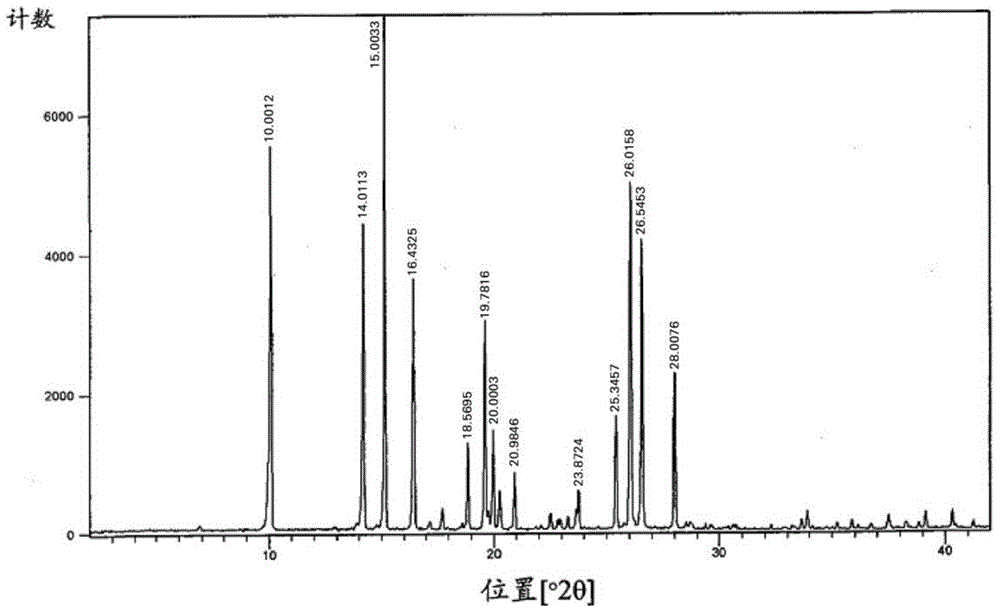
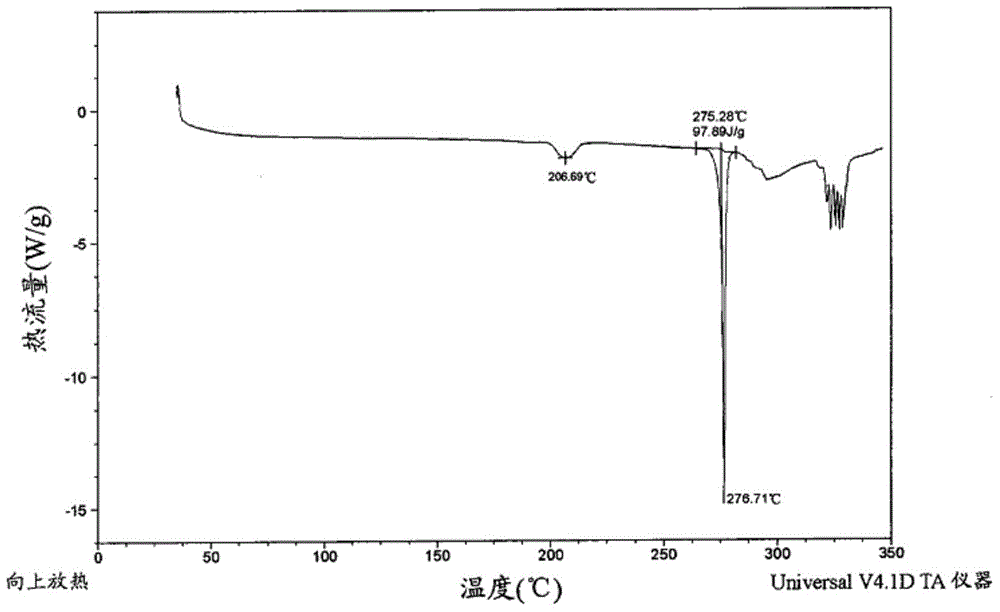
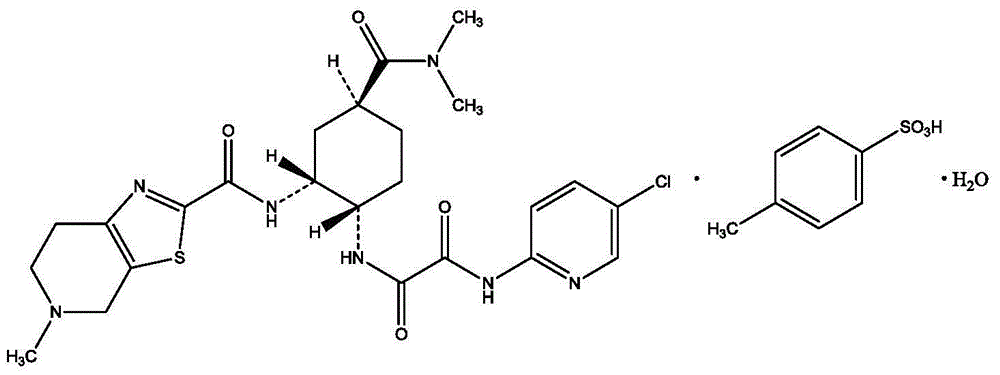
Edoxaban tosilate hydrate
A technology of p-toluenesulfonic acid and edoxaban, applied in organic chemistry, drug combination, blood diseases, etc., can solve the problem of weak inhibitory activity and achieve the effect of low impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0022] The formulation of embodiment 2-quality research and quality standard of compound of the present invention
[0023] The specific degradation impurities contained in edoxaban p-toluenesulfonate are impurities L, M, N, O. Among them, impurities L and M are hydrolysis products of edoxaban, which are easier to produce under alkaline conditions; impurities N and O are oxidation products.
[0024] According to the results of influencing factor tests and stability tests, the main impurities produced by self-produced and commercially available products during the storage process are oxidized impurities N and O, which will increase under high temperature and high humidity conditions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com