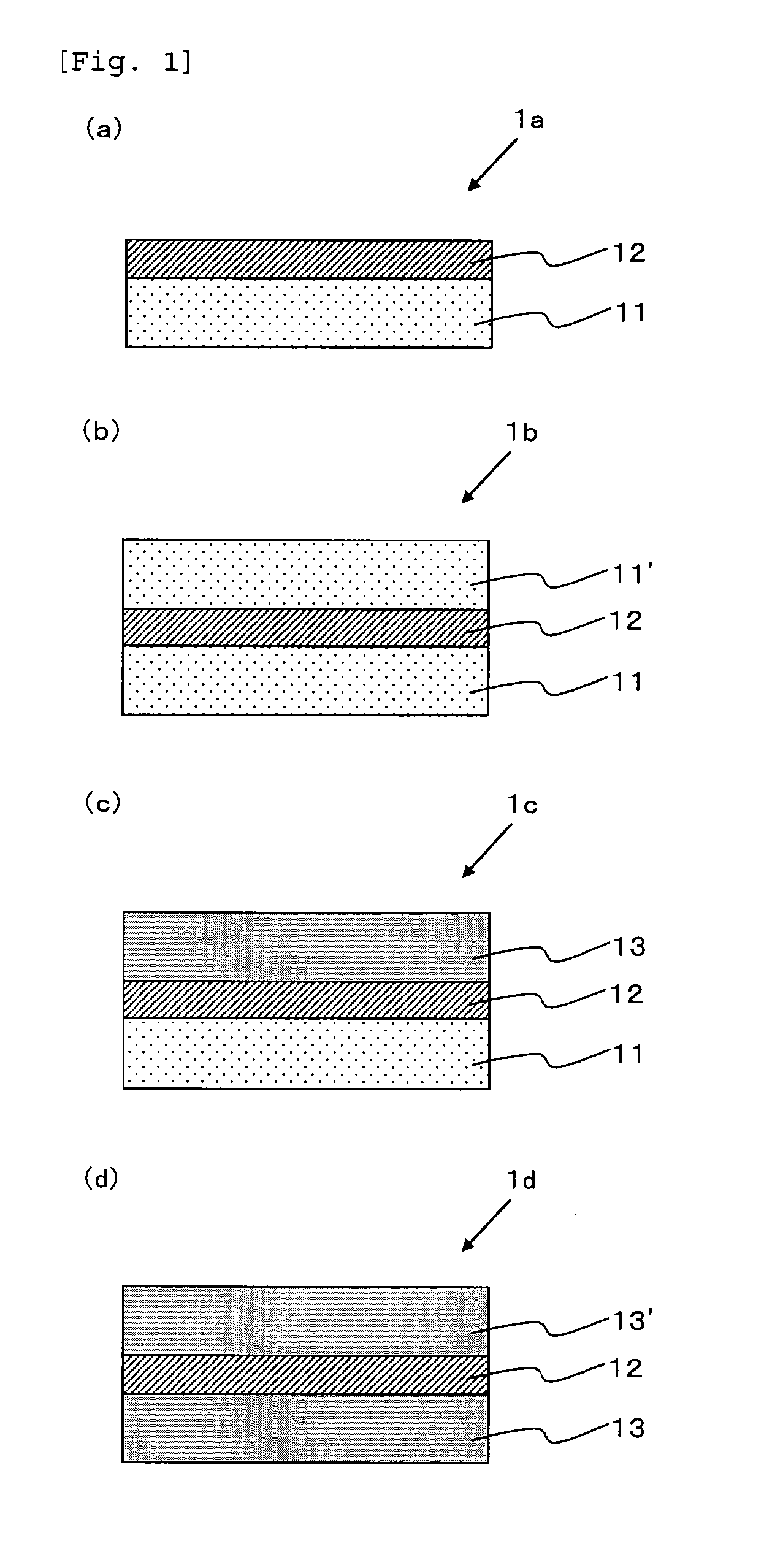
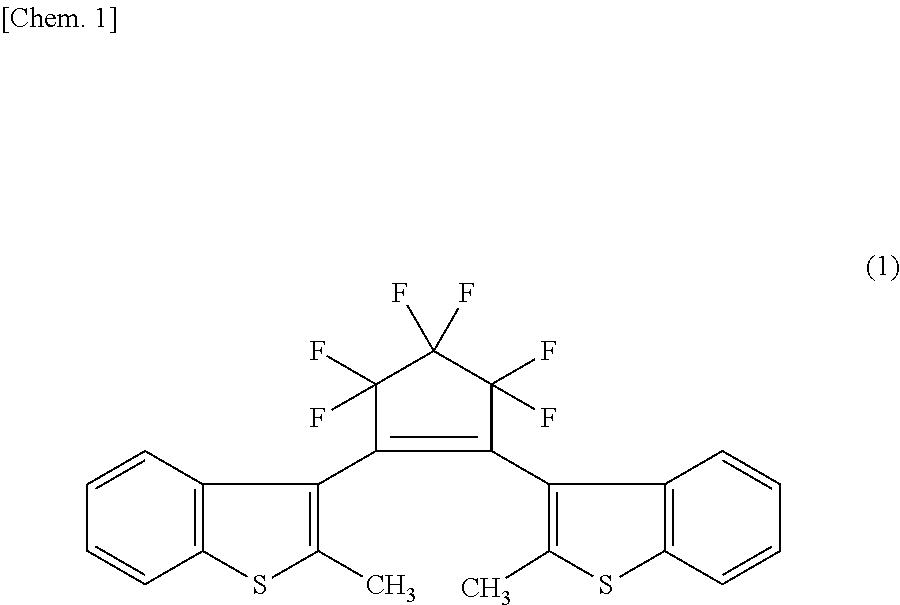
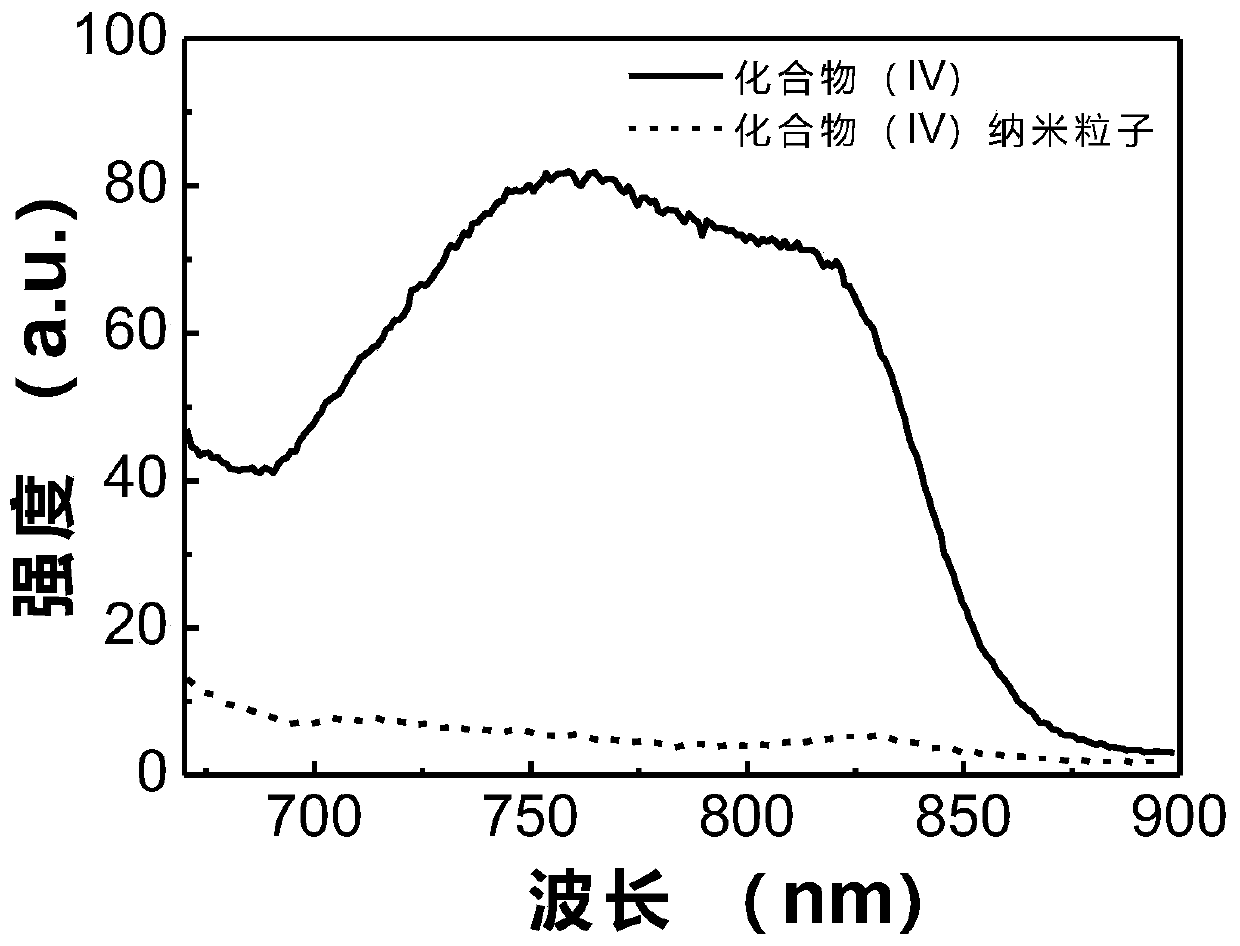
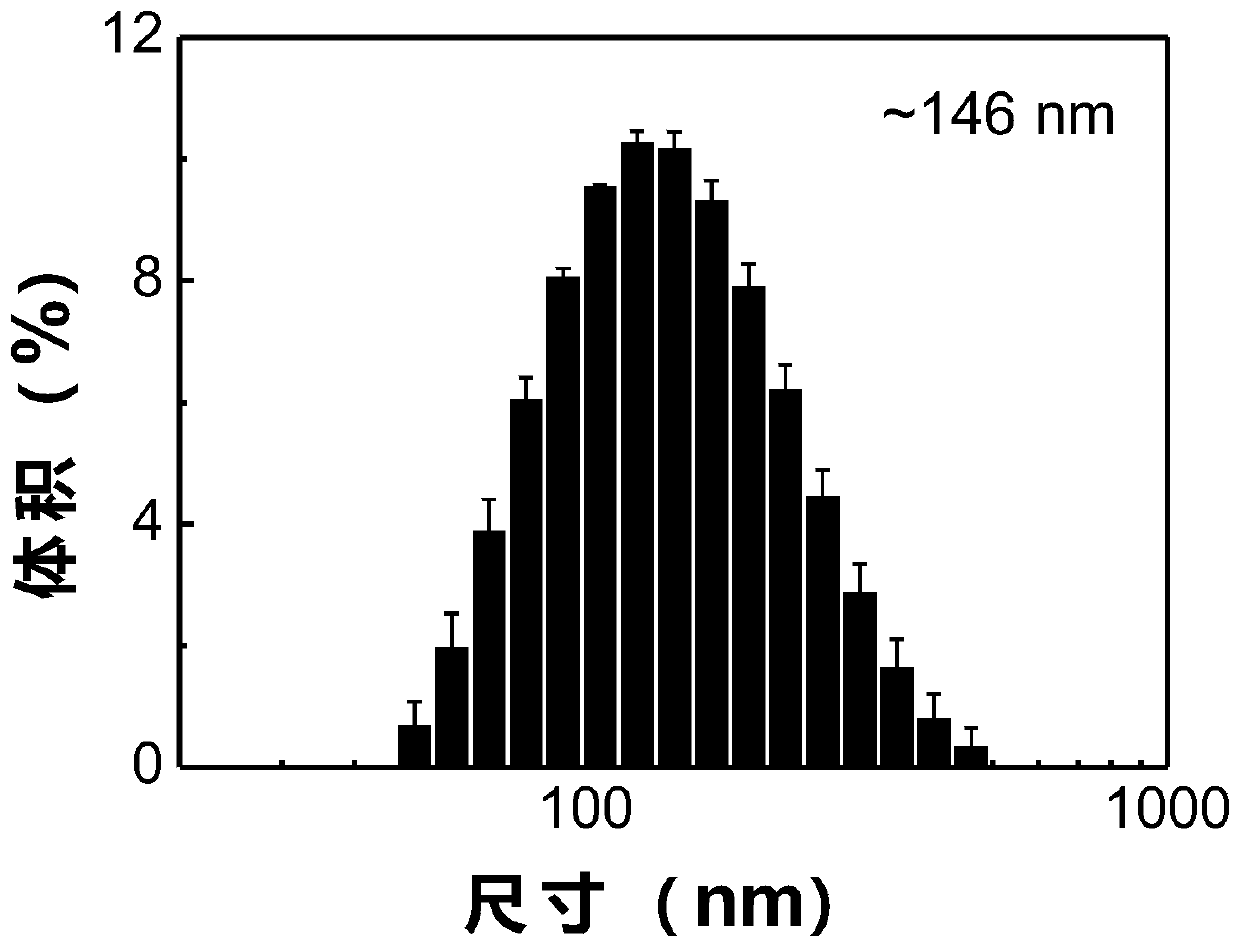
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
124 results about "Dithiane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A dithiane is a heterocyclic compound composed of a cyclohexane core structure wherein two methylene bridges (-CH₂- units) are replaced by sulfur centres. The three isomeric parent heterocycles are 1,2-dithiane, 1,3-dithiane and 1,4-dithiane.
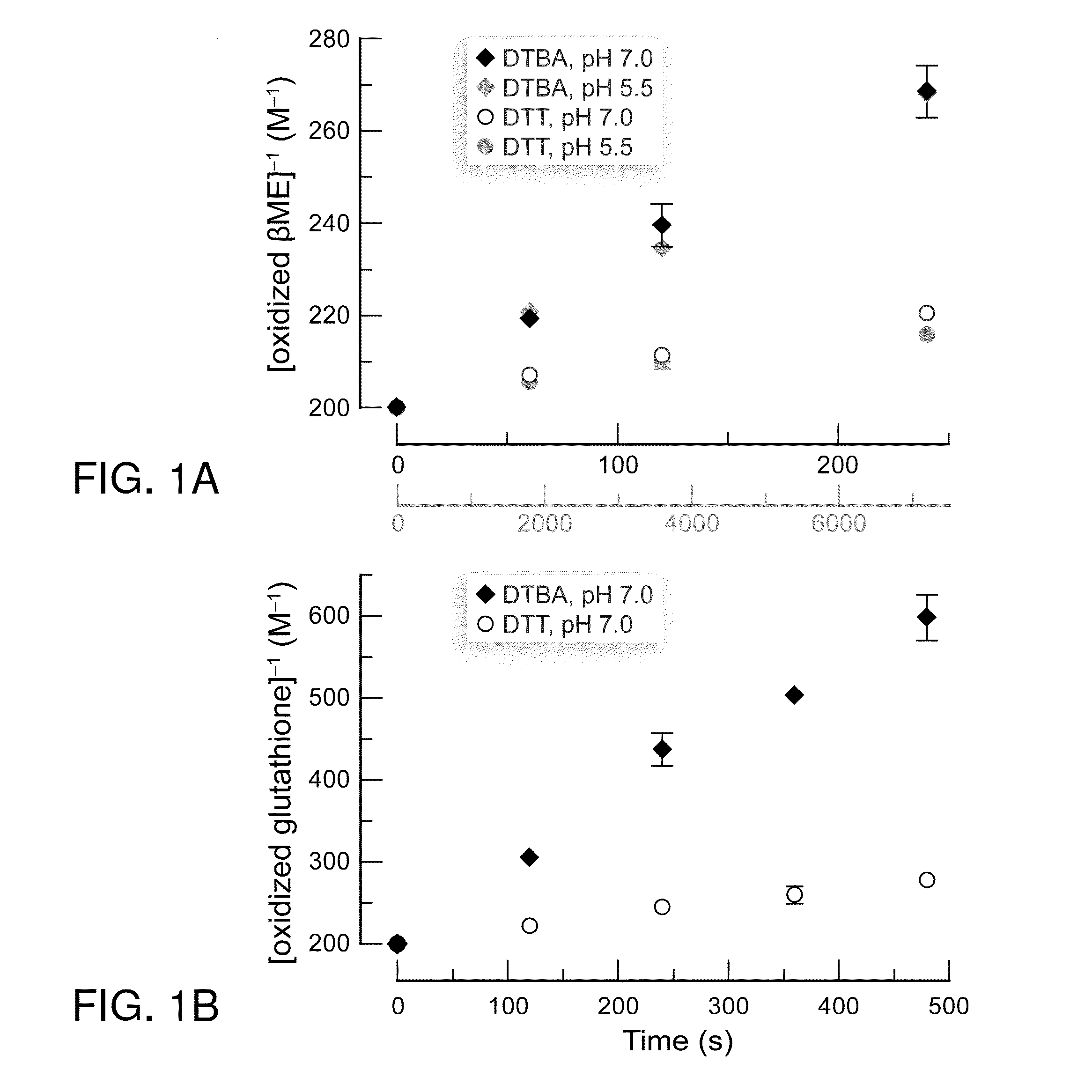
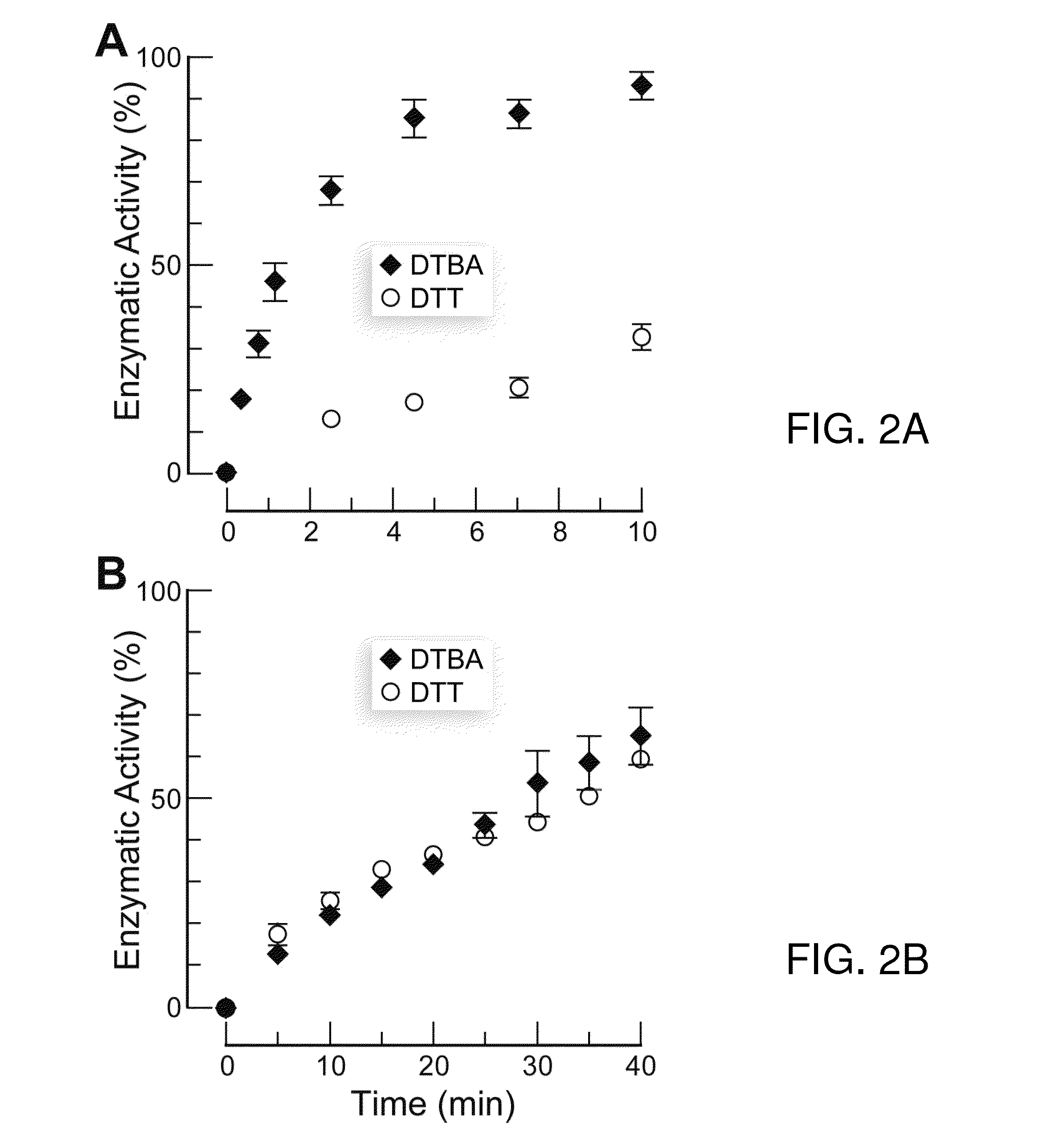
Dithioamine reducing agents
ActiveUS20130211055A1Reduce disulfide bondPrevent formation of disulfide bondSugar derivativesOrganic chemistry methodsDithianeNanoparticle
Dithioamine reducing agents useful for the reduction of disulfide bonds. The reducing agents of this invention are useful, for example, to reduce disulfide bonds, particularly in proteins, or to prevent the formation of disulfide bonds, particularly in proteins and other biological molecules. Reducing agents of this invention are useful and suitable for application in a variety of biological applications, particularly as research and synthetic reagents. The invention provides S-acylated dithioamines which can be selectively activated reducing agents by removal of the S-acyl groups enzymatically or chemically. The invention further provides dithiane precursors of thioamino reducing agents. The invention provides dithioamine reducing agents, S-acylated dithioamines and dithianes which are immobilized on surfaces, including among others, glass, quartz, microparticles, nanoparticles and resins.
Owner:WISCONSIN ALUMNI RES FOUND
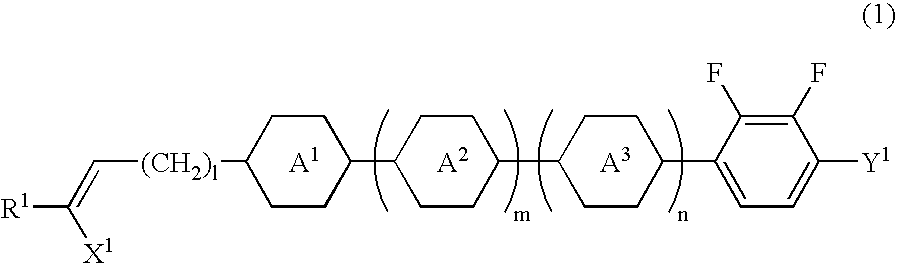
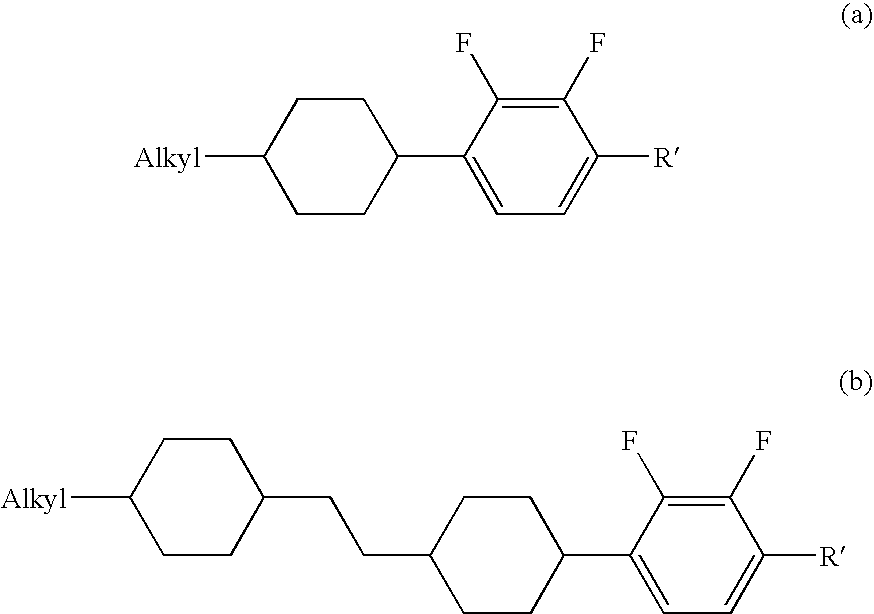
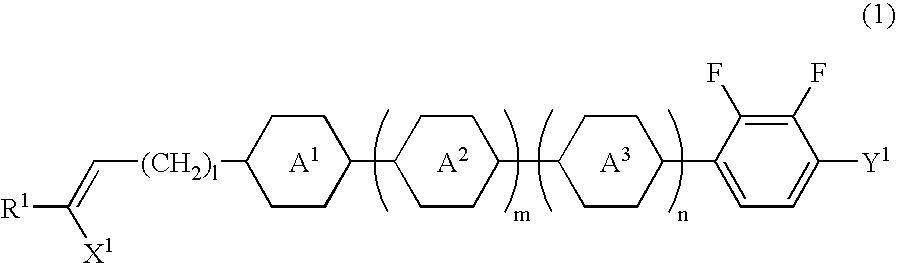
Alkenyl compound having a negative delta epsilon value, liquid crystal composition, and liquid crystal display device
InactiveUS20040065866A1Low viscosityImprove solubilityLiquid crystal compositionsSilicon organic compoundsLiquid crystallineDithiane
Owner:JNC CORP
Polymerizable composition for optical material, optical material, and method for preparing the optical material
ActiveUS20100075154A1High refractive indexGood light fastnessSynthetic resin layered productsCoatingsDithianePropane
The polymerizable composition for an optical material of the present invention comprises a phenylene diisocyanate, at least one polythiol compound selected from the group consisting of 4-mercaptomethyl-1,8-dimercapto-3,6-dithiaoctane, 4,8-, 4,7- or 5,7-dimercaptomethyl-1,11-dimercapto-3,6,9-trithiaundecane, 2,5-bis(mercaptomethyl)-1,4-dithiane, bis(mercaptoethyl)sulfide, 1,1,3,3-tetrakis(mercaptomethylthio)propane, 4,6-bis(mercaptomethylthio)-1,3-dithiane, 2-(2,2-bis(mercaptomethylthio)ethyl)-1,3-dithietane, 1,1,2,2-tetrakis(mercaptomethylthio)ethane 3-mercaptomethyl-1,5-dimercapto-2,4-dithiapentane and tris(mercaptomethylthio)methane.
Owner:MITSUI CHEM INC
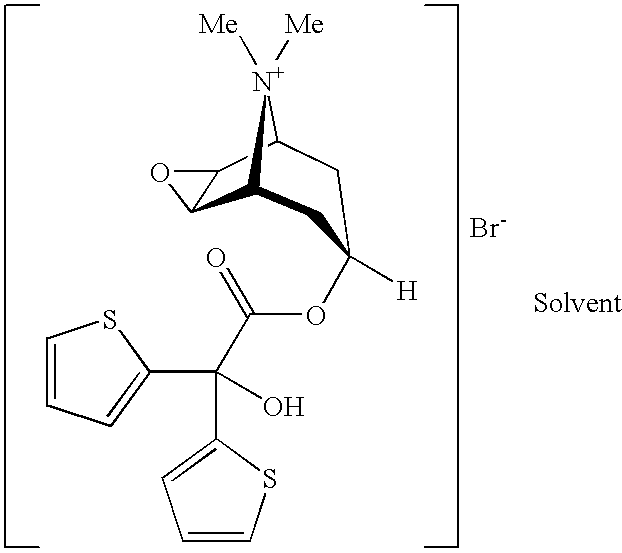
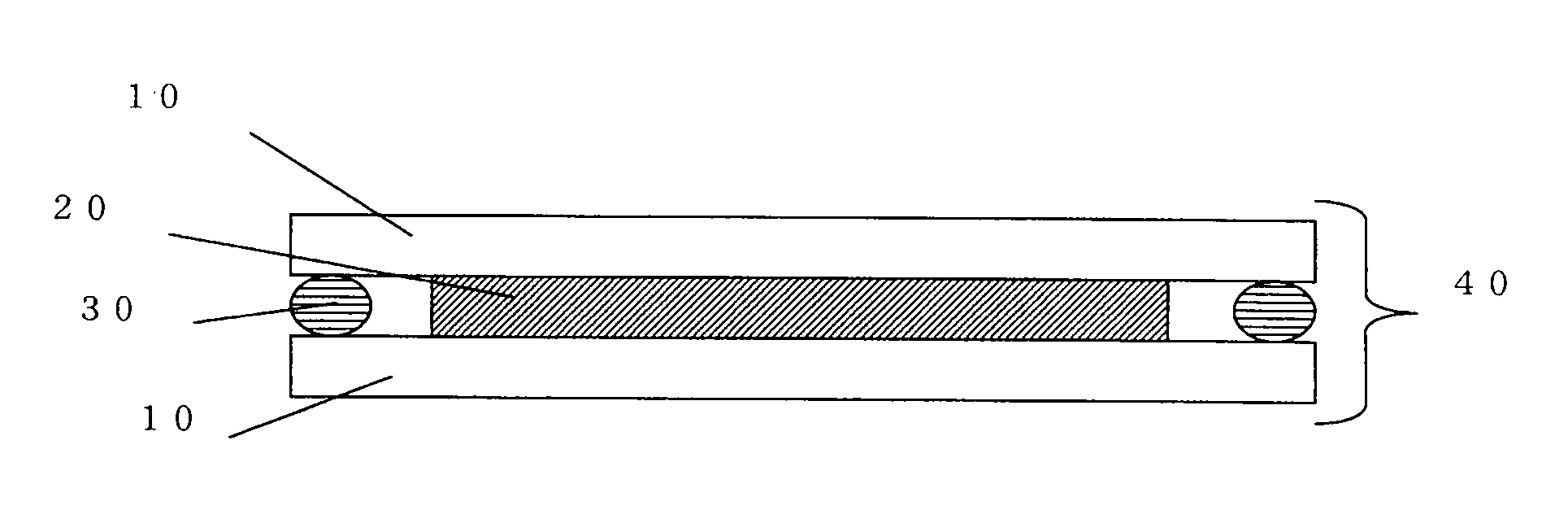
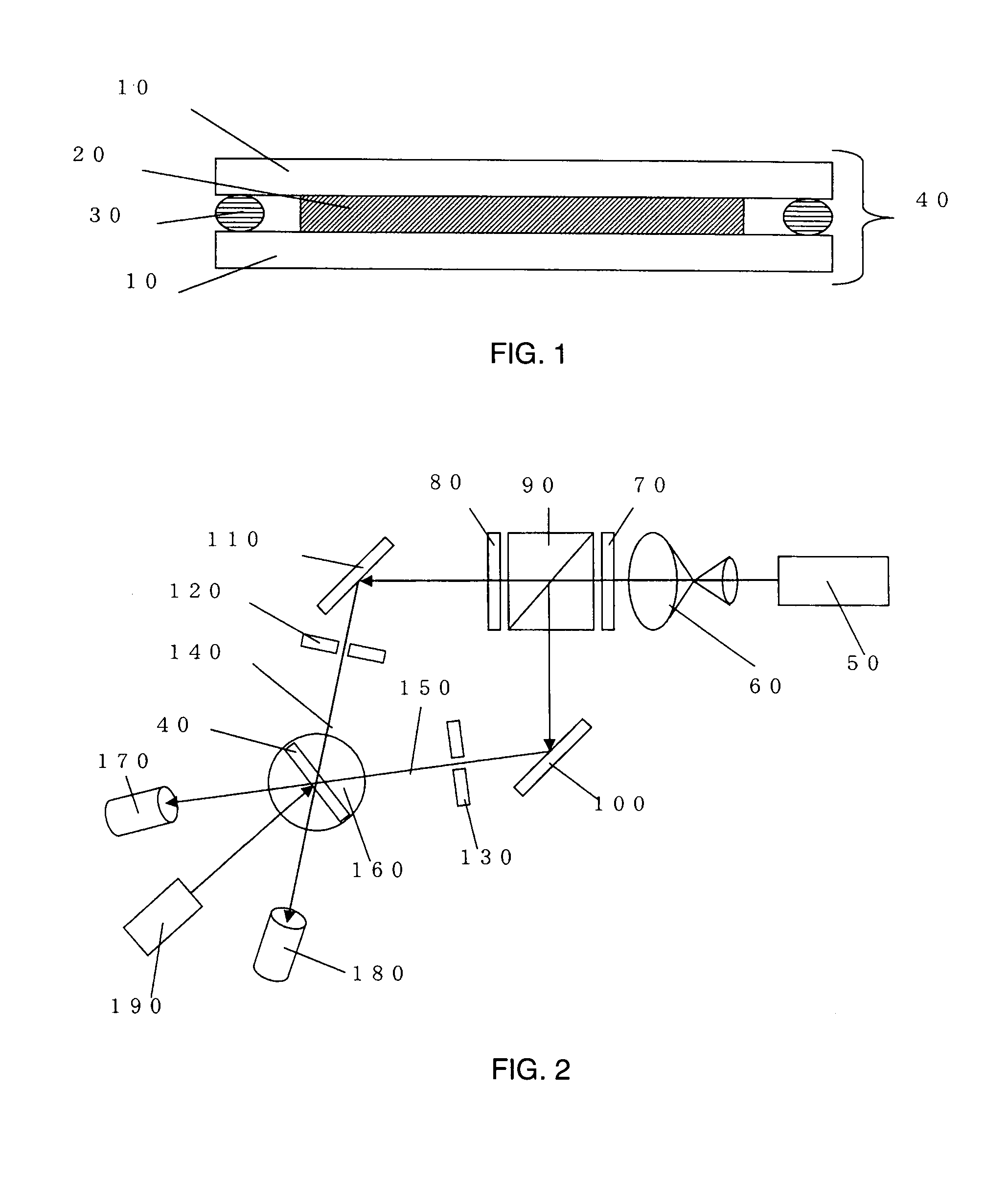
Pure and stable tiotropium bromide
This invention relates to solvates of tiotropium bromide having a purity of at least 99%, process for preparing such pure solvates, and their use in pharmaceutical formulations. This invention also provides tiotropium bromide solvates containing less than about 0.15% area by HPLC of 2,2-dithienyl glycolic acid.
Owner:SICOR SOC ITAL CORTICOSTEROIDI SPA
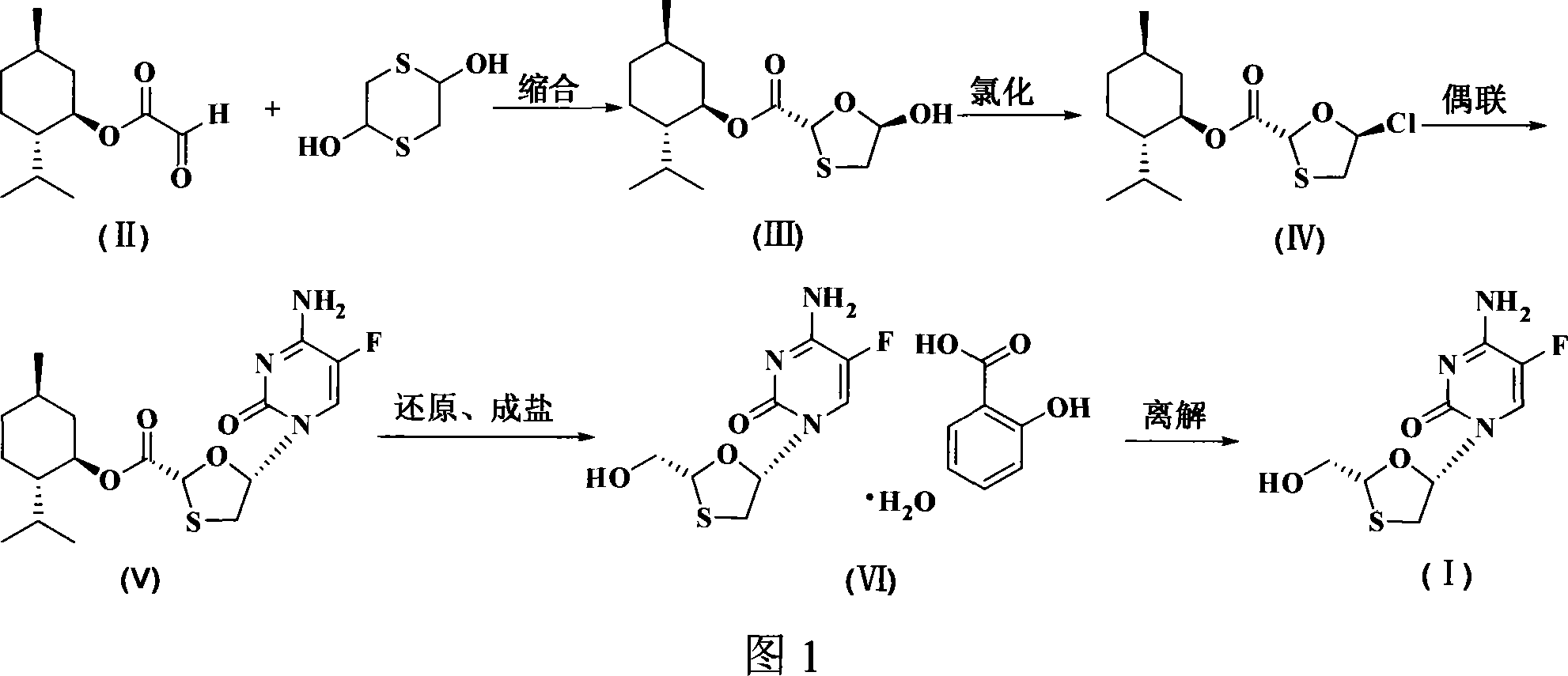
Non-enantioselective prepn process of emtricitabine
InactiveCN101066971AMild reaction conditionsSimple and fast operationOrganic chemistryDithianeAlcohol
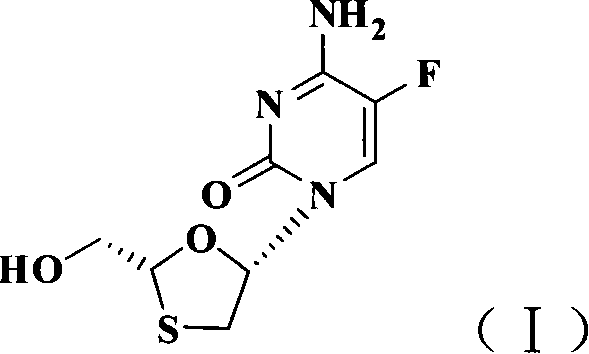
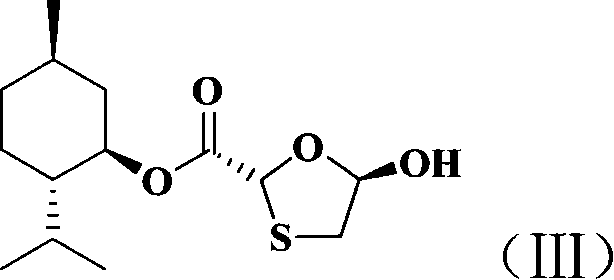
The present invention discloses non-enantioselective preparation process of emtricitabine. The preparation process includes condensation of glyoxalic acid as the initial material and 2, 5-dihydroxy-1, 4-dithiane under the asymmetric induction of optically active alcohol ester to obtain trans-5-hydroxy-1, 3-oxythiacyclopentane-2-carboxylate; halogenating and coupling with silylated 5-flucytosine, and reducing to obtain initial product; reaction with salicylic acid to form salt, purification and separation to obtain optically pure emtricitabine. The present invention has mild reaction condition, simple operation, low cost, less environmental pollution and high product purity reaching medicinal standard, and is suitable for industrial production. The product is used in treating hepatitis B and AIDS.
Owner:葛建利
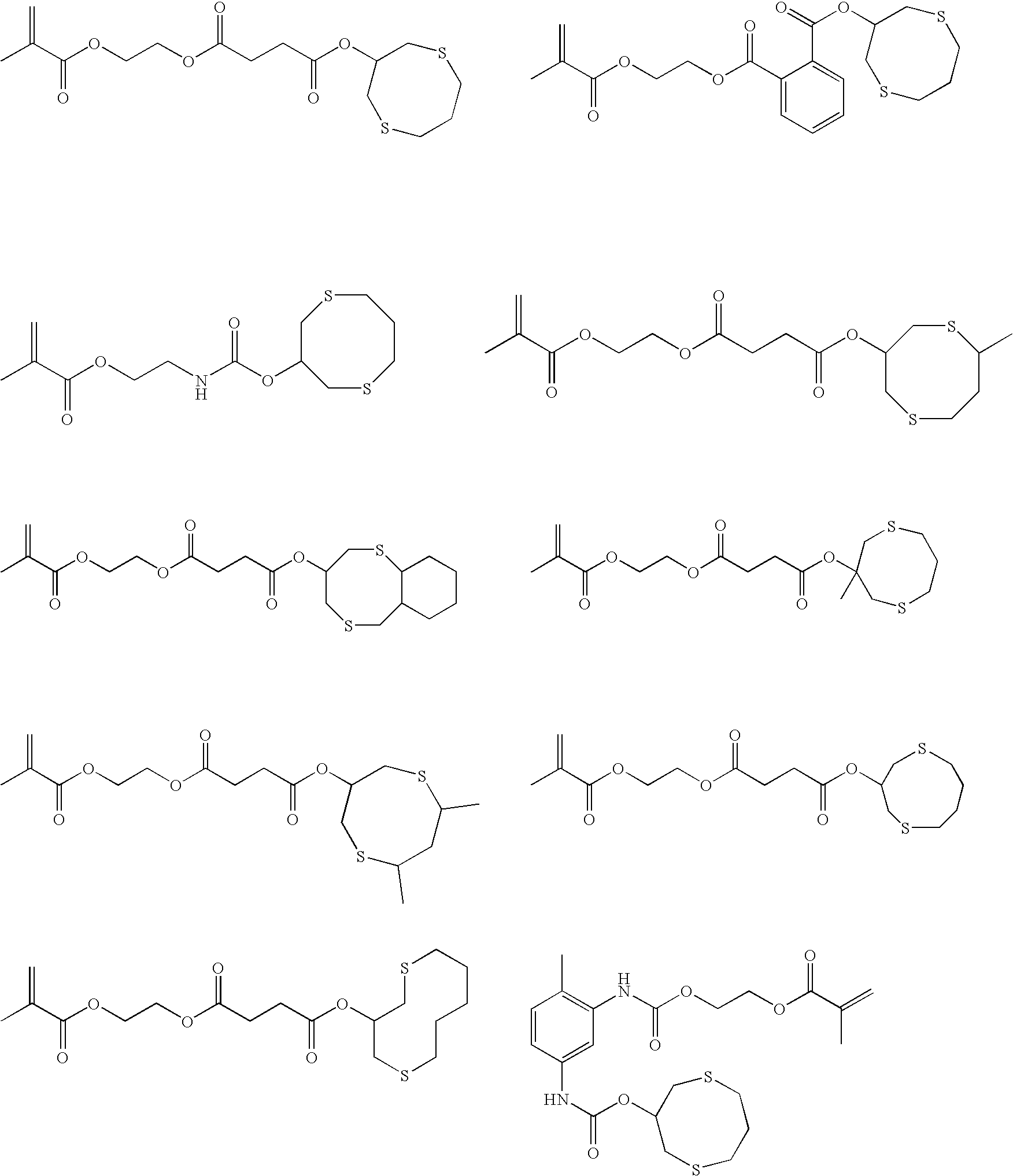
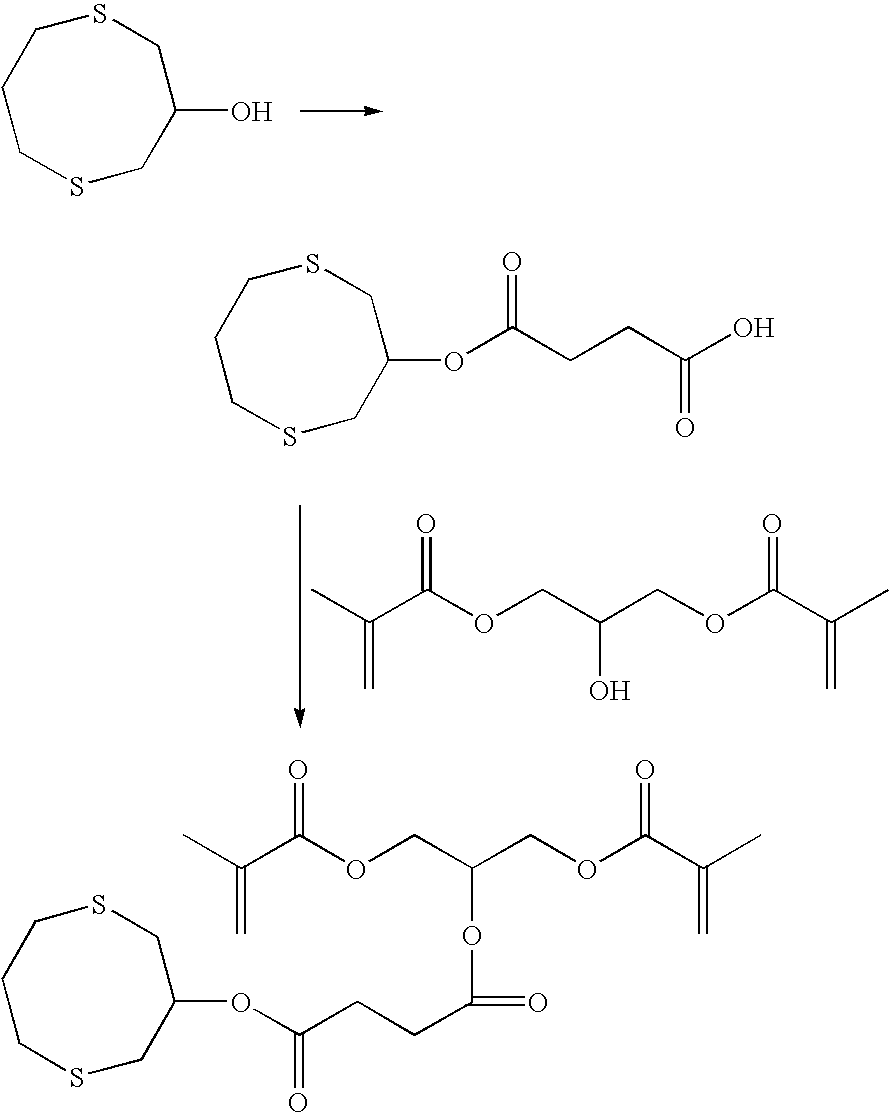
Curable compositions containing dithiane monomers
InactiveUS20070066748A1Low shrinkageImprove mechanical propertiesOrganic chemistryDental impression compositionsDithianeMeth-
The invention features compositions comprising at least one monomer that comprises a cyclic dithiane moiety attached to a (meth)acryloyl moiety. The composition may optionally contain additional polymerizable compounds, such as ethylenically unsaturated compounds, that are typically used in dental compositions.
Owner:3M INNOVATIVE PROPERTIES CO
Polymerizable composition for optical material, optical material and method for producing optical material
Disclosed is a polymerizable composition for o an optical material containing tolylene diisocyanate, hexamethylene diisocyanate, and one or more polythiol compounds selected from the group consisting of 4-mercaptomethyl-1,8-dimercapto-3,6-dithiaoctane,4,8-, 4,7- or 5,7-dimercaptomethyl-1,11-dimercapto-3,6,9-trithiaundecane, pentaerythritol tetrakismercaptoacetate, pentaerythritol tetrakismercaptopropionate, 2,5-bis(mercaptomethyl)-1,4-dithiane, bis(mercaptoethyl)sulfide, 1,1,3,3-tetrakis(mercaptomethylthio)propane, 4,6-bis(mercaptomethylthio)-1,3-dithiane and 2-(2,2-bis(mercaptomethylthio)ethyl)-1,3-dithietane.
Owner:MITSUI CHEM INC
Holographic recording medium
InactiveUS20100221646A1Record information storagePhotomechanical exposure apparatusDithianeThianthrene
A holographic recording medium includes a recording layer. The recording layer includes a framework being expressed with the following general formula (1),In the above formula (1), Ar represents a substituted or unsubstituted group selected from benzothiophene group, naphthothiophene group, dibenzothiophene group, thienothiophene group, dithienobenzene group, benzothiazole group, naphthothiazole group, benzoisothiazole group, naphthoisothiazole group, phenothiazine group, phenoxathiin group, dithianaphthalene group, thianthrene group, thioxanthene group, and bithiophene group. In addition, n is an integer from 1 to 4.
Owner:KK TOSHIBA
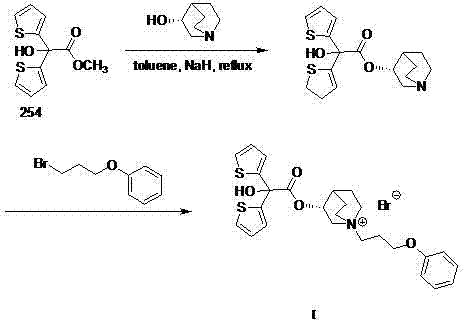
Technology for preparing aclidinium bromide employing one-pot process
The invention belongs to the field of medicinal chemistry, and discloses a technology for preparing aclidinium bromide. By adopting the technology, (R)-3-quinuclidinol, (C1)methyl-2,2-dithienyl glycolate and 3-phenoxy propyl bromide are taken as raw materials, and the aclidinium bromide is prepared by adopting a one-pot process. The method is simple to operate, and convenient for post-treatment, and has good application value.
Owner:AVENTIS PHARMA HAINAN
Method for preparing tiotropium bromide
The invention discloses a method for preparing tiotropium bromide, which comprises the following steps of: preparing scopine-2,2-dithienyl glycolate from scopine and methyl 2,2-dithienyl glycolate, reacting the scopine-2,2-dithienyl glycolate with methyl bromide to prepare a tiotropium bromide crude product, and refining the tiotropium bromide crude product to obtain a tiotropium bromide finishedproduct. The method is characterized in that: the scopine and the methyl 2,2-dithienyl glycolate undergo ester exchange reaction under the action of dimethylbenzene, and the mixed catalysts of sodiumand sodium methoxide, and after the reaction, reaction solution is post-treated to obtain the scopine-2,2-dithienyl glycolate. In the method, in the process of preparing the scopine-2,2-dithienyl glycolate, the sodium and the sodium methoxide are simultaneously taken as the catalysts, and scopine isomer content after the reaction is less than 0.1 percent which completely meets specifications in the trial standards of European pharmacopoeia; and the method solves a big problem for the conventional preparation of the tiotropium bromide and is easy to realize industrial production.
Owner:ANHUI DEXINJIA BIOPHARM
Curable compositions containing dithiane monomers
InactiveUS7495054B2Low shrinkageImprove mechanical propertiesOrganic chemistryDental impression compositionsDithianeMeth-
The invention features compositions comprising at least one monomer that comprises a cyclic dithiane moiety attached to a (meth)acryloyl moiety. The composition may optionally contain additional polymerizable compounds, such as ethylenically unsaturated compounds, that are typically used in dental compositions.
Owner:3M INNOVATIVE PROPERTIES CO
Benzodithiophene based copolymer containing thieno [3,4-B] thiophene units and preparing method and applications thereof
InactiveUS9365679B2High optical and thermal and environmental stabilityDecreases electron-rich numberOrganic chemistryFinal product manufactureDithianePolymer science
The present invention relates to a benzodithiophene based copolymer containing thieno[3,4-b]thiophene units and a preparing method and applications thereof. The polymer has a structural formula (I), wherein R1 and R2 are respectively selected from H, and alkyl groups of C1 to C16; R3 and R4 are respectively selected from H, alkyl groups of C1 to C16, alkoxy groups of C1 to C16, or thiophene groups substituted by alkyl groups of C1 to C16; R5 is selected from alkyl groups of C1 to C16; n is a natural number from 7 to 80. Applications of the benzodithiophene based copolymer containing thieno[3,4-b]thiophene units in polymer solar cells, polymer organic light-emission, polymer organic field effect transistors, polymer organic optical storage, polymer organic nonlinear materials or polymer organic laser are also provided.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +1
Aggregation-induced emission near-infrared emission diketopyrrolopyrrole compound and preparation method thereof
InactiveCN108912126AAdjustment rangeAdjust the fluorescence intensityOrganic chemistryLuminescent compositionsN dimethylformamideKetone
The invention discloses an aggregation-induced emission near-infrared emission diketopyrrolopyrrole compound and a preparation method thereof. The method comprises the steps that 3,6-dithiophene diketopyrrolopyrrole reacts with twice molar weight of alkyl bromide so as to obtain 2,5-dialkyl-3,6-dithienopyrrolopyrrole, then the 2,5-dialkyl-3,6-dithienopyrrolopyrrole sequentially undergoes Vilsmeierreaction with phosphorus oxychloride / N,N-dimethylformamide and undergoes substitution reaction with N-bromosuccinimide so as to obtain 2,5-dialkyl-3-(5-formyl)thienyl-6-(5-bromine)thienyl diketopyrrolopyrrole, and then the 2,5-dialkyl-3-(5-formyl)thienyl-6-(5-bromine)thienyl diketopyrrolopyrrole undergoes Suzuki reaction with tetraphenyl ethylene boric acid ester so as to obtain 2,5-dialkyl-3(4-((E)-2-phenyl-1,2-disubstituted styryl)phenyl)thienyl-6-(5-formyl)thienyl diketopyrrolopyrrole. The compound has the emission range greater than 650 nm and has the aggregation-induced emission property.
Owner:SOUTH CHINA UNIV OF TECH
Synthetic method of 1,3-dithiane structure-containing polysubstituted olefin derivatives
The invention relates to a synthetic method of 1,3-dithiane structure-containing polysubstituted olefin derivatives. The method comprises the following steps: carrying out an oxyradical coupling reaction on 1,3-dithiane and polysubstituted olefins in a solvent in the presence of a catalyst Lewis acid and an activator N-chlorosuccimide with air or oxygen as an oxidant, separating, and purifying to obtain the 1,3-dithiane structure-containing polysubstituted olefin derivatives. The method has the advantages of avoiding of use of a metal catalyst due to cheap and easily available substrates used in the invention, environmental protection, simple reaction process and mild conditions, the 1,3-dithiane structure-containing polysubstituted olefin derivatives have good function group tolerance and can be directly obtained through a one-kettle process, the method has beneficial technical effects, and can be well applied in scientific researches and industrial production.
Owner:LANZHOU UNIVERSITY
Method for preparing 2,5-dimercapto-methyl-1,4-dithiane
InactiveCN101787014AHigh refractive indexLow refractive indexOrganic chemistryDithianeHeat resistance
The invention relates to a method for preparing 2,5-dimercapto-methyl-1,4-dithiane, which belongs to the field of optical materials. A reaction formula is as follows: Na2S.9H2O+S->Na2S22CH2=CHCH2Cl+Na2S2->CH2=CHH2-S-S-CH2CH=CH2. The method comprises the following steps of: (1) synthesizing diallyl disulfide; (2) synthesizing 2,5-bis (chloromethyl)-1,4-dithiane; (3) and synthesizing the 2, 5-dimercapto-methyl-1,4-dithiane. The 2,5-dimercapto-methyl-1,4-dithiane provided by the invention is used to prepare novel optical materials, and has refractive indexes with high dispersion and low dispersion. The 2,5-dimercapto-methyl-1,4-dithiane has the advantages of light weight, colorless, transparence and optical distortion, excellent weatherability, dye affinity, heat resistance, impact resistance and machining performance. The method provided by the invention is simple and practicable, and the yield can be up to 65%.
Owner:JIANGSU POLYTECHNIC UNIVERSITY
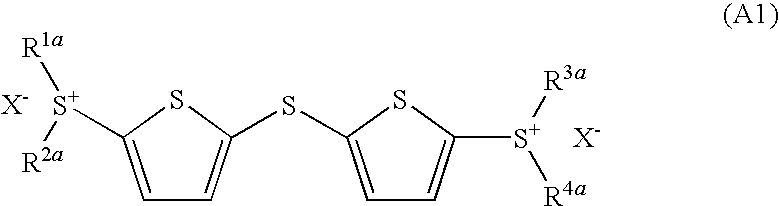
Photoacid generator and photoreactive composition
ActiveUS20100233621A1Reduce yieldHigh sensitivityOrganic chemistryPhotosensitive materialsDithianeReaction rate
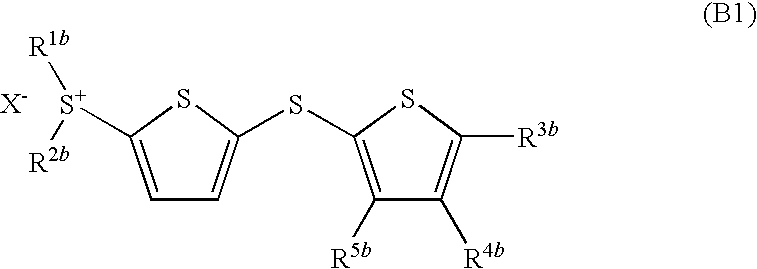
An object of the present invention is to provide photoacid generator, which shows very high sensitivity in the near ultraviolet range of about 300 to 400 nm, and also can remarkably increase a reaction rate of a photoreactive composition using the same, and to provide a photoreactive composition which can initiate the reaction even by irradiation with near ultraviolet light within a short time and also can obtain a desired reaction product. More particularly, the present invention provides a dithienyl sulfide disulfonium salt represented by the formula (A1):wherein R1a to R4a each independently represents an optionally substituted monocyclic carbon ring group, an optionally substituted condensed polycyclic carbon ring group or an optionally substituted monocyclic heterocyclic group, and X− represents an inorganic acid ion or an organic acid ion; a photoacid generator containing the dithienyl sulfide disulfonium salt, and a photoreactive composition containing the photoacid generator and an acid reactive compound; a dithienyl sulfide sulfonium salt represented by the formula (B1):wherein R1b and R2b each independently represents an optionally substituted monocyclic carbon ring group, an optionally substituted condensed polycyclic carbon ring group or an optionally substituted monocyclic heterocyclic group, R3b to R5b each independently represents a hydrogen atom, an alkyl group having 1 to 10 carbon atoms, an alkoxy group having 1 to 4 carbon atoms, an acyl group or a hydroxyl group, and X− represents an inorganic acid ion or an organic acid ion, a photoacid generator containing the same, and a photoreactive composition containing the photoacid generator; and a phenylthiothiophene sulfonium salt represented by the formula (C1):wherein R1c and R2c each independently represents an optionally substituted monocyclic carbon ring group, an optionally substituted condensed polycyclic carbon ring group or an optionally substituted monocyclic heterocyclic group, and R3c to R7c each independently represents a hydrogen atom, an alkyl group having 1 to 10 carbon atoms, alkoxy group having 1 to 4 carbon atoms, an acyl group or a hydroxyl group, and X− represents an inorganic acid ion or an organic acid ion, a photoacid generator containing the same, and a photoreactive composition containing the photoacid generator and an acid reactive compound.
Owner:SUMITOMO SEIKA CHEM CO LTD
Pyridinium derivative used as M3 muscarinic receptor antagonist and application of pyridinium derivative to pharmacy
ActiveCN103965178AEnsuring strength of efficacyLittle side effectsOrganic active ingredientsOrganic chemistryDithianeDisease
The invention relates to the field of pharmacy, particularly to 3R-1,1-dimethyl-3-(2-hydroxyl-2,2-dithienyl-2-acetoxyl) pyrrolidine bromide, 3S-1,1-dimethyl-3-(2-hydroxyl-2,2-dithienyl-2-acetoxyl) pyrrolidine bromide and applications of the 3R-1,1-dimethyl-3-(2-hydroxyl-2,2-dithienyl-2-acetoxyl) pyrrolidine bromide and the 3S-1,1-dimethyl-3-(2-hydroxyl-2,2-dithienyl-2-acetoxyl) pyrrolidine bromide to preparation of medicines for preventing or treating respiratory system diseases, urinary system diseases or gastrointestinal tract diseases.
Owner:JIANGSU LIANHUAN PHARMA
Benzodithiophene based copolymer containing pyridino [2,1,3] thiadiazole units and preparing method and applications thereof
InactiveUS20150284505A1High molecular weightImprove solubilityOrganic chemistryFinal product manufactureDithianePolymer science
The present invention relates to a benzodithiophene based copolymer containing pyridino[2,1,3]thiadiazole units and a preparing method and applications thereof. The polymer has a structural formula (I), wherein R1 and R2 are respectively selected from H or alkyl groups of C1 to C16; R3 and R4 are respectively selected from H, alkyl groups of C1 to C16, alkoxy groups of C1 to C16, or thiophene groups substituted by alkyl groups of C1 to C16; X is N and Y is CH, or X is CH and Y is N; and n is a natural number of 7 to 80. Applications of the benzodithiophene based copolymer containing pyridino[2,1,3]thiadiazole units in polymer solar cells, polymer organic light-emitting, polymer organic field effect transistors, polymer organic optical storage, polymer organic nonlinear materials or polymer organic laser are also provided.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +1
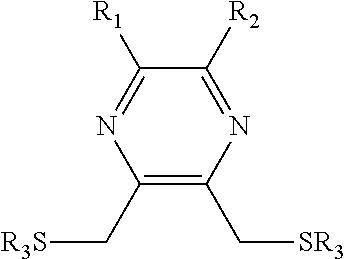
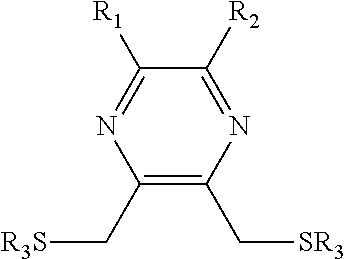
Substituted pyrazine dithiol reducing agents
ActiveUS20150376591A1Reduce disulfide bondPrevent formation of disulfide bondOrganic chemistryTransferasesDithianePyrazine
Substituted dithiol pyrazine compounds useful as reducing agents in biologically relevant media having formula:where variables are defined herein and corresponding oxidized pyrazine dithianes. Reducing agents useful to reduce disulfide bonds, particularly in proteins, or to prevent the formation of disulfide bonds, particularly in proteins, and other biological molecules. Reducing agents useful to regulate protein function in proteins in which a sulfhydryl group is associated with biological activity. Reducing agents useful and suitable for application in a variety of biological applications, particularly as research and synthetic reagents. S-acylated dithiol pyrazine compounds, where R3 is an acyl group, are selectively activated as reducing agents by removal of the S-acyl groups enzymatically or chemically. Dithiol pyrazine reducing agents and corresponding S-acylated dithiol pyrazines, immobilized on surfaces or conjugated to other chemical species, are provided.
Owner:WISCONSIN ALUMNI RES FOUND
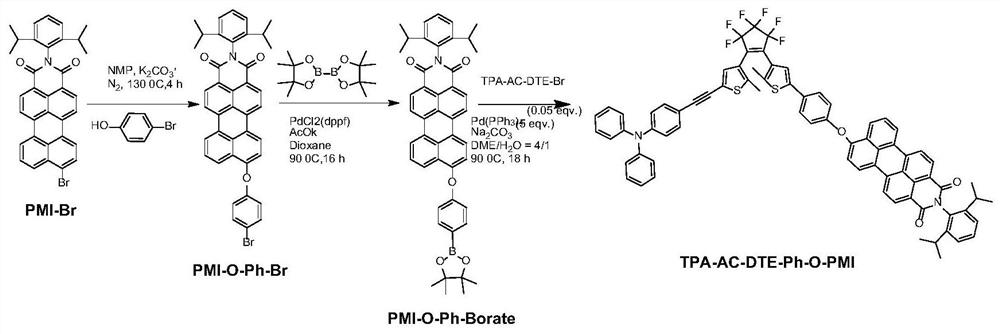
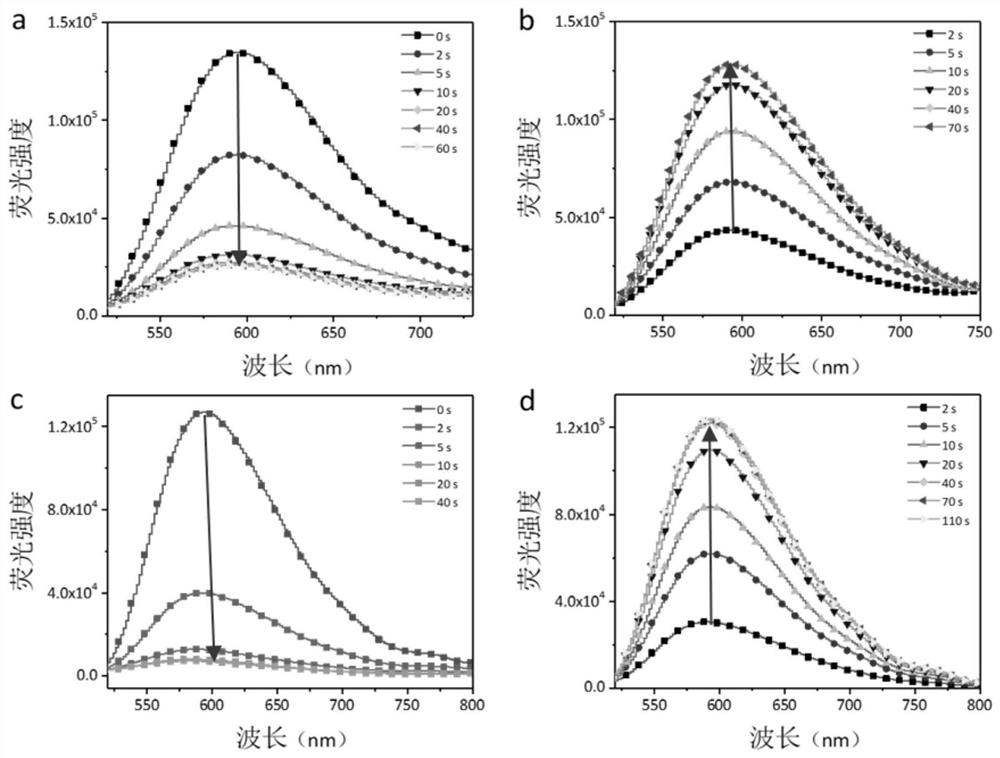
Visible-light-regulated dithienyl ethylene fluorescent molecular switch and preparation method and application thereof
ActiveCN112300142AHigh fluorescence quenching rateImprove fatigue resistanceOrganic chemistryFluorescence/phosphorescenceDithianeAryl
The invention belongs to the field of new materials, and particularly relates to a visible-light-regulated dithienyl ethylene fluorescent molecular switch and a preparation method and application thereof. The dithienyl ethylene derivative takes aniline alkynyl as a photosensitization group and perylene monoimide (PMI) as a fluorescent group, and comprises a structural unit shown as a formula (I) or a formula (II), wherein R1 and R2 are respectively and independently alkyl of C1-C10, alkyl alcohol of C1-C10 or aryl of C6-C20. When the derivative is used as a fluorescent molecular switch, the introduction of an aniline alkynyl group enables the fluorescence response wavelength of the molecular switch to be redshifted to a visible light region, and the derivative has the advantages of good thermal stability, good fatigue resistance, high cyclization rate, high fluorescence switch ratio and the like.
Owner:HUAZHONG UNIV OF SCI & TECH
Preparation method of anti-sulfur poisoning platinum complex
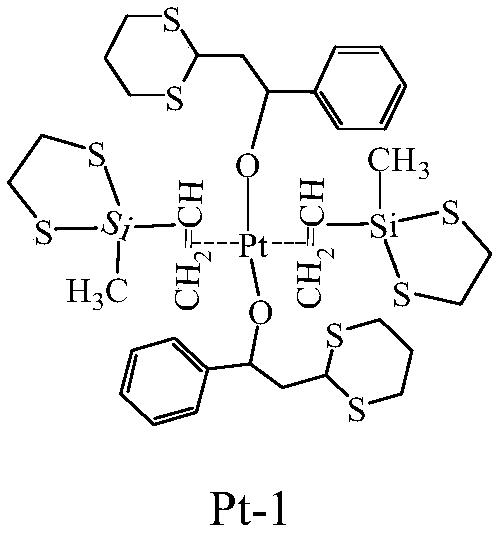
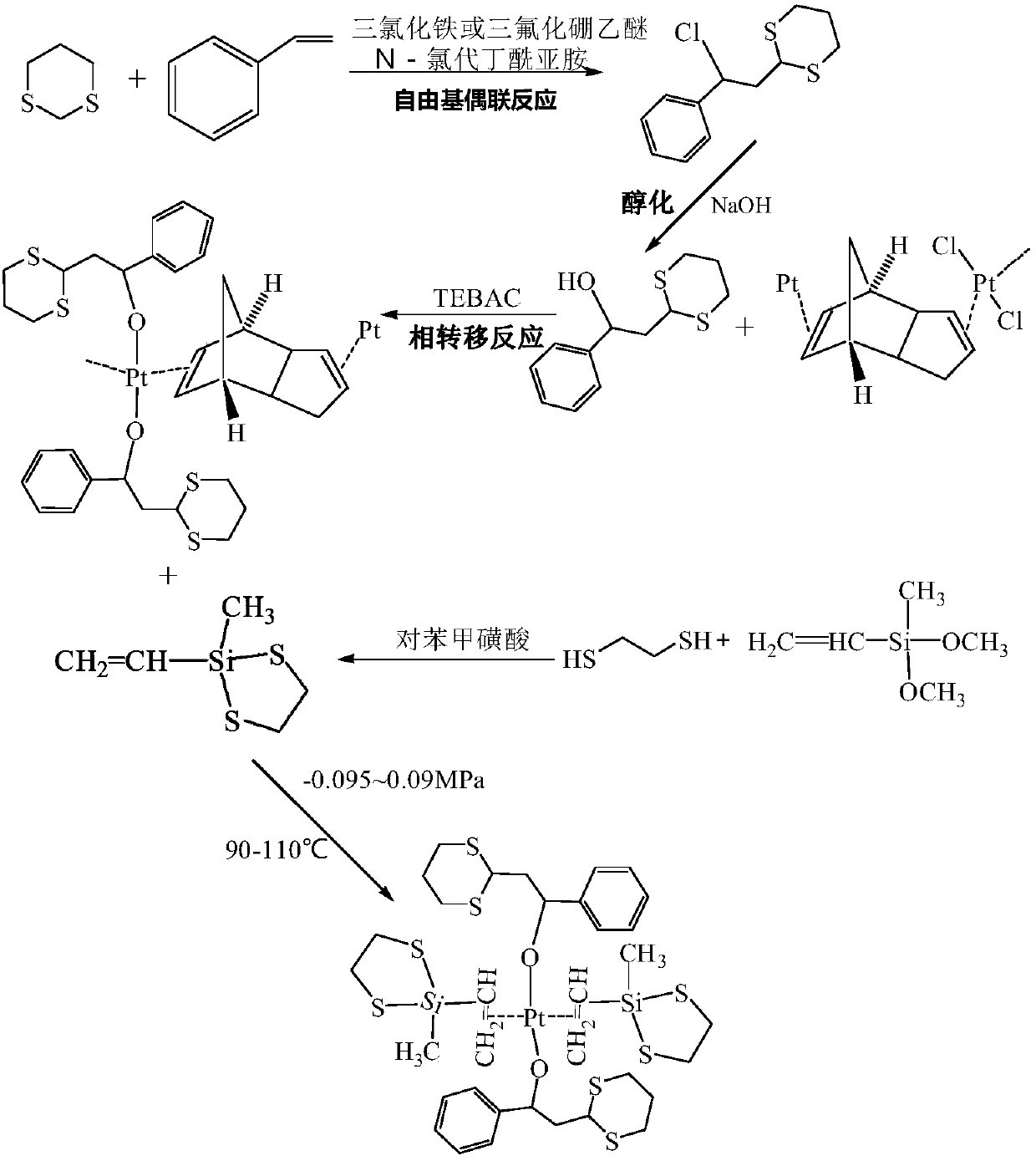
InactiveCN108794537AHigh activityLong storage periodOrganic-compounds/hydrides/coordination-complexes catalystsPlatinum organic compoundsDithianeBenzene
The invention provides a preparation method of an anti-sulfur poisoning platinum complex. The preparation method comprises the following steps: preparing a 1,3-dithiane benzene containing derivative intermediate A, preparing an unsaturated symmetrical epithio-silane intermediate B, connecting the 1,3-dithiane benzene containing derivative intermediate A to Pt through a phase transfer substitutionbond, then firstly substituting and then chelating the unsaturated symmetrical epithio-silane intermediate B to Pt, and obtaining the anti-sulfur poisoning platinum complex. The preparation method issimple and convenient, the reaction condition is mild, the production efficiency is high, the cost is low, and the industrial production is realized. A prepared catalyst has a highly symmetric complexstructure, the anti-sulfur poisoning platinum complex is stable at the room temperature, can be stored for long and has an excellent anti-sulfur poisoning catalytic effect, the catalytic activity ismore than 92%, the curing degrees of a sulfur-containing system and a non-sulfur system are basically the same and both reach more than 90%, and the anti-sulfur poisoning platinum complex has an excellent anti-sulfur poisoning capability, and is widely applied to the hydrosilylation.
Owner:GUANGDONG UNIV OF TECH
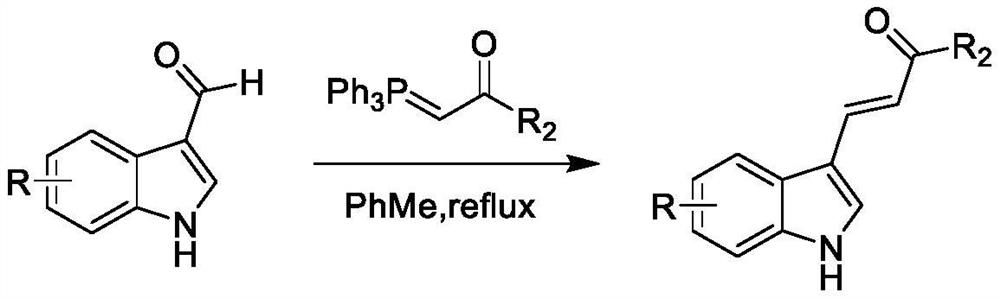
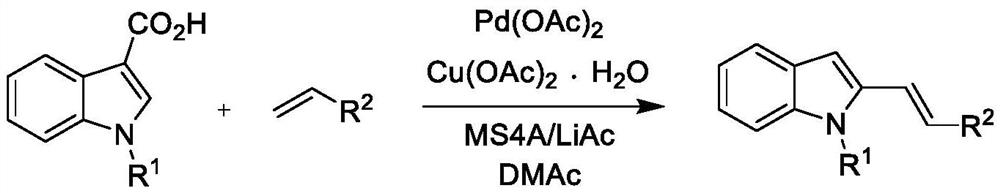
Synthetic method of alkenyl indole derivative
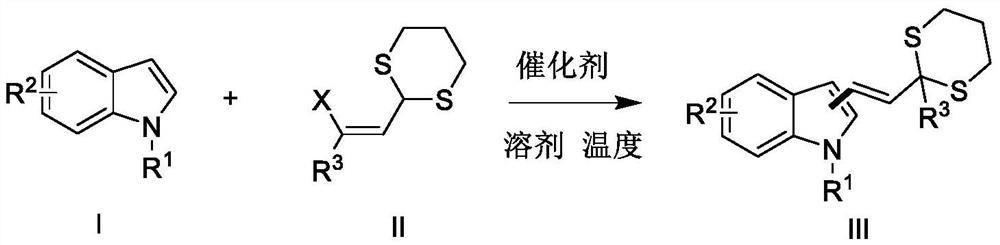
PendingCN114085216ASimple structureEasy to separate and purifyOrganic chemistry methodsPeptidesDithianePtru catalyst
The invention belongs to the field of organic synthesis, and relates to a synthetic method of alkenyl indole derivatives. The alkenyl indole compound has important research value in the aspects of synthesis and pharmaceutical activity, but conventional synthesis steps are tedious, conditions are harsh, and use of expensive heavy metals and positioning groups is involved. The invention provides a synthetic method of the alkenyl indole derivative, which comprises the following steps: by taking an alkenyl-1, 3-dithiane derivative and substituted indole as raw materials, synthesizing the alkenyl indole derivative through migration of a thiane ring under the action of a catalyst. The method is an efficient and simple synthesis method, has the advantages of mild reaction, high efficiency, simplicity in operation and economical raw materials, and has very high practicability.
Owner:LANZHOU UNIVERSITY
Adhesive composition and adhesive sheet
InactiveUS20150031785A1Faster rateGood Photochromic PropertiesOrganic chemistryAntifouling/underwater paintsDithianePolymer science
A pressure-sensitive adhesive composition containing an acrylic copolymer (A) having a structural unit (a1) derived from an alkyl (meth)acrylate (a1)′ and a structural unit (a2) derived from a functional group-containing monomer (a2)′, a crosslinking agent (B), and a photochromic dye (C) selected from a group consisting of dithienylethene-based compounds, oxazine-based compounds and naphthopyran-based compounds has a relatively rapid rate of color change from colored to colorless after termination of irradiation with UV rays, has excellent photochromic performance and can be a pressure-sensitive adhesive layer of a colorless pressure-sensitive adhesive sheet.
Owner:LINTEC CORP
Polymorphic substance of pyridinium derivative used as M3 muscarinic receptor antagonist as well as preparation method and medicine composition of polymorphic substance
ActiveCN103965179AMeet the requirements for the preparation of preparations for inhalation administrationMeets requirements for inhaled drug product formulationsOrganic active ingredientsOrganic chemistryDiseasePyridinium
The invention relates to the field of pharmacy, particularly to a polymorphic substance of 3R-1,1-dimethyl-3-(2-hydroxyl-2,2-dithienyl-2-acetoxyl) pyrrolidine bromide, a preparation method of the polymorphic substance and applications of the polymorphic substance to preparation of medicines for preventing or treating respiratory system diseases, urinary system diseases or gastrointestinal tract diseases.
Owner:JIANGSU LIANHUAN PHARMA
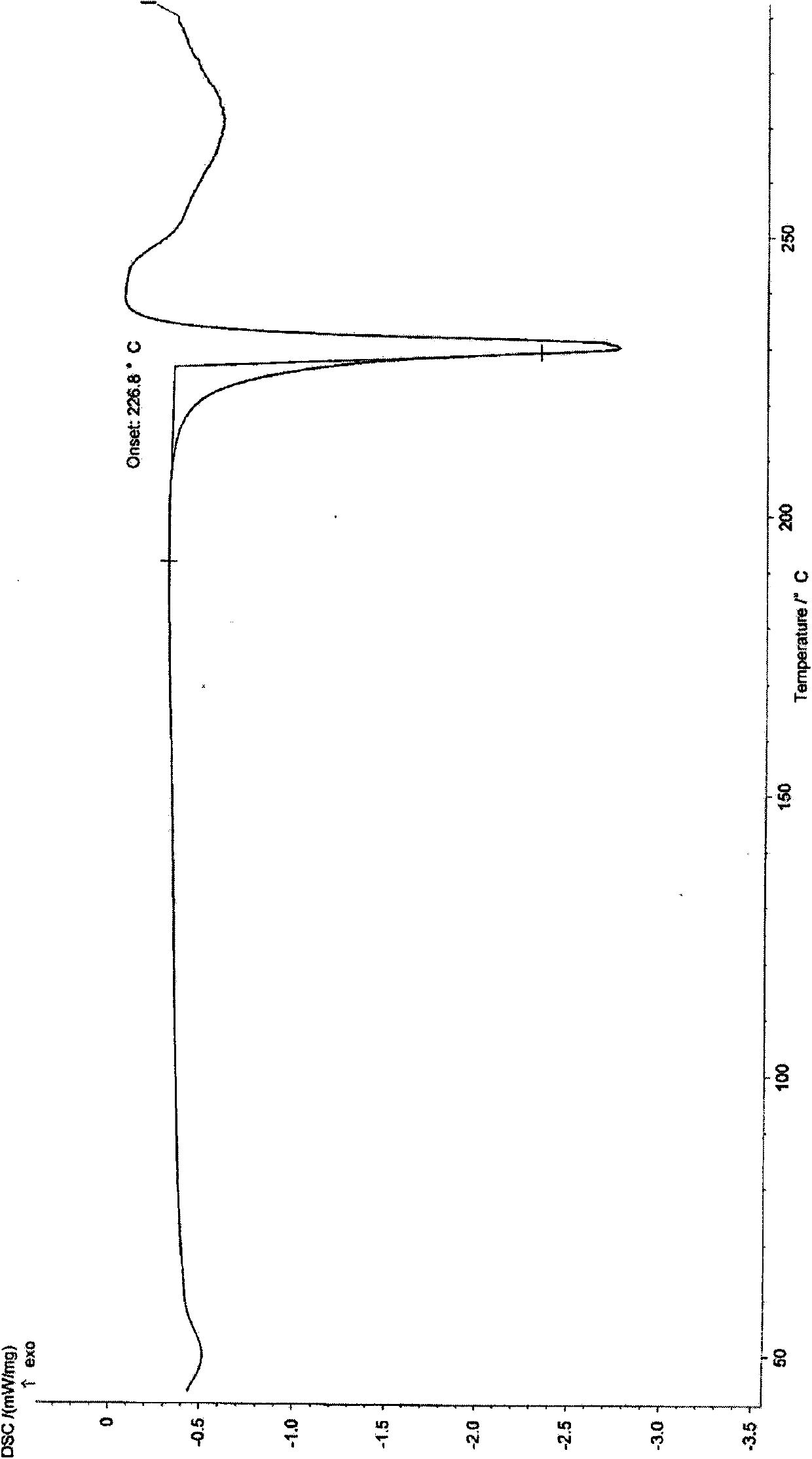
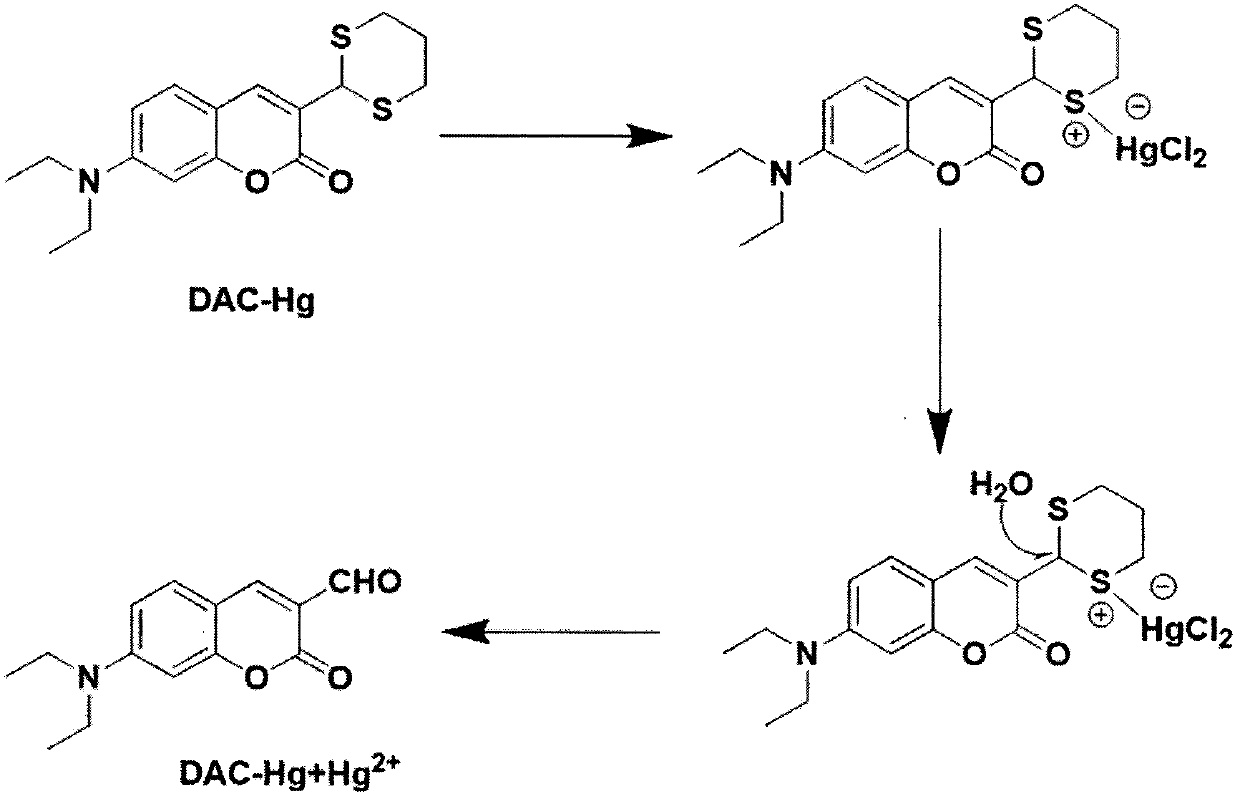
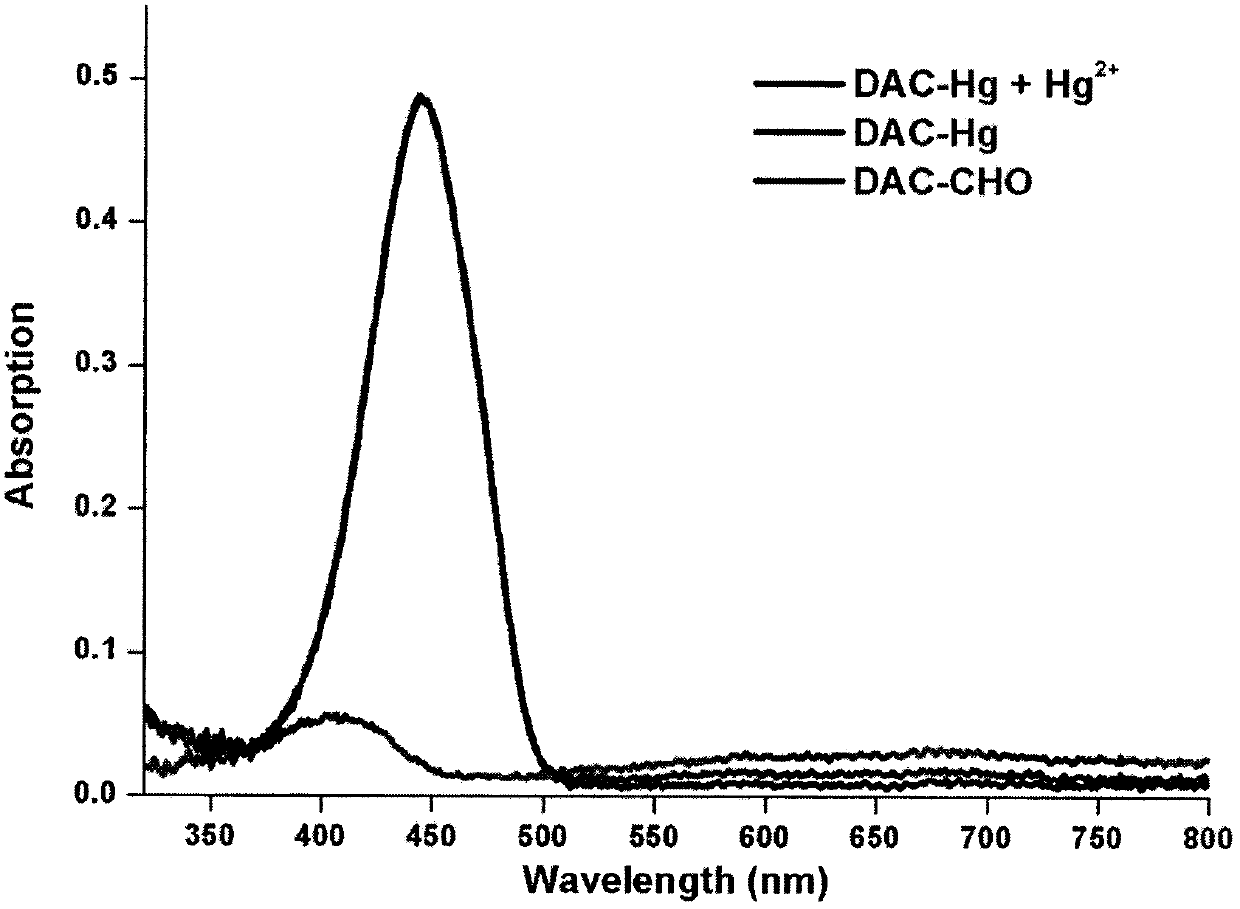
Preparation and application of coumarin type fluorescent probe for detecting Hg<2+>
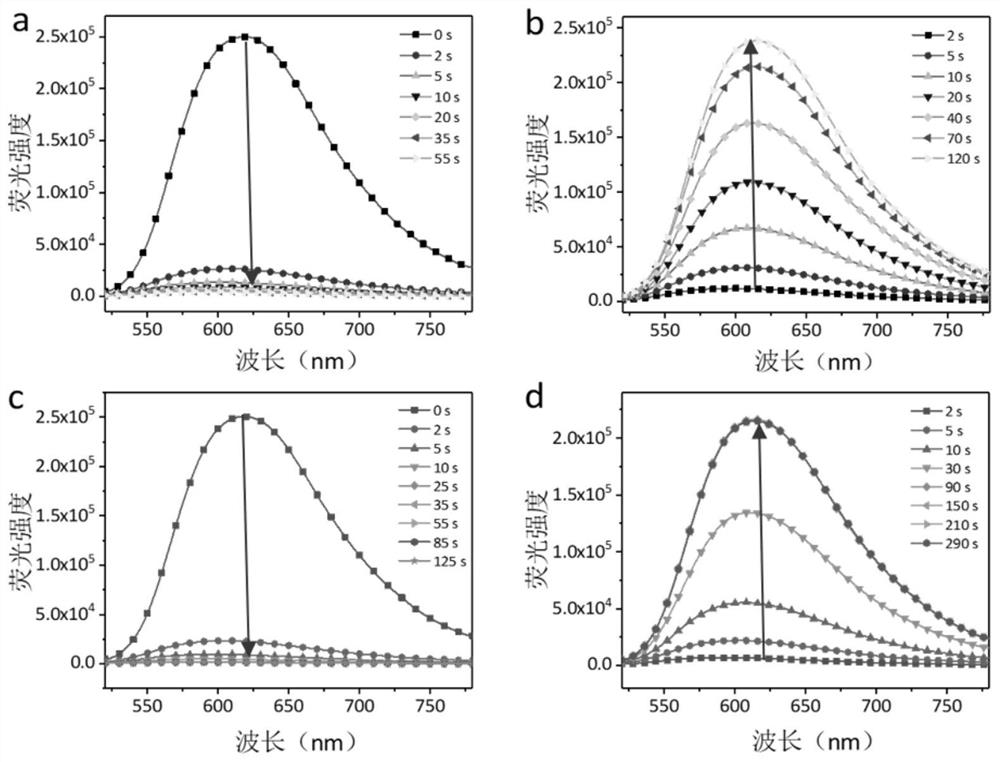
InactiveCN110551498AGood choiceHigh sensitivityOrganic chemistryFluorescence/phosphorescenceDithianeFluorescence
The invention relates to a preparation method and application of a coumarin type fluorescent probe; the compound is named as 7-(diethylamino)-3-(1,3-dithiane-2-yl)-2H-pyran-2-one. The compound has small molecular weight, simple structure and quite sensitive effect on Hg<2+>, shows excellent selectivity and sensitivity, is suitable for naked eye detection of Hg<2+>, has the detection limit of 5.0 nM which is the same as the most stringent Hg<2+> measurement standard, and has the potential of in-situ detection. The application of the established method in analysis of environment and seafood samples provides satisfactory results. Therefore, the compound provided by the invention provides a promising method for Hg<2+> detection and stimulates the development of induction of other heavy metal ions and transition metal ions. In a variety of water media, such as water, soil and seafood products, the probe further shows the potential of measuring and managing HTM ions, and has a broad application prospect.
Owner:NANJING UNIV
Pressure sensitive adhesive composition and pressure sensitive adhesive sheet
ActiveUS20140077139A1Good weather resistanceChange color quicklyFilm/foil adhesivesOptical elementsDithianePolymer science
A pressure-sensitive adhesive composition containing a pressure-sensitive adhesive that contains from 40 to 95% by mass of a rubber-based resin not containing a styrene-derived constituent unit, and a photochromic dye of a dithienylethene-based compound, wherein the content of the photochromic dye is from 0.40 to 8.00 parts by mass relative to 100 parts by mass of the pressure-sensitive adhesive, and a pressure-sensitive adhesive sheet having a pressure-sensitive adhesive layer formed of the pressure-sensitive adhesive composition have excellent weather resistance that can withstand long-term use, a relatively rapid rate of color change from colored to colorless, and excellent peelability to release sheets.
Owner:LINTEC CORP
D-A-D type organic photo-thermal small molecular material and preparation method thereof
InactiveCN110950894AGood dispersionStrong light-to-heat conversion abilityOrganic chemistryDithianePolymer science
The invention provides a D-A-D type organic photo-thermal small molecular material and a preparation method thereof. The D-A-D type organic photo-thermal small molecular material contains an electronwithdrawing group 2,1,3-benzothiadiazole and an electron donating group 3,6-di(thiophen-2-yl)-2,5-dihydropyrrolo[3,4-c] pyrrole-1,4-dione. The preparation method comprises the steps: substituting H ona thiophene ring of bromo-isooctane substituted 3,6-di(thiophen-2-yl)-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione with N-bromo-succinimide, and carrying out a reaction with 2,1,3-benzothiadiazole-4,7-bis(pinacol borate) through Suzuki reaction to obtain the target product. With the introduction of an alkyl chain on the side chain of the electron donating group, the solubility of the organic photo-thermal material in the organic solvent is improved, the problem of poor solubility of the organic polymer is overcome, and favorable light stability is achieved.
Owner:SHANXI UNIV
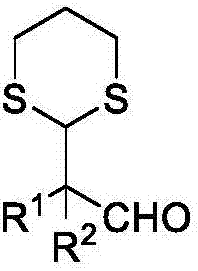
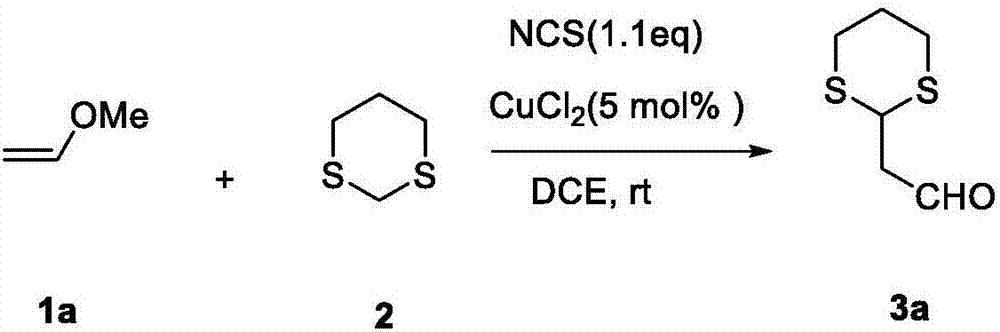
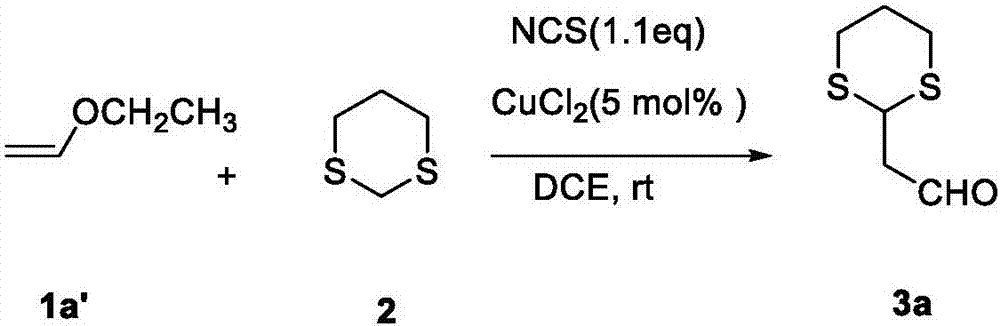
Method for synthesizing alpha-1,3-dithiane substituted aldehyde compound
The invention relates to a method for synthesizing alpha-1,3-dithiane substituted aldehyde compound. The preparation method comprises the following steps: under existence of an activating agent of N-chloro succinimide and a catalyst of Cu(II), dissolving 1,3-dithiane and substituted vinyl ether into an organic solvent, reacting for 1 to 4 hours under room temperature to 50 DEG C and separating and purifying to obtain alpha-1,3-dithiane substituted aldehyde compound. The method disclosed by the invention has moderate operation condition and avoids rigorous dewatering operation and device, the utilized commercialized vinyl ether compound is cheap and easy to obtain, a reaction process is simple, good functional group tolerance is obtained, a beneficial technological effect is obtained, and the method can be well applied to scientific researches and industrial production.
Owner:LANZHOU UNIVERSITY
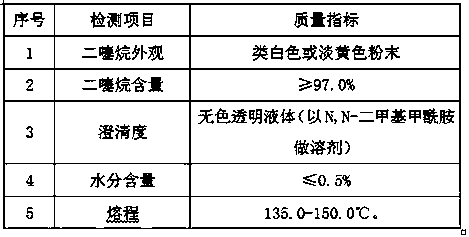
Preparation method for 2,5-dihydroxyl-1,4-dithiane
The invention provides a preparation method for 2,5-dihydroxyl-1,4-dithiane. The preparation method comprises the steps of adjusting pH of chloroacetaldehyde, preparing a sodium hydrosulfide solution,dropwise adding part of sodium hydrosulfide solution, carrying out a dropwise adding reaction, and carrying out aging. The 2,5-dihydroxyl-1,4-dithiane prepared by the method has a yield not lower than 90.0%; the appearance of the 2,5-dihydroxyl-1,4-dithiane prepared by the method is white-like or yellowish powder, and the content of dithiane is not lower than 97.0%; the moisture content is not higher than 0.5%; the clarity is of colorless transparent liquid (N,N-dimethylformamide serves as a solvent); and a melting range is 135.0 DEG C to 150.0 DEG C.
Owner:WEIFANG HUITAO CHEM
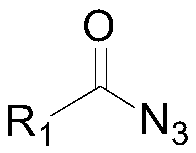
Preparation method of 4-hydroxyl-3-substuted-thiazolane-2-ketone compound
The invention discloses a preparation method of a 4-hydroxyl-3-substuted-thiazolane-2-ketone compound and relates to the technical field of medicine. The preparation method of the 4-hydroxyl-3-substuted-thiazolane-2-ketone compound includes the following steps of adding an acyl nitrine compound and 2,5-dyhydroxyl-1,4-dithiane into an organic solvent for a ring-closure reaction to obtain a reactionliquid; filtering the reaction liquid, obtaining a filtrate, removing impurities in the filtrate and then distilling the filtrate to obtain a concentrated liquid; conducting silica gel column chromatography on the concentrated liquid to obtain 4-hydroxyl-3-substuted-thiazolane-2-ketone. The provided preparation method can achieve efficient synthesis of 4-hydroxyl-3-substuted-thiazolane-2-ketone and increase the yield.
Owner:WUHAN POLYTECHNIC UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com






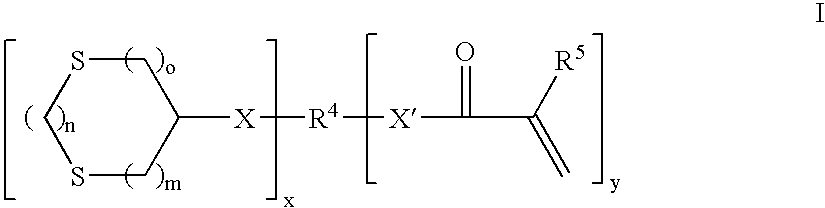



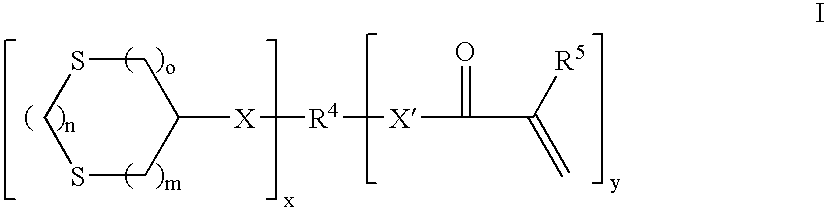

![Benzodithiophene based copolymer containing thieno [3,4-B] thiophene units and preparing method and applications thereof Benzodithiophene based copolymer containing thieno [3,4-B] thiophene units and preparing method and applications thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2547cb5f-d9b8-46a5-a979-45f785cc2439/US09365679-20160614-D00000.PNG)
![Benzodithiophene based copolymer containing thieno [3,4-B] thiophene units and preparing method and applications thereof Benzodithiophene based copolymer containing thieno [3,4-B] thiophene units and preparing method and applications thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2547cb5f-d9b8-46a5-a979-45f785cc2439/US09365679-20160614-D00001.PNG)
![Benzodithiophene based copolymer containing thieno [3,4-B] thiophene units and preparing method and applications thereof Benzodithiophene based copolymer containing thieno [3,4-B] thiophene units and preparing method and applications thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2547cb5f-d9b8-46a5-a979-45f785cc2439/US09365679-20160614-D00002.PNG)









![Benzodithiophene based copolymer containing pyridino [2,1,3] thiadiazole units and preparing method and applications thereof Benzodithiophene based copolymer containing pyridino [2,1,3] thiadiazole units and preparing method and applications thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/40297f05-4f47-43c8-81fa-30fe4b2d4aad/US20150284505A1-20151008-D00000.PNG)
![Benzodithiophene based copolymer containing pyridino [2,1,3] thiadiazole units and preparing method and applications thereof Benzodithiophene based copolymer containing pyridino [2,1,3] thiadiazole units and preparing method and applications thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/40297f05-4f47-43c8-81fa-30fe4b2d4aad/US20150284505A1-20151008-D00001.PNG)
![Benzodithiophene based copolymer containing pyridino [2,1,3] thiadiazole units and preparing method and applications thereof Benzodithiophene based copolymer containing pyridino [2,1,3] thiadiazole units and preparing method and applications thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/40297f05-4f47-43c8-81fa-30fe4b2d4aad/US20150284505A1-20151008-D00002.PNG)