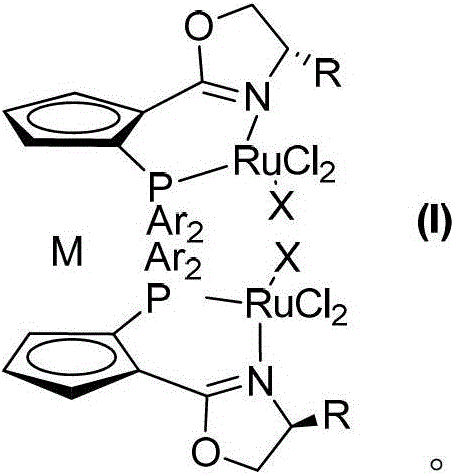
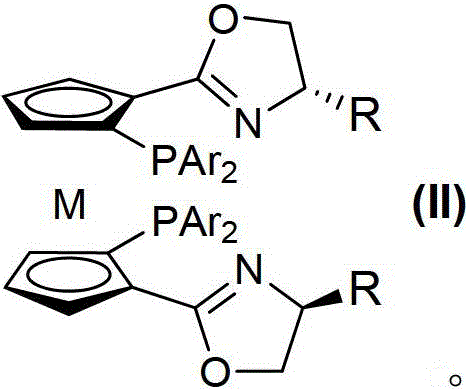
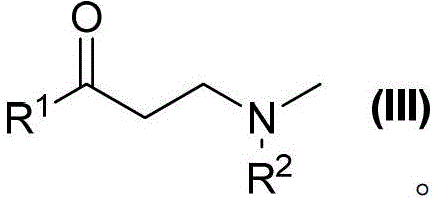
A kind of chiral double reaction center ruthenium catalyst and its synthesis and application
A ruthenium catalyst and double-reaction technology, applied in the face-chiral double-reaction center ruthenium catalyst and its synthesis, the application of asymmetric hydrogenation of β-amino ketone compounds, ruthenium catalyst and its synthesis and application field, can solve the problem of catalyst Ligand synthesis is complicated, stable storage is not possible, and the cost of metal rhodium is high, so as to achieve good industrial application prospects, convenient storage and use, and good catalytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Synthesis of Hilarochiral Double Reaction Center Ruthenium Catalysts
[0059] Tris(triphenylphosphine)ruthenium dichloride (3.8 mg, 4 μmol) and chiral ligand (M=Ru, R=i-Pr, Ar=4-MeC 6 h 4 -, 2 μmol) was dissolved in methanol (3 mL), heated and stirred at 0°C for 1 hour. Cool to room temperature, remove the solvent under reduced pressure, and separate by column chromatography (using a silica gel column, eluent: ethyl acetate / petroleum ether = 1 / 5) to obtain 3.21 mg of a dark green solid with a yield of 92%.
[0060] Preparation of 3-benzylmethylamino-1-phenylpropanol from 3-benzylmethylamino-1-propiophenone
[0061]
[0062] Under nitrogen atmosphere, 3-benzylmethylamino-1-propiophenone (0.4mmol), methanol (2.6mL), potassium hydroxide in methanol (0.4mL, 0.08M) and catalyst 1.73mg (TON=400) were added. The reaction system was placed in an autoclave at 25 °C and H 2 (20atm) and stirred for 6 hours. The solvent was removed under reduced pressure, separated by colum...
Embodiment 2
[0064] Synthesis of Hilarochiral Double Reaction Center Ruthenium Catalysts
[0065] Tris(triphenylphosphine)ruthenium dichloride (3.8 mg, 4 μmmol) and chiral ligands (M=Ru, R=s-Bu, Ar=C 6 h 5 -, 2.8 μmmol) was dissolved in ethanol (3 mL), heated and stirred at 30°C for 2 hours. Cool to room temperature, remove the solvent under reduced pressure, and separate by column chromatography (using a silica gel column, eluent: ethyl acetate / petroleum ether = 1 / 5) to obtain 3.31 mg of a dark green solid with a yield of 94.8%.
[0066] Preparation of 3-benzylmethylamino-1-phenylpropanol from 3-benzylmethylamino-1-propiophenone
[0067]
[0068] Under nitrogen atmosphere, 3-benzylmethylamino-1-propiophenone (0.4mmol), ethanol (2.6mL), potassium hydroxide in ethanol (0.4mL, 0.09M) and catalyst 1.715mg (TON=400) were added. The reaction system was placed in an autoclave at 25 °C and H 2 (20atm) and stirred for 12 hours. The solvent was removed under reduced pressure, separated by col...
Embodiment 3
[0070] Synthesis of Hilarochiral Double Reaction Center Ruthenium Catalysts
[0071] Tris(triphenylphosphine)ruthenium dichloride (3.8 mg, 4 μmmol) and chiral ligand (M=Ru, R=Me, Ar=3,5-(CF 3 ) 2 C 6 h 3 -, 2.2 μmmol) was dissolved in toluene (3 mL), heated and stirred at 100°C for 2 hours. Cool to room temperature, remove the solvent under reduced pressure, and separate by column chromatography (using a silica gel column, eluent: ethyl acetate / petroleum ether=1 / 5) to obtain 3.56 mg of a dark green solid with a yield of 96.1%.
[0072] Preparation of 3-benzylmethylamino-1-phenylpropanol from 3-benzylmethylamino-1-propiophenone
[0073]
[0074] Under nitrogen atmosphere, 3-benzylmethylamino-1-propiophenone (0.4mmol), toluene (2.6mL), potassium hydroxide aqueous solution (0.4mL, 0.1M) and catalyst 1.715mg (TON=400) were added. The reaction system was placed in an autoclave at 10 °C and H 2 (20atm) and stirred for 12 hours. The solvent was removed under reduced pressur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| enantiomeric excess | aaaaa | aaaaa |
| enantiomeric excess | aaaaa | aaaaa |
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com