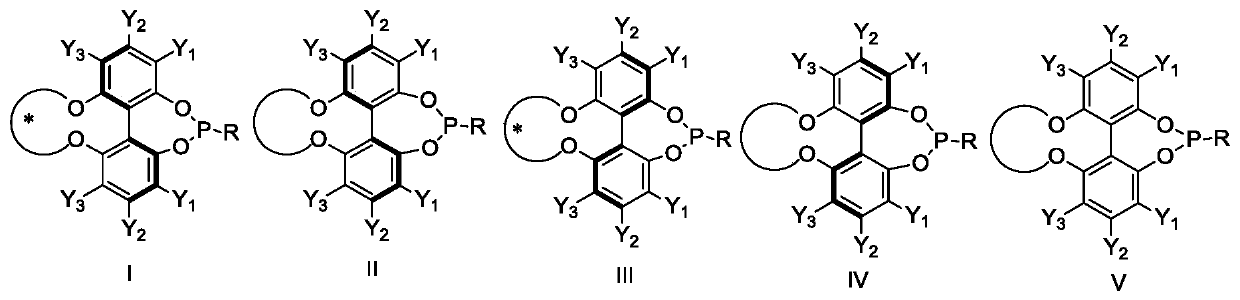
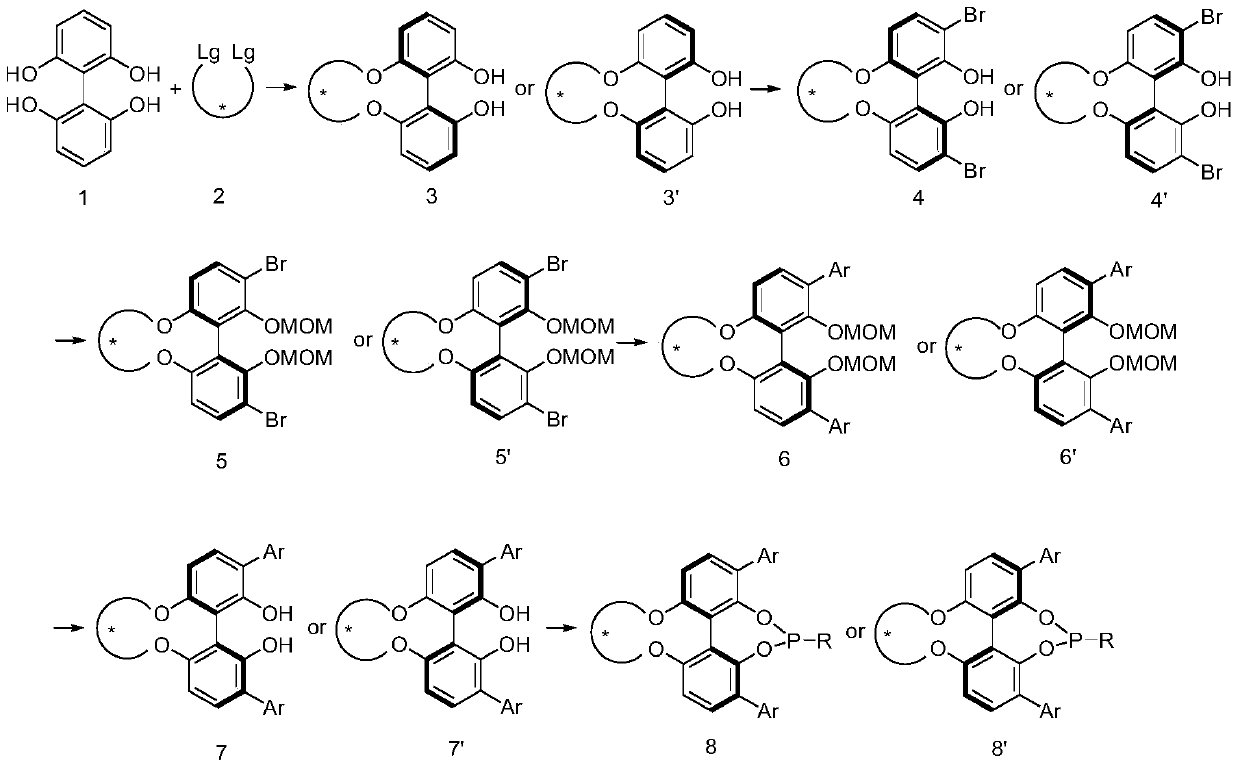
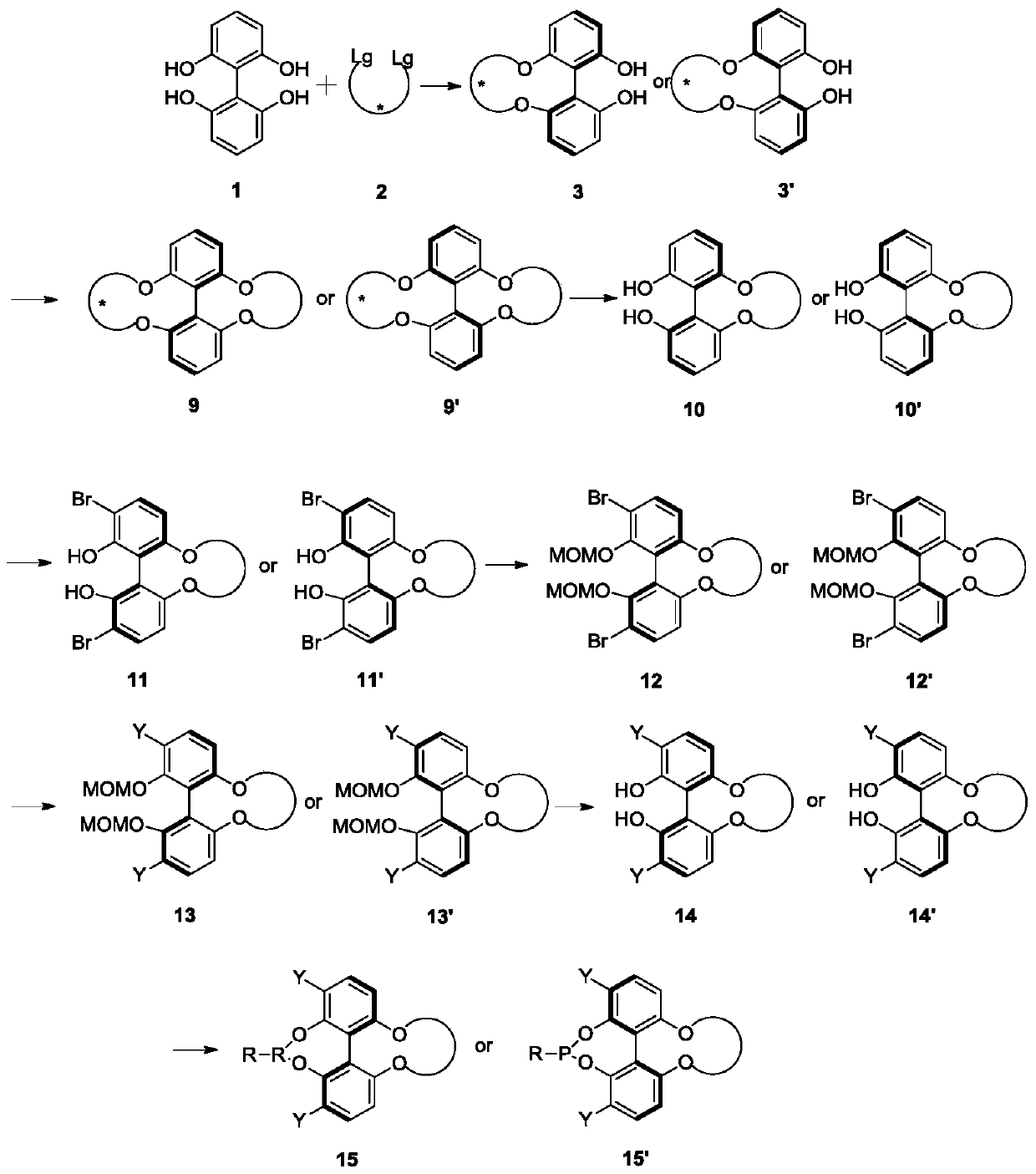
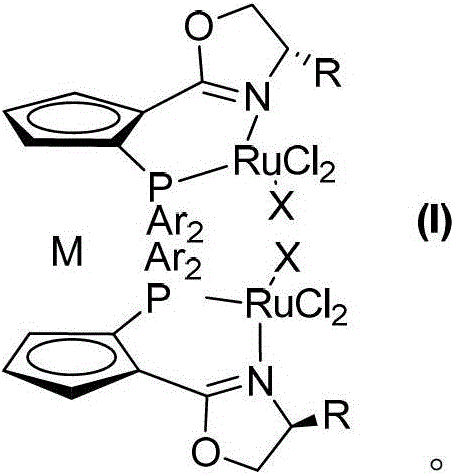
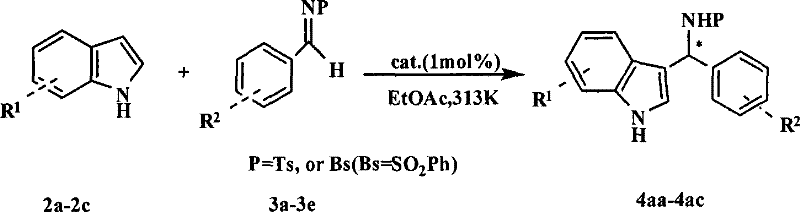Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
37 results about "Asymmetric induction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
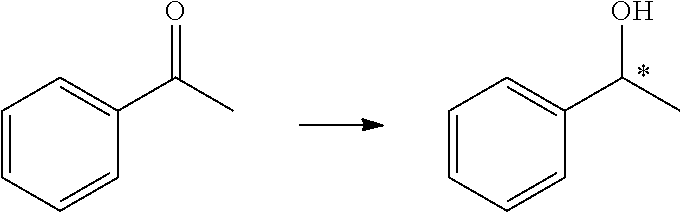
Asymmetric induction (also enantioinduction) in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.
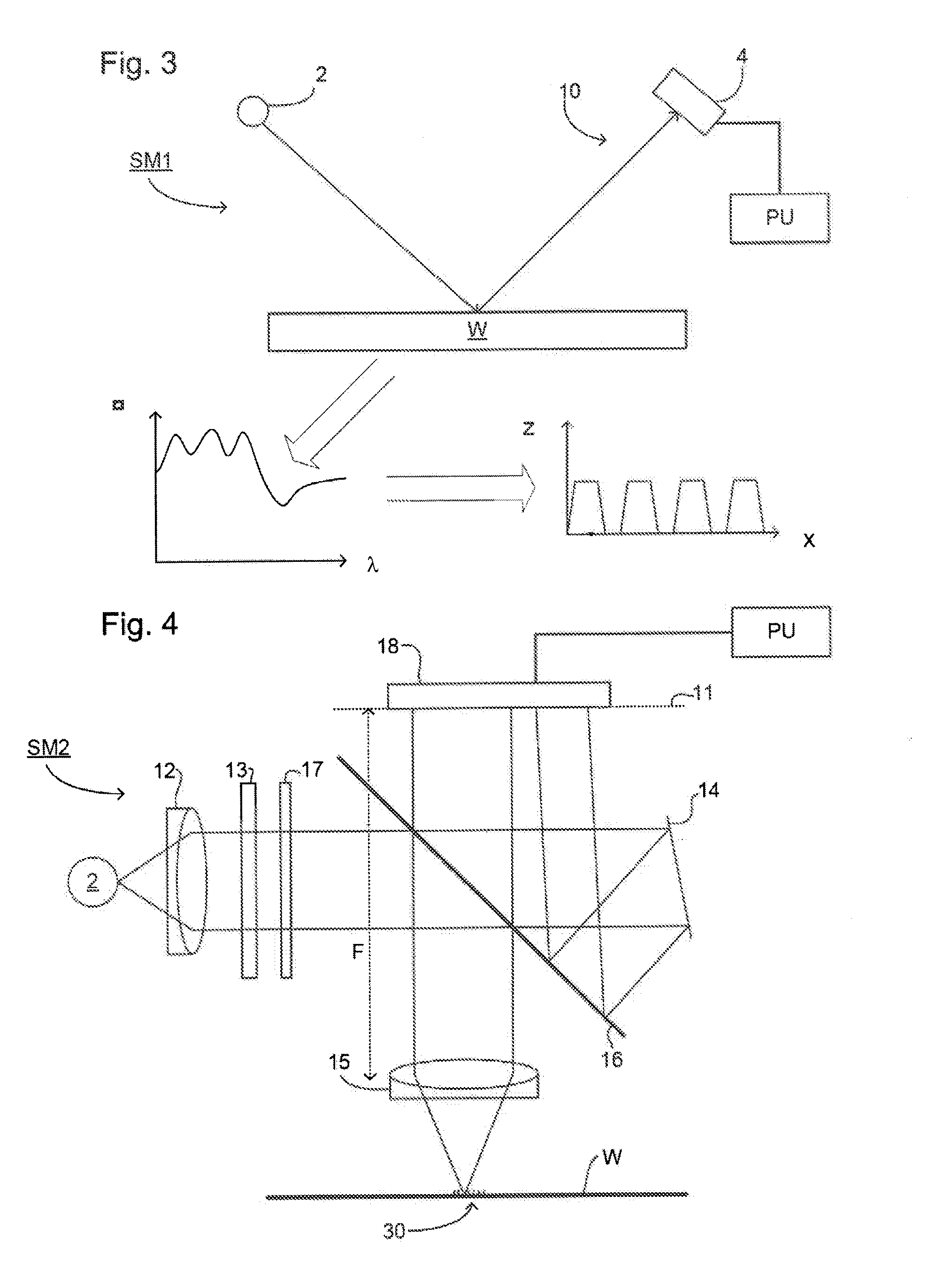
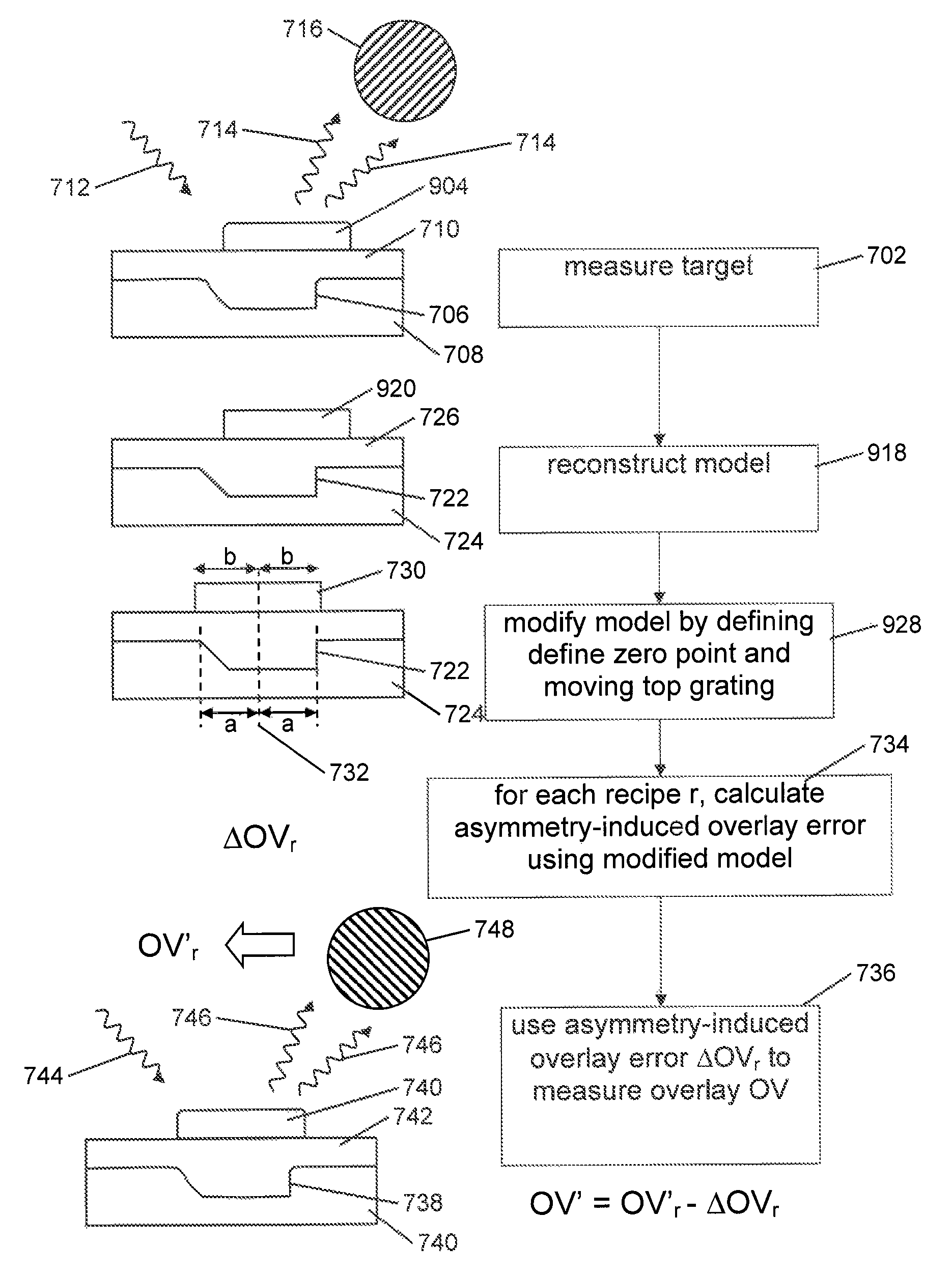
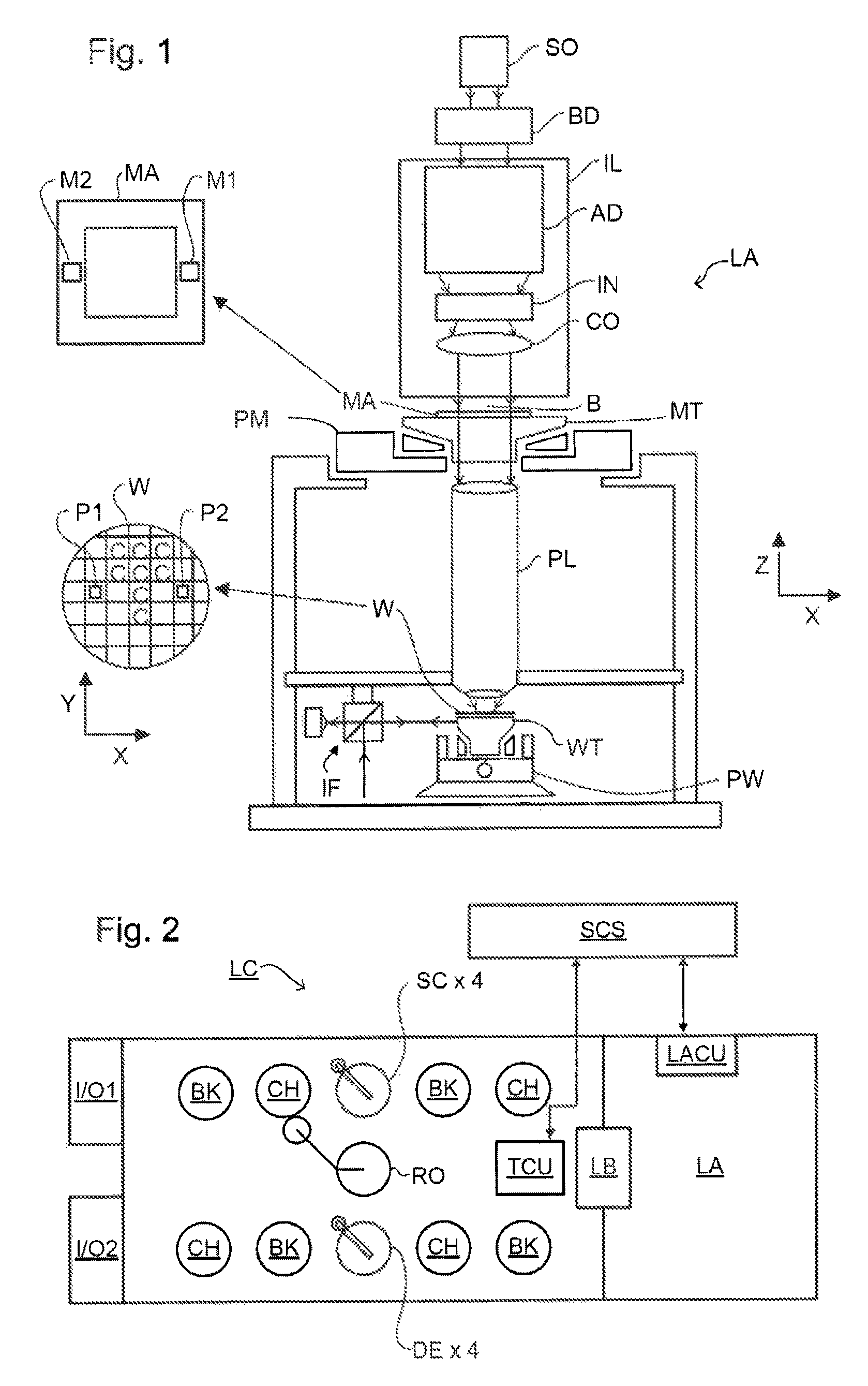
Method and Apparatus for Determining an Overlay Error
ActiveUS20120013881A1Scattering properties measurementsPhotomechanical apparatusAsymmetric inductionScatterometer
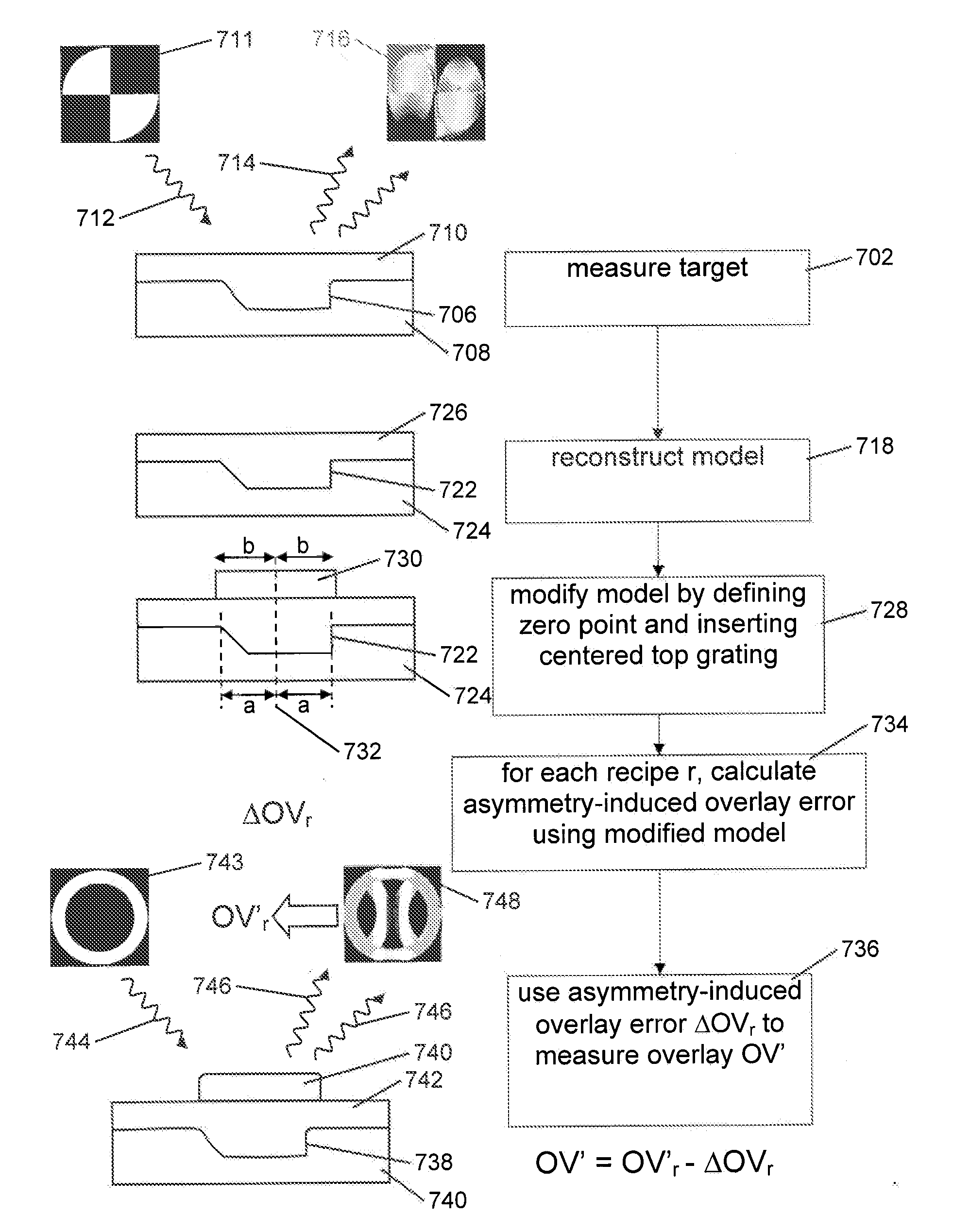
A method of determining an overlay error. Measuring an overlay target having process-induced asymmetry. Constructing a model of the target. Modifying the model, e.g., by moving one of the structures to compensate for the asymmetry. Calculating an asymmetry-induced overlay error using the modified model. Determining an overlay error in a production target by subtracting the asymmetry-induced overlay error from a measured overlay error. In one example, the model is modified by varying asymmetry p(n′), p(n″) and the calculating an asymmetry-induced overlay error is repeated for a plurality of scatterometer measurement recipes and the step of determining an overlay error in a production target uses the calculated asymmetry-induced overlay errors to select an optimum scatterometer measurement recipe used to measure the production target.
Owner:ASML NETHERLANDS BV
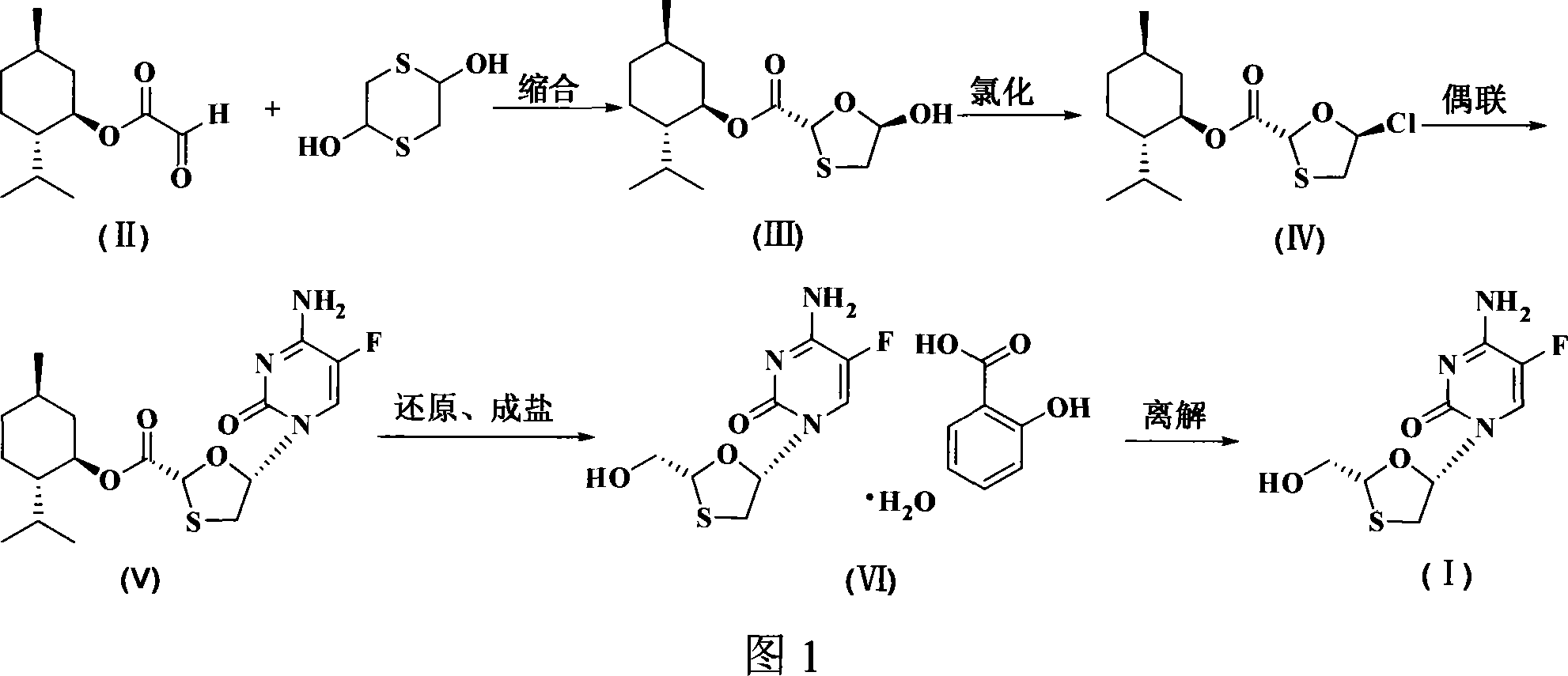
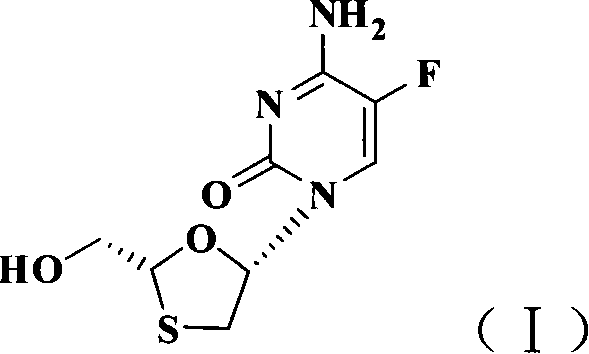
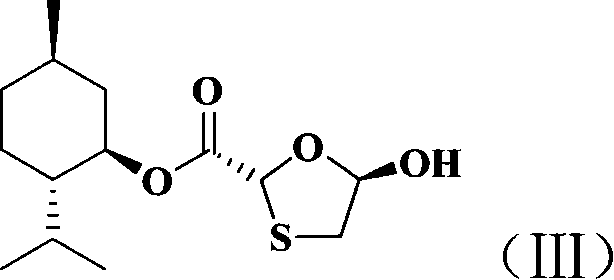
Non-enantioselective prepn process of emtricitabine
InactiveCN101066971AMild reaction conditionsSimple and fast operationOrganic chemistryDithianeAlcohol
The present invention discloses non-enantioselective preparation process of emtricitabine. The preparation process includes condensation of glyoxalic acid as the initial material and 2, 5-dihydroxy-1, 4-dithiane under the asymmetric induction of optically active alcohol ester to obtain trans-5-hydroxy-1, 3-oxythiacyclopentane-2-carboxylate; halogenating and coupling with silylated 5-flucytosine, and reducing to obtain initial product; reaction with salicylic acid to form salt, purification and separation to obtain optically pure emtricitabine. The present invention has mild reaction condition, simple operation, low cost, less environmental pollution and high product purity reaching medicinal standard, and is suitable for industrial production. The product is used in treating hepatitis B and AIDS.
Owner:葛建利
Process for producing optically active sulfoxide derivative
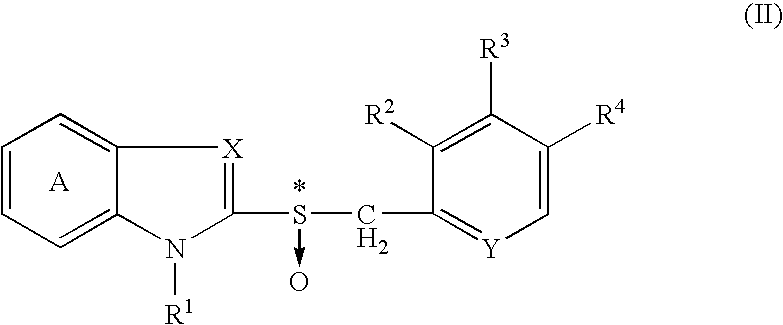
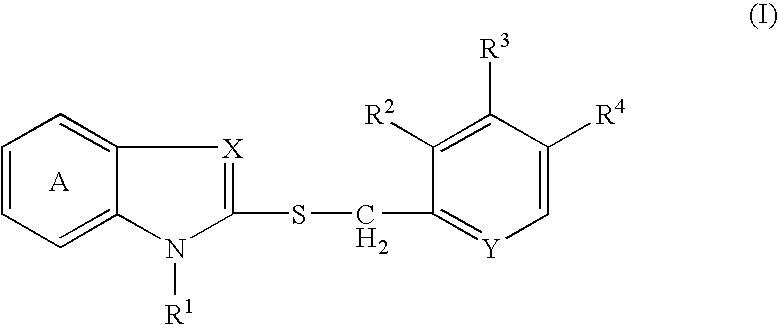
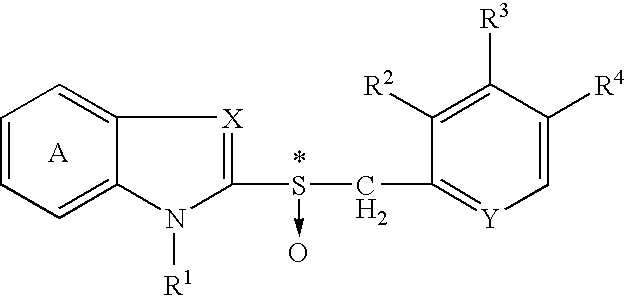
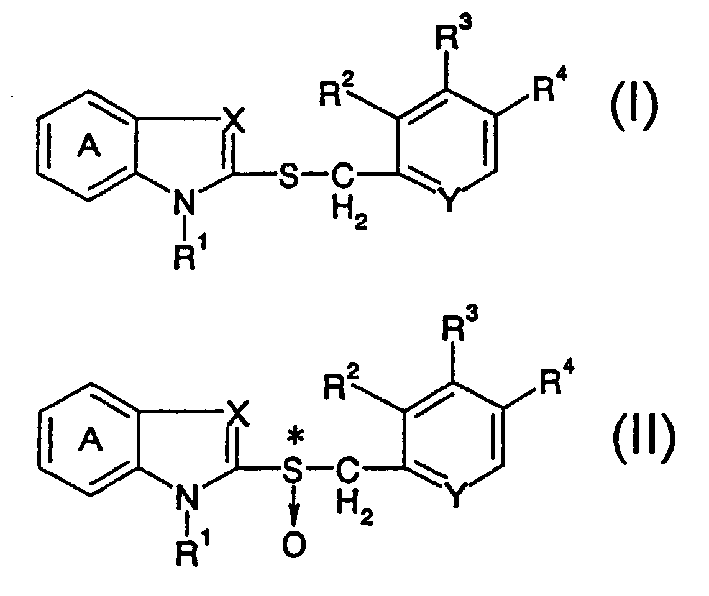
The present invention relates to a production method of an optically active form of a compound represented by formula (II) wherein ring A is a benzene ring optionally having substituent(s); R1 is H, a hydrocarbon group optionally having substituent(s), an acyl group or an acyloxy group; R2, R3 and R4 are each H, an alkyl group optionally having substituent(s), an alkoxy group optionally having substituent(s) or an amino group optionally having substituent(s); X is N or CH; Y is N or CH; and * shows an asymmetric center, or a salt thereof, which includes reacting a compound represented by the formula (I) wherein each symbol is as defined above, or a salt thereof, with an excess amount of an oxidizing agent in the presence of a catalyst for asymmetric induction, and provides an efficient production method of an optically active sulfoxide derivative in high yield on an industrial large scale by a convenient method, while achieving an extremely high enantiomer excess.
Owner:TAKEDA PHARMA CO LTD
Iridium catalyzed enantiotropic hydrosubstituting process of aromatic pyridine ring and pyrazine ring
InactiveCN1468852AHigh enantiomeric excessMild reaction conditionsOrganic chemistry methodsOrganic-compounds/hydrides/coordination-complexes catalystsIridiumQuinoxaline
The iridium catalyzed enantiotropic hydrosubstituting process of aromatic pyridine ring and pyrazine ring uses catalyst system comprising chiral coordination compound of iridium and additive compound. The reaction conditions includes temperature of 0-80 deg.c; solvent of dichloromethane, toluene, tetrahydrofuaran, 1, 2-dichloro ethane, isopropanol, etc.; additive compound of tetrabutyl ammonium iodide, iodine, amine, etc.; pressure of 1-100 atm; substrate / catalyst ratio up to 5000; and chiral coordination compound of diphosphine, N-P compound, S-P compound, etc. The catalyst system is prepared through reaction of iridium precursor and chiral compound in the said solvent and the addition of the additive compound while stirring; and can produce asymmetrical induction up to 96 %. The process of the present invention is environment friendly.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Nanometer structure cinchona alkaloid thiourea multi-phase bionic catalyst and preparation method thereof
InactiveCN101168134AOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsThioureaActive component
Provided is a nanostructured polyphase biomimetic catalyst of quinine and a process for preparing same, belonging to the catalytic field of preparing technology of biomimetic catalyst. The method for preparation of the novel catalyst is that mesoporous material with a nanometer-sized bore diameter is employed to be a vector, a functional group provided by the vector is employed to be a first platform and reacts with substance which has active primitives, and further a second reactive platform is provided and is assembled in situ inside a nanostructured space, thereby forming the objective catalyst. The catalyst has the characteristics that active components are assembled via high dispersion in a one-dimensional or three-dimensional straight-through nanometer-sized pore passage, forming a nanostructured chiral molecule reactor, and displaying the high asymmetric inductivity. The value of the catalyst is higher than the homogeneous phase catalytic level, and the catalyst has the high reutilization property and important adaptation value. Further, the invention is easy to be commercially developed.
Owner:BEIJING UNIV OF CHEM TECH
Process for producing optically active sulfoxide devivative'
InactiveCN1426406AHigh overdoseLess quantityOrganic active ingredientsOrganic chemistryHydrogenEnantiomer
A process for producing an optically active isomer of a compound represented by the formula (II) (wherein ring A represents an optionally substituted benzene ring; R<1> represents hydrogen, an optionally substituted hydrocarbon group, acyl, or acyloxy; R<2>, R<3>, and R<4> each represents hydrogen, optionally substituted alkyl, optionally substituted alkoxy, or optionally substituted amino; X represents nitrogen or CH; Y represents nitrogen or CH; and * indicates an asymmetric center) or of a salt of the compound, characterized by reacting a compound represented by the formula (I) (wherein the symbols have the same meanings as the above) or a salt thereof with an excess of an oxidizing agent in the presence of a catalyst for asymmetry induction. This process is a simple process by which an optically active sulfoxide derivative can be efficiently and industrially mass-produced in high yield while attaining an extremely high enantiomer excess.
Owner:TAKEDA PHARMA CO LTD
Method for preparing chiral beta-amino arylbutyric acid derivatives
InactiveCN102212015AHigh optical purityEasy to operateOrganic compound preparationAmino-carboxyl compound preparationArylAsymmetric induction
The invention relates to a method for preparing chiral beta-amino arylbutyric acid derivatives (as shown in a structural formula I). The method comprises the following steps: condensing aryl acetoacetic ester with chiral alkamine; carrying out asymmetric induction reduction so as to obtain annular chiral beta-amino arylbutyric acid derivatives (as shown in a structural formula II); and hydrogenating the compound II so as to prepare chiral beta-amino arylbutyric acid derivatives (as shown in the structural formula I). The method is convenient for operation, low in cost and suitable for large-scale production, and the optical purity of the obtained product is high (more than 99.0% ee (enantiomeric excess)).
Owner:UNITRIS BIOPHARMA
Phosphoramidite ligand as well as preparation method and application thereof
ActiveCN104610363AHigh reactivityHigh enantioselectivityGroup 5/15 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsIridiumCatalytic effect
The invention relates to the field of chemical catalysis and discloses a phosphoramidite ligand as well as a preparation method and an application thereof. The synthesized phosphoramidite ligand and an enantiomer or a racemate of the phosphoramidite ligand take biphenyl as a framework and are subjected to an asymmetric induced reaction of axial chirality to realize complete transfer from central chirality to axial chirality. A synthetic method for the phosphoramidite ligand and the enantiomer or the racemate of the phosphoramidite ligand is simple and economic, and a common and complex chiral resolution process is avoided during chiral ligand preparation. The phosphoramidite ligand and the enantiomer or the racemate of the phosphoramidite ligand is employed to form the ligand together with active metal-iridium for catalyzing an asymmetric addition reaction of N-protected isatin and arylboronic acid, a very good catalytic effect is achieved, and a product can obtain the yield of more than 96% and the enantiomeric excess (ee) value of more than 92%.
Owner:SUN YAT SEN UNIV
Method and apparatus for determining an overlay error
ActiveUS8908147B2Material analysis by optical meansPlant cultivationAsymmetric inductionScatterometer
A method of determining an overlay error. Measuring an overlay target having process-induced asymmetry. Constructing a model of the target. Modifying the model, e.g., by moving one of the structures to compensate for the asymmetry. Calculating an asymmetry-induced overlay error using the modified model. Determining an overlay error in a production target by subtracting the asymmetry-induced overlay error from a measured overlay error. In one example, the model is modified by varying asymmetry p(n′), p(n″) and the calculating an asymmetry-induced overlay error is repeated for a plurality of scatterometer measurement recipes and the step of determining an overlay error in a production target uses the calculated asymmetry-induced overlay errors to select an optimum scatterometer measurement recipe used to measure the production target.
Owner:ASML NETHERLANDS BV
Method for antipodal selective synthesis of (R)-lansoprazole
InactiveCN104177336AHigh optical purityHigh chemical purityOrganic chemistryLansoprazoleAsymmetric induction
The invention relates to a preparation method for efficient synthesis of (R)-lansoprazole, and according to the method, in the presence of an asymmetric induction catalyst, an oxidant with 1.3 to 1.5 times molar equivalent relative to prochiral compound lansoprazole thioether is used for selective catalytic oxidation to obtain the (R)-lansoprazole. The method has the advantages of being economic, environmentally friendly, high efficiency, high in optical purity and high in chemical purity of products, and is a method suitable for industrialized production.
Owner:SHANGHAI HUILUN BIOLOGICAL TECH CO LTD
Cinchona alkaloid quaternary ammonium salt derivatives as well as preparation method and application thereof
InactiveCN101531658AHigh catalytic efficiencyHigh activityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsSynthesis methodsHigh activity
The invention relates to cinchona alkaloid quaternary ammonium salt derivatives and a synthesis method thereof as well as application of the derivatives in synthetizing high optical purity natural amino acid and non-natural amino acid. A structural general formula (I) represents a chiral cinchona alkaloid quaternary ammonium salt, wherein R1 is methoxyl, hydroxyl, hydrosulfuryl or hydrogen; R2 is alkyl with 3 to 20 carbon atoms; X is halogen; and n is a positive integer from 1 to 4. The cinchona alkaloid quaternary ammonium salt catalyst has high activity in an alkylation reaction, good stereoselectivity, high catalysis efficiency, a wide substrate range and good asymmetric induction.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Asymmetric induction catalytic synthesis method for L-muscone
InactiveCN101863749AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsSynthesis methodsAsymmetric induction
The invention discloses an asymmetric induction catalytic synthesis method for L-muscone. The L-muscone is prepared by performing asymmetric induction catalytic synthesis on N-3-methyl-cyclopentadecane alkenyl benzylamine which is obtained by asymmetrically converting a racemate compound for multiple times and used as raw materials in the presence of an organo-transition metal complex inducing catalyst. The method has the prominent advantages of high economic benefit, simple synthesis process and low synthesis temperature.
Owner:DALIAN ZHAOYI BIOLOGY KETONE TECH
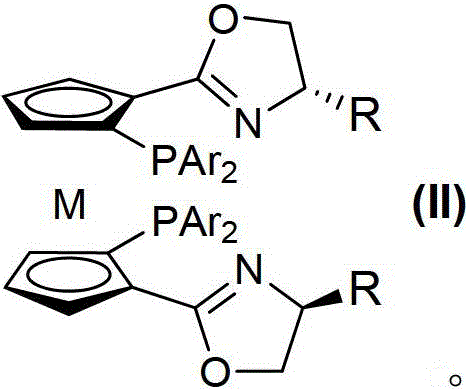
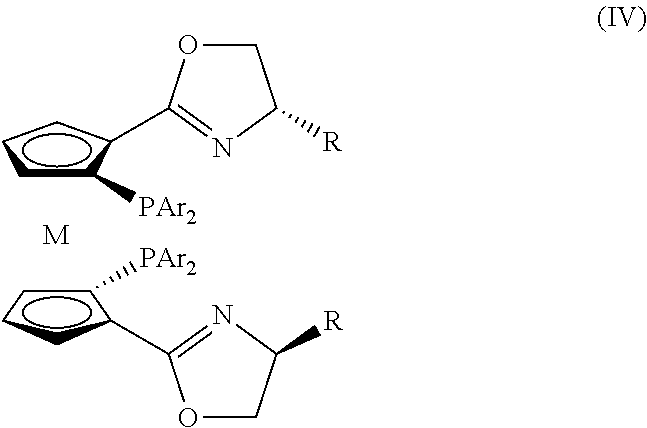
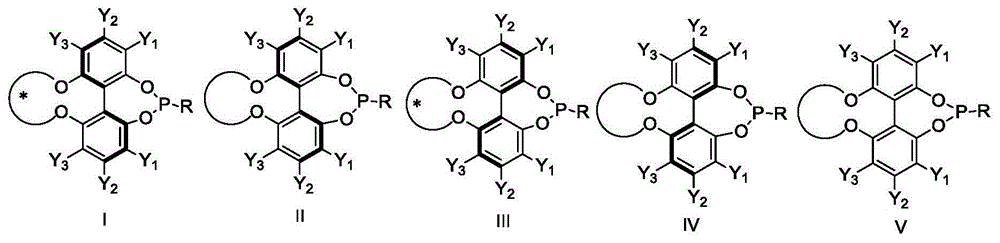
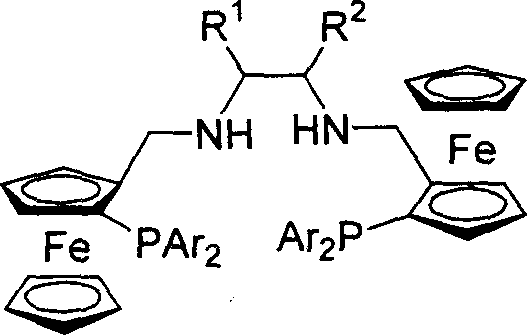
Planar chiral [2,2]-p-cyclo-aralkyl oxazolinyl phosphine ligand and its synthesis and use
InactiveCN1398865AGood chemical stabilityEasy to adjustGroup 5/15 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsPlanar chiralityAlloy
The present invention relates to a kind of planar chiral [2,2]-p-cyclo-aralkyl oxazolinyl phosphine ligand and its synthesis and use. The compound is prepared through the reaction between chiral [2,2]-p-cyclo- oxazolinyl bromide and dibasic phosphine chloride R2'PCl in the presence of alkali. The ligand has two chiral factors of planar chirality and central chirality as well as convertibility of phosphine substituent and oxazolinyl ring substituent, and thus strong adaptability to different asymmetrical catalytic reaction. For example, it has good asymmetrical induction result in Pd catalyzed asymmetrical alloy substituting reaction and copper catalytic asymmetrical 1,4-addition reaction. The ligand has high chemical stability, simple synthesis and is suitable for use in industrial production.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
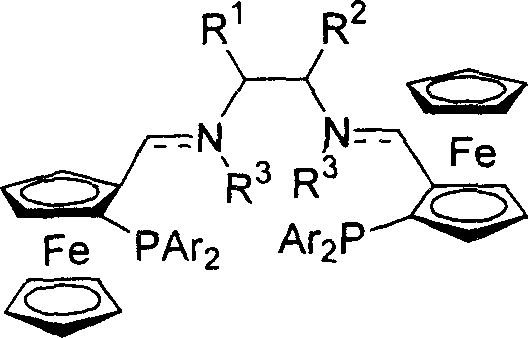
Dicyclopentadienyl iron pocket ligand possessing central chirality and planar chirality, synthesis method and uses
InactiveCN1733782AHigh yieldHigh enantioselectivityGroup 5/15 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsSynthesis methodsPlanar chirality
The invention relates to a ferrocene ligand having central chirality and planar chirality, method for synthesizing and use thereof. The ligand has the central chirality of diamines, and is obtained through condensation of ferrocene phosphine aldehyde and chiral diamine, and further reduction, the ligand can be purified through recrystallization and column chromatography. The ligand can be used as catalyst in asymmetric alkylation reaction.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Synthesis method of cinacalcet hydrochloride and intermediate thereof
ActiveCN104829460AHigh purityHigh ee valueOrganic compound preparationPreparation by reductive alkylationSynthesis methodsAsymmetric induction
The invention discloses a synthesis method of cinacalcet hydrochloride and an intermediate thereof. The cinacalcet hydrochloride is prepared by carrying out asymmetric reduction ammonification on 3-(3-(trifluoromethyl)phenyl)propyl-1-amine and 1-(naphthyl-1-yl)ethyl ketone. The asymmetric reduction ammonification is performed by carrying out asymmetric induction by combining a Hantzsch ester and a chiral phosphine ligand. The 3-(3-(trifluoromethyl)phenyl)propyl-1-amine is prepared by the following steps: carrying out Heck coupling reaction on 3-bromo-1-trifluoromethyl benzene and acrylonitrile to obtain 3-(3-(trifluoromethyl)phenyl)acrylonitrile, and carrying out catalytic hydrogenation. The catalyst of the Heck coupling reaction is tetra(triphenylphosphine) palladium, DBA palladium or palladium acetate. The method for synthesizing cinacalcet hydrochloride greatly lowers the production cost, and is suitable for industrialized mass production. The method for synthesizing the 3-(3-(trifluoromethyl)phenyl)propyl-1-amine greatly lowers the production cost, and has higher yield.
Owner:CHANGZHOU SUNLIGHT PHARMA
Method for using palladium homogeneous system in enantioselective catalysis of ketone hydride
InactiveCN1899695AEasy to operateRaw materials are easy to getOrganic-compounds/hydrides/coordination-complexes catalystsReaction temperatureKetone
The method of catalyzing hydrogenation of ketone enantioselectively with homogeneous Pt catalyst system has chiral diphosphorus complex of Pt as catalyst system, reaction temperature of 25-75deg.c, solvent of 2, 2, 2-trifluoroethanol, reaction pressure of 3-70 atm and chiral diphosphorus ligand used. The homogeneous Pt catalyst system is prepared through mixing metal precursor of Pt and chiral diphosphorus ligand at room temperature and the subsequent vacuum concentrating. The catalyst can result in asymmetrical inducing as high as 92 % on alpha-one-benzamide substituted ketone. The present invention has simple operation, facile material, high reaction selectivity, high yield and environment friendship.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
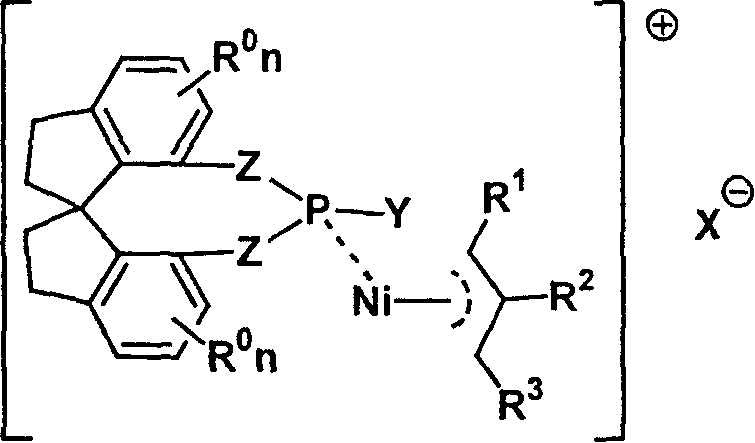
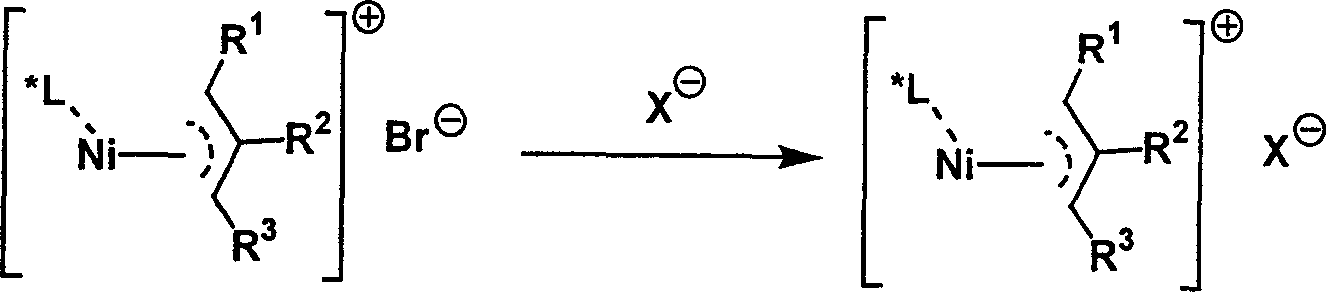
Metal complex catalyst of chiral spirocyclo mono-phosphorus (phosphine) ligand and nickel, its prepn. method and application
InactiveCN1792452AEasy to synthesizeHigh chemoselectivityOrganic-compounds/hydrides/coordination-complexes catalystsAsymmetric inductionHydrogenation reaction
A metal complex as catalyst with wide range of application is prepared from chiral spirocyclic mono-phosphorus (mono-phosphine) ligand and Ni. It can be used in asymmetrical olefinic hydrogenation reaction to obtain a compound with chiral center. Its preparing process is also disclosed.
Owner:NANKAI UNIV
Carbon-phosphorus chiral dialkyl oxygen phosphine and synthesis method thereof
InactiveCN103665038AClear operational advantageReduce stepsGroup 5/15 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsAsymmetric inductionPhenyl group
The invention provides (RP)-menthyl phenyl oxygen phosphine and a synthesis method thereof. The synthesis method comprises the following steps of (1) preparing a menthyl magnesium halide or a menthyl lithium solution; (2) enabling the menthyl magnesium halide or the menthyl lithium solution to react with phenyl phosphine dichloride; (3) hydrolyzing the phenyl menthyl phosphine chloride; (4) performing post-treatment. According to the method, the obtained phenyl menthyl oxygen phosphine has a better asymmetric induction effect as containing a large-volume of mint group; besides, the chiral menthyl facilitates the stabilization of phosphorus atom configuration.
Owner:LIAOCHENG UNIV
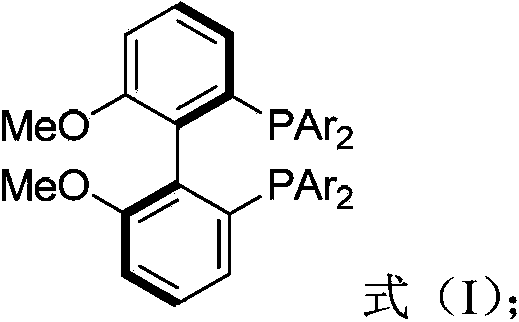
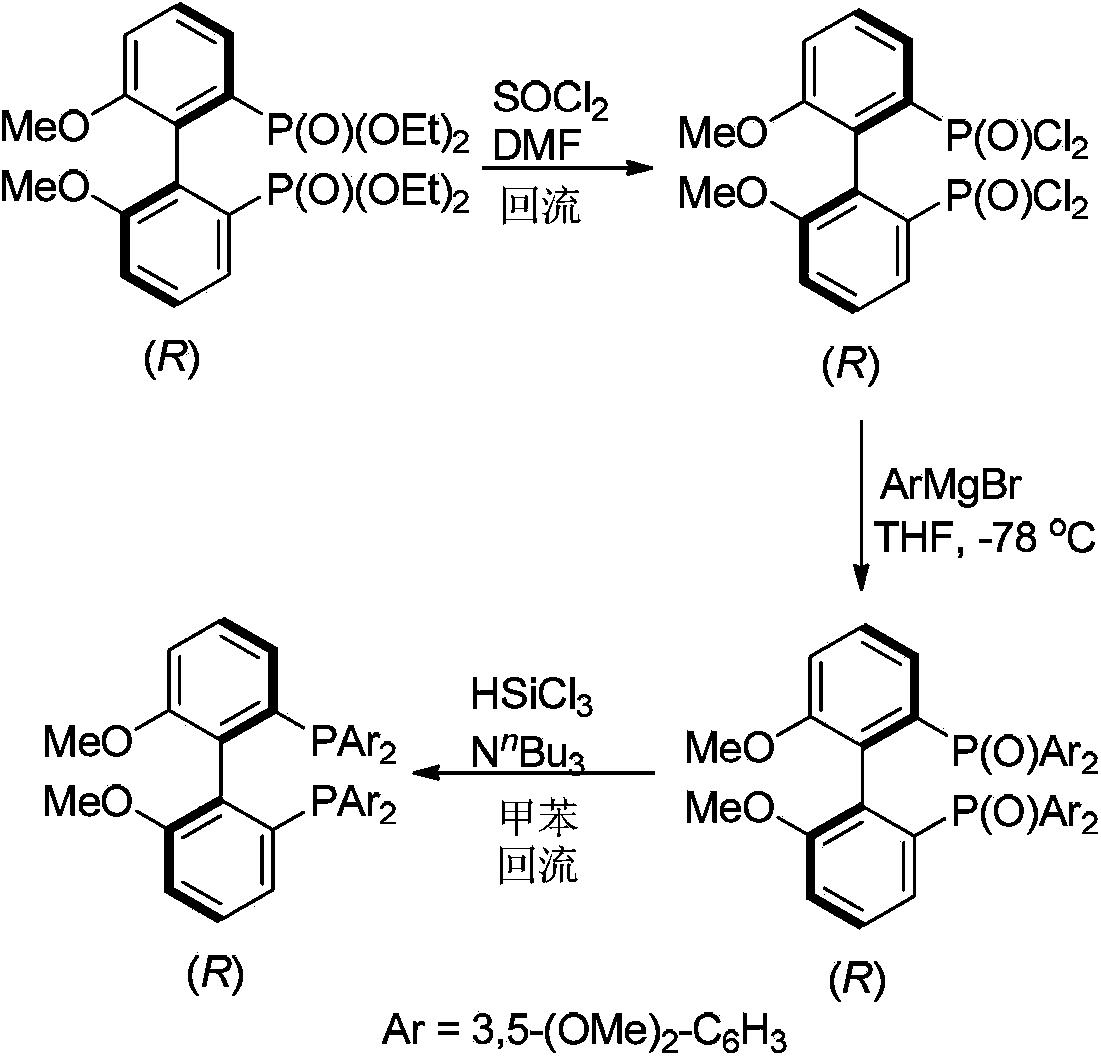
Biphenyl ligand, synthetic method thereof and application thereof in methoxyl carbonylation reaction of racemized propargyl alcohol carbonate
ActiveCN104109174AIncrease steric hindranceMeet needsGroup 5/15 element organic compoundsPreparation by carbon monoxide or formate reactionAsymmetric inductionCarbonylation
The invention discloses a biphenyl ligand, a synthetic method thereof and an application thereof in an asymmetric methoxyl carbonylation reaction, catalyzed by palladium, of racemized propargyl alcohol carbonate, wherein the biphenyl ligand is R-(+)-2,2'-bis(di(3,5,dimethoxyphenyl)-6,6'-dimethoxy-1,1'-biphenyl. The biphenyl can achieve asymmetric induction to the methoxyl carbonylation reaction of the racemized propargyl alcohol carbonate and 2,3-allenoate having higher optical purity can be prepared in high efficiency.
Owner:EAST CHINA NORMAL UNIV
Asymmetric oxidation method for dexlansoprazole
InactiveCN104610226ALess quantityAvoid excessive oxidationAsymmetric synthesesOver oxidationOrganic solvent
The invention relates to an asymmetric oxidation method for dexlansoprazole. The invention provides a method for producing 2-[[3-methyl-4-(2,2,2-trifluoroethoxyl)pyridine-2-radical]methylsulfinyl]-1H-benzimidazole. The method comprises the step of: in an organic solvent, performing the oxidation reaction on 2-[3-methyl-4-(2,2,2-trifluoroethoxyl)-2-pyridine]methylsulfonyl-1H-benzimidazole and an oxidizing agent under the existence of an asymmetric induction effect catalyst and alkali. The method is characterized in that the quantity of the oxidizing agent is about 1.2-1.4 molar equivalents relative to the 2-[3-methyl-4-(2,2,2-trifluoroethoxyl)-2-pyridine]methylsulfonyl-1H-benzimidazole. According to the production method disclosed by the invention, by controlling the use quantity of the oxidizing agent, raw materials can be completely converted and over oxidation is also avoided, so that target optical rotation sulfoxide derivatives can be effectively and industrially prepared under high yield and in a large scale by the convenient method, meanwhile, quite high enantioselectivty is realized and the quantity of over oxidation byproducts is very small.
Owner:SUNSHINE LAKE PHARM CO LTD
Asymmetric hydrogenation method for ketone compound
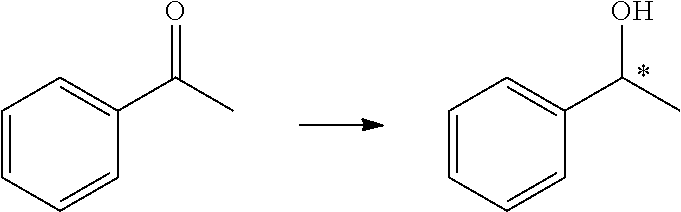
InactiveUS20130053574A1Effectively synthesize enantiomerically pure alcoholsSynthetic is simpleOrganic compound preparationOxygen compounds preparation by reductionHydrogen atmosphereAsymmetric induction
The invention relates to an asymmetric hydrogenation method for ketone compounds, comprising the step of: under hydrogen atmosphere, in the presence of an in situ catalyst derived from a chiral ligand and a ruthenium salt, adding a ketone compound and a base into a second solvent to carry out an asymmetric hydrogenation for the ketone compound. The invention can obtain a conversion of 100% and a highest asymmetric inducement effect of 99.7% for the ketone compound. The invention has the advantages including simple procedure, high conversion and selectivity, good atom economy and good prospect of industrial application.
Owner:NIPPON CHECMICAL IND CO LTD
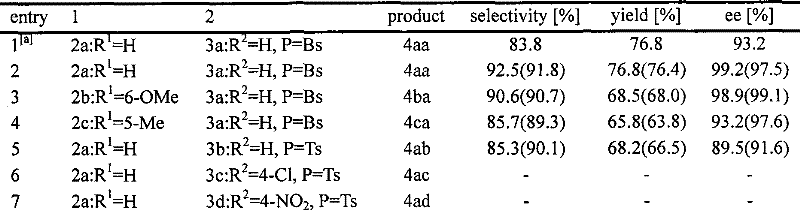
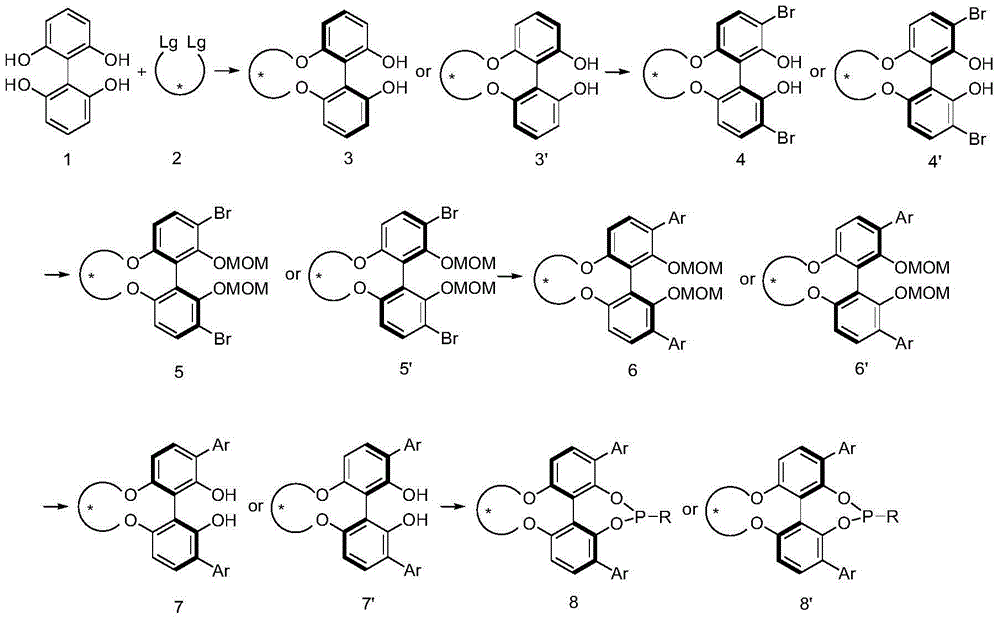
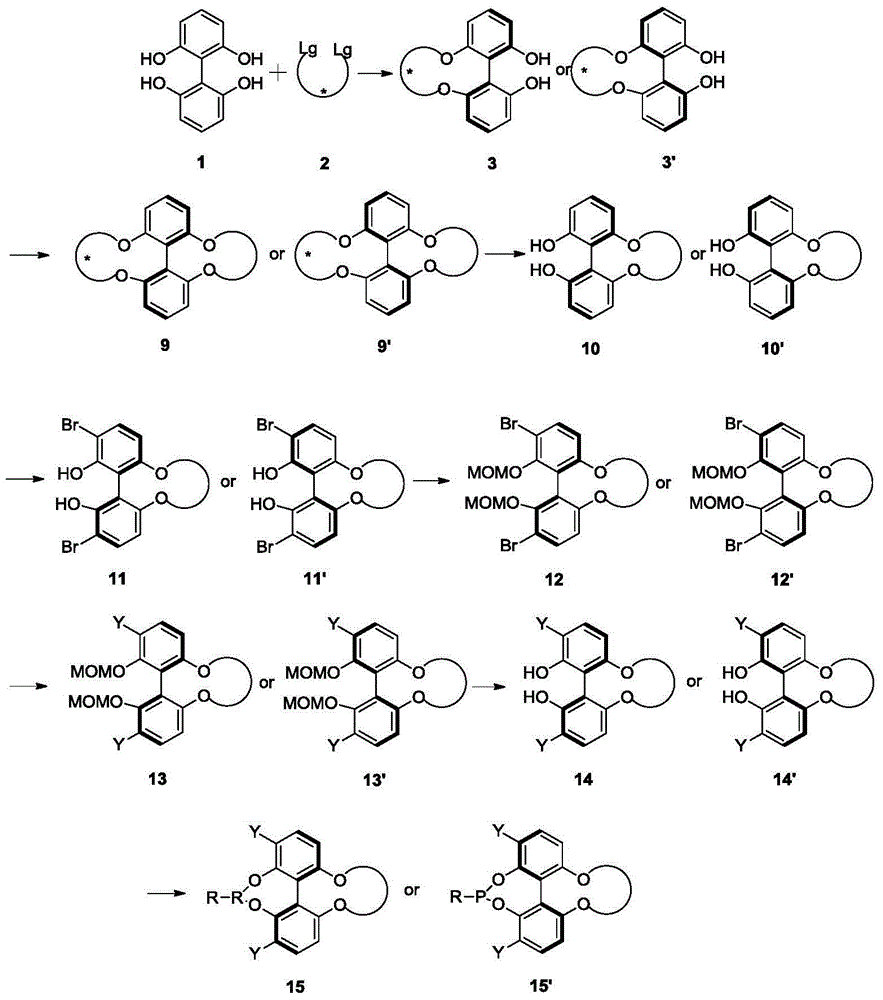
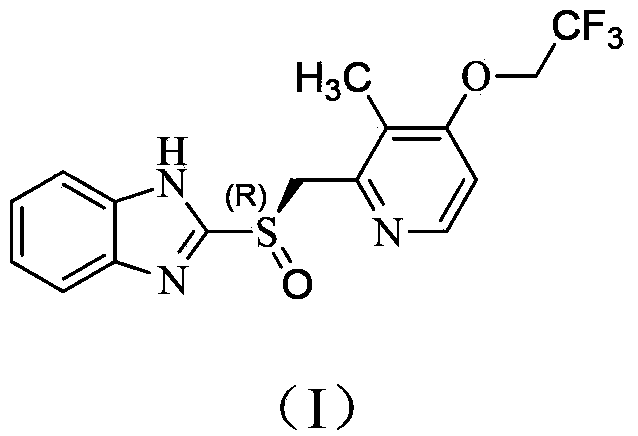
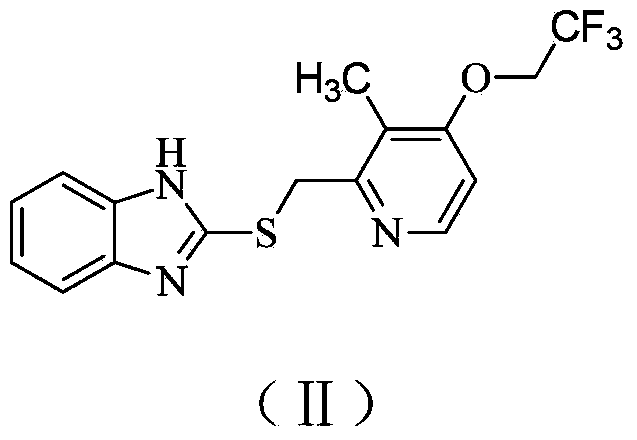
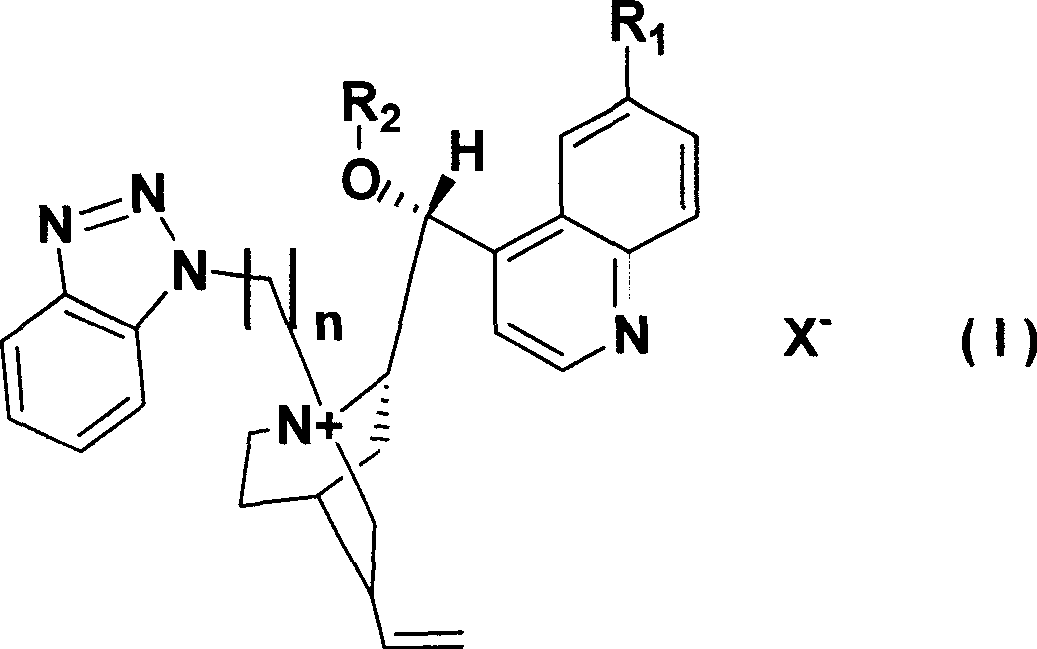
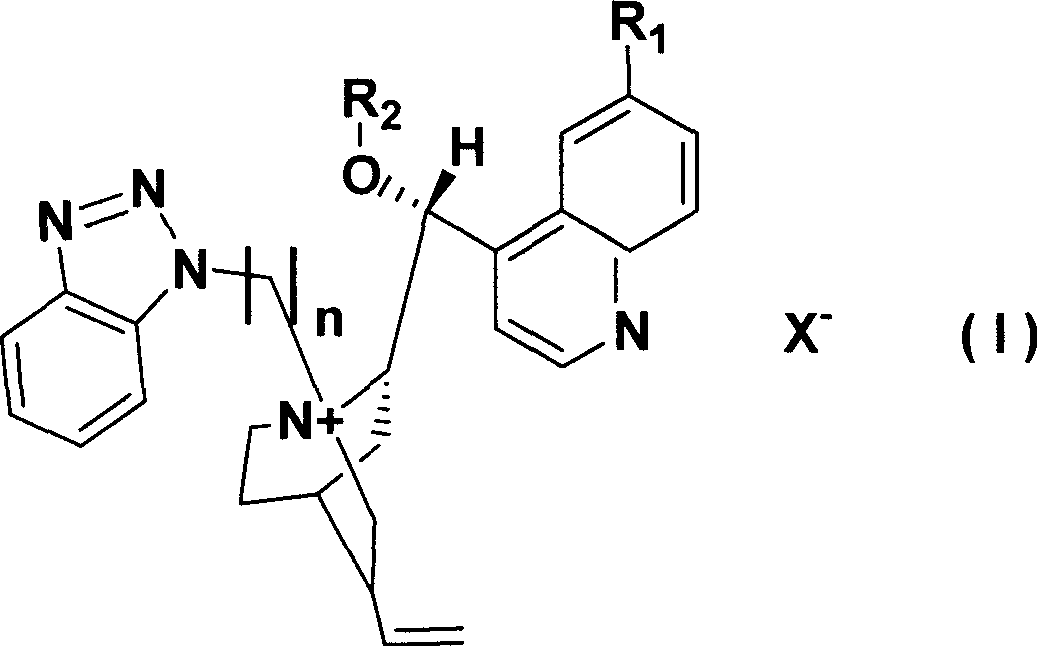
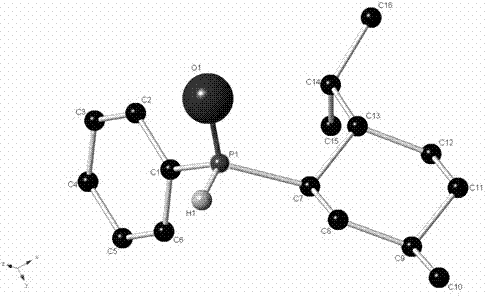
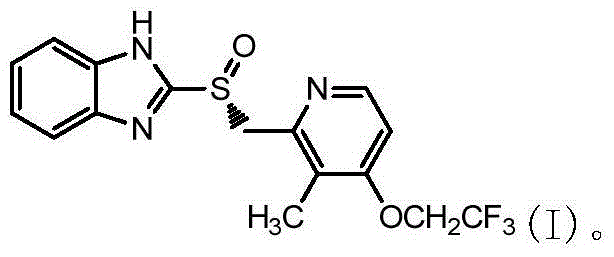
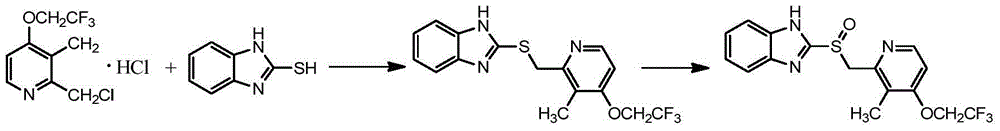
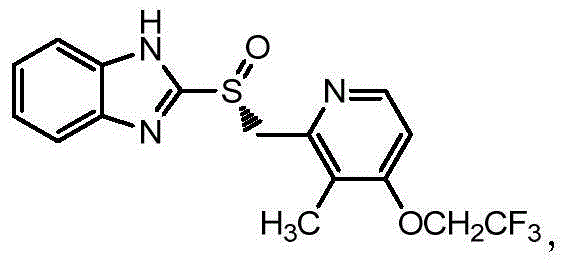
Preparation method of (R)-2-[[[3[methyl-4-nitro-2-pyridyl]methyl]sulfinyl]benzimidazole
The invention discloses a preparation method of (R)-2-[[[3[methyl-4-nitro-2-pyridyl]methyl]sulfinyl]benzimidazole. The preparation method is characterized in that the preparation method comprises a step that 2-[[[3[methyl-4-nitro-2-pyridyl]methyl]thio]benzimidazole or its salt reacts with an excess oxidant in a solvent in the presence of an asymmetric induction effect catalyst, and the molar ratio of the 2-[[[3[methyl-4-nitro-2-pyridyl]methyl]thio]benzimidazole or its salt to the oxidant is 1:1-1:2.0. The optical purity and the yield of (R)-2-[[[3[methyl-4-nitro-2-pyridyl]methyl]sulfinyl]benzimidazole prepared through the method of the invention are extremely high.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Device and method for evaluating organic material for organic solar cell
ActiveUS20140213800A1High enantioselectivityHigh reactionRhodium organic compoundsCarboxylic acid amides optical isomer preparationOrganic solar cellAsymmetric induction
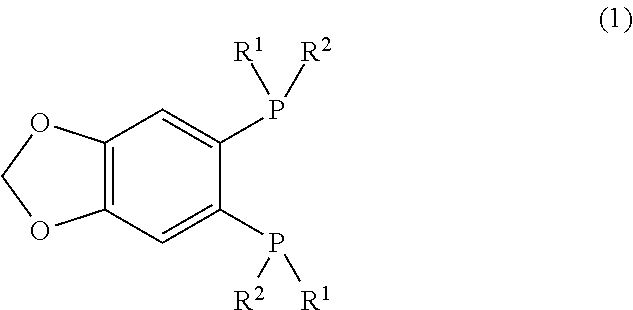
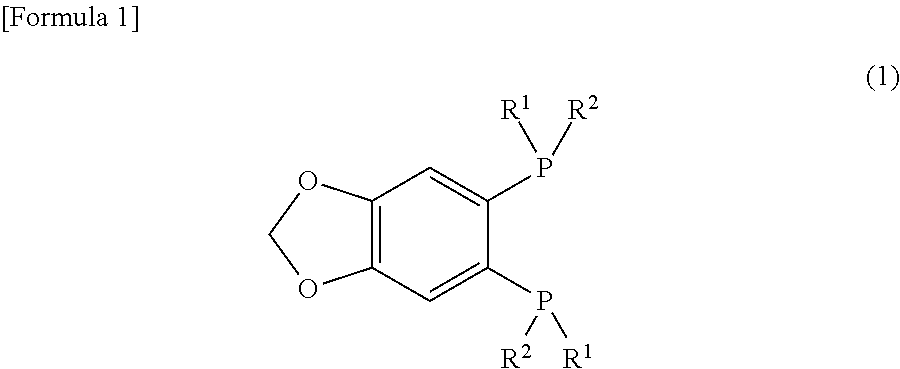
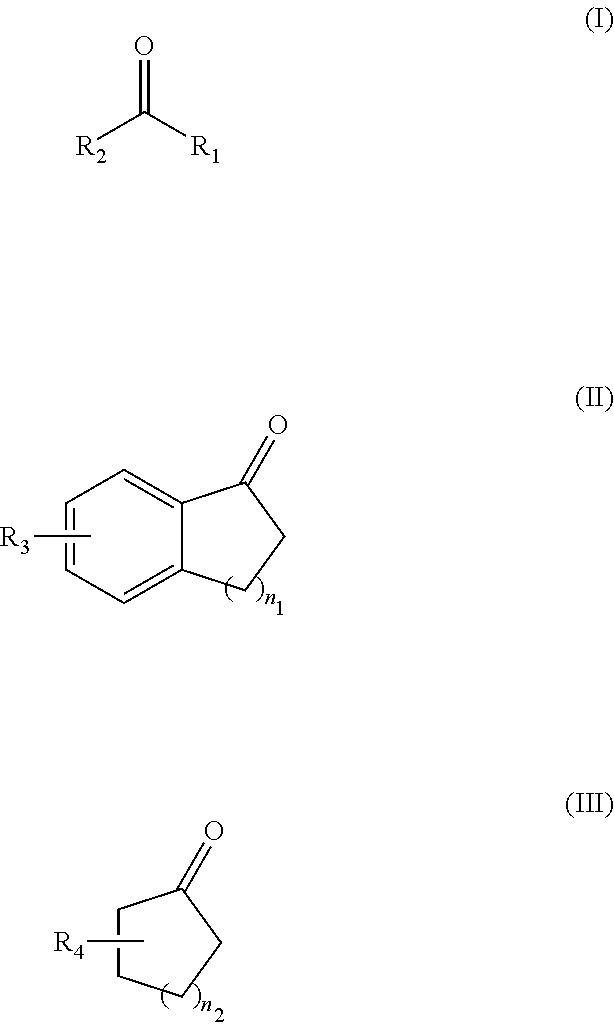
Provided are a novel 1,2-bis(dialkylphosphino)-4,5-(methylenedioxy)benzene derivative that forms a metal complex having particularly high asymmetry induction capacity and catalytic activity on β-dehydroamino acids, a method for manufacturing the same, a metal complex having this 1,2-bis(dialkylphosphino)-4,5-(methylenedioxy)benzene derivative as a ligand, and an asymmetric hydrogenation method using this metal complex. A 1,2-bis(dialkylphosphino)-4,5-(methylenedioxy)benzene derivative represented by general formula (1). (In the formula, R1 and R2 represent an alkyl group having 1-10 carbon atoms, and R1 and R2 have different numbers of carbon atoms.)
Owner:NIPPON CHECMICAL IND CO LTD
Planar chiral double-reactive center ruthenium catalyst and synthesis and application thereof
ActiveCN104056663AEasy to synthesizeHigh yieldOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystAsymmetric induction
The invention discloses a planar chiral double-reactive center ruthenium catalyst, synthesis and an application thereof. The planar chiral double-reactive center ruthenium catalyst provided by the invention not only is simple in synthesis, but also has double reactive centers, and can be used efficiently for asymmetric catalytic hydrogenation of beta-aminoketone compounds; and finally a conversion rate of 100% and an asymmetric induction effect of more than 99.9% can be achieved. The synthesis of the catalyst of the invention is simple in operation, and high in selectivity and yield, and has good atom economy and good industrial application prospects.
Owner:SHANGHAI JIAO TONG UNIV
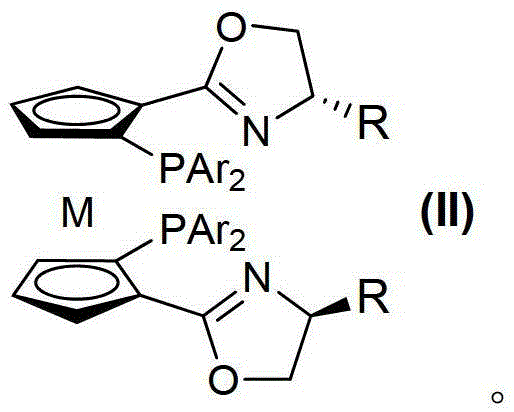
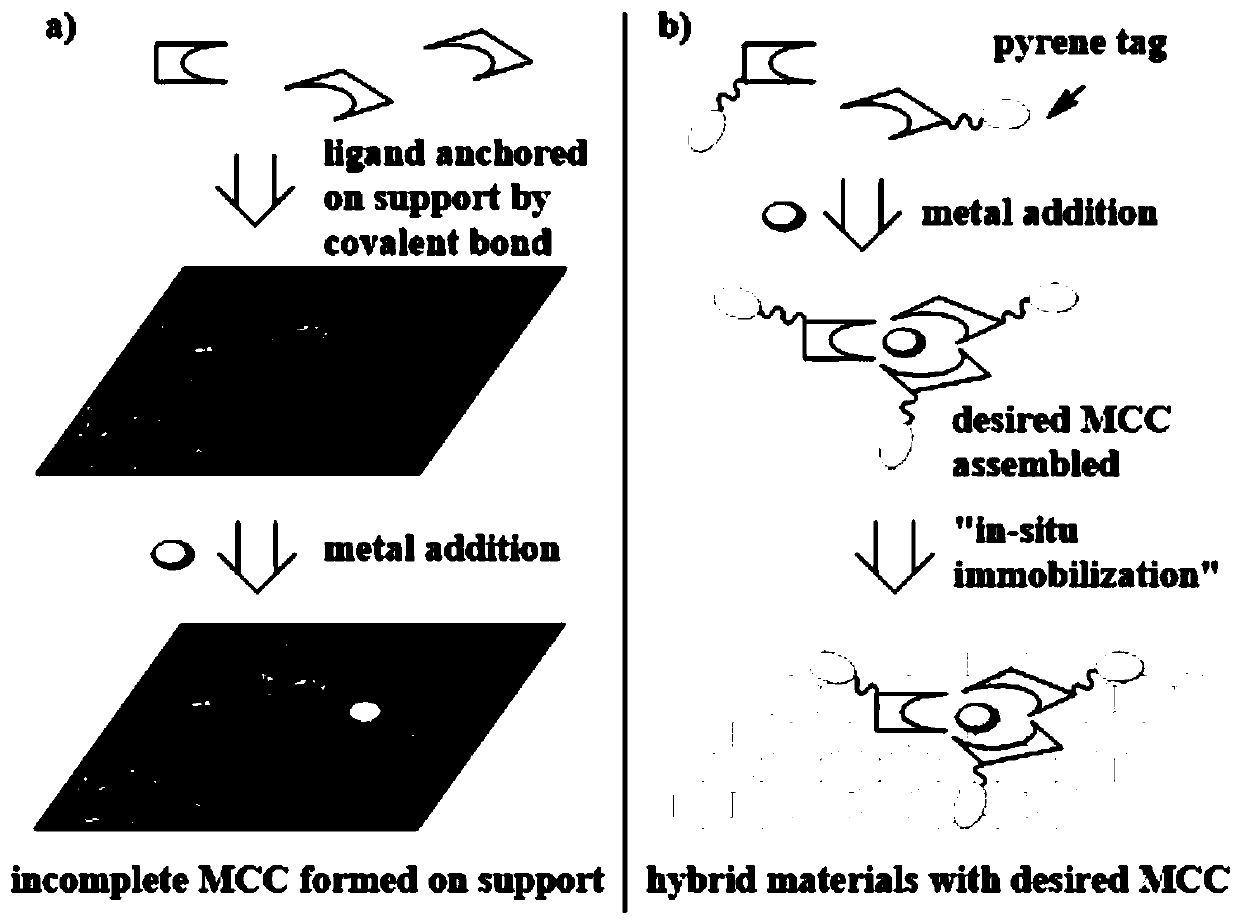
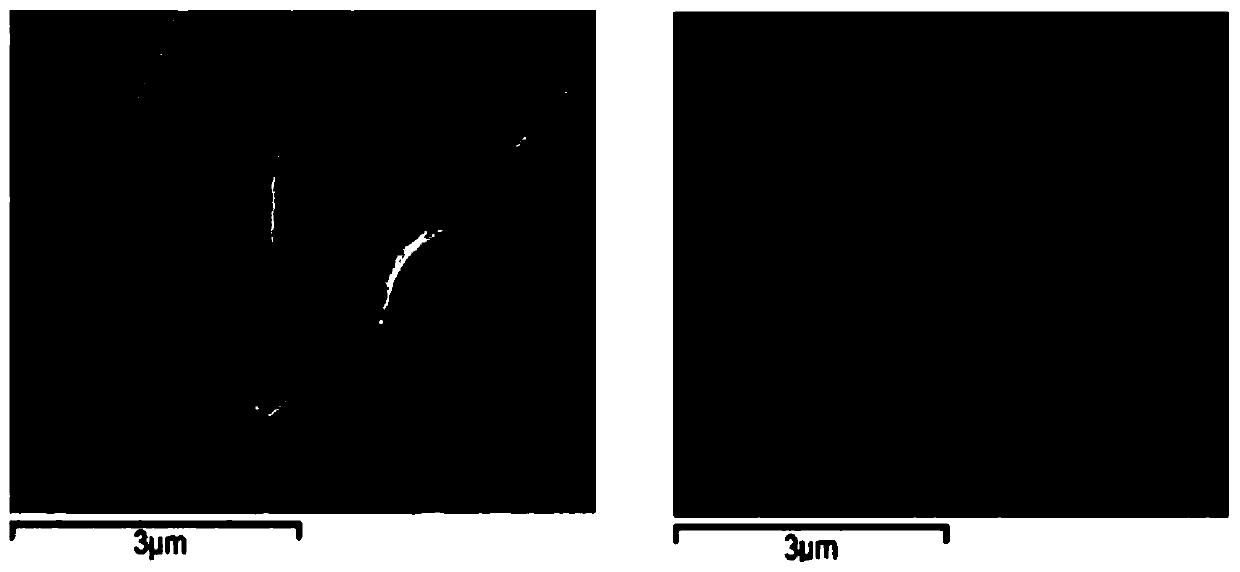
Graphene adsorption multi-component chiral catalyst and application thereof in asymmetric hydrogenation
ActiveCN110841720AAvoid the need for additional chemical modificationsImprove responseOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystAsymmetric induction
The invention discloses a graphene adsorption multi-component chiral catalyst and application thereof in asymmetric hydrogenation, and belongs to the field of organic chemistry. A hybrid material multi-component chiral catalyst 2a @ graphene adsorbed on graphene is prepared by using an in-situ immobilization strategy, and when the catalyst is applied to hydrogenation of a dehydroamino acid derivative, a good asymmetric induction effect is shown, and the conversion rate reaches 99% or above and the highest ee is 96%. And after the reaction is finished, recycling can be realized through simple filtration. The graphene adsorption multi-component chiral catalyst provides a good reference for developing other heterogeneous hybrid chiral catalysts based on non-covalent interaction asymmetric reaction.
Owner:HENAN NORMAL UNIV
A kind of phosphoramidite ligand and its preparation method and application
ActiveCN104610363BHigh reactivityHigh enantioselectivityGroup 5/15 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsIridiumSynthesis methods
The invention relates to the field of chemical catalysis, and discloses a kind of phosphoramidite ligand, its preparation method and application. The synthesized phosphoramidite ligands and their enantiomers or their racemates use biphenyl as the backbone, and undergo a central chiral asymmetric induction reaction to achieve complete central chirality to axial chirality transfer. The synthesis method of the phosphoramidite ligand and its enantiomer or its raceme is simple and economical, and the common and complicated chiral resolution process is avoided when preparing the chiral ligand. The present invention uses phosphoramidite ligands and their enantiomers or their racemates to form complexes with active metal iridium to catalyze the asymmetric addition reaction of N-protected isatin and arylboronic acid, and obtains a good catalytic effect. The product can be obtained in a yield of more than 96% and an enantiomeric excess (ee) value of more than 92%.
Owner:SUN YAT SEN UNIV
A kind of chiral double reaction center ruthenium catalyst and its synthesis and application
ActiveCN104056663BEasy to synthesizeHigh yieldOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsSurface reactionAsymmetric induction
The invention discloses a chiral double reaction center ruthenium catalyst as well as its synthesis and application. The chiral double-reaction center ruthenium catalyst provided by the present invention is not only easy to synthesize, but also the double-reaction center ruthenium catalyst it has can be efficiently applied to the asymmetric catalytic hydrogenation of β-aminoketone compounds; finally, 100% conversion can be obtained rate and more than 99.9% asymmetry induction effect. The synthesis operation of the catalyst described in the invention is simple and convenient, the selectivity and yield are high, the catalyst has good atom economy and good industrial application prospect.
Owner:SHANGHAI JIAOTONG UNIV
Nanometer structure cinchona alkaloid thiourea multi-phase bionic catalyst and preparation method thereof
InactiveCN101168134BEasy to implementGood repeatabilityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsThioureaActive component
Provided is a nanostructured polyphase biomimetic catalyst of quinine and a process for preparing same, belonging to the catalytic field of preparing technology of biomimetic catalyst. The method for preparation of the novel catalyst is that mesoporous material with a nanometer-sized bore diameter is employed to be a vector, a functional group provided by the vector is employed to be a first platform and reacts with substance which has active primitives, and further a second reactive platform is provided and is assembled in situ inside a nanostructured space, thereby forming the objective catalyst. The catalyst has the characteristics that active components are assembled via high dispersion in a one-dimensional or three-dimensional straight-through nanometer-sized pore passage, forming a nanostructured chiral molecule reactor, and displaying the high asymmetric inductivity. The value of the catalyst is higher than the homogeneous phase catalytic level, and the catalyst has the high reutilization property and important adaptation value. Further, the invention is easy to be commercially developed.
Owner:BEIJING UNIV OF CHEM TECH
Indoline compound with optical activity and preparation method thereof
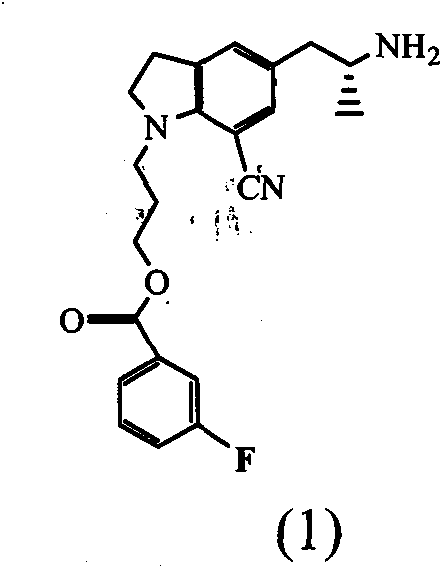
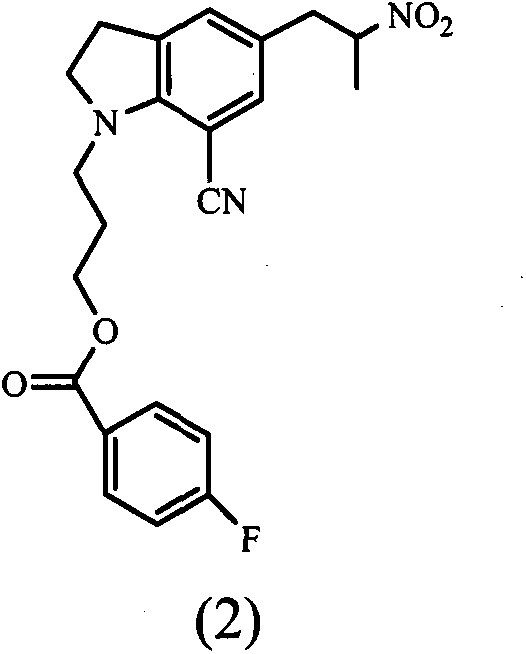
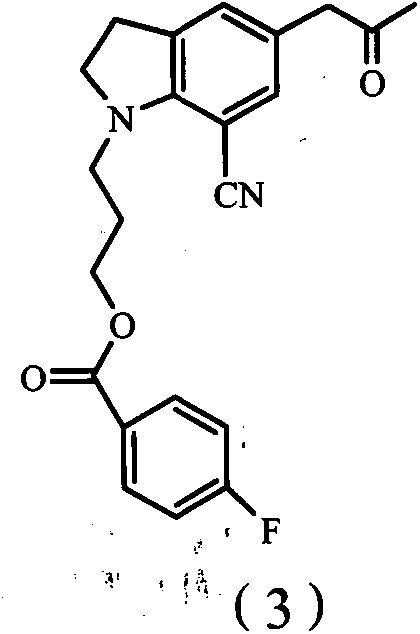
ActiveCN101993406BHigh rate of asymmetric inductionOrganic chemistryBulk chemical productionAsymmetric inductionIndoline
The invention provides an indoline compound with optical activity, namely R-5-(2-amidopropyl)-1-(3-(4-fluorine benzoyl oxygen group) propyl)-7-cyan indoline. The compound can be used as a key intermediate and used for preparing a chiral drug, namely, Silodosin. The invention also provides a preparation method of R-5-(2-amidopropyl)-1-(3-(4-fluorine benzoyl oxygen group) propyl)-7-cyan indoline, which is characterized by comprising the following steps: taking 1-(3-(4-fluorine benzoyl oxygen group) propyl)-5-(2-nitrylpropyl)-7-cyan indoline as a raw material to obtain the 1-(3-(4-fluorine benzoyl oxygen group) propyl)-5-(2-oxopropyl)-7-cyan indoline, carrying out asymmetric reaction on the 1-(3-(4-fluorine benzoyl oxygen group) propyl)-5-(2-oxopropyl)-7-cyan indoline and a cheap chiral auxiliary agent, namely alpha-phenylethylamine, wherein asymmetric inductivity is 6:1, and finally removing protective group.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD +1
Asymmetric hydrogenation method for ketone compound
InactiveUS8962852B2Effectively synthesize enantiomerically pure alcoholsSynthetic is simpleOrganic compound preparationGroup 8/9/10/18 element organic compoundsHydrogen atmosphereAsymmetric induction
The invention relates to an asymmetric hydrogenation method for ketone compounds, comprising the step of: under hydrogen atmosphere, in the presence of an in situ catalyst derived from a chiral ligand and a ruthenium salt, adding a ketone compound and a base into a second solvent to carry out an asymmetric hydrogenation for the ketone compound. The invention can obtain a conversion of 100% and a highest asymmetric inducement effect of 99.7% for the ketone compound. The invention has the advantages including simple procedure, high conversion and selectivity, good atom economy and good prospect of industrial application.
Owner:NIPPON CHECMICAL IND CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com



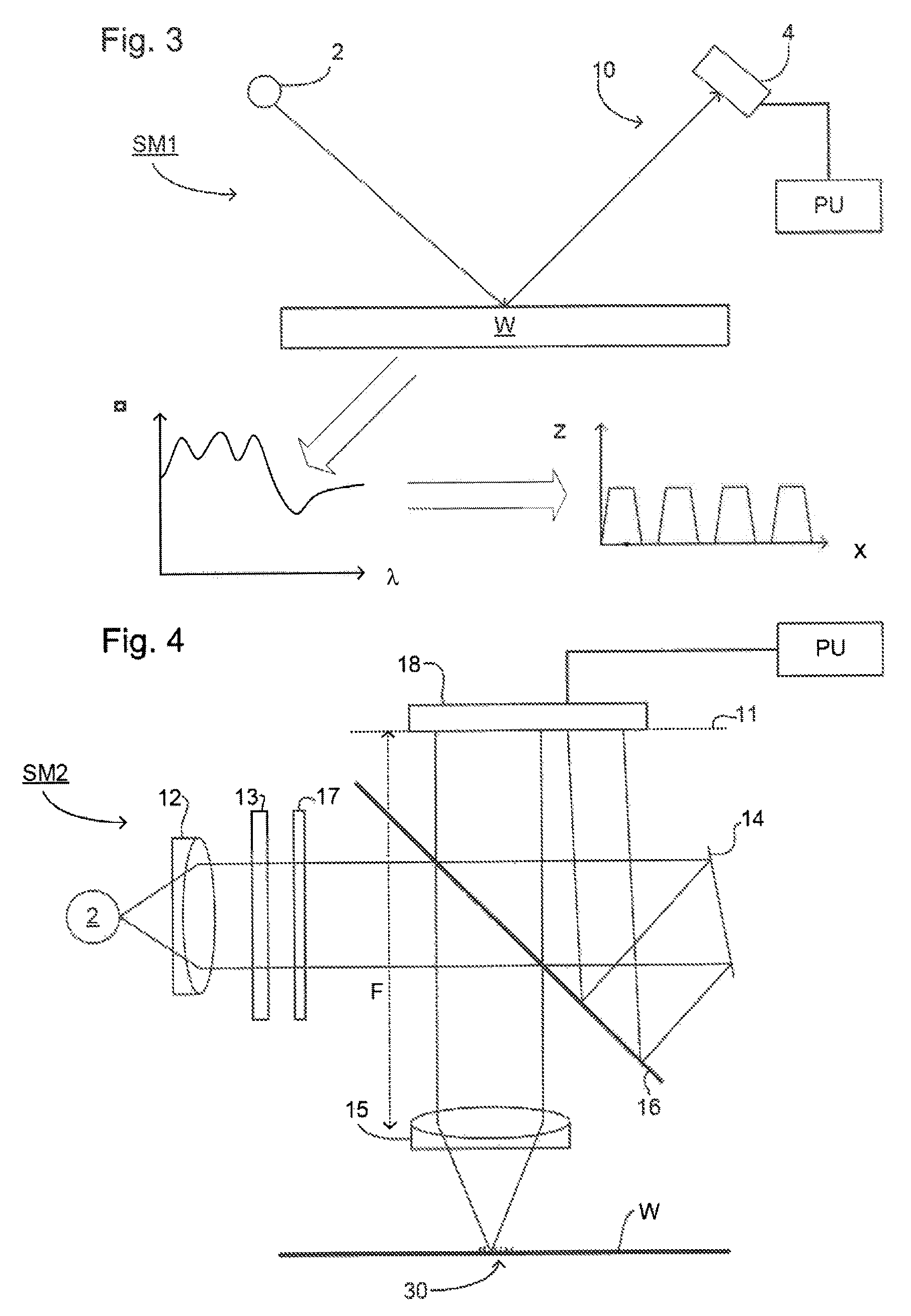

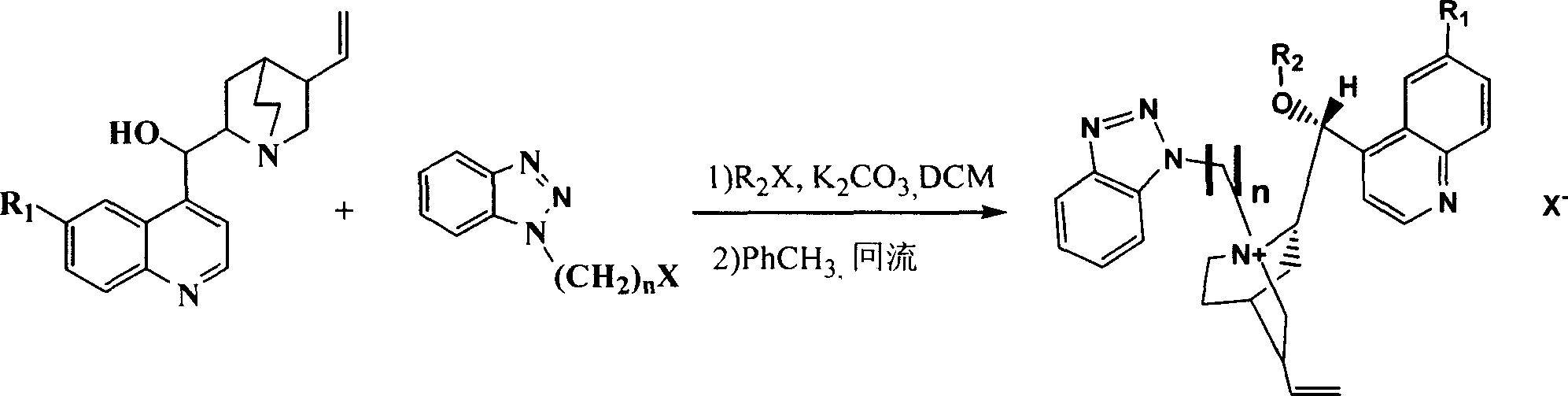



![Planar chiral [2,2]-p-cyclo-aralkyl oxazolinyl phosphine ligand and its synthesis and use Planar chiral [2,2]-p-cyclo-aralkyl oxazolinyl phosphine ligand and its synthesis and use](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/191fd211-4d7e-48ea-b405-cbacbb942dab/02136741.PNG)

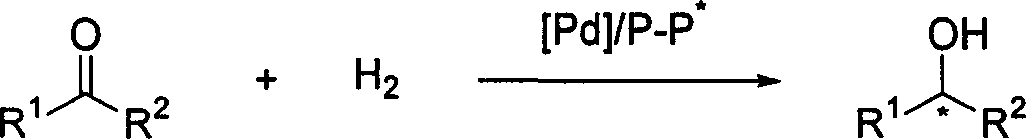
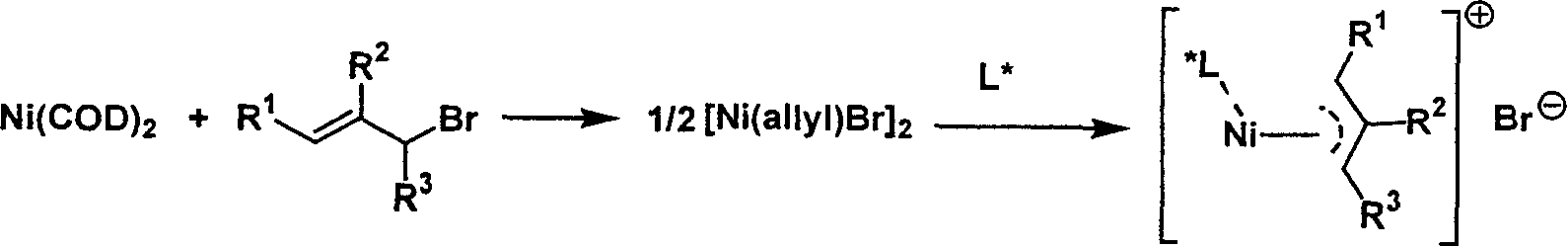

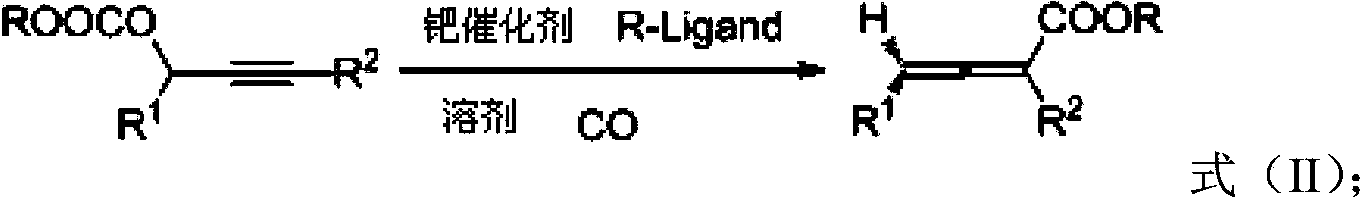
![Preparation method of (R)-2-[[[3[methyl-4-nitro-2-pyridyl]methyl]sulfinyl]benzimidazole Preparation method of (R)-2-[[[3[methyl-4-nitro-2-pyridyl]methyl]sulfinyl]benzimidazole](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d9607a21-3767-472b-a41d-4949c82fafea/HDA0000054676630000011.PNG)
![Preparation method of (R)-2-[[[3[methyl-4-nitro-2-pyridyl]methyl]sulfinyl]benzimidazole Preparation method of (R)-2-[[[3[methyl-4-nitro-2-pyridyl]methyl]sulfinyl]benzimidazole](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d9607a21-3767-472b-a41d-4949c82fafea/HDA0000054676630000012.PNG)
![Preparation method of (R)-2-[[[3[methyl-4-nitro-2-pyridyl]methyl]sulfinyl]benzimidazole Preparation method of (R)-2-[[[3[methyl-4-nitro-2-pyridyl]methyl]sulfinyl]benzimidazole](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d9607a21-3767-472b-a41d-4949c82fafea/BDA0000054676620000011.PNG)