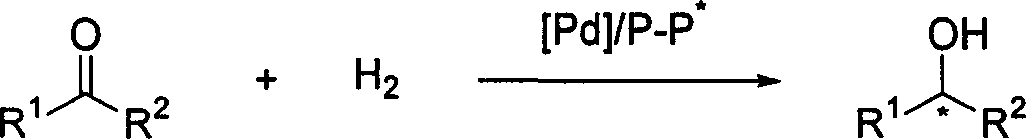Method for using palladium homogeneous system in enantioselective catalysis of ketone hydride
A technology for catalytic hydrogenation of ketones and catalysts, which is applied in chemical instruments and methods, physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, etc., to achieve the effects of convenient preparation, easy availability of raw materials, and good yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: optimization of conditions
[0039] Put palladium precursor (0.005 mmol) and chiral ligand (0.006-0.0075 mmol) into a reaction bottle, add 1 ml of acetone after nitrogen replacement, and stir at room temperature for 1 hour. Then concentrate in vacuo, add 1-1.2 milliliters of 2,2,2-trifluoroethanol under nitrogen, transfer this solution to the reaction kettle with the substrate (0.25 mmol) in advance, and feed hydrogen (14-70 atmospheric pressure) , and put it into an oil bath of specified temperature to react for 12 hours. Slowly release hydrogen, remove the solvent, and directly separate the pure product by column chromatography. The reaction formula and ligand structure are as follows:
[0040]
[0041] The conversion rate was determined by proton nuclear magnetic resonance spectroscopy, and the enantiomeric excess of the product was determined by chiral HPLC, see Table 2.
[0042] serial number
Embodiment 2
[0043] Example 2: Asymmetric hydrogenation of α-o-benzamide substituted ketones
[0044] Pd(CF 3 CO 2 ) 2 (1.7 mg, 0.005 mmol) and the chiral bisphosphorus ligand (R, R)-Me-DuPhos (1.8 mg, 0.006 mmol), after nitrogen replacement, 1 ml of acetone was added and stirred at room temperature for 1 hour. Then concentrate in vacuo, add 1-1.2 milliliters of 2,2,2-trifluoroethanol under nitrogen, transfer this solution to the reaction kettle with the substrate (0.25 mmol) in advance, and feed hydrogen (14-28 atmospheric pressure) , Put it into the specified temperature oil bath to react for 12-18 hours. Slowly release the hydrogen, remove the solvent and obtain the pure product directly by column chromatography, the reaction formula is as follows:
[0045]
[0046] The catalyst used in No. 5 is Pd(OTf) 2 / (S)-SYNPHOS pure complex, the enantiomeric excess of the product was determined by chiral HPLC, see Table 3.
[0047] serial number
Embodiment 3
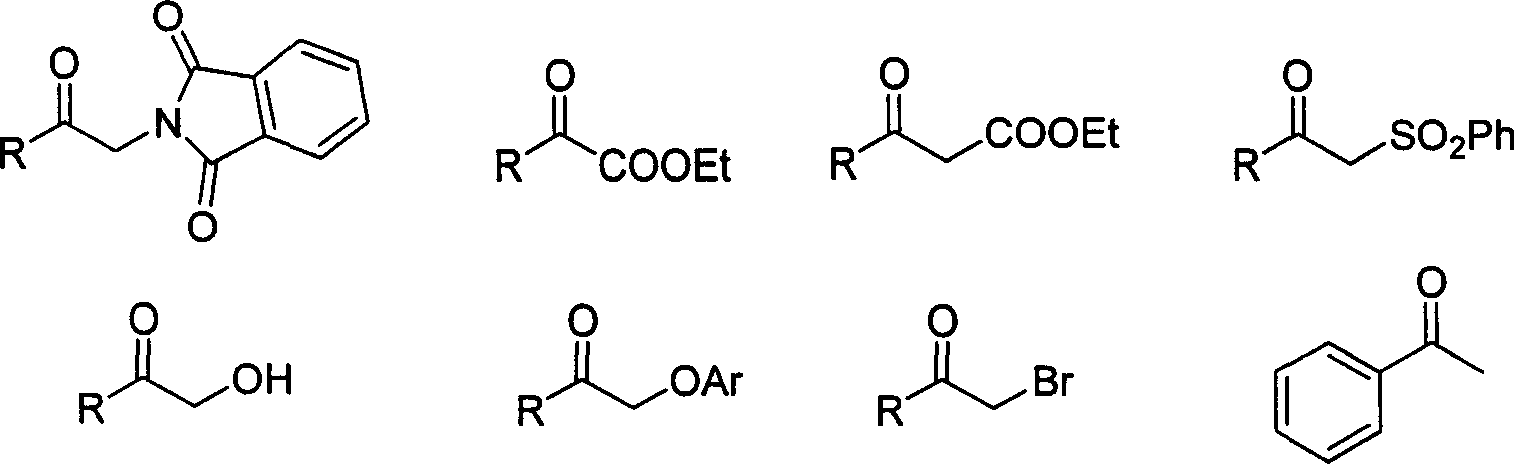
[0048] Example 3: Asymmetric hydrogenation of additionally functionalized ketones
[0049] Pd(CF 3 CO 2 ) 2 (1.7mg, 0.005mmol) and chiral bisphosphorus ligand (0.006-0.0075mmol), add 1ml of acetone after nitrogen replacement, and stir at room temperature for 1 hour. Then concentrate in vacuo, add 1-1.2 milliliters of 2,2,2-trifluoroethanol under nitrogen, transfer this solution to the reaction kettle with the substrate (0.25 mmol) in advance, feed hydrogen to the specified pressure, and put Reaction in an oil bath at specified temperature for 12-18 hours. Slowly release the hydrogen, remove the solvent and obtain the pure product directly by column chromatography, the reaction formula is as follows:
[0050]
[0051] The enantiomeric excess of the product was determined by chiral HPLC or GC, see Table 4.
[0052] Table 4: Asymmetric hydrogenation of functionalized ketones
[0053]
[0054] 9
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com