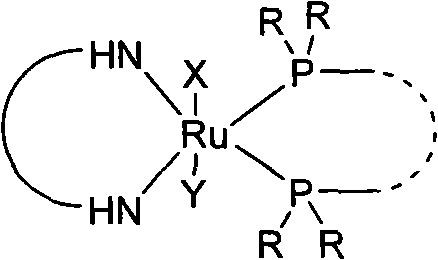
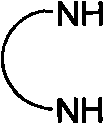
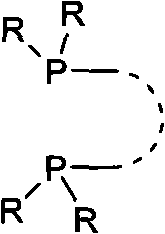
Chiral ruthenium catalyst and use thereof in asymmetric hydrogenation of ketone
A technology of ruthenium catalyst and hydrogenation reaction, which is applied in the field of chiral metal ruthenium catalyst and its application in ketone asymmetric hydrogenation reaction, can solve the problems of high cost and high catalyst cost, and achieve simple structure, low cost and strong practicability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Preparation of (s)-1-phenylethyl alcohol 2a from acetophenone 1a
[0040]
[0041] Under an argon atmosphere, the catalyst Cat.1 (0.1%, 0.0025mmol), acetophenone 1a (300mg, 2.5mmol) and KO t Bu (28 mg, 0.25 mmol) was dissolved in n-PrOH (2.5 ml) and placed in an autoclave. Fill the autoclave with 10 atm hydrogen and stir at room temperature for 10 h. After the reaction, the solvent was drained and separated by column chromatography (using a silica gel column; eluent: petroleum ether / ethyl acetate=5 / 1) to obtain pure product 2a. The product was a colorless liquid (98% ee, >99% yield).
Embodiment 2
[0042] Example 2: Preparation of (s)-1-phenylethyl alcohol 2a from acetophenone 1a
[0043]
[0044] Under argon atmosphere, the above-mentioned catalyst (0.1%, 0.0025mmol), acetophenone 1a (300mg, 2.5mmol) and KOtBu (28mg, 0.25mmol) prepared by the preparation method of type A catalyst were dissolved in n-PrOH ( 2.5ml) and placed in a sealed tube. Use liquid nitrogen to freeze the solution in the sealed tube into a solid and evacuate the sealed tube into a vacuum with a vacuum pump, then heat the sealed tube to melt the solid inside and pour 1 atm of hydrogen into the sealed tube, and stir at room temperature for 30 h. After the reaction, the solvent was drained and separated by column chromatography (using a silica gel column; eluent: petroleum ether / ethyl acetate=5 / 1) to obtain pure product 2a. The product was a colorless liquid (96% ee, >99% yield).
Embodiment 3
[0045] Example 3: Preparation of (s)-1-phenylethyl alcohol 2a from acetophenone 1a
[0046]
[0047] Under an argon atmosphere, the above-mentioned catalyst (0.1%, 0.0025mmol), acetophenone 1a (300mg, 2.5mmol) and KO t Bu (28 mg, 0.25 mmol) was dissolved in n-PrOH (2.5 ml) and placed in an autoclave. Fill the autoclave with 20 atm hydrogen and stir at room temperature for 10 h. After the reaction, the solvent was drained and separated by column chromatography (using a silica gel column; eluent: petroleum ether / ethyl acetate=5 / 1) to obtain pure product 2a. The product was a colorless liquid (98% ee, >99% yield).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com