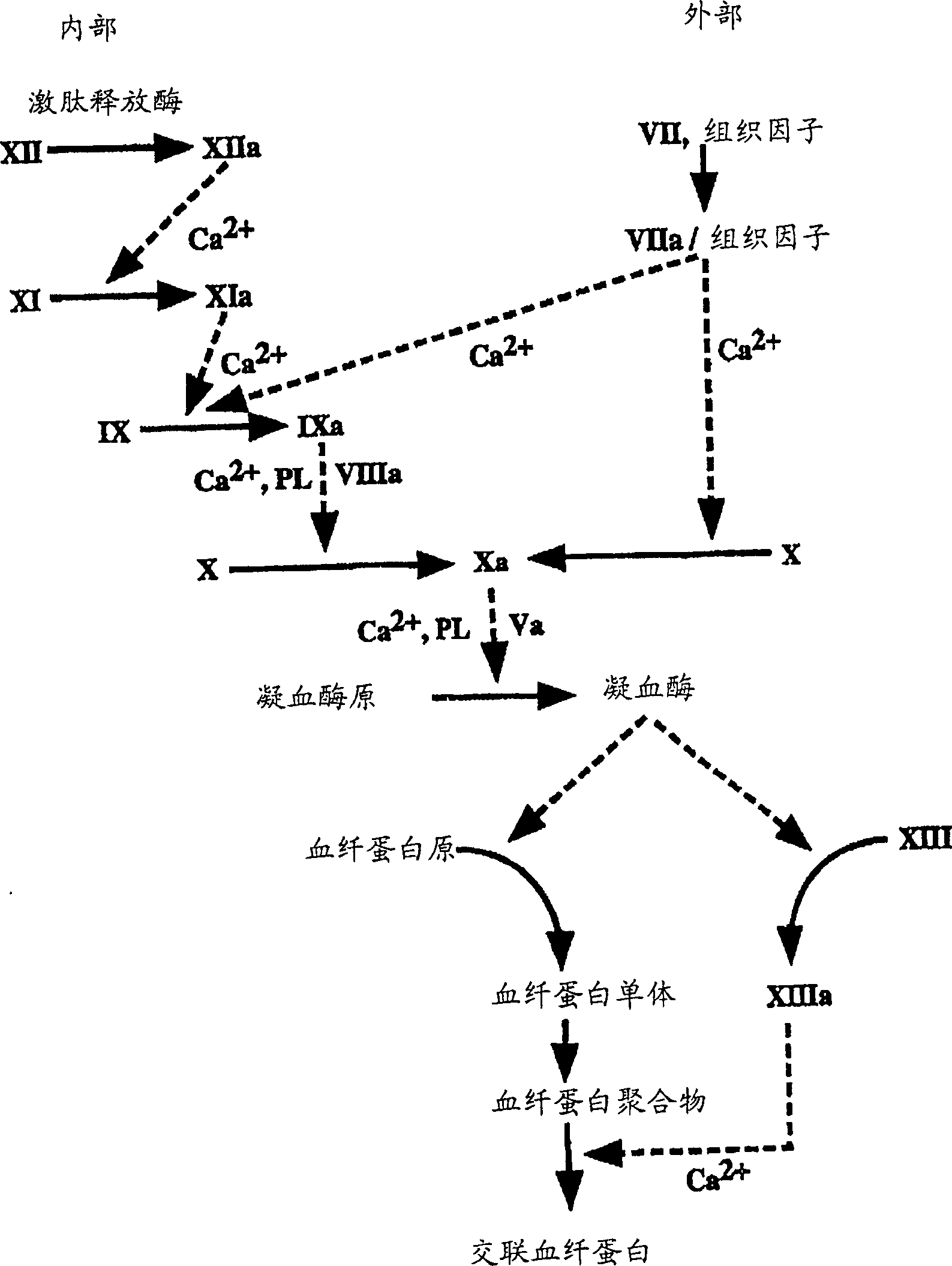
Pharmaceutically stable hemostatic compositions
A composition and stable technology, applied in the field of medicine, can solve the problems of unsatisfactory safety, unsatisfactory efficacy and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
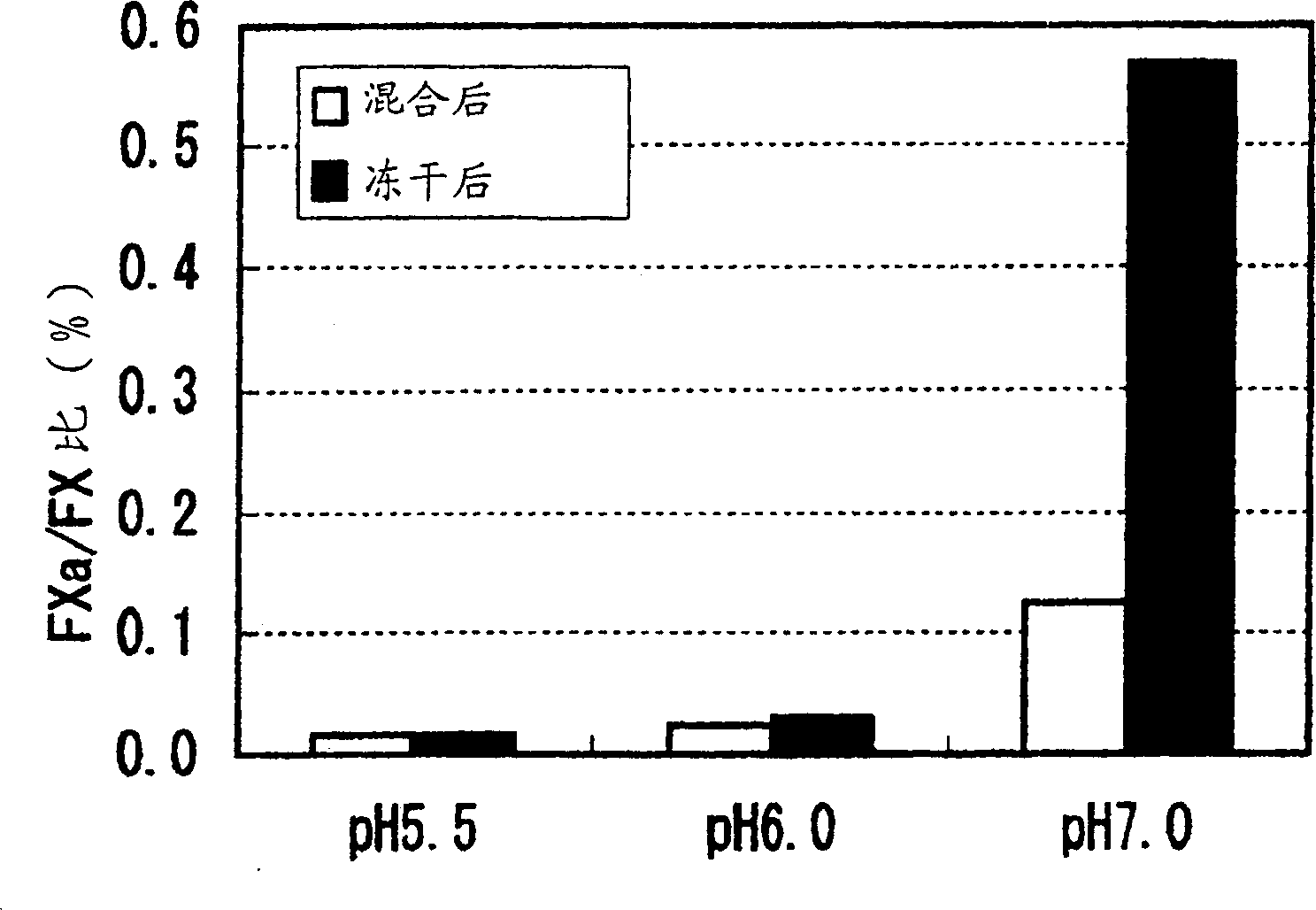
[0024] In order to study the stability of the FVIIa / FX mixture in the buffer solution, the buffer solution (without CaCl 2 MES buffer: 100mM MES, 100mM NaCl) 0.4mg / mL FVIIa and 1.0mg / mL FX were mixed together, and the mixture was incubated at 37°C. The respective activities of FVIIa, FX, and FXa in the sample are measured at each prescribed time, and the measurement is performed in a system in which the aforementioned factors do not affect each other. FVIIa used here is a blood product prepared as described in Japanese Patent Publication 155797 / 1991.
[0025] As a result, after 24 hours of incubation at each pH of the buffer tested, both FVIIa and FX retained greater than 90% of their activity. The FXa content is calculated based on its specific hydrolysis activity to a synthetic substrate (S2222). The molar ratio of FXa content to FX is figure 2 Shown. No increase in FXa content was observed in pH 5.6 and 6.0 buffers, while a significant increase in FXa content was measured in p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com