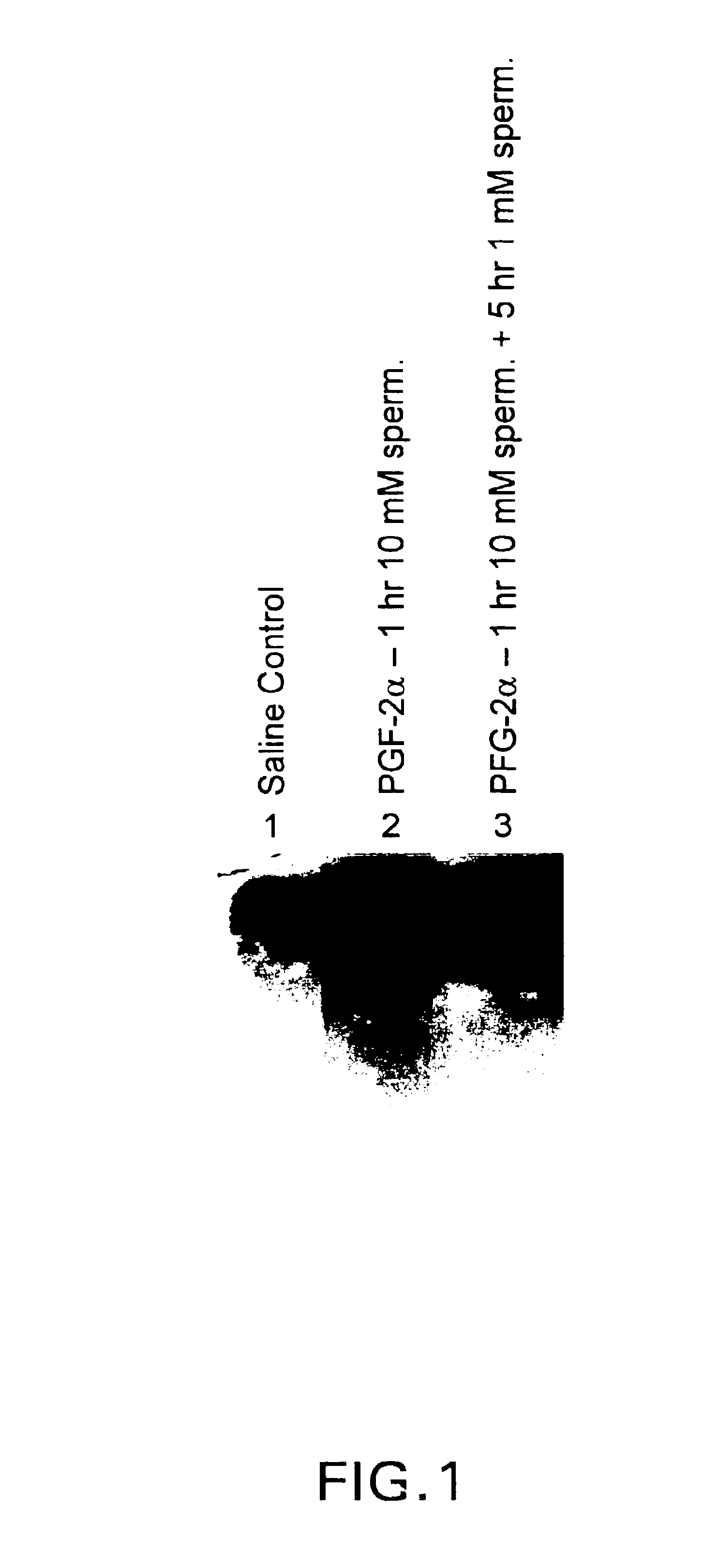
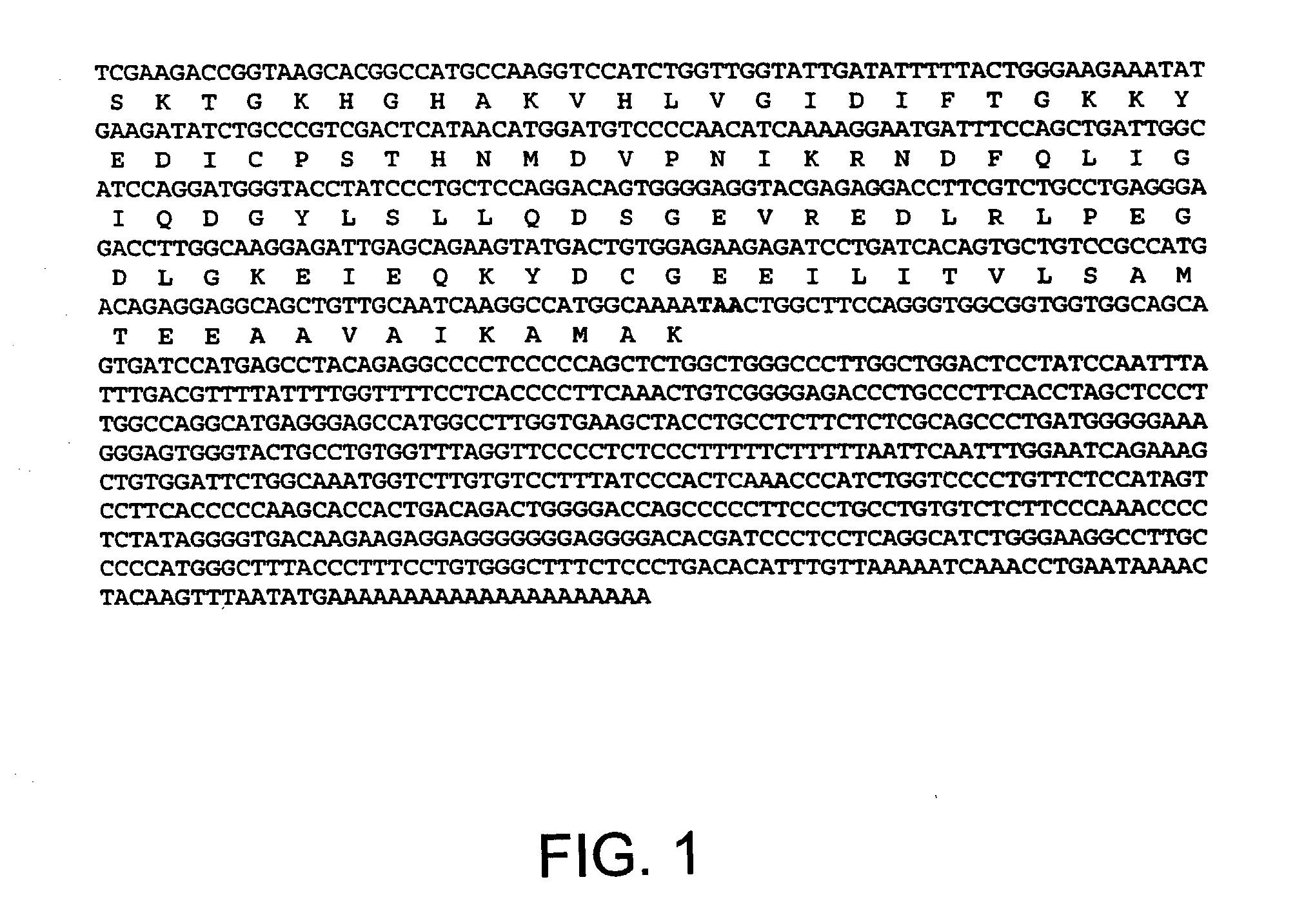
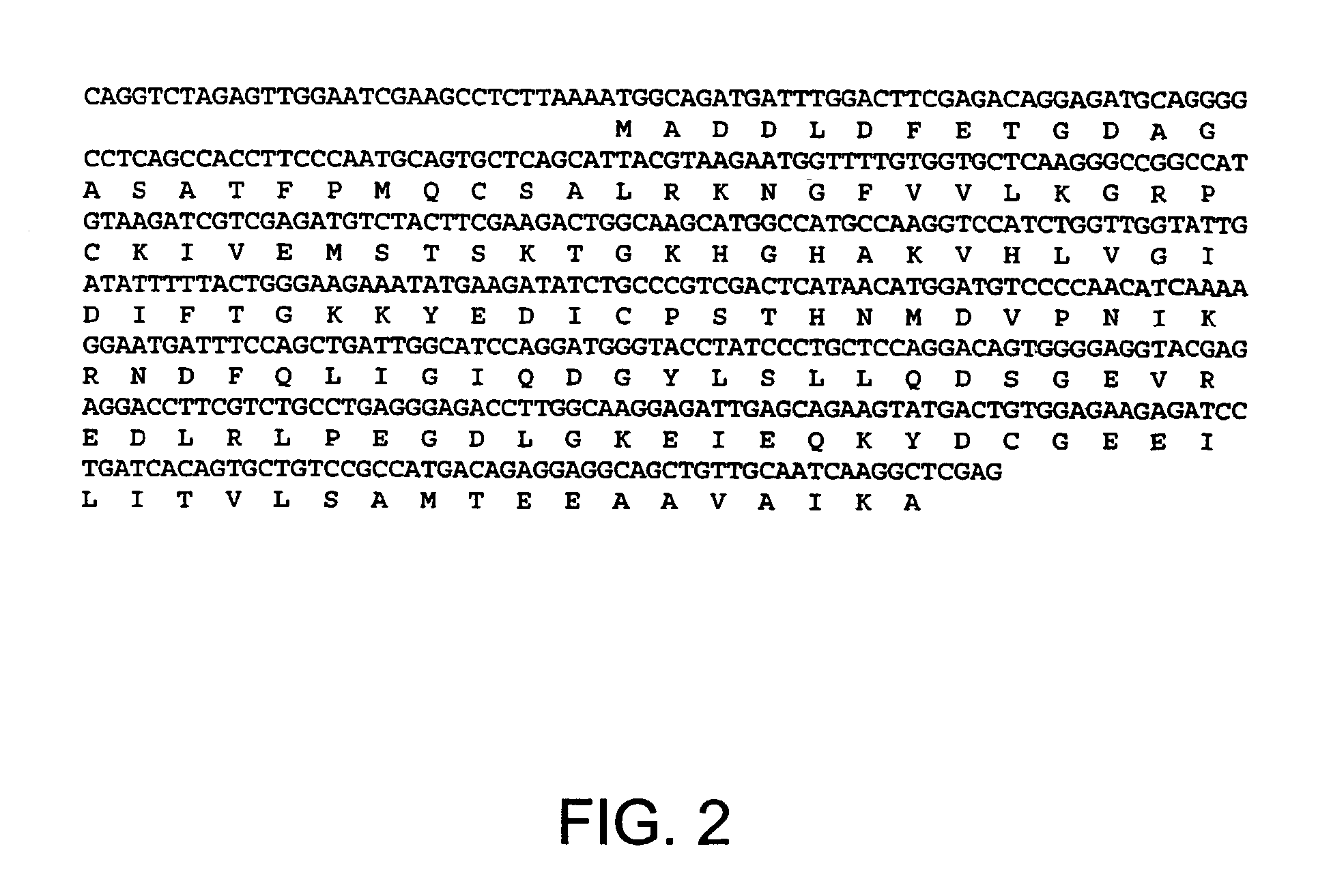
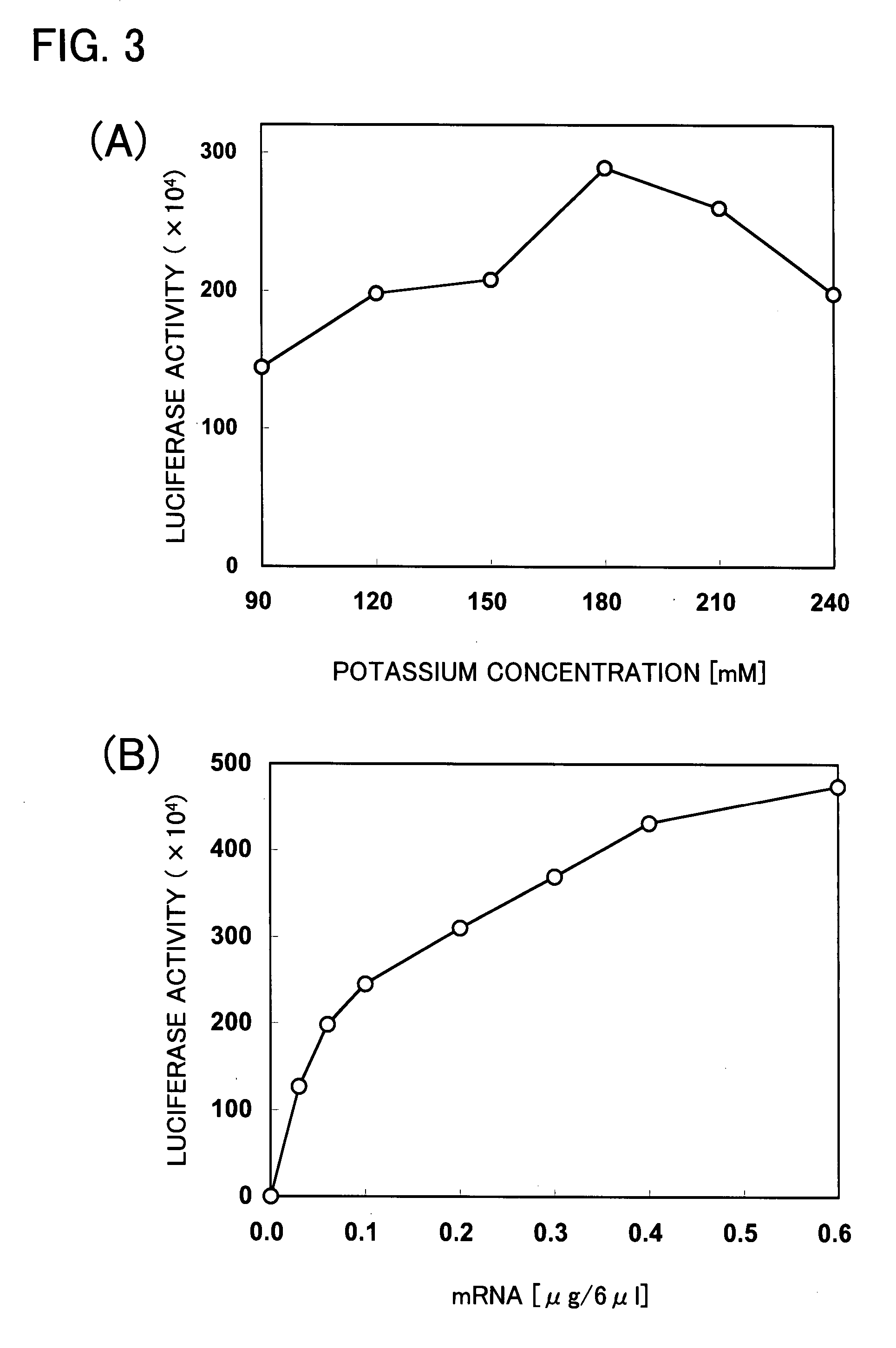
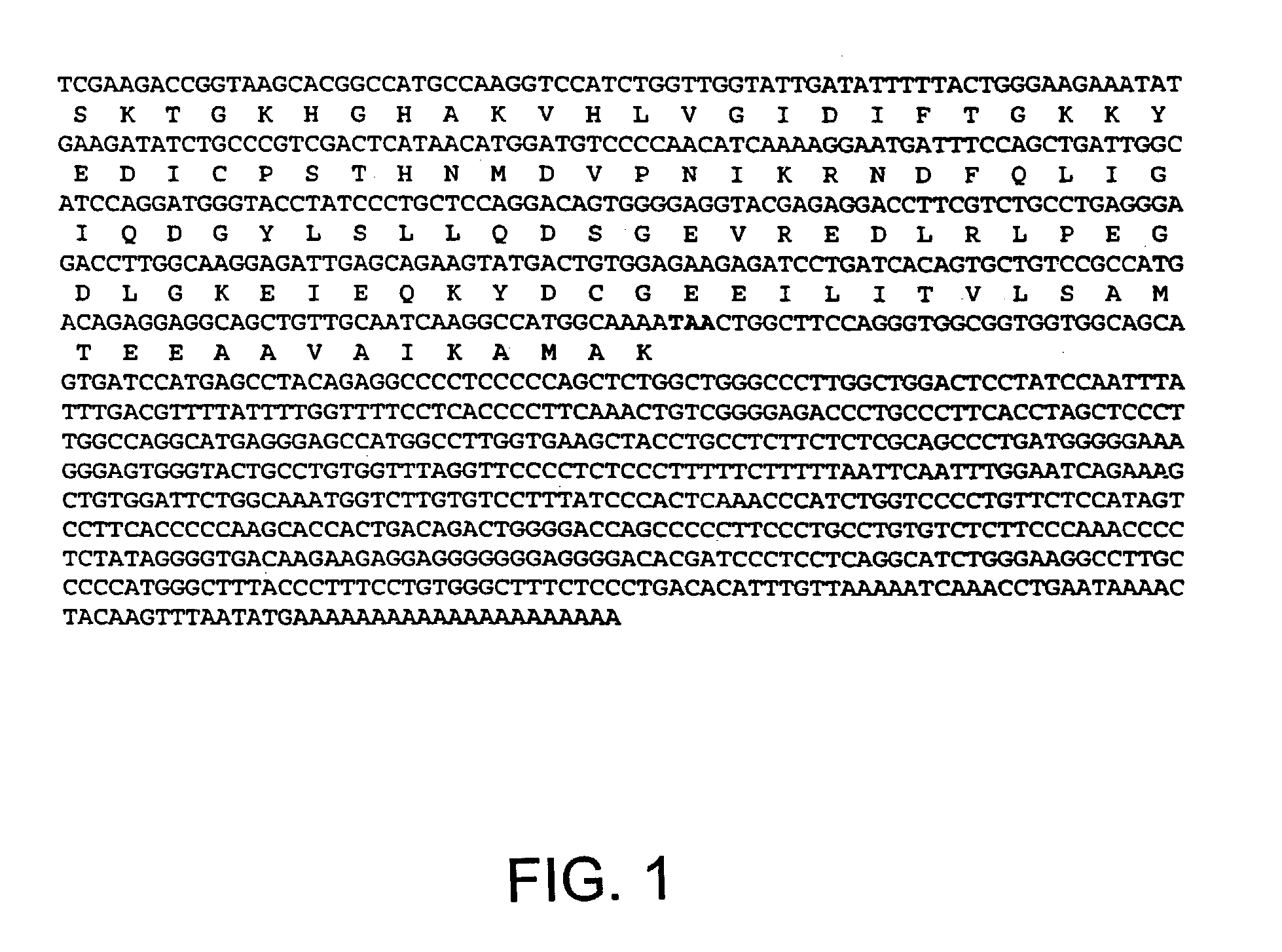
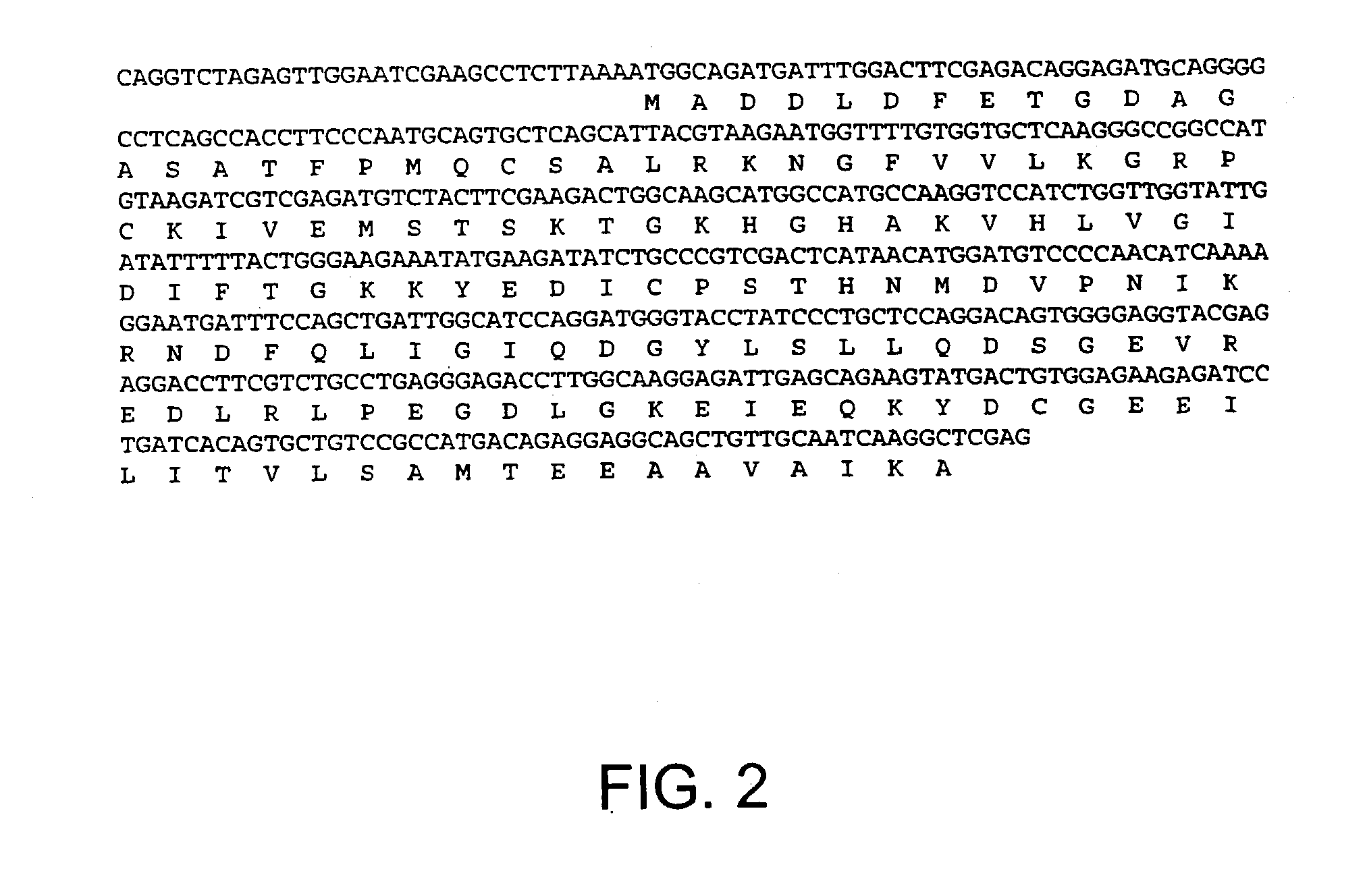
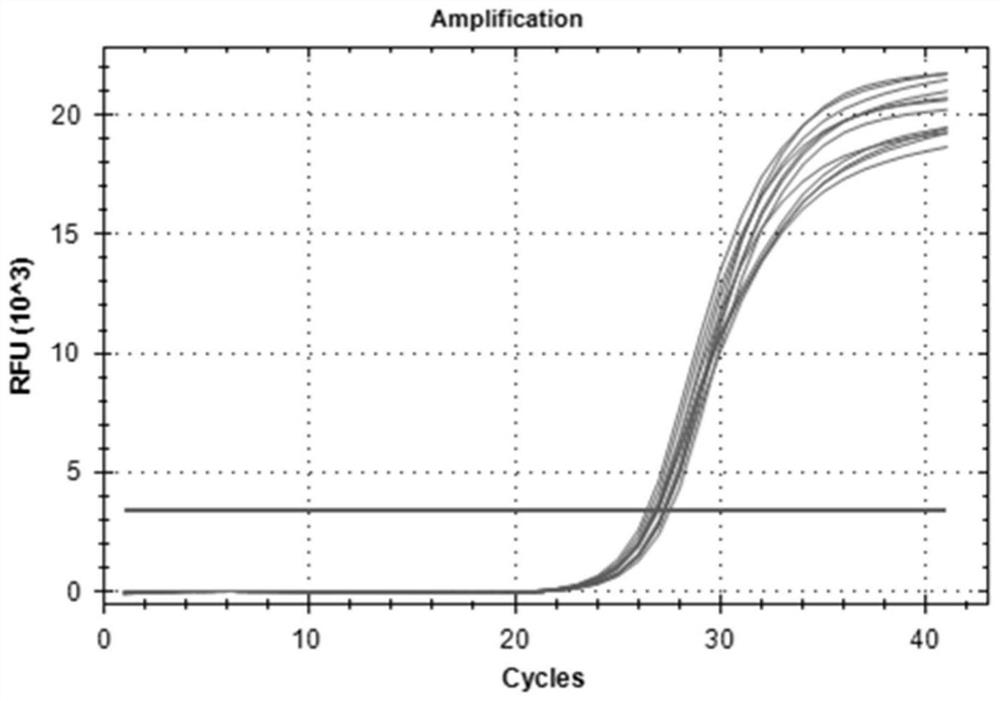
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
72 results about "Initiation factor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Initiation factors are proteins that bind to the small subunit of the ribosome during the initiation of translation, a part of protein biosynthesis. Initiation factors can interact with repressors to slow down or prevent translation or they can interact with activators to help them start or increase the rate of translation. In bacteria they are simply called Ifs (i.e.., IF1, IF2, & IF3) and in eukaryotes they are known as eIFs (i.e.., eIF1, eIF2, eIF3). In eukaryotic transcription these are also called the general transcription factors, or σ factors...
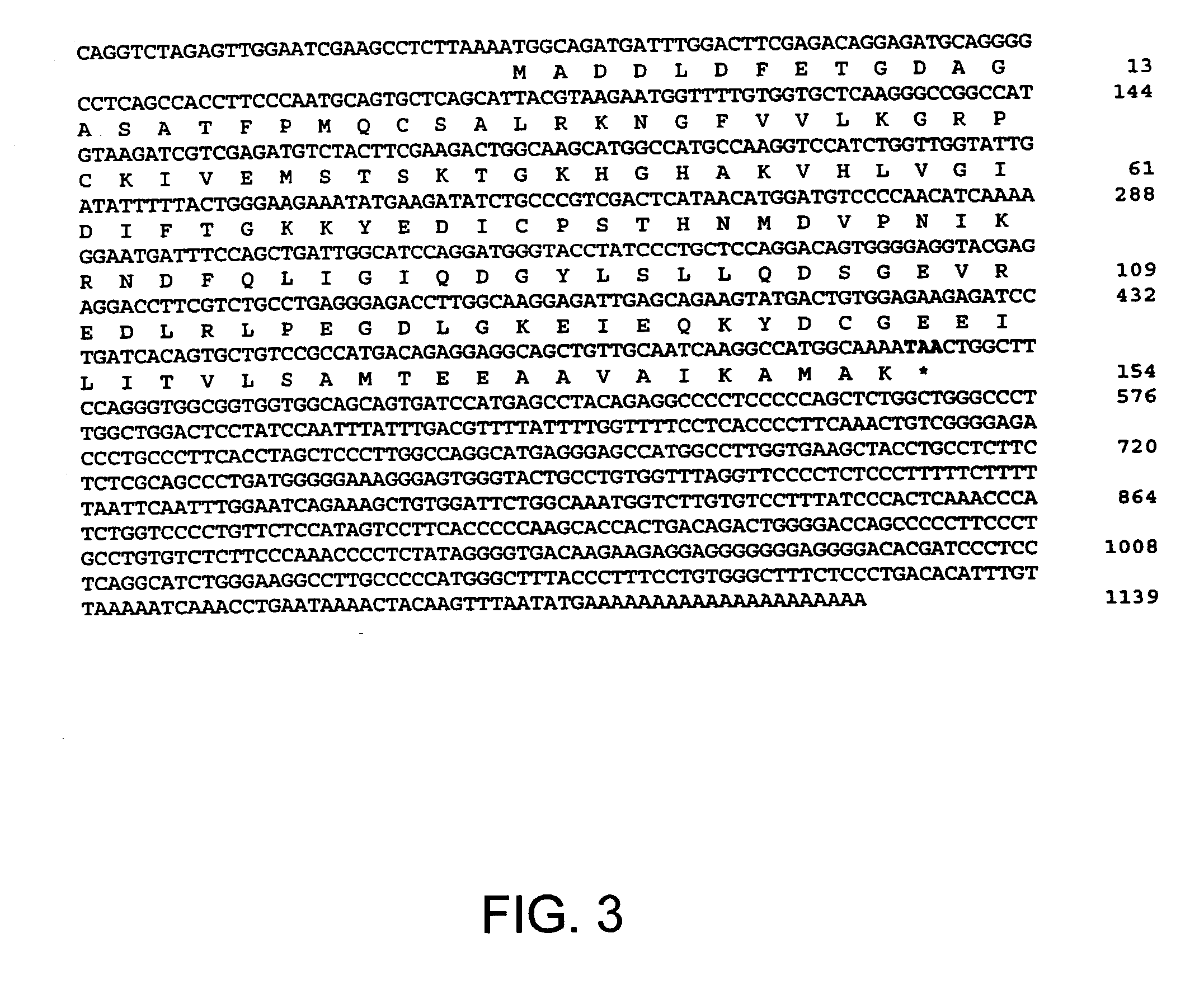
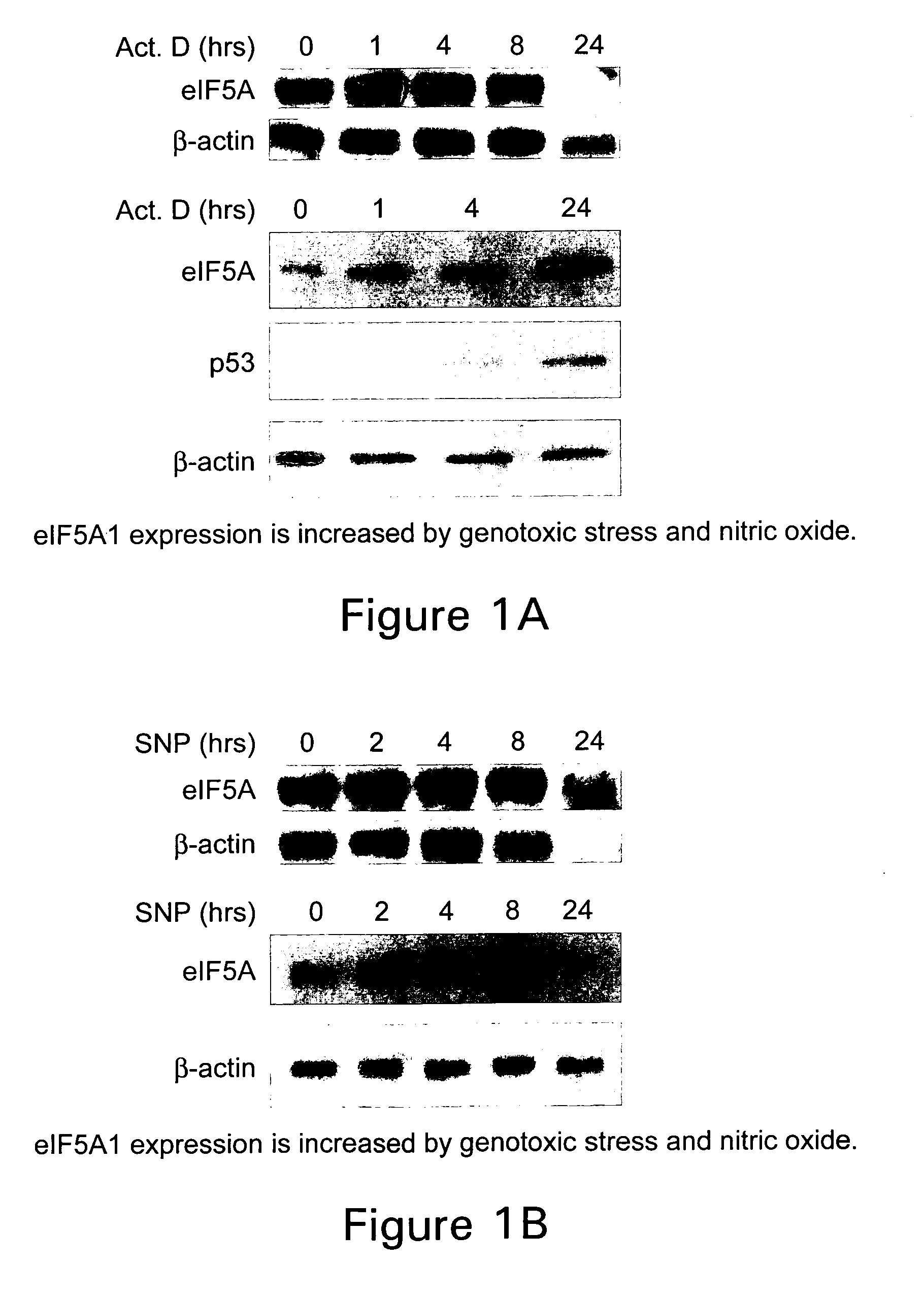
DNA encoding apoptosis-induced eucaryotic initiation factor-5A and deoxyhypusine synthase and a method for controlling apoptosis in animals and humans
InactiveUS6867237B1Reduce the amount requiredReduce translationBiocideLaser detailsAntisense OrientationInitiation factor
Genes encoding an apoptosis-induced eukaryotic initiation Factor-5A (eIF-5A) and an apoptosis-induced deoxyhypusine synthase (DHS), whose expressions are induced by the onset of apoptosis, are identified. The DHS gene and the eIF-5A gene, alone or in combination, as well as inhibitors of the DHS reaction, are used to modulate apoptosis in animals and humans. For example, modulation of apoptosis in cells of animals and humans is achieved by introduction of a gene or gene fragment encoding apoptosis-induced eIF-5A, apoptosis-induced DHS, or both into the animal or human cell in antisense orientation.
Owner:SENESCO TECHNOLOGIES INC
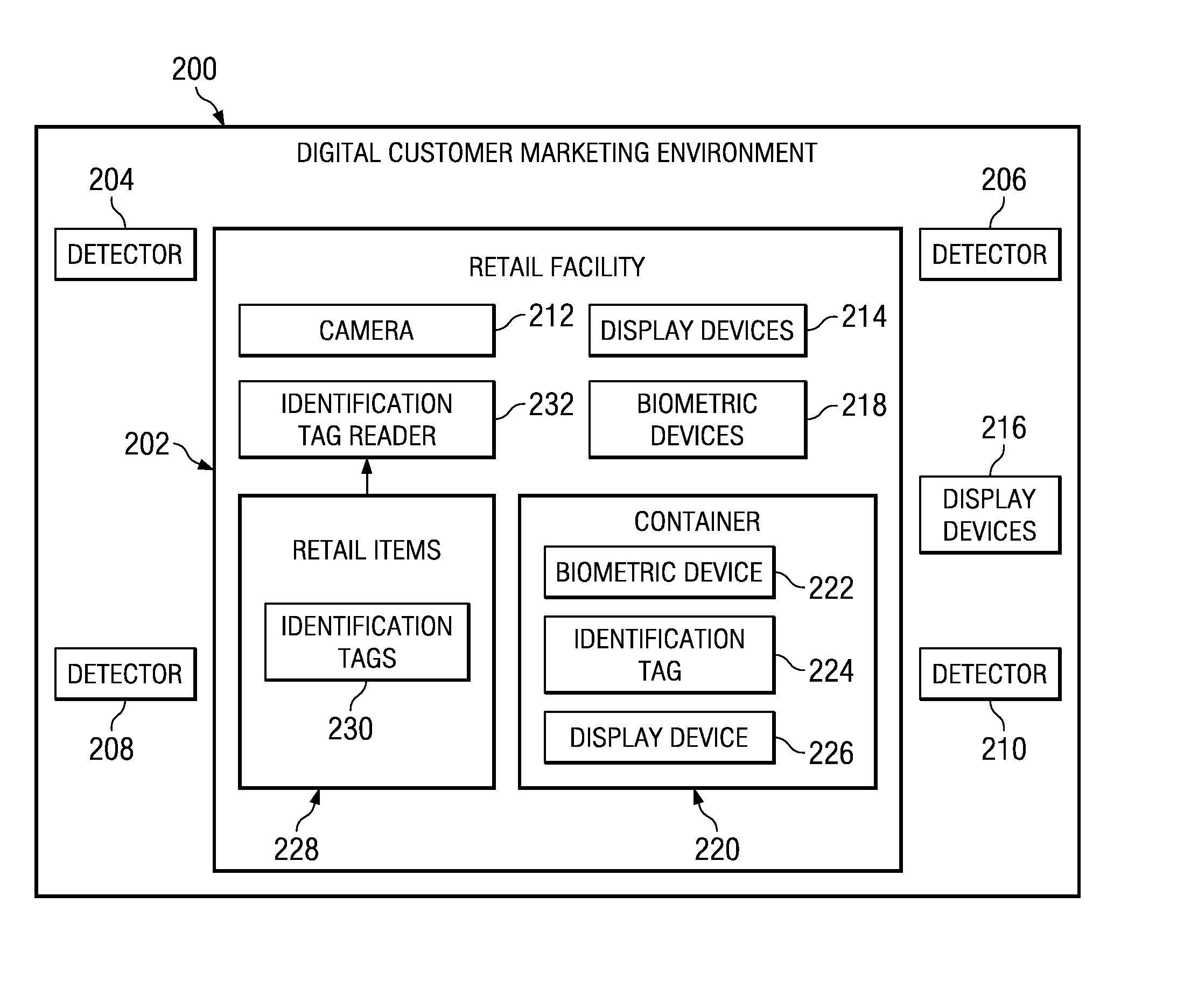
Preferred customer marketing delivery based on dynamic data for a customer
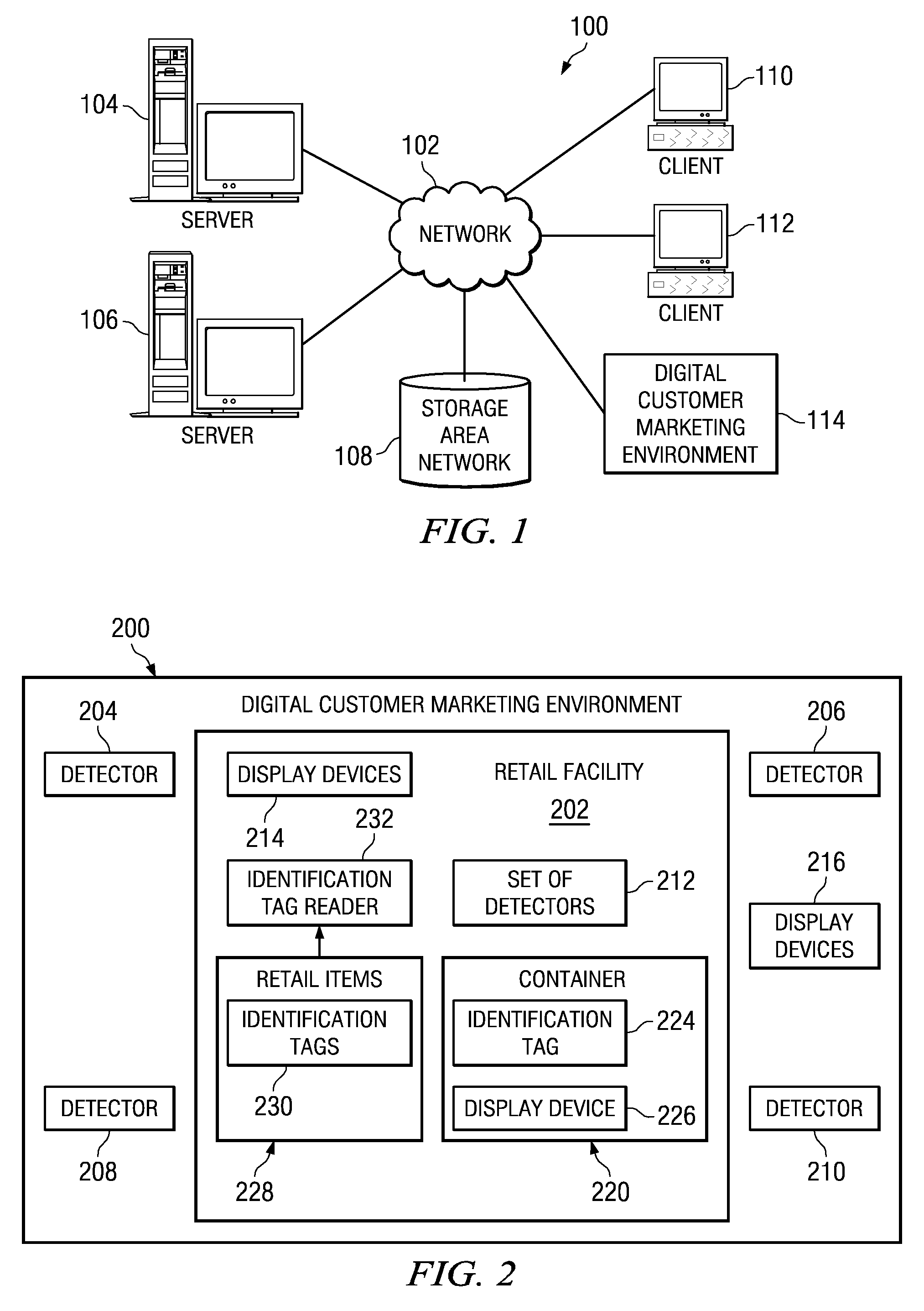
A computer implemented method, apparatus, and computer usable program product for automatically determining a marketing status for a customer. In one embodiment, detection data is received from a set of detectors associated with the retail facility. The detection data is processed to form dynamic data. The dynamic data is analyzed using a set of data models to identify marketing initiation factors. In response to the marketing initiation factors indicating initiation of marketing to the customer, a customized marketing message is generated for the customer and dynamically transmitted to a display device associated with the customer. The customized marketing message is displayed to the customer in real-time as the customer is shopping at the retail facility.
Owner:TERRACE LICENSING LLC
Method and apparatus for preferred customer marketing delivery based on dynamic data for a customer
InactiveUS20080249868A1AdvertisementsBuying/selling/leasing transactionsInitiation factorDisplay device
A computer implemented method, apparatus, and computer usable program product for automatically determining a marketing status for a customer. In one embodiment, detection data is received from a set of detectors associated with the retail facility. The detection data is processed to form dynamic data. The dynamic data is analyzed using a set of data models to identify marketing initiation factors. In response to the marketing initiation factors indicating initiation of marketing to the customer, a customized marketing message is generated for the customer and dynamically transmitted to a display device associated with the customer. The customized marketing message is displayed to the customer in real-time as the customer is shopping at the retail facility.
Owner:TERRACE LICENSING LLC
Nucleic acids, polypeptides, and methods for modulating apoptosis
InactiveUS20030050272A1Sure easyReduce the amount requiredAntibacterial agentsOrganic active ingredientsInitiation factorDeoxyhypusine synthase
The present invention relates to isolated and / or purified rat apoptosis-specific eucaryotic initiation Factor-5A (eIF-5A) and deoxyhypusine synthase (DHS) nucleic acids and polypeptides. The present invention also relates to methods of modulating apoptosis using apoptosis-specific eIF-5A and DHS, and antisense oligonucleotides and expression vectors of apoptosis-specific eIF-5A and DHS useful in such methods.
Owner:SENESCO TECHNOLOGIES INC
Method and apparatus for preferred customer marketing delivery based on biometric data for a customer
A computer implemented method, apparatus, and computer usable program product for automatically determining a marketing status for a customer. Biometric readings for the customer are received from a set of biometric devices associated with a retail facility to form biometric data describing a set of physiological responses of the customer. The biometric data is analyzed to identify a set of marketing initiation factors that indicate a degree of receptivity of the customer to marketing messages. In response to the set of marketing initiation factors indicating initiation of marketing to the customer, a customized marketing message is generated for the customer. The customized marketing message is transmitted to a display device for display to the customer in real-time as the customer is shopping.
Owner:TERRACE LICENSING LLC
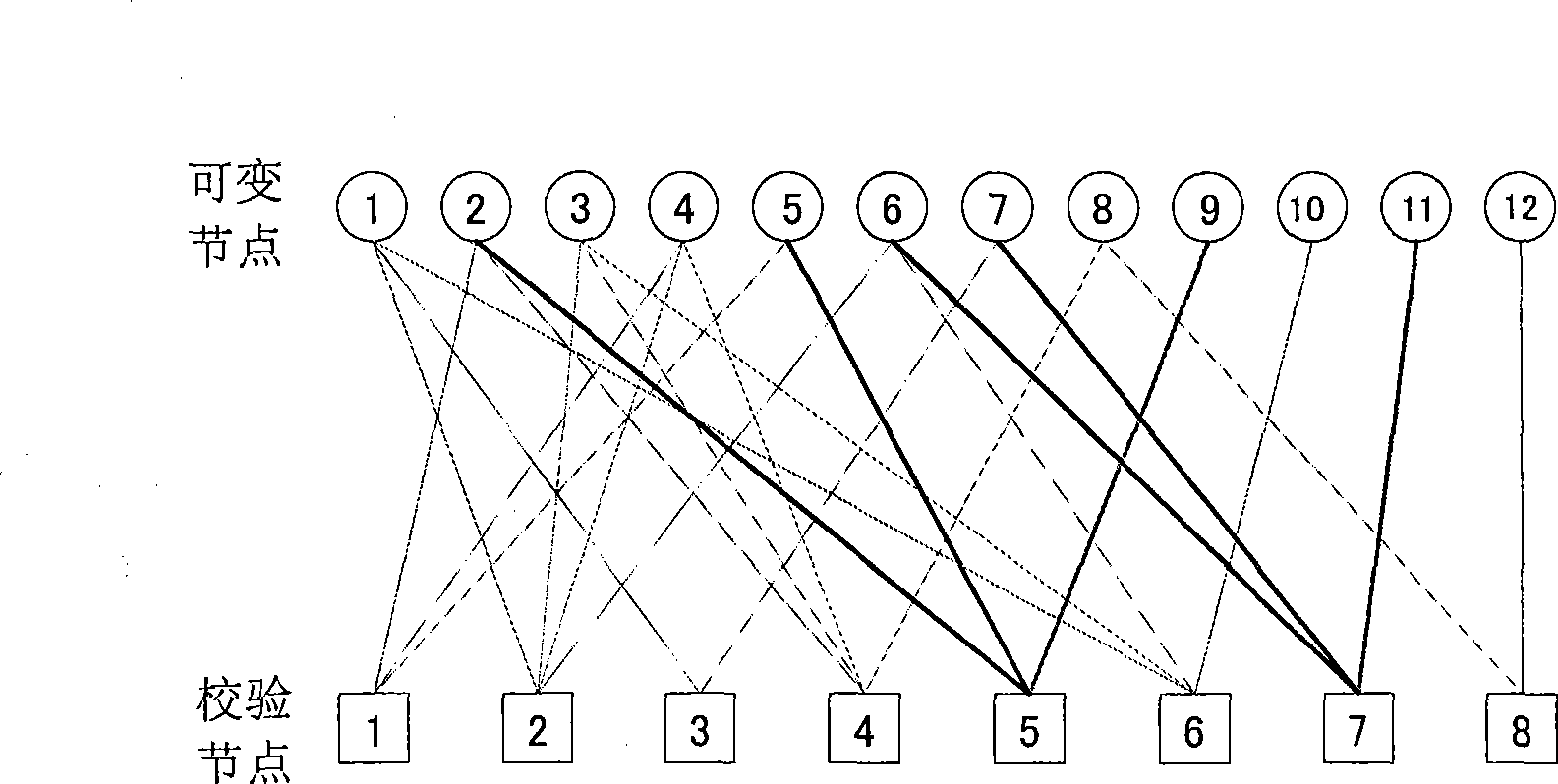
Method for improving code parallelism degree and implementing coding delay
InactiveCN101431337AError correction/detection using multiple parity bitsCode conversionInitiation factorLeft direction
The invention discloses a coding delay method for reducing double diagonal quasi-cyclic low density parity check code, comprising the steps: based on the set extended code rate of 1 / k, the double diagonal of basis matrix at the m line and n row can be extended along the direction of the double diagonal to form an expended matrix of the double diagonal structure, wherein, k is equal to 3, 4, 5...k0, and 1 / k0 is the minimum code rate; a first nonzero element of the i*m+1line check part is moved towards left direction to (n-m+l) line along the i*m+1line, wherein, i is equal to 1, 2, ..., k0-1; a first check relation is taken as initiation factor to calculate check bit of n-m+l row; by utilizing the check relation of moving towards left direction to (n-m+l) line, a plurality of groups of check bits are calculated in parallel by a mode of recursive coding.
Owner:PANASONIC CORP
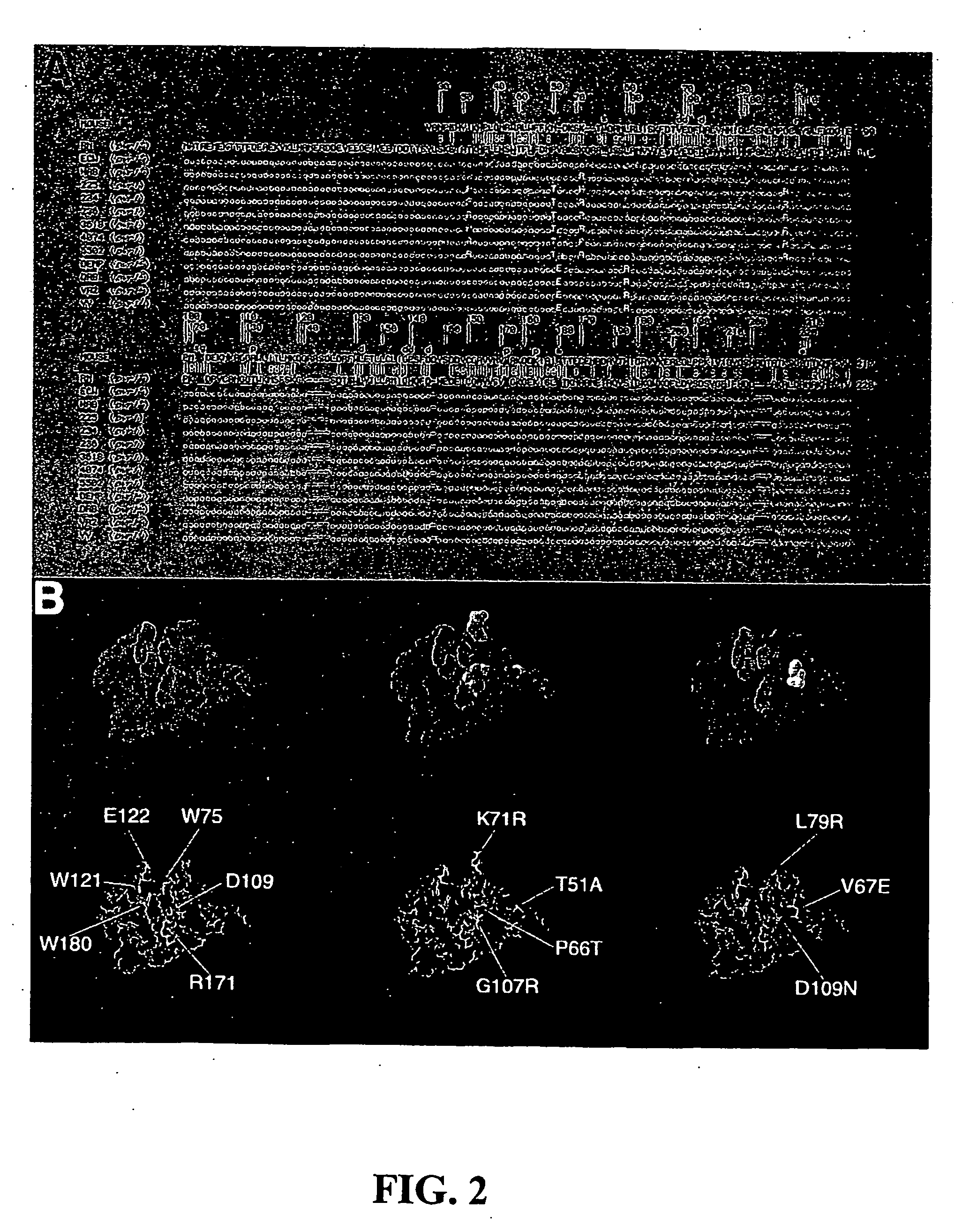
Recessive plant viral resistance results from mutations in translation initiation factor eIf4e
InactiveUS20060294618A1Good antiviral effectBacteriaOther foreign material introduction processesHeterologousInitiation factor
The present invention relates to methods of imparting virus resistance to plants. In one aspect, this method involves silencing a gene encoding a translation initiation factor eIF4E in the plant. In another aspect, this method involves overexpressing a heterologous translation initation factor eIF4E in a plant. The present invention further relates to a genetic construct containing a nucleic acid molecule encoding a heterologous translation initiation factor eIF4E, as well as to an expression system containing the genetic construct and a host cell transformed with the genetic construct. The present invention also relates to transgenic plants, seeds, and plant parts transformed with the genetic construct. The present invention also relates to an isolated nucleic acid molecule encoding a mutant translation initiation factor eIF4E that is effective in imparting virus resistance in plants. The present invention also relates to a mutant translation initiation factor eIF4E and a method for making the mutant.
Owner:CORNELL RES FOUNDATION INC
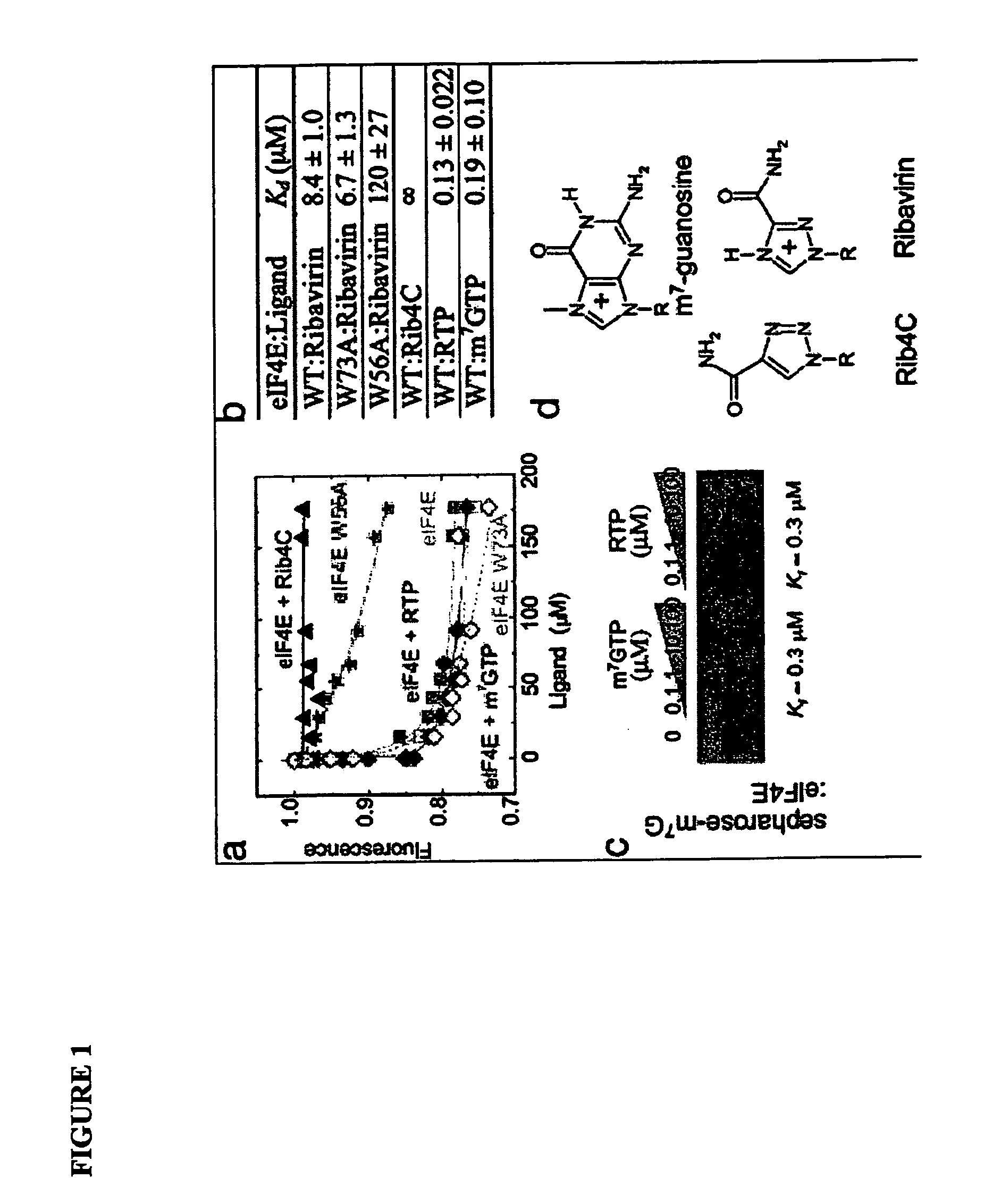
Translational Dysfunction Based Therapeutics
Provided are methods and compositions for inhibiting eukaryotic translation initiation factor eIF4E. Such methods and compositions may be used alone or in conjunction with other therapies, such as gene therapies, for inhibiting cell proliferation and / or treating cancer.
Owner:TRANSLATIONAL THERAPEUTICS
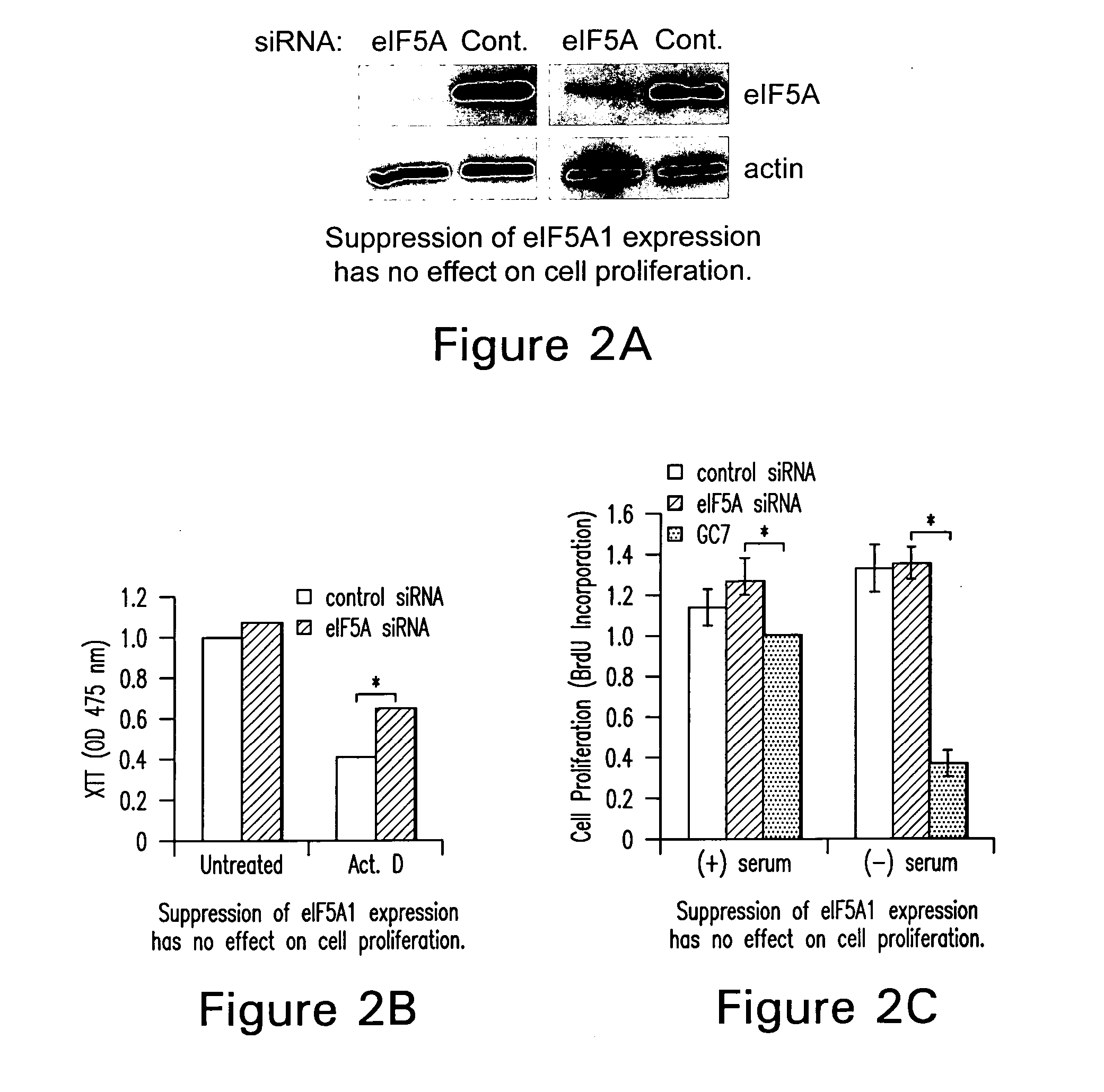
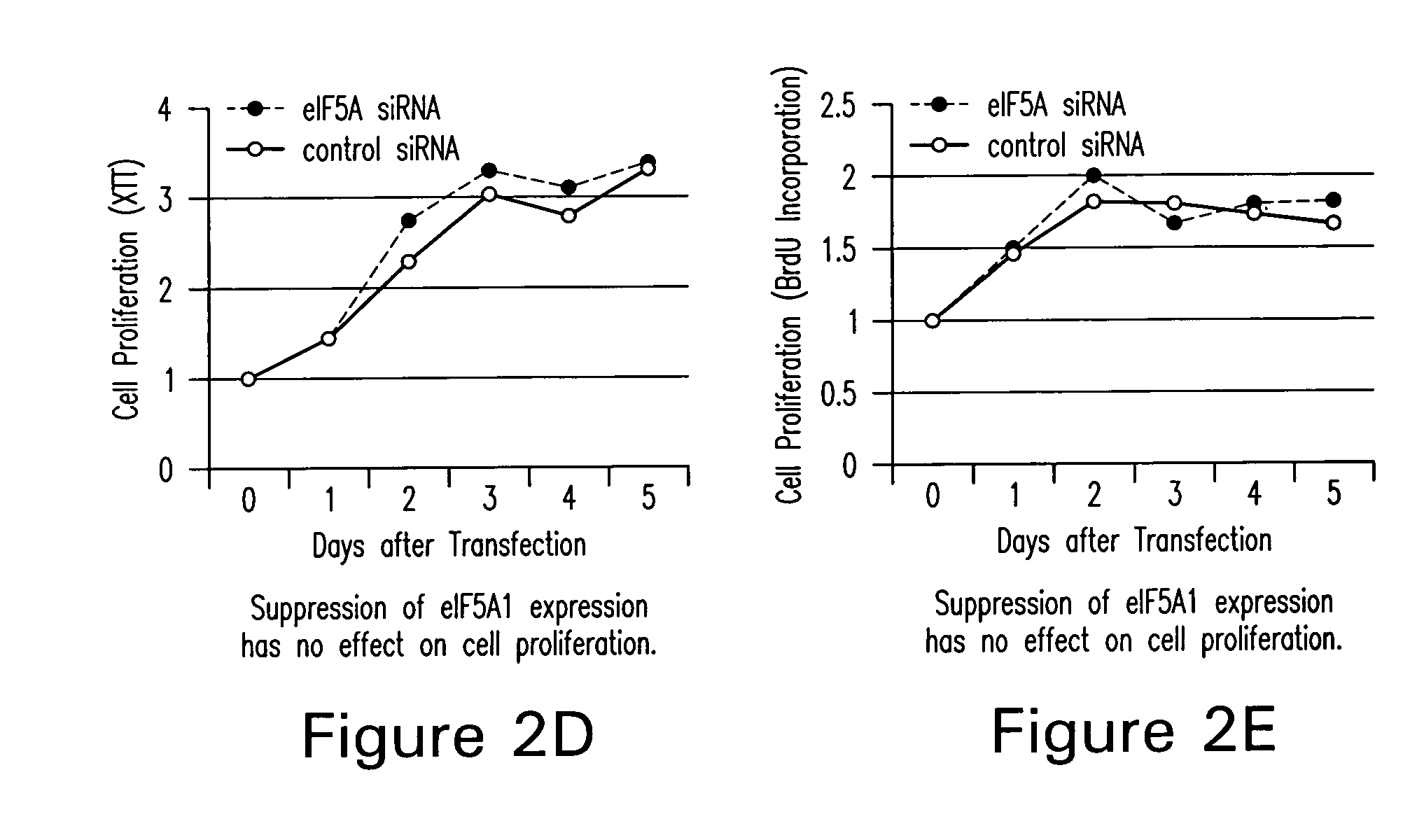
Inhibition of apoptosis-specific eIF-5A ("eIF-5A1") with antisense oligonucleotides and siRNA as anti-inflammatory therapeutics
InactiveUS20060094677A1Suppressing and inhibiting expressionIncreasing Bcl- expressionSenses disorderNervous disorderInitiation factorApoptosis
The present invention relates to apoptosis specific eucaryotic initiation factor 5A (eIF-5A), referred to as apoptosis-specific eIF-5A or eIF5A1, nucleic acids and polypeptides and methods for inhibiting or suppressing apoptosis in cells using antisense nucleotides or siRNAs to inhibit expression of apoptosis-specific eIF-5A. The invention also relates to suppressing or inhibiting expression of pro-inflammatory cytokines or inhibiting activation of NFKB by inhibiting expression of apoptosis-specific eIF-5A.
Owner:SENESCO TECHNOLOGIES INC
Use of eIF-5A to kill multiple myeloma cells
InactiveUS20070154457A1Shrink tumorSlow tumor growthBiocideOrganic active ingredientsInitiation factorLymphatic Spread
The present invention relates to eucaryotic initiation factor 5A and the use of polynucleotides encoding the same to inhibit cancer cell growth and inhibit metastases. In a preferred embodiment, eIF-5A1 is used to kill multiple myeloma cells.
Owner:SENESCO TECHNOLOGIES INC
Use of apoptosis-specific eIF-5A siRNAs and antisense polynucleotides to inhibit/suppress an inflammatory response
InactiveUS20060154887A1Suppressing and inhibiting expressionIncreasing Bcl- expressionSenses disorderNervous disorderInitiation factorApoptosis
The present invention relates to apoptosis specific eucaryotic initiation factor 5A (eIF-5A), referred to as apoptosis-specific eIF-5A or eIF5-A1, nucleic acids and polypeptides and methods for inhibiting or suppressing apoptosis in cells using antisense nucleotides or siRNAs to inhibit expression of apoptosis-specific eIF-5A. The invention also relates to suppressing or inhibiting expression of pro-inflammatory cytokines or inhibiting activation of NFkB by inhibiting expression of apoptosis-specific eIF-5A.
Owner:SENESCO TECHNOLOGIES INC
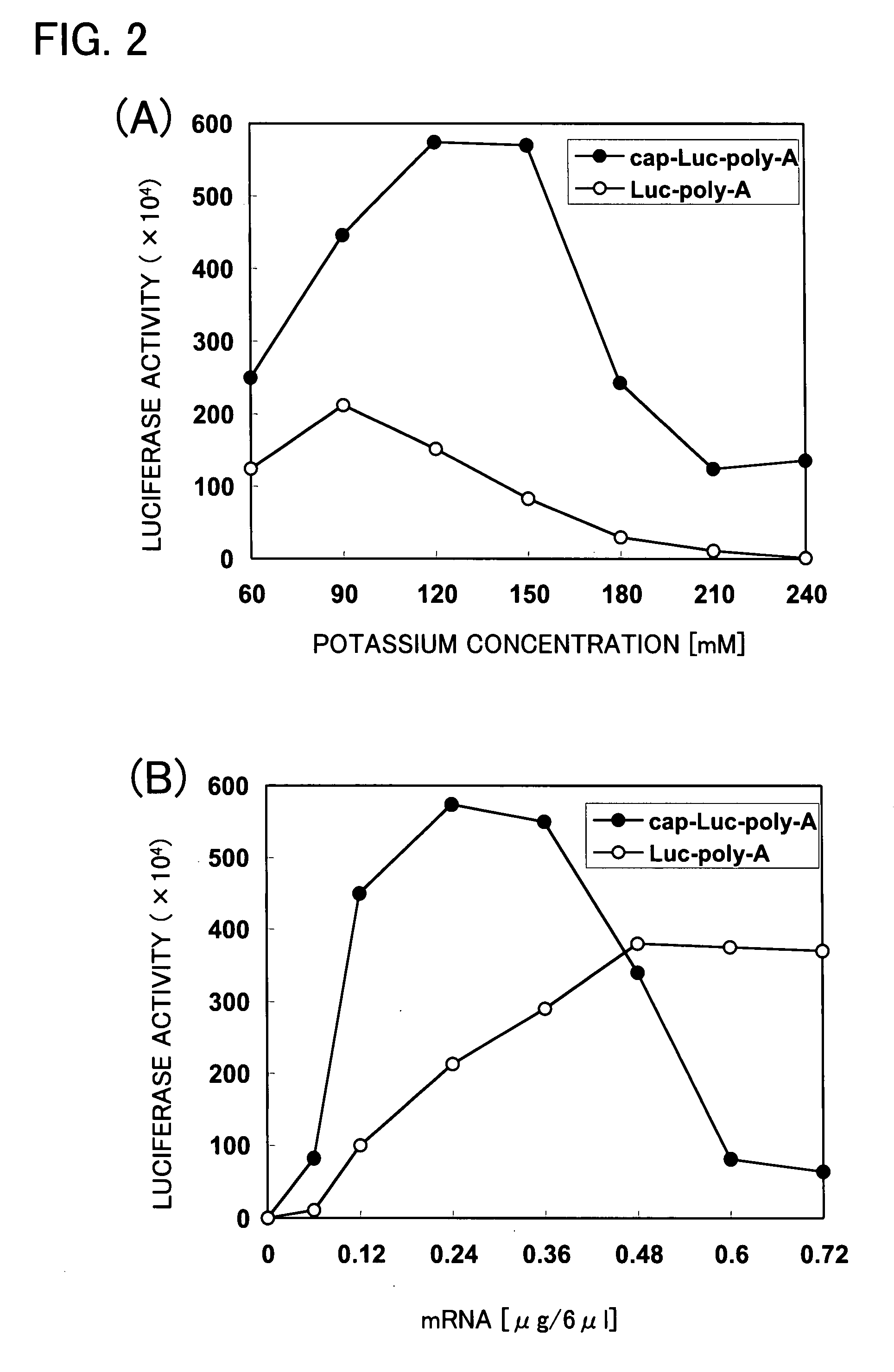
Cell-free system for synthesis of proteins derived from cultured mammalian cells
InactiveUS20070281337A1High synthetic activityLow costVertebrate cellsPeptidesBiotechnologyInitiation factor
Prepared is an extract composition having an improved protein synthetic activity in a cell-free protein synthesis system using a mammalian cultured cell extract. An eukaryotic translation initiation factor and / or translational regulator are added to a cell-free protein synthesis system comprising an extract prepared from cultured mammalian cells and a template mRNA. These factors are one or more selected from the group consisting of eukaryotic translation initiation factors 4E (eIF4E), 2 (eIF2) and 2B (eIF2B), and eukaryotic translational regulator p97.
Owner:RIKEN
Use of antisense oligonucleotides or siRNA to suppress expression of eIF-5A1
InactiveUS20060178330A1Suppressing and inhibiting expressionInhibiting and suppressing expressionOrganic active ingredientsSenses disorderInitiation factorApoptosis
The present invention relates to apoptosis specific eucaryotic initiation factor 5A (eIF-5A), referred to as apoptosis factor 5A1 or simply factor 5A1, apoptosis factor 5A1 nucleic acids and polypeptides and methods for inhibiting or suppressing apoptosis in cells using antisense nucleotides or siRNAs to inhibit expression of factor 5A1. The invention also relates to suppressing or inhibiting expression of pro-inflammatory cytokines by inhibiting expression of apoptosis factor 5A.
Owner:SENESCO TECHNOLOGIES INC
Nucleic acids, polypeptides, compositions, and methods for modulating apoptosis
InactiveUS20030064952A1Convenient amountEfficient screeningAntibacterial agentsOrganic active ingredientsInitiation factorDeoxyhypusine synthase
The present invention relates to isolated and / or purified rat apoptosis-specific eucaryotic initiation Factor-5A (eIF-5A) and deoxyhypusine synthase (DHS) nucleic acids and polypeptides. The present invention also relates to methods of modulating apoptosis using apoptosis-specific eIF-5A and DHS, and antisense oligonucleotides and expression vectors of apoptosis-specific eIF-5A and DHS useful in such methods.
Owner:SENESCO TECHNOLOGIES INC
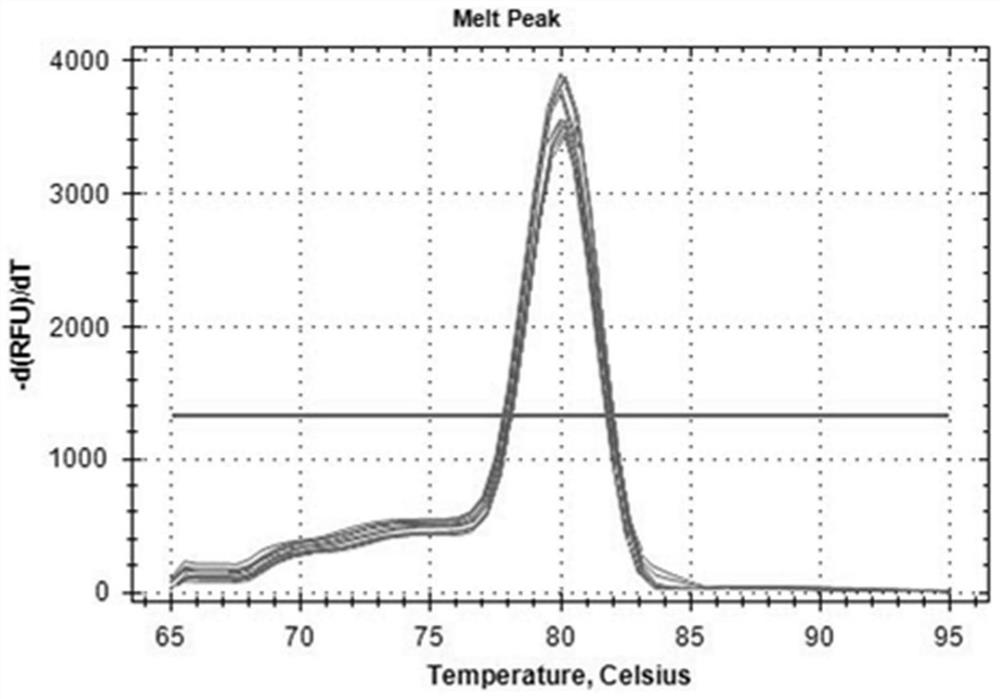
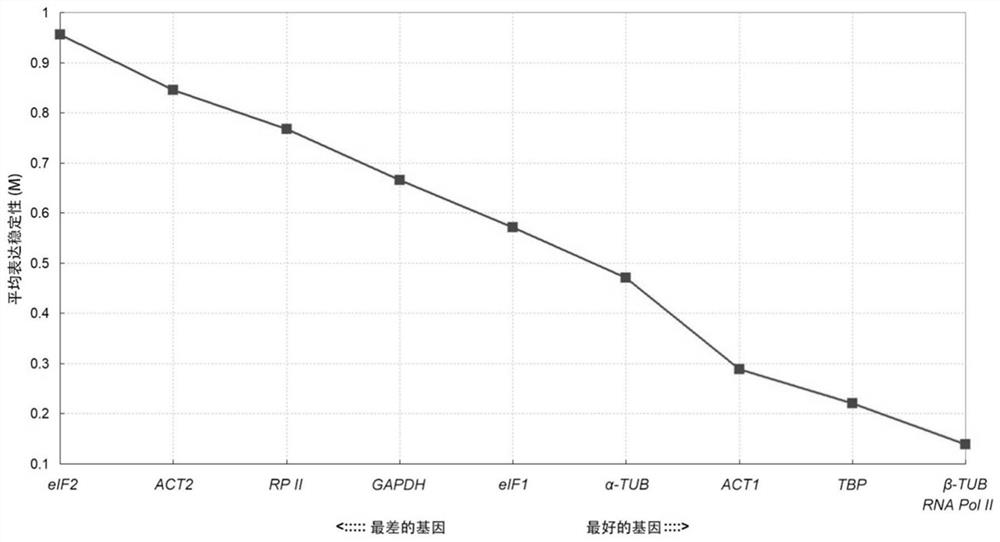
Paeonia ostii reference gene under drought stress, and special primer and application thereof
ActiveCN111733168AImprove stabilityImprove reliabilityMicrobiological testing/measurementOxidoreductasesBiotechnologyInitiation factor
The invention relates to a paeonia ostii reference gene under drought stress, and a special primer and application thereof. The reference gene is a TATA box binding protein TBP gene, an actin ACT1 gene, an actin ACT2 gene, a glyceraldehyde-3-phosphate dehydrogenase GAPDH gene, an eukaryote translation initiation factor eIF1 gene, a eukaryote translation initiation factor eIF2 gene, a tubulin alpha-TUB gene, a tubulin beta-TUB gene, an RNA polymerase II RNA Pol II gene or an RNA polymerase II transcription factor RP II gene. The nucleotide sequence of the gene is disclosed as the sequence table. The invention aims to provide a gene which can be stably expressed in the paeonia ostii gene expression profile under drought stress, and the gene is used as a paeonia ostii drought stress referencegene.
Owner:YANGZHOU UNIV
Cell penetrating peptides to target eif4e
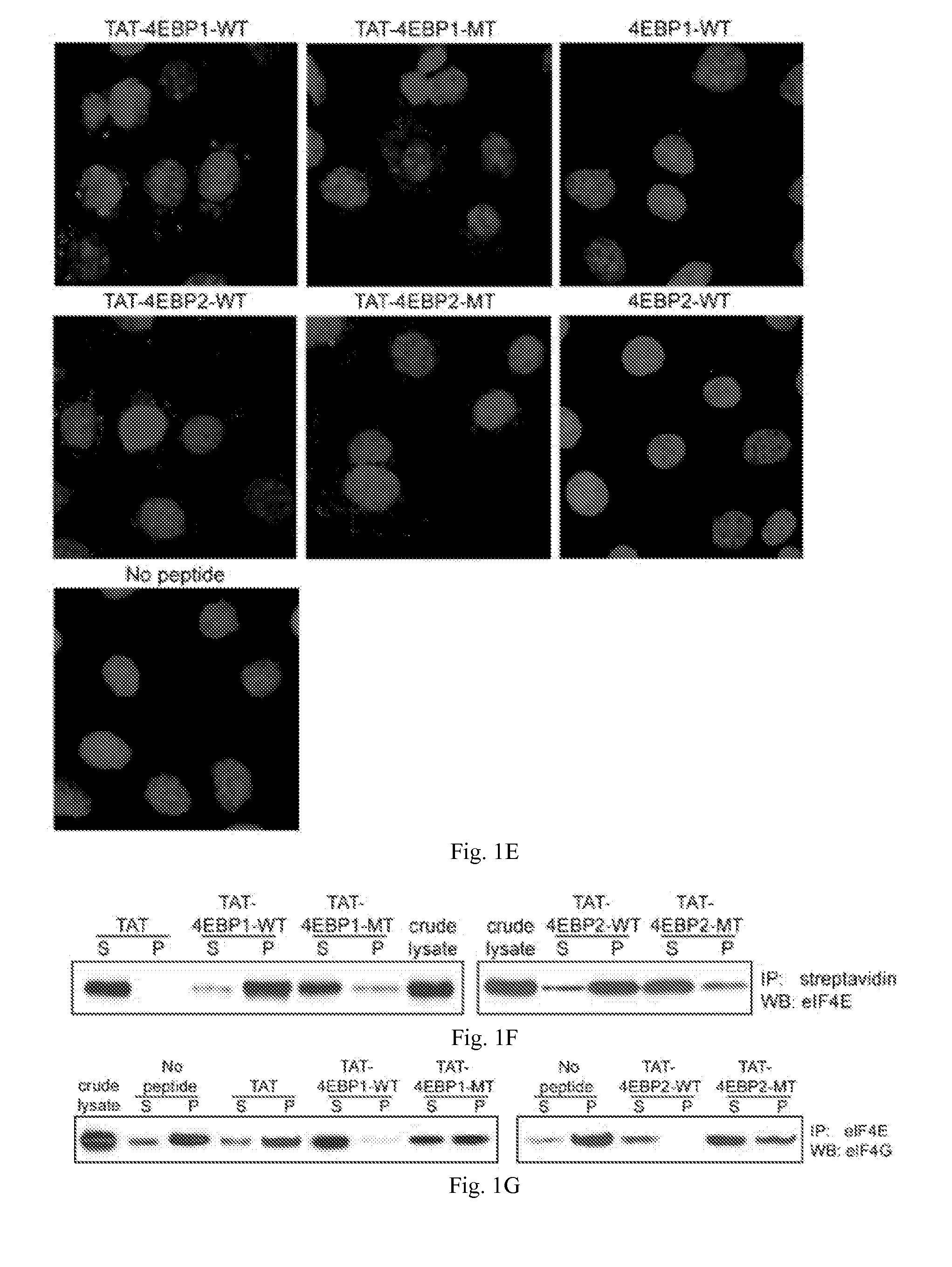
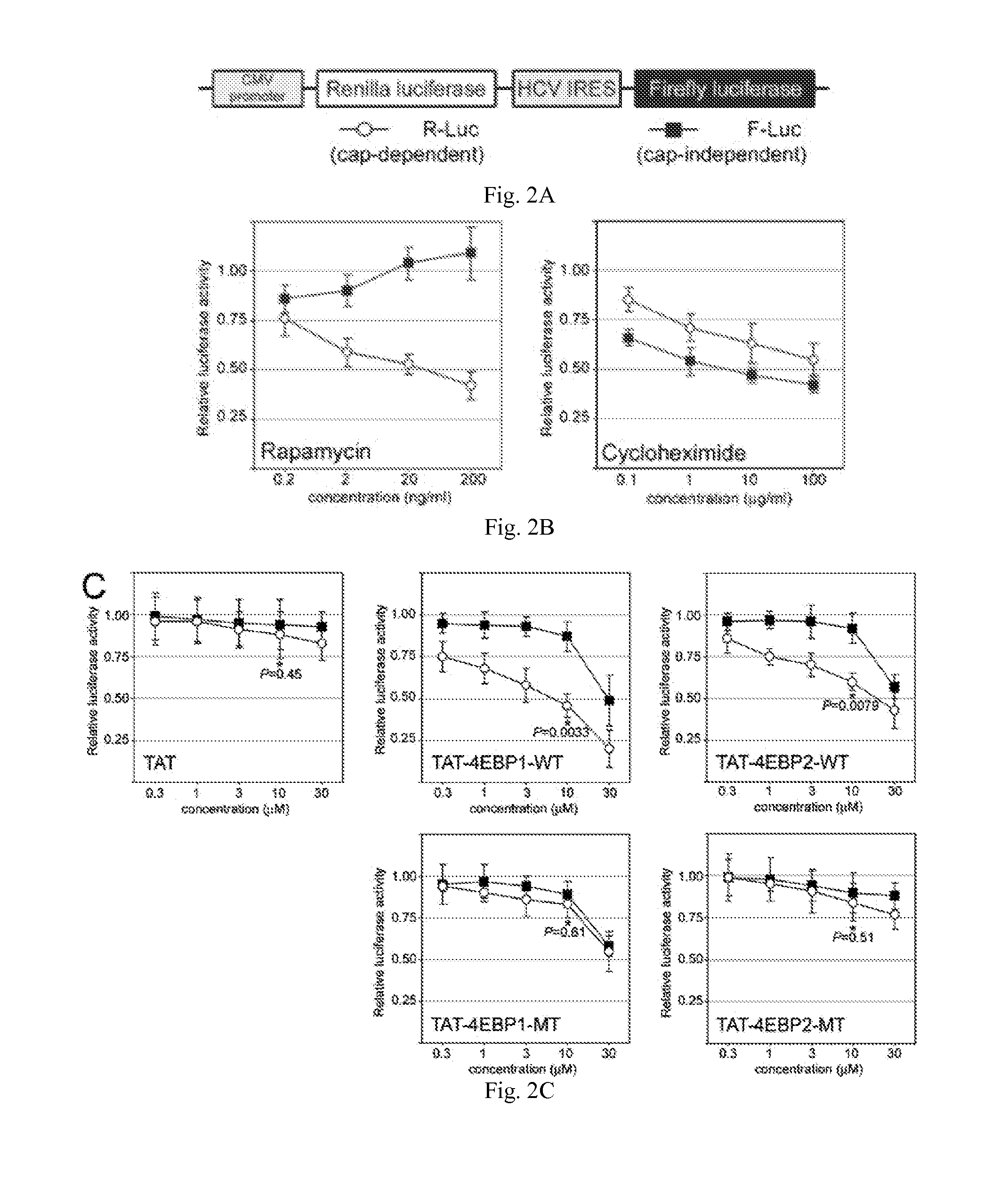
InactiveUS20150166621A1Polypeptide with localisation/targeting motifPeptide/protein ingredientsInitiation factorEIF4E
The present invention provides compounds to disrupt the eIF4E-eIF4G interaction and pharmaceutically acceptable salts of such compounds. Generally, the compounds are cell-penetrating peptides which bind mammalian initiation factor eIF4E (CPP-eIF4E), wherein the peptide comprises an amino acid sequence selected from the group consisting of SEQ ID NOS: 7, 9-11, 12-26, 27-44 and 45-51. More preferably, the amino acid sequence comprises at least 9 to about 40 amino acids and the mammalian initiation factor eIF4E is human initiation factor eIF4E.
Owner:F HOFFMANN LA ROCHE & CO AG
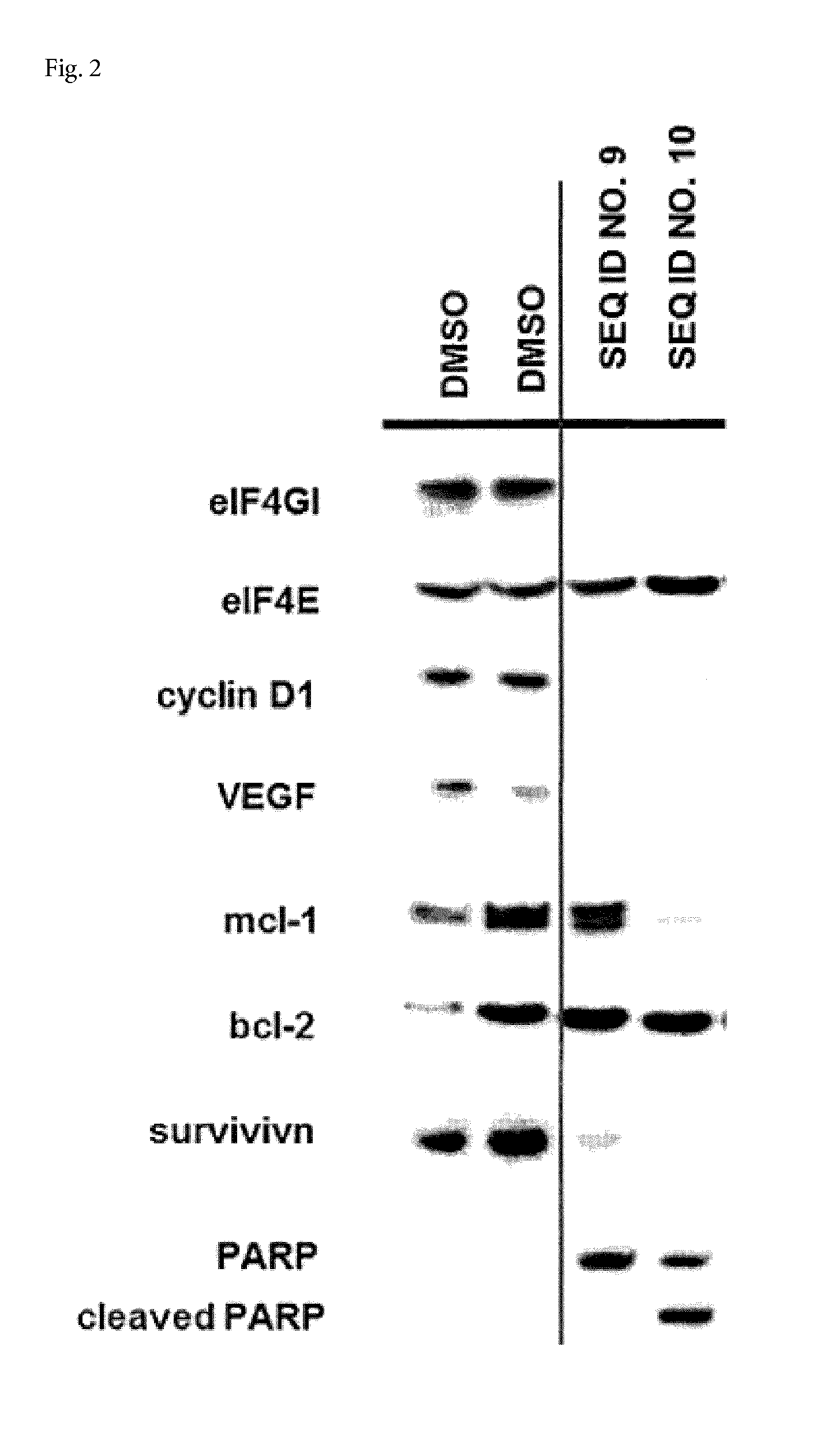
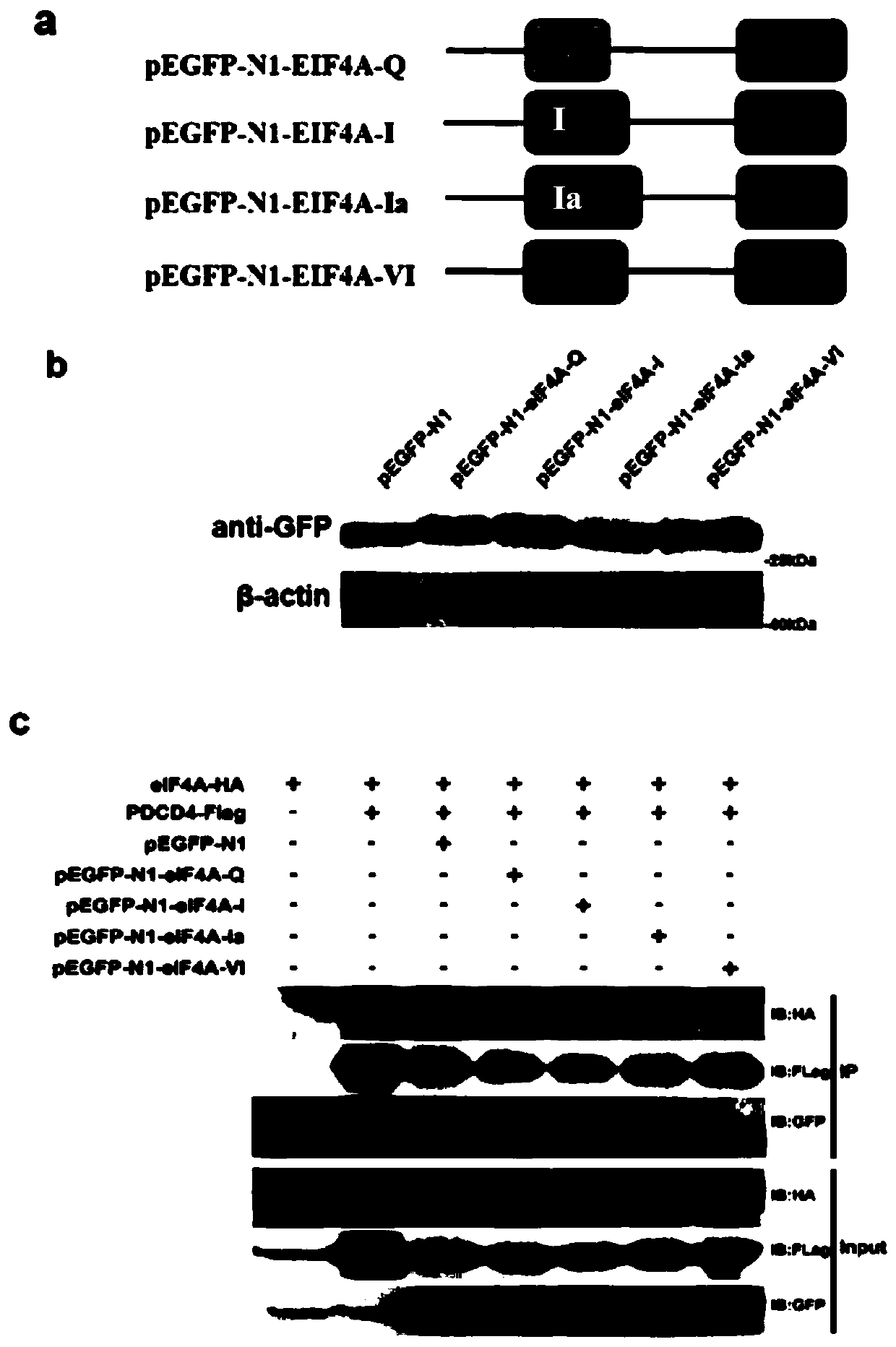
Fusion polypeptide and application of fusion polypeptide in preparing medicine for anti-depression and neurodegenerative disease
ActiveCN110256574AHigh expressionAdjust Healing EffectsPolypeptide with localisation/targeting motifNervous disorderDiseaseInitiation factor
The invention belongs to the technical field of biology, and specifically relates to a 9R-eIF4A-VI fusion polypeptide and an application of fusion polypeptide in preparing a medicine for anti-depression. The studies confirm that PDCD4 can bind to a eukaryotic translation initiation factor (eIF4A) to inhibit the expression of a brain-derived neurotrophic factor (BDNF), impedes the repair and synaptic transmission of damaged neurons, and aggravates the symptoms of the depressed patients. The application screens a number of domains in which PDCD4 binds to eIF4A, by fusion of a transmembrane sequence and a domain on eIF4A, the fusion polypeptide having the interference effect is obtained, the experiments verify that the fusion polypeptide can effectively interfere with the binding of PDCD4 and eIF4A, has good transmembrane efficiency and stability, and can be applied to the preparation of antidepressant drugs, which has great significance.
Owner:SHANDONG UNIV
Method for detecting activated blood coagulation factor XI in human intravenous immunoglobulin
ActiveCN103185710AAvoid preactivationIndirectly reflect changes in production contentFluorescence/phosphorescenceInitiation factorFactor ii
The invention discloses a method for detecting an activated blood coagulation factor XI in human intravenous immunoglobulin. The method comprises the steps as follows: (1) mixing an IVIG (intravenous immunoglobulin) sample with specially-preprocessed platelet-poor plasma (PPP); (2) adding a thrombin specific fluorogenic substrate with a phospholipid agent into a reaction system; (3) adding an initiation factor for starting a TGT (thrombin generation test), wherein the initiation factor comprises a calcium ion source and the phospholipid agent; (4) obtaining a thrombin generation curve providing parameters by the optimized reaction system; and (5) negatively correlating the peak time of thrombin (TTP) of the detected sample with the FXIa (activated blood coagulation factor XI) level in the sample. The FXIa level in the detected sample can be indirectly obtained by comparing the TTP of the detected sample with that of an FXIa standard sample. The method is suitable for detecting residual micro FXIa in human intravenous immunoglobulin (IVIG) products and is used for extracorporal evaluation of thrombosis risk of related products.
Owner:BLOOD TRASFUSION INST CHINESE ACAD OF MEDICAL SCI +1
Cell-free system for synthesis of proteins derived from cultured mammalian cells
InactiveUS20110300575A1High synthetic activityLow costHydrolasesPeptide/protein ingredientsInitiation factorFree protein
Prepared is an extract composition having an improved protein synthetic activity in a cell-free protein synthesis system using a mammalian cultured cell extract. An eukaryotic translation initiation factor and / or translational regulator are added to a cell-free protein synthesis system comprising an extract prepared from cultured mammalian cells and a template mRNA. These factors are one or more selected from the group consisting of eukaryotic translation initiation factors 4E (eIF4E), 2 (eIF2) and 2B (eIF2B), and eukaryotic translational regulator p97.
Owner:RIKEN
Nucleic acids, polypeptides, compositions, and methods for modulating apoptosis
InactiveUS7166467B2Antibacterial agentsOrganic active ingredientsInitiation factorDeoxyhypusine synthase
The present invention relates to isolated and / or purified rat apoptosis-specific eucaryotic initiation Factor-5A (eIF-5A) and deoxyhypusine synthase (DHS) nucleic acids and polypeptides. The present invention also relates to methods of modulating apoptosis using apoptosis-specific eIF-5A and DHS, and antisense oligonucleotides and expression vectors of apoptosis-specific eIF-5A and DHS useful in such methods.
Owner:SENESCO TECHNOLOGIES INC
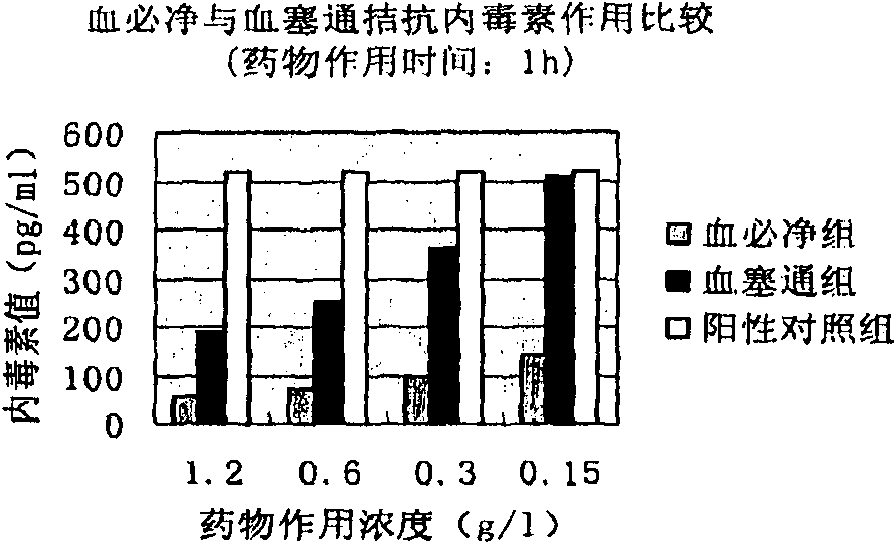
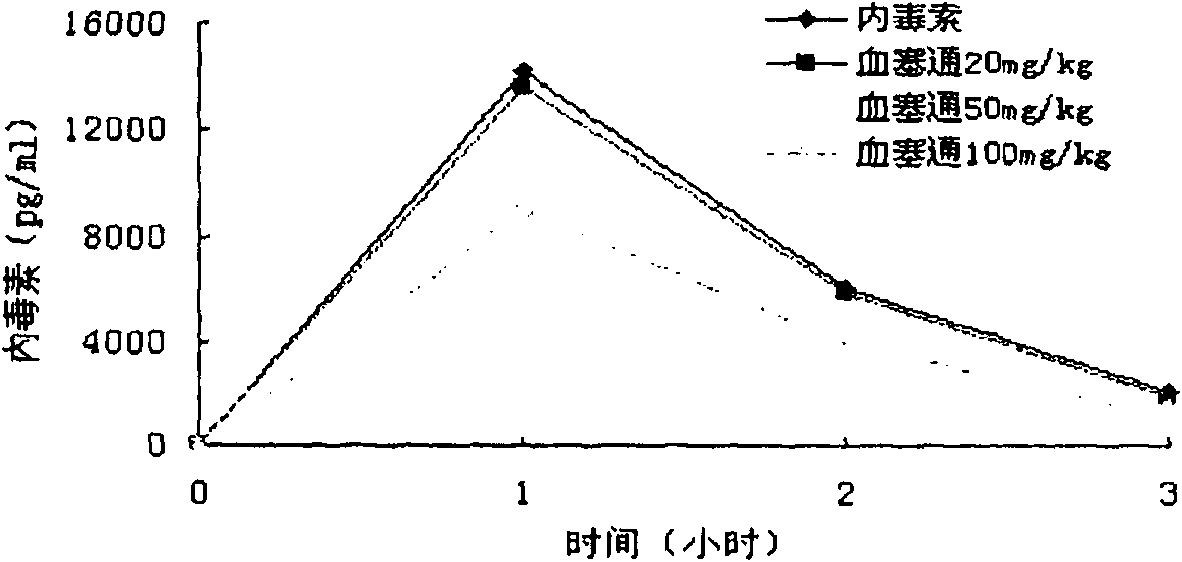
Application of pseudoginseng root total saponins and preparations thereof in adjuvant treatment of 'systemic inflammatory response syndrome'
The invention relates to application of pseudoginseng root total saponins and preparations thereof in adjuvant treatment of 'systemic inflammatory response syndrome' (SIRS). The SIRS is effectively removed aiming at an initiation factor of the SIRS, namely endotoxin; the SIRS is inhibited aiming at main damage factors, namely inflammatory mediators, in the development process of the SIRS; and the damage of lung, liver, kidney, small intestines and other important organs caused by the endotoxin is effectively prevented and treated. The damage of vascular endothelial cells caused by the endotoxin is resisted, and the vascular endothelial cells are protected. Severe circulatory disturbance in an SIRS pathological state is improved, and the microcirculation blood flow is increased; therefore, the adjuvant treatment effect on the SIRS is exerted.
Owner:KPC PHARM INC
Peptides that bind eukaryotic translation initiation factor 4e
InactiveUS20110319338A1Increase serum stabilityEffective cell penetrationPeptide/protein ingredientsVirus peptidesInitiation factorADAMTS Proteins
Methods, compositions and kits for treating proliferative and non-proliferative diseases associated with abnormal protein synthesis. Chimeric peptide constructs are comprised in compositions and kits for use in the treatment of proliferative diseases, such as ovarian cancer, and for inhibiting protein synthesis in a tumor cell compared to a non-tumor cell.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Papaya with novel traits and methods for producing such papaya plants
ActiveUS20190110418A1Improve the immunityMicrobiological testing/measurementMutant preparationBiotechnologyPapaya family
The present application relates to papaya plants having increased resistance to the papaya ringspot virus as compared to a wild type plant due to a mutation in the eukaryotic translation initiation factor 4e and / or eukaryotic translation initiation factor iso4e gene leading to non-functional eukaryotic translation initiation factor 4e and / or eukaryotic translation initiation factor iso4e proteins. Methods of producing such papaya plants having increased resistance to the papaya ringspot virus are described.
Owner:BENCHBIO PVT LTD +2
Polypeptide synthetic condensation agent 3-hydroxy-4-oxy-3,4-dihydro-1,2,3-benzotriazozine synthesizing method
This invention discloses a composing method of polypeptide synthesis condensing agent that is 3-hydroxide radical-4-oxygen-3,4-dihydro-1,2,3- benzotriazin. This method uses methyl anthranilate as initiation factors. Target product is obtained by three step reaction with easily obtained raw material that is low poison. This invention has characteristics such as raw material is easily obtained, route is simple, noxious property is low, side reaction is little, it easily attributes, overall yield is suitable.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
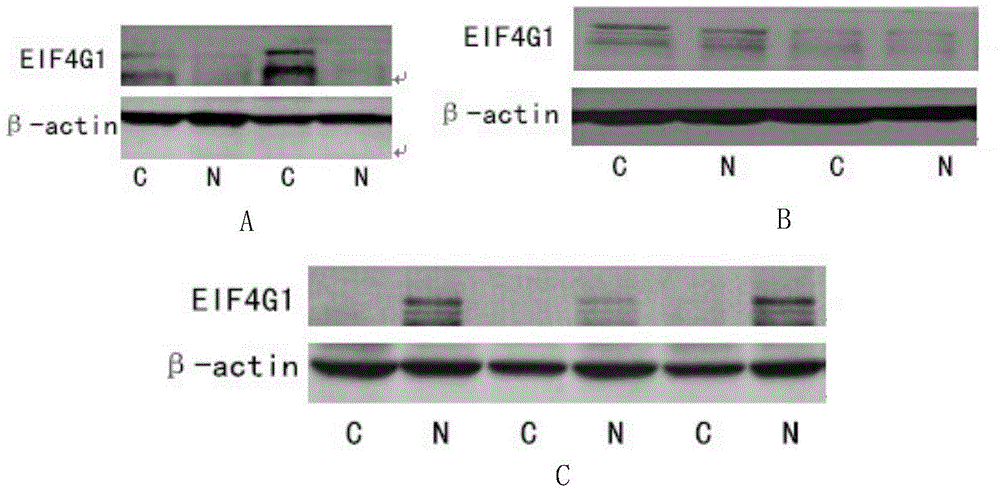
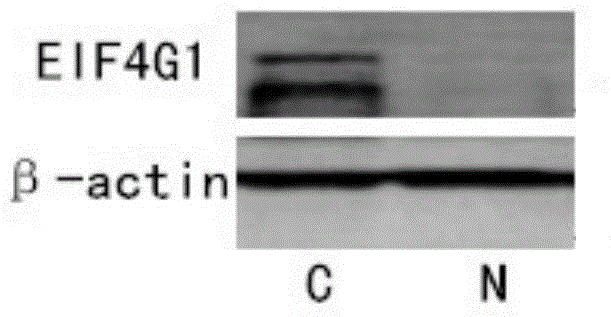
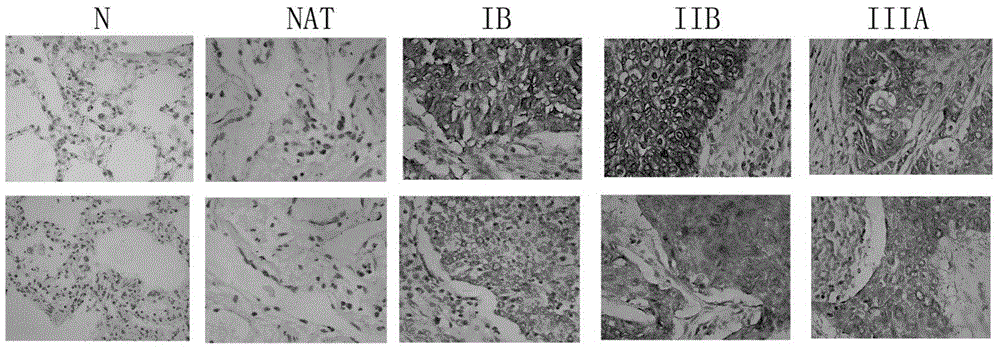
Applications of EIF4G1 in diagnosis and treatment of squamous cell carcinomas
ActiveCN105624275AGenetic material ingredientsMicrobiological testing/measurementInitiation factorSquamous Carcinomas
The present invention discloses applications of EIF4G1 in diagnosis of squamous cell carcinomas, particularly uses of a eukaryotic translation initiation factor 4G1 (EIF4G1) gene or protein, or a detection reagent thereof, wherein the eukaryotic translation initiation factor 4G1 (EIF4G1) gene or protein, or detection reagent thereof is used for preparing a kit for determining whether tumors are non-small cell lung squamous cell carcinomas. In addition, experiment results show that EIF4G1 has a certain high expression in squamous cell carcinomas with different sources, such that EIF4G1 can be adopted as the marker of the squamous cell carcinoma pathological type so as to provide the accurate method for typing of different sources of cancers.
Owner:SHANGHAI EAST HOSPITAL
Virus-resistant tobacco and breeding method therefor
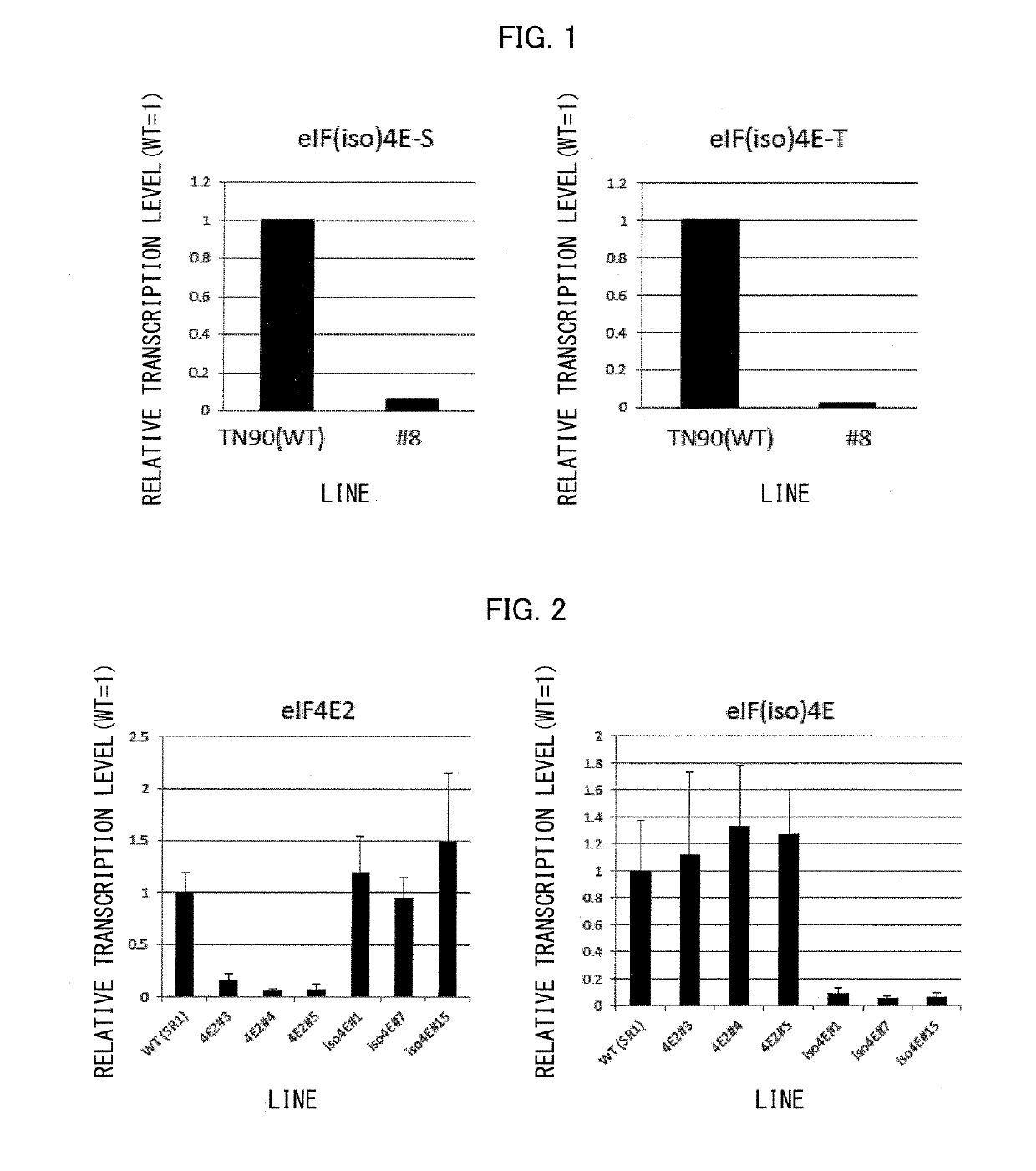
ActiveUS20190174706A1Tobacco preparationMicrobiological testing/measurementInitiation factorNicotiana tabacum
In order to provide tobacco resistant to a virus, tobacco in accordance with the present invention is arranged such that: (i) a translation initiation factor eIF(iso)4E protein which is non-functional with respect to a virus is produced or (ii) expression of a translation initiation factor eIF(iso)4E gene is suppressed; and (a) a translation initiation factor eIF4E2 protein, which is non-functional with respect to a virus, is produced or (b) expression of a translation initiation factor eIF4E2 gene is suppressed.
Owner:JAPAN TOBACCO INC
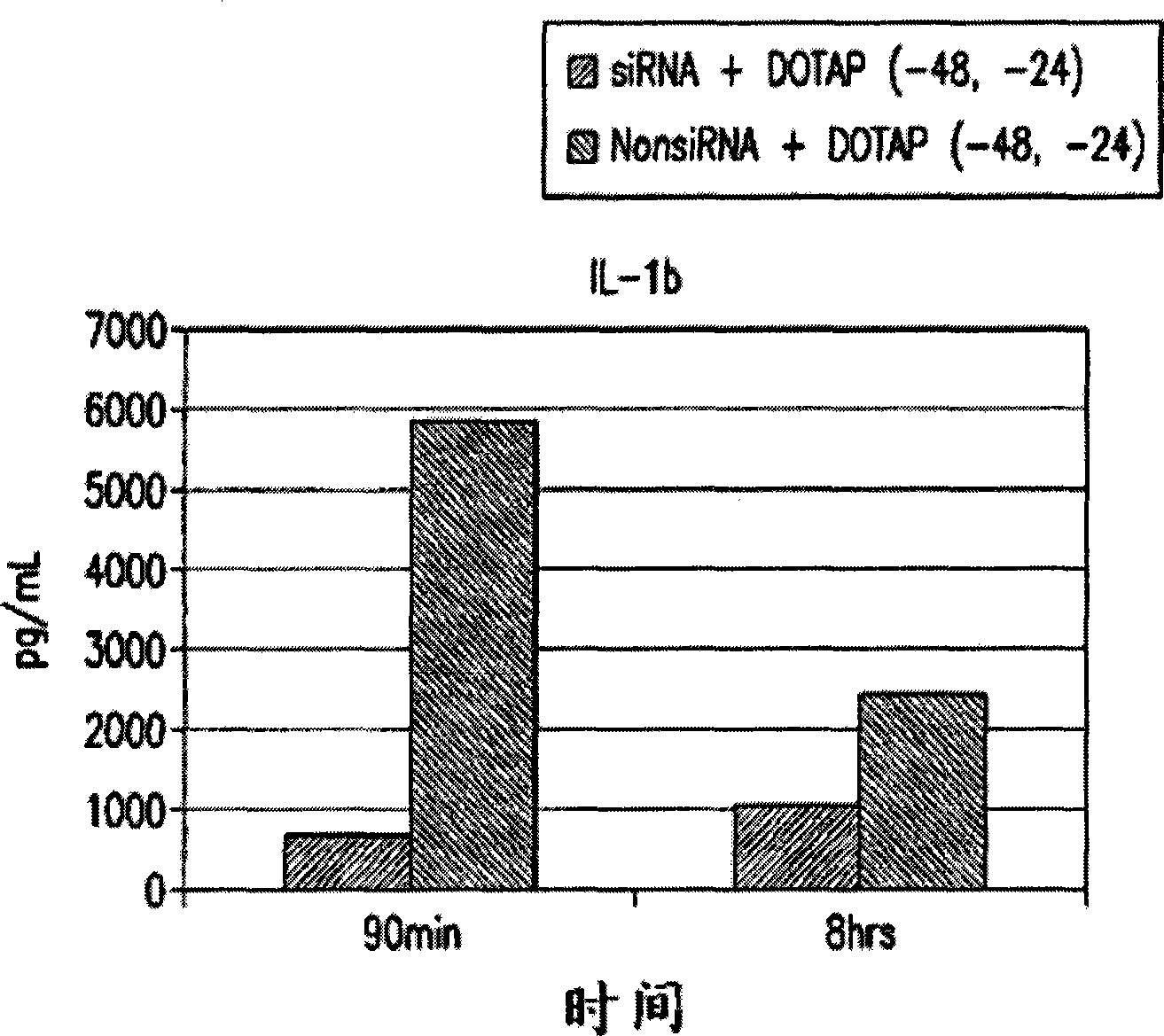
Use of apoptosis-specific eIF-5A siRNA to down regulate expression of proinflammatory cytokines to treat sepsis
InactiveUS20090118207A1Suppressing and inhibiting expressionReduce expressionAntibacterial agentsOrganic active ingredientsInitiation factorMammal
The present invention relates to apoptosis specific eucaryotic initiation factor 5A (eIF-5A), referred to as apoptosis-specific eIF-5A or eIF5-A1, nucleic acids and polypeptides and methods for down regulating pro-inflammatory cytokines in a mammal by administering siRNA against eIF-5A1 to the mammal to treat / prevent sepsis and / or hemorrhagic shock.
Owner:SENESCO TECHNOLOGIES INC
Salt responsive genes useful for generating salt resistant transgenic plants
ActiveUS20090217411A1Confer salt-resistanceLimited successBryophytesSugar derivativesInitiation factorPlant cell
The present invention relates to a transgenic plant comprising one or more plant cells transformed with exogenous nucleic acid encoding a Dunaliella salt-inducible or salt-responsive protein selected from the group consisting of elongation initiation factor 3 (eIF3), NADPH dependent quinone reductase (QOR), aldo-keto reductase (AKR), bifunctional aspartate kinase-homoserine reductase (AK-HSD) and mitochondrial import membrane translocase subunit (TIM9), or a fragment, homolog or variant thereof. The transgenic plant has increased tolerance to salt as compared to a corresponding non-transgenic plant. The present invention further relates to nucleic acids, vectors and constructs encoding the Dunaliella salt-inducible or salt-responsive proteins, and to a method of producing a transgenic plant having an increased tolerance to salt, a method of modifying a plant capacity to survive salt shock, and a method of modifying plant recovery after exposure to salt stress, by introducing the nucleic acids, constructs and / or vectors into one or more cells of the plant.
Owner:HAZERA GENETICS LTD +2
Use of apoptosis-specific eif-5a sirna to down regulate expression of proinflammatory cytokines to treat sepsis
The present invention relates to apoptosis specific eucaryotic initiation factor 5A (eIF-5A), referred to as apoptosis-specific eIF-5A or eIF5-A1, nucleic acids and polypeptides and methods for down regulating pro-inflammatory cytokines in a mammal by administering siRNA against eIF-5A1 to the mammal to treat / prevent sepsis and / or hemorrhagic shock.
Owner:SENESCO TECHNOLOGIES INC
Nucleic acids, polypeptides, and methods for modulating apoptosis
The invention discloses a nucleic acids, polypeptides, and methods for modulating apoptosis. The present invention relates to isolated and / or purified rat apoptosis-specific eucaryotic initiation Factor-5A (eIF-5A) and deoxyhypusine synthase (DHS) nucleic acids and polypeptides. The present invention also relates to methods of modulating apoptosis using apoptosis-specific eIF-5A and DHS, and antisense oligonucleotides and expression vectors of apoptosis-specific eIF-5A and DHS useful in such methods.
Owner:SENESCO TECHNOLOGIES INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com