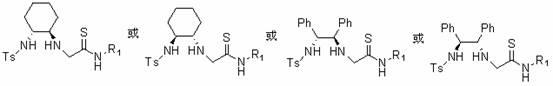Novel method for preparing chiral dicarbonyl derivative by catalysis
A technology for catalytic preparation and derivatives, applied in chemical instruments and methods, preparation of organic compounds, chemical/physical processes, etc., can solve problems such as difficult and difficult hydroxyl groups
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
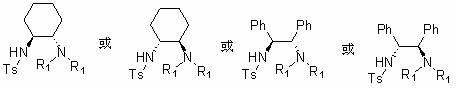
[0067] Dissolve 5.68mmol of cyclohexanediamine tartrate in 75ml of 2N NaOH solution, add 7.6mmol of triethylamine and 55ml of dichloromethane, cool to 0°C, slowly add 38ml of dichloromethane solution with 3.8mmol of TsCl dropwise at room temperature After the reaction was completed, the aqueous phase was extracted with 25ml×3, 2N HCl, and the pH value was adjusted to 9 with 2N NaOH, and the organic phase was extracted with dichloromethane, anhydrous MgSO 4 Dry, and remove the solvent under reduced pressure to obtain a pale yellow solid catalyst a-1 or catalyst a-2 (R 1 =Ts), yield 90%.
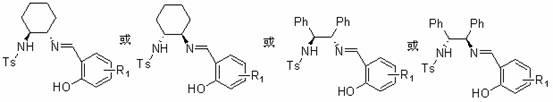
[0068] Add 500ml toluene and 0.015mol catalyst in 1L reaction flask a-1 or catalyst a-2 (R 1 = Ts), after stirring and dissolving, add 0.1mol of carboindanone ester and 0.1mol of β-nitrostyrene, stir at room temperature until the reaction is complete, and remove the solvent by rotary evaporation to obtain chiral dicarbonyl derivatives, Yield 80%, e.e value 75%, dr value 2:1.
Embodiment 2
[0070] Dissolve 4.69mmol of diphenylethylenediamine in 30ml of dichloromethane, add 4.5mmol of triethylamine, cool to 0°C, slowly add 10ml of dichloromethane solution in which 4.69mmol of TsCl is dissolved, and complete the reaction at constant temperature until complete. After finishing washing with 10ml×2 water, then wash with 10ml saturated NaCl salt, anhydrous NaCl 2 SO 4 Dry, remove the solvent under reduced pressure to obtain a white or light yellow solid product, and recrystallize with ethyl acetate to obtain the catalyst a-3 or catalyst a-4 (R 1 =Ts), productive rate 94%.
[0071] Add 500ml toluene and 0.01mol catalyst in 1L reaction flask a-3 or catalyst a-4 (R 1 = Ts), after stirring and dissolving, add 0.1mol of carboindanone ester and 0.1mol of β-nitrostyrene, stir at room temperature until the reaction is complete, and remove the solvent by rotary evaporation to obtain chiral dicarbonyl derivatives, The yield was 87%, the e.e. value was 84%, and the dr valu...
Embodiment 3
[0073] Dissolve 3.6mmol of diphenylethylenediamine (or cyclohexanediamine) and 0.3mmol of DMAP in 40ml of dichloromethane, then add 12mmol of triethylamine, cool to 0°C, slowly add 17mmol of TFAA dropwise, and stir at room temperature to complete the reaction After the reaction was completed, it was extracted with 50ml of dichloromethane, washed with 50ml of 2NHCl, 50ml of saturated sodium bicarbonate solution and 50ml of saturated brine, and the organic phase was washed with anhydrous Na 2 SO 4 Dry, remove the solvent under reduced pressure to obtain the catalyst a-1 or catalyst a-2( or catalyst a-3 or catalyst a-4 ) (R 1 =DMB), yield 85%.
[0074] Add 500ml toluene and 0.012mol catalyst in 1L reaction flask a-1 or catalyst a-2( or catalyst a-3 or catalyst a-4 ) (R 1 =DMB), after stirring and dissolving, add 0.1mol of carboindanone ester and 0.1mol of β-nitrostyrene, stir at room temperature until the reaction is complete, and remove the solvent by rotary evaporatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com