Dicyclopentadienyl iron pocket ligand possessing central chirality and planar chirality, synthesis method and uses
A ferrocene, chiral technology, applied in the field of synthesis of ferrocene pocket ligands
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
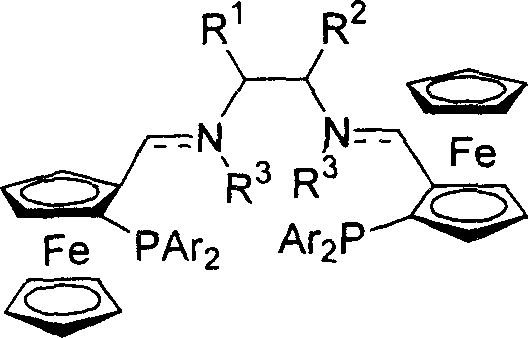
[0023] Embodiment 1: the synthesis of ferrocene bis-imide phosphine ligand
[0024] At room temperature, add (1.0mmol) ferrocene aldehyde phosphine and (0.5mmol) chiral diamine and 5ml toluene to the reaction flask, then add molecular sieves and catalytic amount of 0.05mmol p-toluenesulfonic acid, reflux and stir for five hours, diatoms Filtrate with soil, remove the solvent under reduced pressure, and recrystallize from petroleum ether to obtain the corresponding bis-imidophosphine ligand.
[0025] (R,R,Sp,Sp)-P1(R 1 and R 2 =-(CH 2 ) 4 -, Ar=Ph)
[0026] Yield: 62%; mp: 184°C; [α] D 20 =+276.6° (c=0.34, CHCl 3 ); 1 H NMR (300MHz, CDCl 3 ): δ1.23-1.42(m, 6H), 1.61-1.64(m, 2H), 3.01-3.04(m, 2H), 3.78(s, 2H), 4.11(s, 10H), 4.37-4.39(m , 2H), 4.92(s, 2H), 7.14-7.59(m, 20H), 8.23(d, J=1.8Hz, 2H); 31 P NMR (161.92MHz, CDCl 3 ): δ-20.11; MS (EI) m / z (rel) 875 (M + +1, 15.25), 810 (100.00), 397 (18.39), 121 (10.51). IR (KBr): 2924, 2854, 1649, 1640, 1433, 102...
Embodiment 2
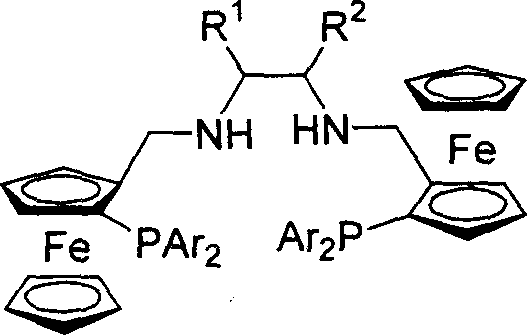
[0035] Embodiment 2: synthesis of ferrocene diamine phosphine ligand
[0036] At room temperature, dissolve 10 mmol of bis-imidophosphine ligand in 20 ml of ethanol, add 12 mmol of sodium borohydride to reflux for 6 hours, add water to treat unreacted sodium borohydride, extract with ether, wash the organic phase with saturated ammonium chloride, and the product Separation and purification by column chromatography.
[0037] (R,R,Sp,Sp)-P6(R 1 and R 2 =-(CH 2 ) 4 -, Ar=Ph)
[0038] Yield: 65%; [α] D 20 =-336.1° (c=0.47, CHCl 3 ); 1 H NMR (300MHz, CDCl 3 ): δ1.36-1.61(m, 8H), 1.88(m, 2H), 3.55(d, J=2.1Hz, 4H), 3.63(s, 10H), 3.74(s, 2H), 4.24(s, 2H), 4.37(s, 2H), 7.16-7.56(m, 20H); 31 P NMR (161.92MHz, CDCl 3 ): δ-22.95; MS (EI) m / z (rel) 877 (M + +1, 20.01), 812 (100), 399 (18.56), 121 (9.63). IR (KBr): 3056, 2926, 1587, 1106, 742, 697cm -1 ;Elemental Analysis C 52 h 52 Fe 2 N 2 P 2 : Calculated C, 71.08; H, 5.97; N, 3.19. Found C, 70.80; H, 6...
Embodiment 3
[0046] Example 3: Asymmetric allylation of α-methoxyacetophenone
[0047] Under argon protection, [Pd(C 3 h 5 )Cl] 2 (1.6mg, 0.00437mmol) and ligand (0.0962mmol) were dissolved in 1mL tetrahydrofuran, and refluxed for 0.5 hours. Another reaction tube contained α-methoxyacetophenone (0.174mmol) in 0.5mL tetrahydrofuran, Add LHMDS (0.21mL1M in THF, 0.21mmol) under cooling in a dry ice-acetone bath, keep this temperature for 0.5 hours, add the catalyst prepared on site, allyl acetate (0.262mmol) and additive AgBr (0.0174mmol), stir for 10min, Then the temperature was raised to -20°C for reaction. The reaction was tracked by TLC, the reaction was quenched by adding saturated brine, the layers were separated, the aqueous phase was extracted with ether (3×10 mL), the organic phases were combined, and dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure and purified by column chromatography. Yield: 97%; ee(%): 93%; 1 H NMR (300MHz, CDCl 3 ): δ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com