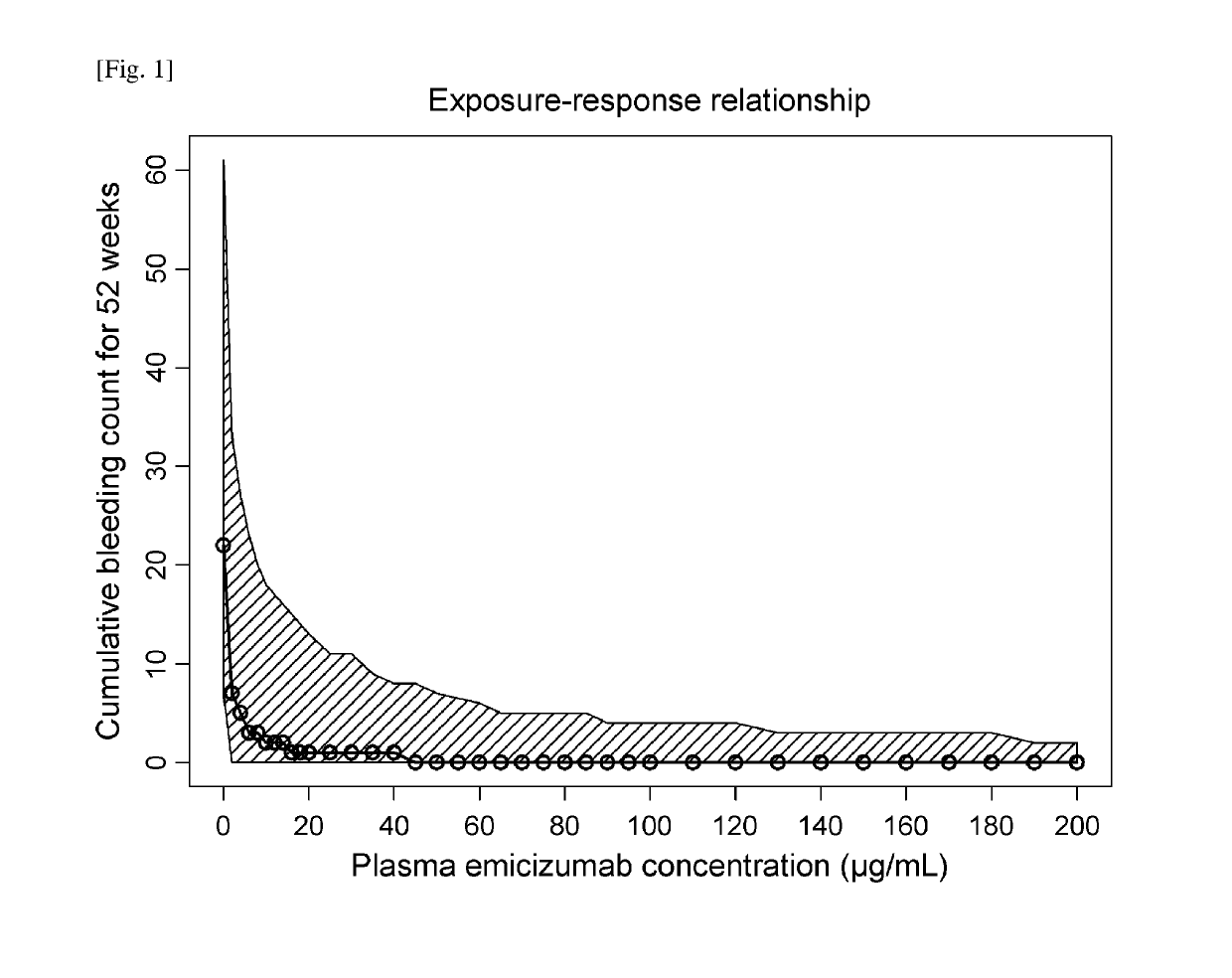
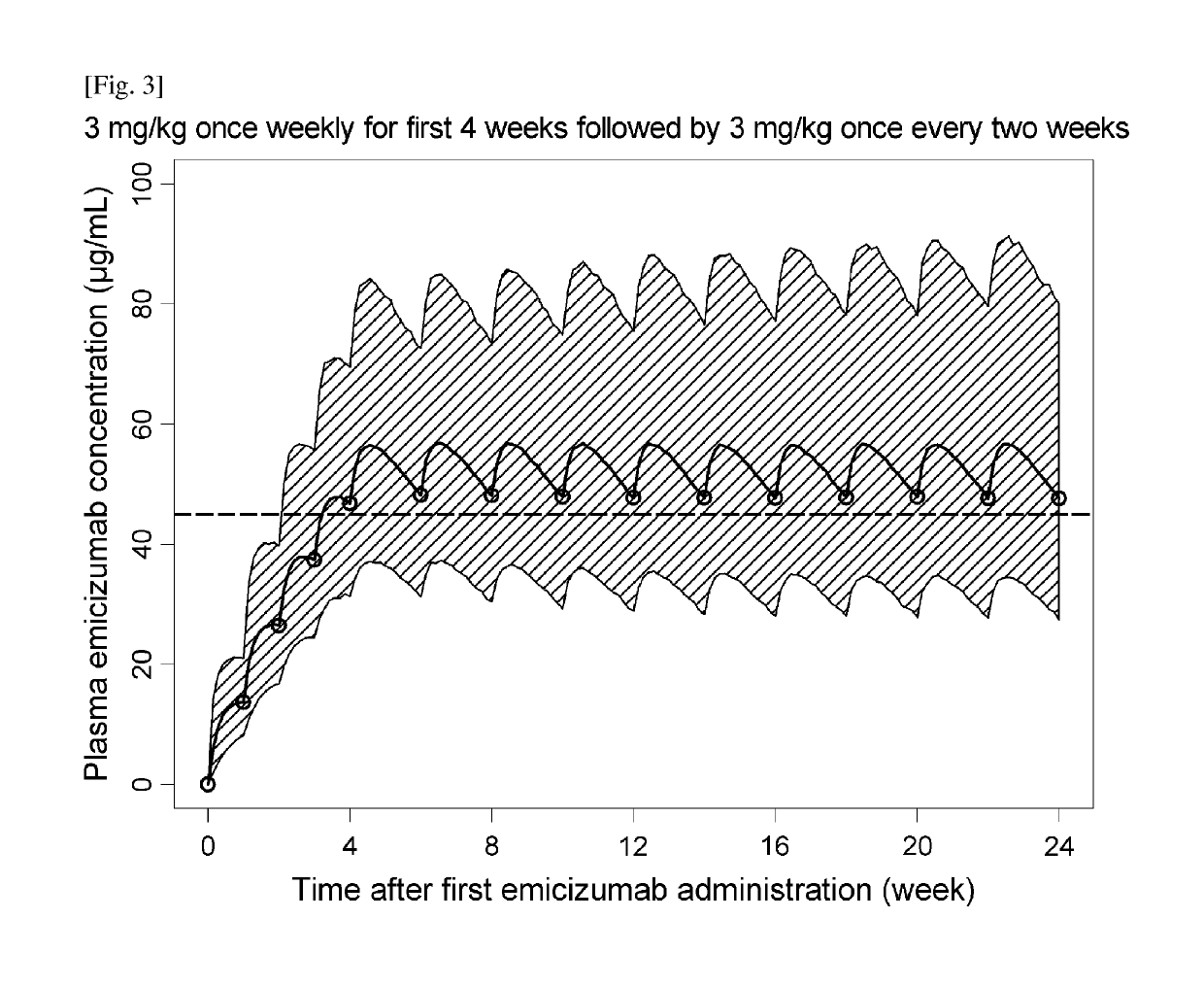
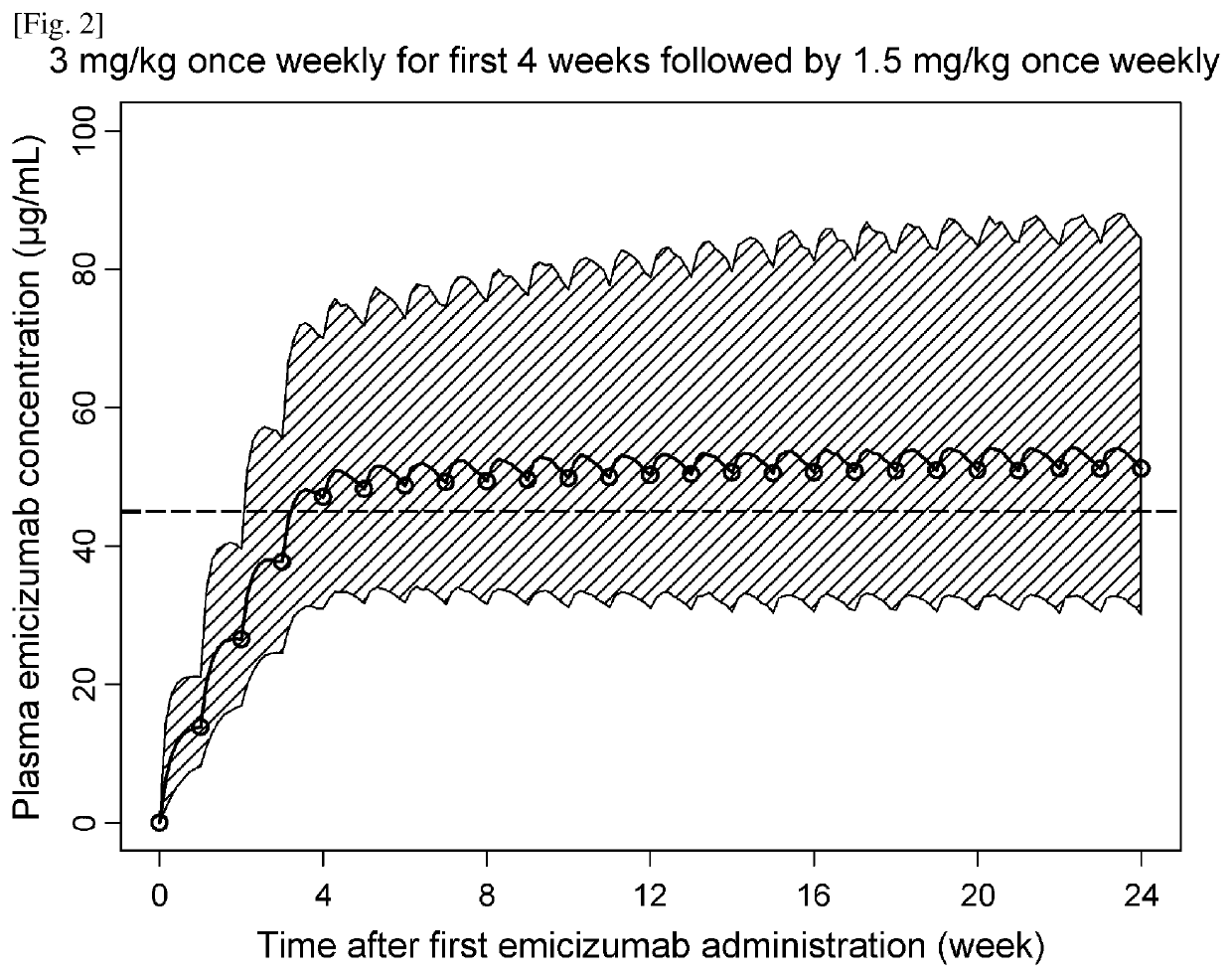
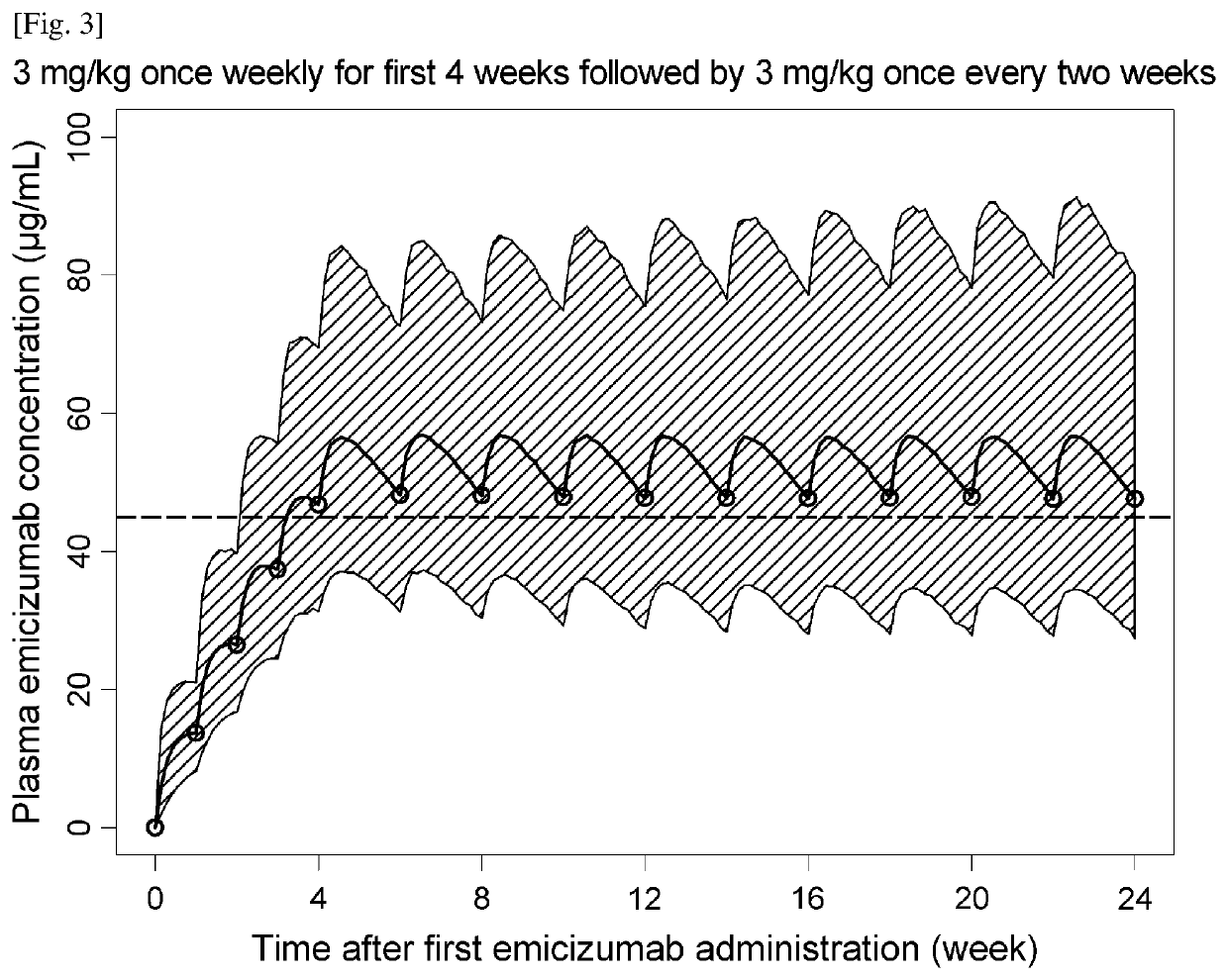
Methods of using a bispecific antibody that recognizes coagulation factor ix and/or activated coagulation factor ix and coagulation factor x and/or activated coagulation factor x
ActiveUS20190194352A1Reduce morbidityImmunoglobulins against blood coagulation factorsAntibody ingredientsRegimenBlood Coagulation Factor X
An objective of the present invention is to provide an effective pharmaceutical composition or a dosage regimen for preventing and / or treating bleeding, a disease accompanying bleeding, or a disease caused by bleeding. The inventors discovered that by administering a pharmaceutical composition comprising a bispecific antigen-binding molecule that recognizes (a) blood coagulation factor IX and / or activated blood coagulation factor IX and (b) blood coagulation factor X and / or activated blood coagulation factor X according to a given dosage regimen, bleeding, a disease accompanying bleeding, or a disease caused by bleeding can be prevented and / or treated more effectively.
Owner:F HOFFMANN LA ROCHE & CO AG +1
Methods of using a bispecific antibody that recognizes coagulation factor IX and/or activated coagulation factor IX and coagulation factor X and/or activated coagulation factor X
ActiveUS11352438B2Reduce morbidityImmunoglobulins against blood coagulation factorsAntibody ingredientsDosing regimenAntigen
An objective of the present invention is to provide an effective pharmaceutical composition or a dosage regimen for preventing and / or treating bleeding, a disease accompanying bleeding, or a disease caused by bleeding. The inventors discovered that by administering a pharmaceutical composition comprising a bispecific antigen-binding molecule that recognizes (a) blood coagulation factor IX and / or activated blood coagulation factor IX and (b) blood coagulation factor X and / or activated blood coagulation factor X according to a given dosage regimen, bleeding, a disease accompanying bleeding, or a disease caused by bleeding can be prevented and / or treated more effectively.
Owner:F HOFFMANN LA ROCHE & CO AG +1
Novel benzamidine compound
InactiveUS20080021065A1Prevent thrombosisBiocideOrganic chemistryBlood Coagulation Factor XBenzamidine
Compounds represented by formula (1) and pharmaceutically acceptable salt thereofs: wherein each symbol is as defined in the specification, are useful as inhibitors of an activated blood coagulation factor X. Compositions which contain, as an active ingredient, an FXa selective low-molecular weight FXa inhibitor having a short serum half-life are particularly useful as anticoagulants for an extracorporeal blood circuit.
Owner:AJINOMOTO CO INC
Use of activated coagulation factor vii for treating thrombolytic therapy-induced major bleedings
Major bleedings induced by thrombolytic / fibrinolytic therapy, including intracranial haemorrhages, are treated by administering to a subject suffering from such bleedings an effective amount of activated coagulation factor VII (VIIa) or a functional derivative thereof.
Owner:BOEHRINGER INGELHEIM PHARM KG
Immobilized snake venom blood coagulation factor activator and preparation method of activated blood coagulation factor
ActiveCN108743924AShorten activation reaction timeRaise factor specific activityPeptide/protein ingredientsUnknown materialsActive componentFactor ii
The invention relates to an immobilized snake venom blood coagulation factor activator and a preparation method of an activated blood coagulation factor. The immobilized snake venom blood coagulationfactor activator comprises an active component and a vector provided with the active component immobilizedly, wherein the active component is a snake venom extract. The preparation method of the activated blood coagulation factor comprises the steps that pig plasma or cow plasma is made through a filtering column filled with the immobilized snake venom blood coagulation factor activator to obtainthe activated blood coagulation factor by collecting effluent or the immobilized snake venom blood coagulation factor activator is added into the pig plasma or the cow plasma for stirring for 1-5 hours to obtain the activated blood coagulation factor through filtering. The immobilized snake venom blood coagulation factor activator can be used repeatedly, and when the primarily-extracted blood coagulation factor is activated, only the immobilized snake venom blood coagulation factor activator needs to be removed, so that the snake venom component in extraction liquid is removed at the same time; moreover, by means of the method, activation reaction time is shortened, the factor specific activity after activation is improved, and the activation specificity is high.
Owner:苏州良辰生物医药科技有限公司
Activated factor X(FXa) stimulants as new antihemorrhagic agents for topical use
The activated coagulation Factor X (FXa) stimulating agents may be used in the treatment of hemorrhaging in a subject. Compounds and combinations are described which are particularly useful for the topical treatment of hemorrhaging in a healthy subject or in a subject with hemorrhagic diathesis.
Owner:THROMBOTARGETS EURO SL
Factor XI antibodies and methods of use
PendingCN114380914AImmunoglobulins against blood coagulation factorsAntibody ingredientsAntigenAntigen Binding Fragment
The present invention relates to monoclonal antibodies and antigen-binding fragments thereof that bind to human coagulation factor XI and activated coagulation factor XI ("coagulation factor XIa"), and pharmaceutical compositions and methods of treatment comprising the same.
Owner:NOVARTIS AG
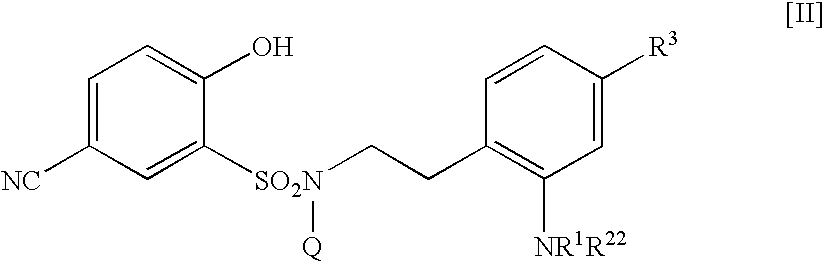
5-amidino-n-(2-aminophenethyl)-n-hydroxy-benzenesulffonamide derivative, medical composition containing the same, pharmaceutical use thereof and intermediate therefor
InactiveUS7022689B2Reduce doseAvoided and declined adverse effectAntibacterial agentsBiocideHydrogen atomChemical composition
The present invention relates to a 5-amidino-N-(2-aminophenethyl)-2-hydroxybenzenesulfonamide derivative represented by the general formula: wherein R1 is a hydrogen atom or a lower alkyl group;R2 represents a hydrogen atom, an optionally substituted lower alkyl group, etc.;R3 is a di(lower alkyl)amino group, a lower alkyl group, a cycloalkyl group, etc.;Q is a hydrogen atom or an optionally substituted lower alkyl group; andZ is a hydrogen atom or a hydroxy group, etc.,or a pharmaceutically acceptable salt thereof, which exerts a potent and selective activated blood coagulation factor X inhibitory activity and is useful as an agent for the prevention or treatment of a disease occurred associating an activated blood coagulation factor X, a pharmaceutical composition comprising the same, a pharmaceutical use thereof and an intermediate thereof.
Owner:KISSEI PHARMA
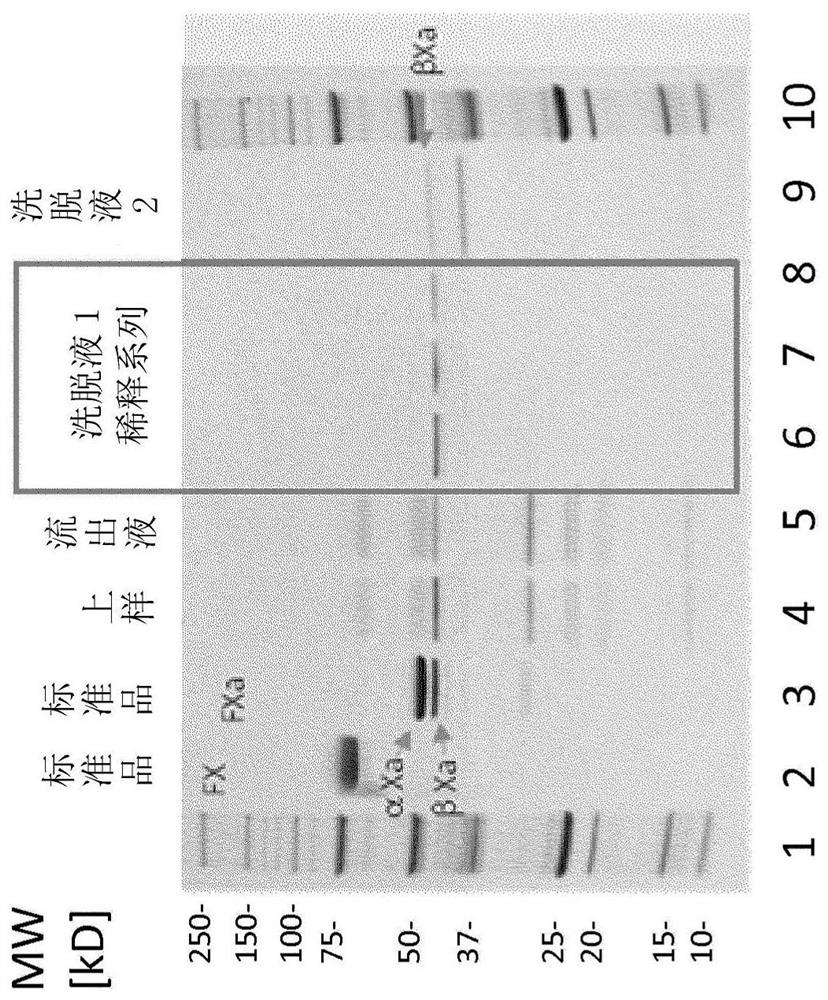
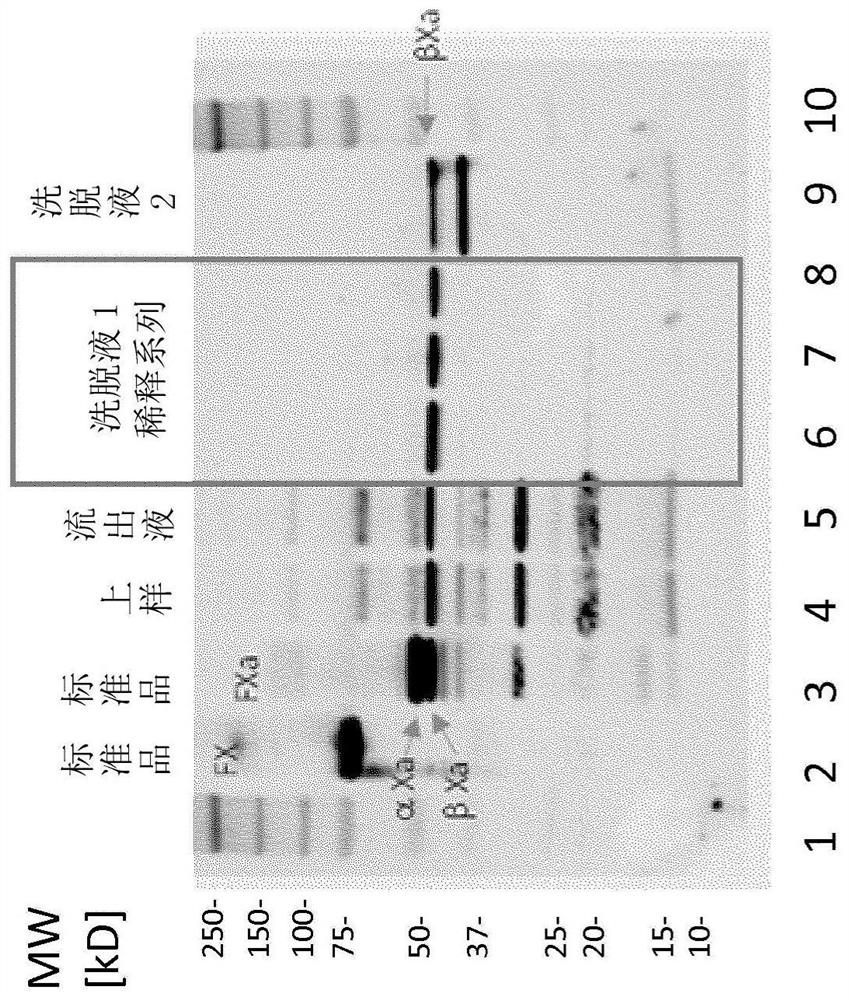
FX ACTIVATION PROCESS AND USE THEREOF IN PREPARATION OF FXa COMPOSITION
PendingCN112423782APeptide/protein ingredientsPeptide preparation methodsProtein activationCoagulation Factor Xa
The invention relates to a high purity coagulation factor Xa (FXa or activated coagulation factor X) preparation and an activation and purification process to obtain the FXa of high purity and high degree of activation without the addition of proteinaceous activators during manufacturing.
Owner:OCTAPHARMA
Method for rapidly extracting human immunoglobulin from blood plasma
ActiveCN112500477AQuality improvementHigh yieldSerum immunoglobulinsPeptide preparation methodsSpecific immunityThrombus
The invention relates to the technical field of biological products, in particular to a method for rapidly extracting human immunoglobulin from blood plasma. According to the invention, plasma is usedas a raw material, octanoic acid with a specific concentration is adopted for direct precipitation, the component reaction steps are reduced, and the final product is good in quality and high in yield and basically does not contain activated blood coagulation factor XI and other impurities, which can effectively avoid the thrombogenic risk in the process of product use, and reduce the athromboticadverse reaction of the product. The production method is simple, the process period is short, component reaction steps in a plasma low-temperature ethanol process are not needed, the immunoglobulincan be rapidly prepared, the method is particularly suitable for preparing specific immunoglobulin for sudden large-scale epidemic infection, and the specific immune antibody preparation with high efficiency and high safety can be quickly provided for patients.
Owner:GUIZHOU TAIBANG BIOLOGICAL PROD
Chimeric clotting factors
ActiveUS10202595B2High activityPeptide/protein ingredientsPharmaceutical non-active ingredientsProcoagulation ActivityClotting factor
Owner:BIOVERATIV THERAPEUTICS INC
Pharmaceutically stable hemostatic compositions
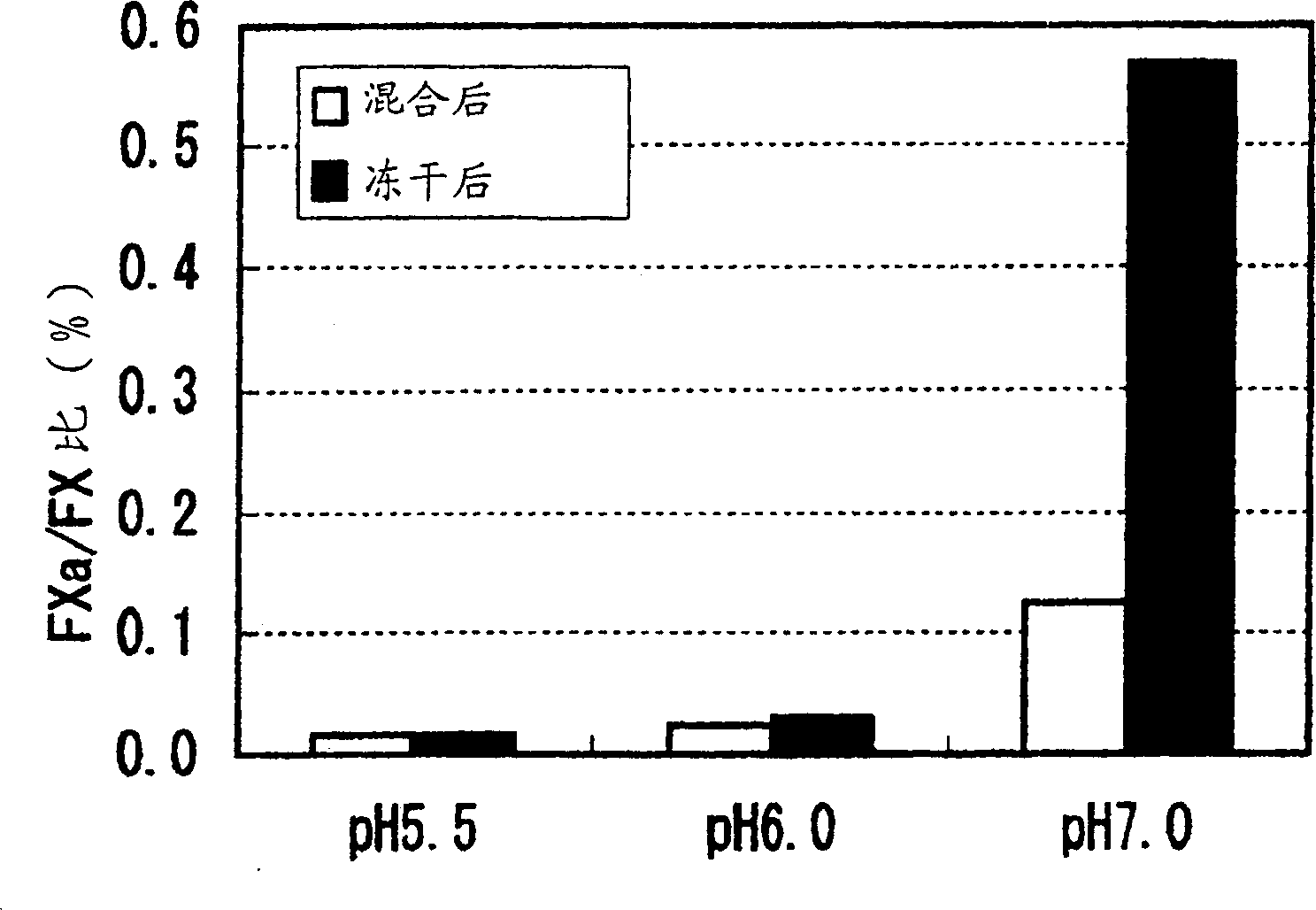
The present application provides novel hemostatic compositions or hemostatic preparations. There is provided a pharmaceutically stable hemostatic composition in solution, characterized by comprising activated coagulation factor VII (FVIIa) and coagulation factor X (FX) mixed together in a single container. The pH value of the mixed solution is maintained at 5.0-6.5.
Owner:KM BIOLOGICS CO
A kind of anti-activated blood coagulation factor V monoclonal antibody and its preparation method and application
ActiveCN108341877BHigh affinityStrong specificityImmunoglobulins against blood coagulation factorsBiological material analysisAntiendomysial antibodiesHeavy chain
The invention discloses an anti-activated coagulation factor V monoclonal antibody, which is characterized in that the amino acid sequence of its light chain is shown in SEQ ID NO: 3, and the amino acid sequence of its heavy chain is shown in SEQ ID NO: 4. The invention also discloses the preparation method and application of the aforementioned monoclonal monomer. The anti-activated blood coagulation factor V monoclonal antibody of the invention has strong binding ability to activated blood coagulation factor V, and can be used for detection of activated blood coagulation factor V.
Owner:吴剑波 +1
Novel benzamidine compound
InactiveUS20100105731A2Prevent thrombosisBiocideOrganic chemistryBlood Coagulation Factor XBenzamidine
Owner:AJINOMOTO CO INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com