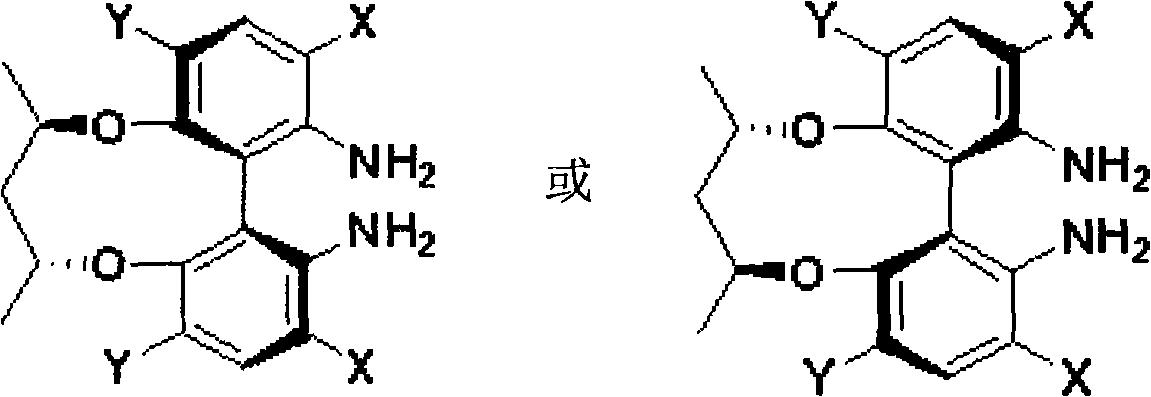
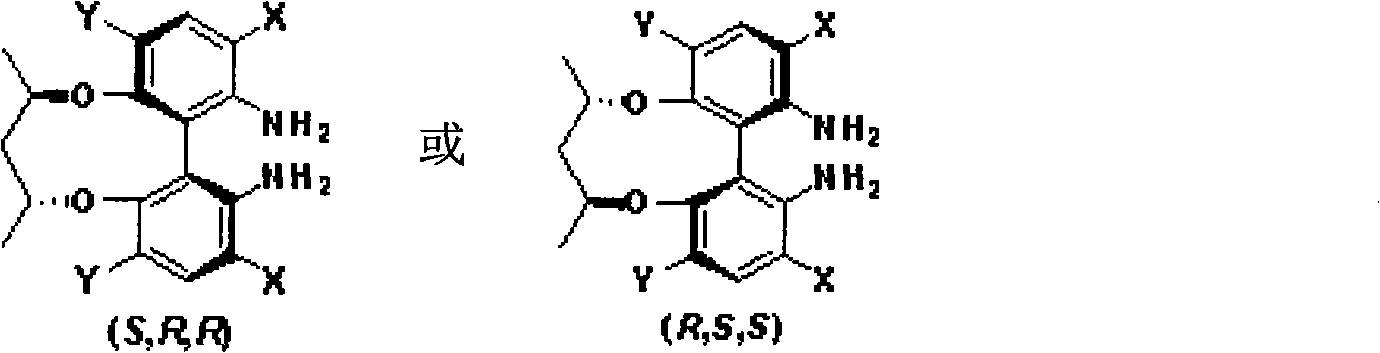
Axial chirality diamine compound induced by central chirality and synthetic method
A bisamine compound and chiral induction technology, applied in the direction of organic chemistry, can solve problems such as limitations in industrialization and application, and achieve the effect of easy raw materials, simplified synthesis process, and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Synthesis of (R, R)-2,4-bis[(2-iodo-3-nitro)phenoxy]pentane (1a)
[0036] Dissolve 2-iodo-3-nitrophenol (2.8g, 0.01mol) in 25mL N, N-dimethylformamide, then add potassium carbonate (4.14g, 0.03mol), (2S, 4S)-2, 4-Pentanediol bis-p-toluenesulfonate (6.00mmol), heated at 80°C until the reaction was complete (TLC tracking), and distilled off most of N,N-dimethylformamide under reduced pressure, after adding water, CH 2 Cl 2 The organic phases were extracted and combined, washed with water and saturated brine respectively, dried over anhydrous sodium sulfate, and purified by column chromatography to obtain 5.26 g of light yellow powder product compound (1a) with a yield of 88%. [α] 25 D =-169.8 (c 0.40, CHCl 3 ); 1 H NMR (CDCl 3 , TMS, 300MHz) δ1.44(d, J=5.7Hz, 6H), 2.13~2.17(m, 2H), 4.83~4.87(m, 2H), 6.82(d, J=8.1Hz, 2H), 7.10 (d, J=8.1Hz, 2H), 7.18~7.7.24(t, 2H); 13 C NMR (CDCl 3 , TMS, 75MHz) δ20.33, 44.78, 73.98, 81.35, 115.97, 116.89, 130.33, 155.58, 158.16; H...
Embodiment 2
[0038] Synthesis of (S, S)-2,4-bis[(2-iodo-3-nitro)phenoxy]pentane (1b)
[0039]2-iodo-3-nitrophenol (2.8g, 0.01mol) was dissolved in 25mL N,N-dimethylformamide, and potassium carbonate (4.14g, 0.03mol) was added, (2R, 4R)-2 , 4-pentanediol bis-p-toluenesulfonate (6.00mmol), heated at 50°C until the reaction was complete (TLC tracking), and distilled off most of N,N-dimethylformamide under reduced pressure, after adding water, CH 2 Cl 2 The organic phases were extracted and combined, washed with water and saturated brine respectively, dried over anhydrous sodium sulfate, and purified by column chromatography to obtain 5.26 g of light yellow powder product compound (1b) with a yield of 88%. [α] 25 D =+169.8 (c 0.40, CHCl 3 ); 1 H NMR (CDCl 3 , TMS, 300MHz) δ1.44(d, J=5.7Hz, 6H), 2.13~2.17(m, 2H), 4.83~4.87(m, 2H), 6.82(d, J=8.1Hz, 2H), 7.10 (d, J=8.1Hz, 2H), 7.18~7.7.24(t, 2H); 13 C NMR (CDCl 3 , TMS, 75MHz) δ20.33, 44.78, 73.98, 81.35, 115.97, 116.89, 130.33, 155.58, ...
Embodiment 3
[0041] Synthesis of (R, R)-2,4-bis([(2-iodo-3-nitro)phenoxy]pentane (1a)
[0042] Dissolve 2-iodo-3-nitrophenol (2.8g, 0.01mol) in 25mL N,N-dimethylformamide, then add potassium carbonate (0.05mol), (2S,4S)-2,4-pentane Diol bis-p-methanesulfonate (0.01mmol), heated at 100°C until the reaction was complete (TLC tracking), and distilled off most of N,N-dimethylformamide under reduced pressure, after adding water, CH 2 Cl 2 The organic phases were extracted and combined, washed with water and saturated brine respectively, dried over anhydrous sodium sulfate, and purified by column chromatography to obtain 5.26 g of light yellow powder product compound (1a) with a yield of 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com