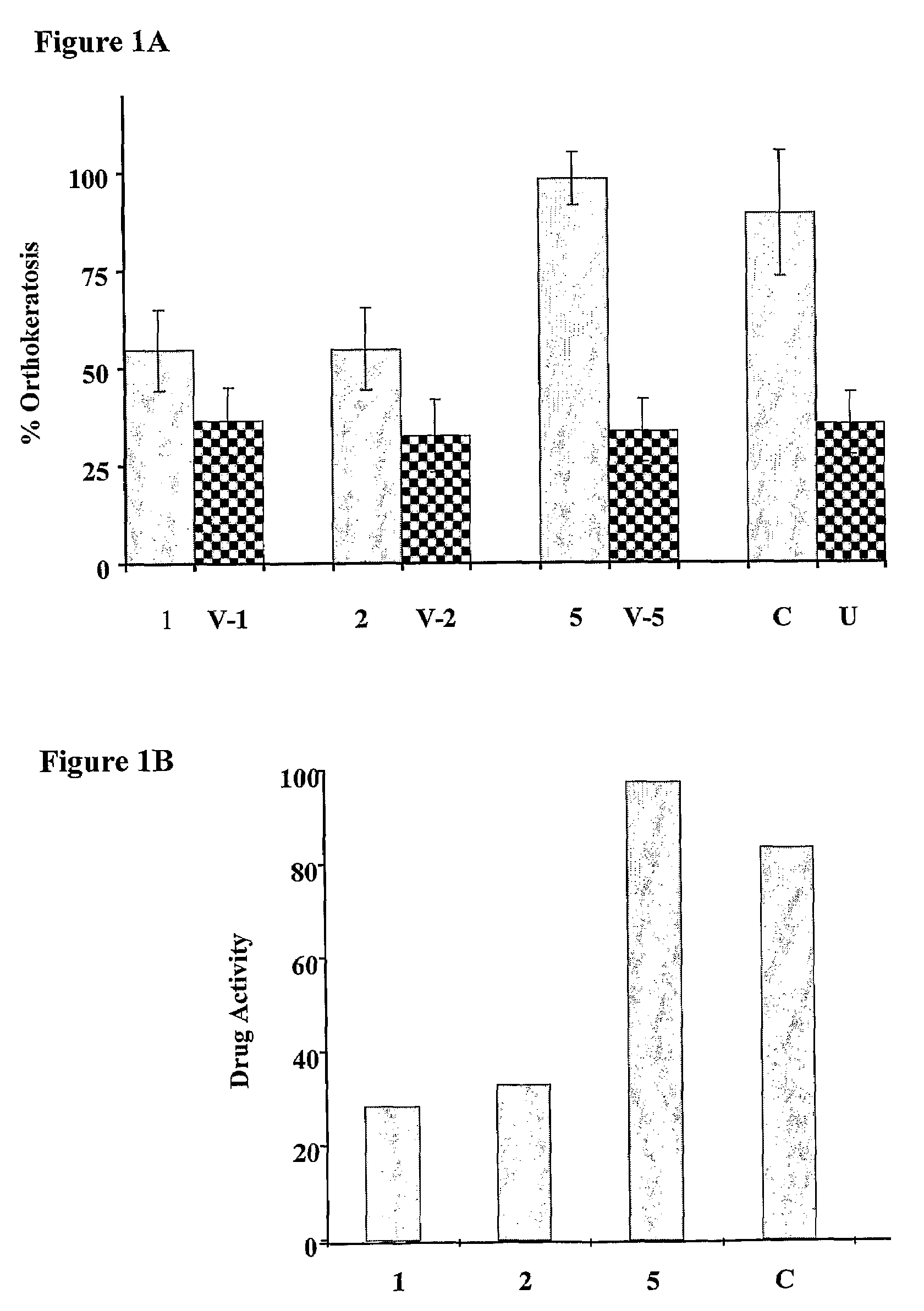
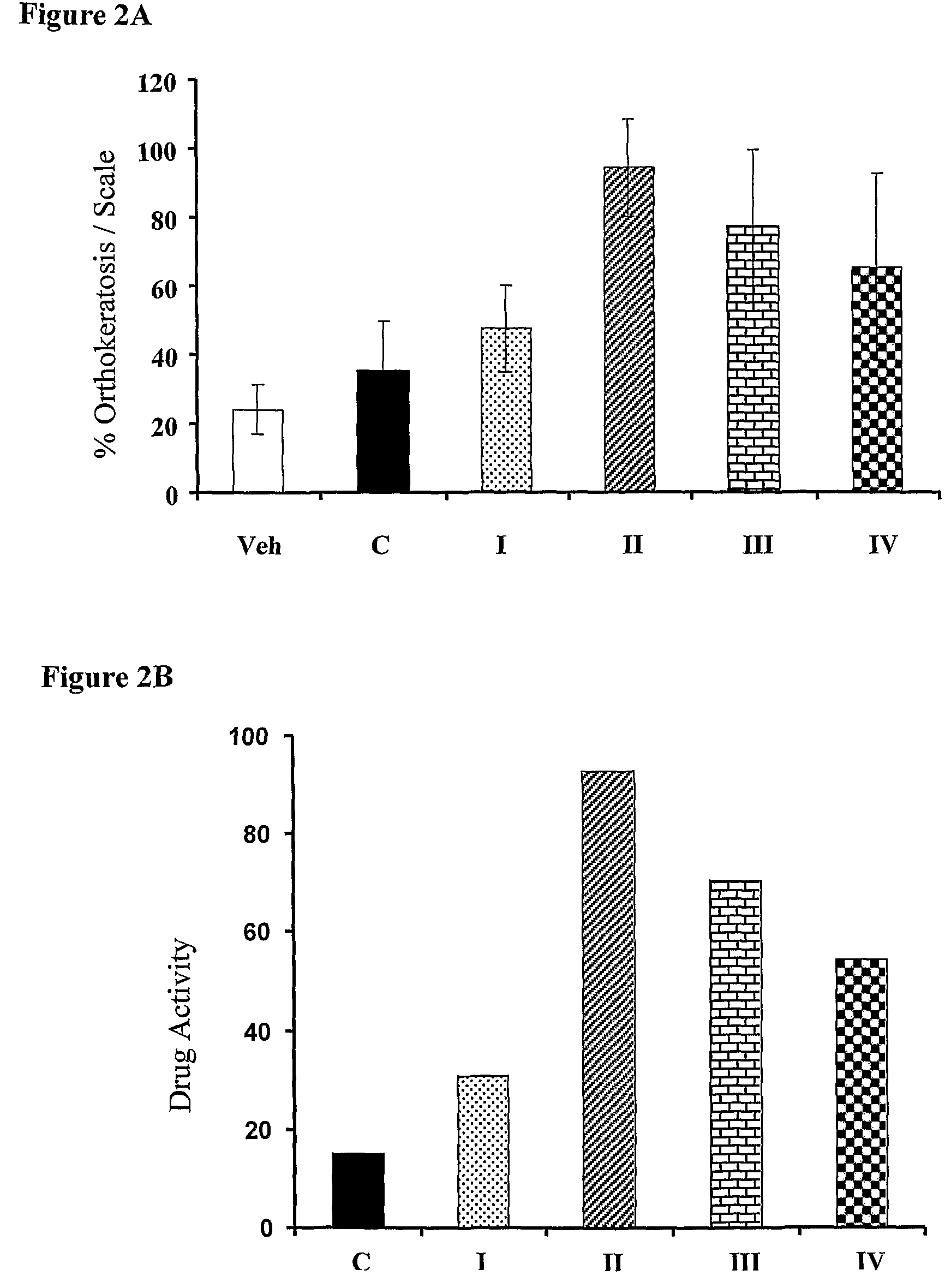
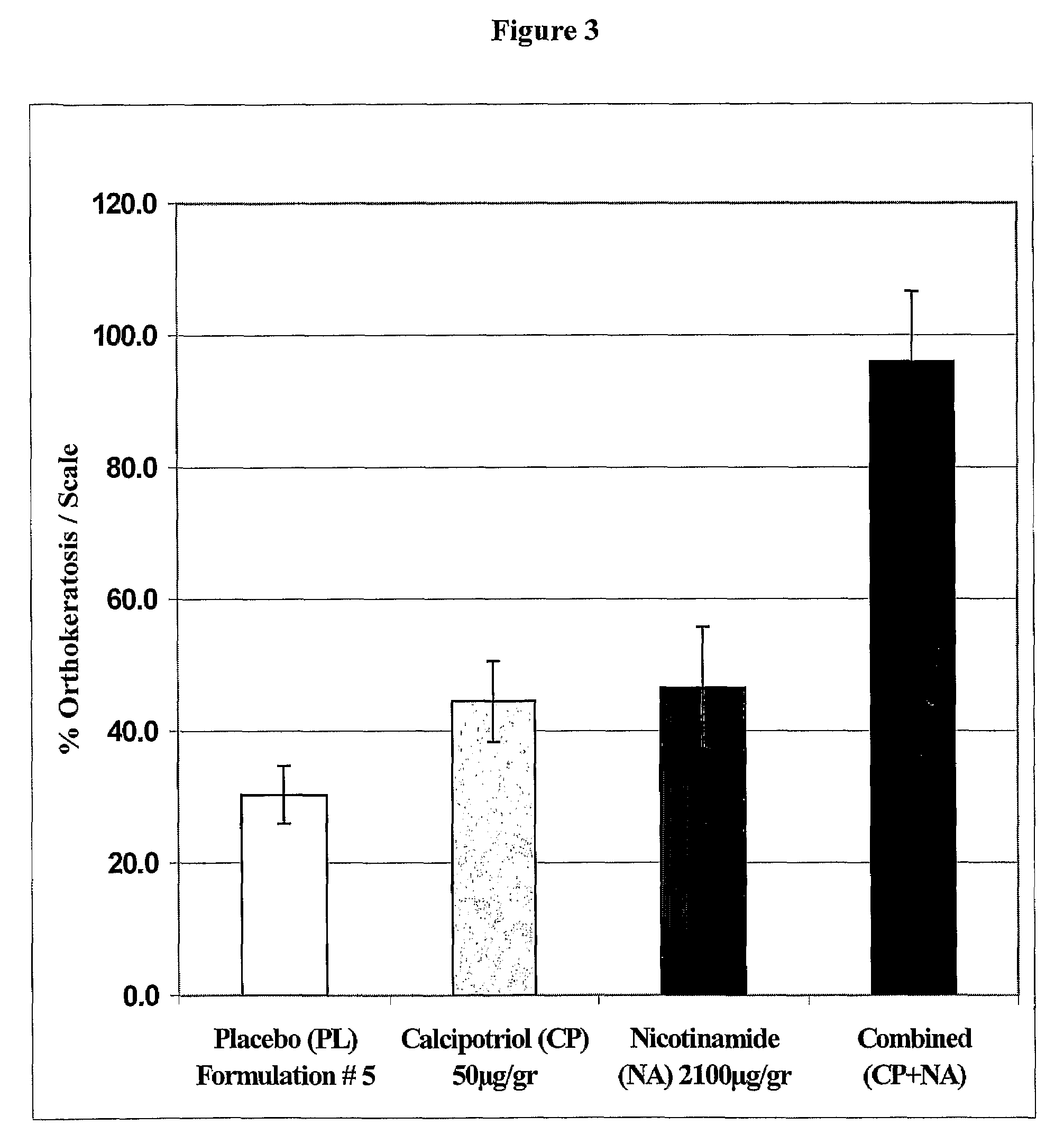
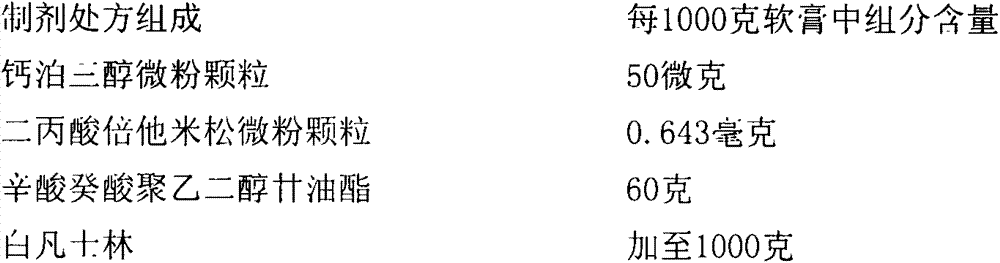Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
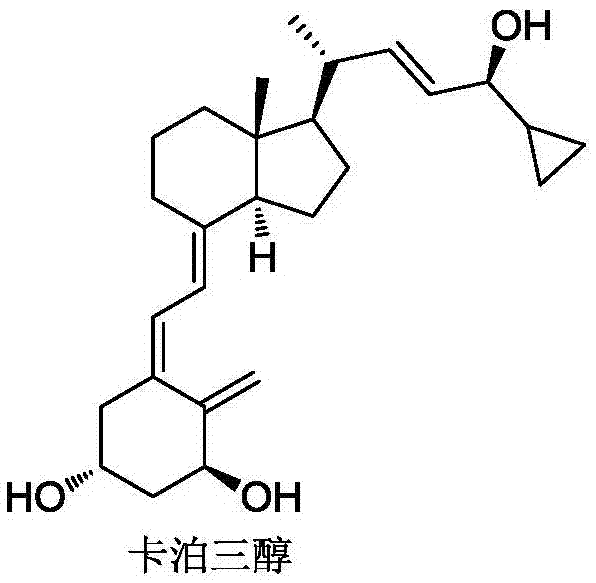
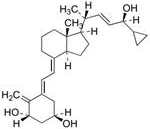
65 results about "Calcipotriol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
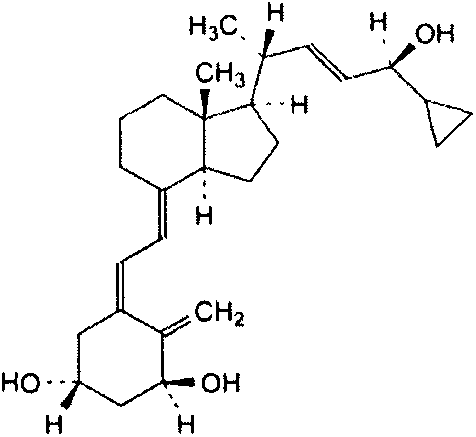
This medication is used to treat psoriasis.
Compositions and methods for treating hyperproliferative epidermal diseases
InactiveUS20090131488A1Relieve symptomsHighly efficaciousBiocideHydroxy compound active ingredientsDiseaseVitamin D metabolism
The present invention provides compositions and methods for use in the treatment of hyperproliferative dermal diseases. Specifically, the present invention teaches pharmaceutical compositions for topical administration where the compositions contain nicotinamide and a vitamin D metabolite, calcipotriol, which are particularly effective in treating and in the maintenance treatment of psoriasis and other related dermal disorders and diseases.
Owner:DERMIPSOR LTD
Pharmaceutical compositions for the treatment of psoriasis
Pharmaceutical compositions for the treatment of skin disorders such as psoriasis, acne and eczema, methods of making the compositions and methods of use thereof are described herein. The composition comprises psorberine, an alcohol-water extract isolated from the Mahonia aquifolium plant, and one or more additional active agents. In a preferred embodiment, the one or more active agents is a vitamin D3 analog, such as calcipotriol. The compositions may also contain excipients such as emollients, surfactants, emulsifiers and buffers. The compositions are formulated into a cream, lotion or ointment for topical administration.
Owner:APOLLO PHARMA
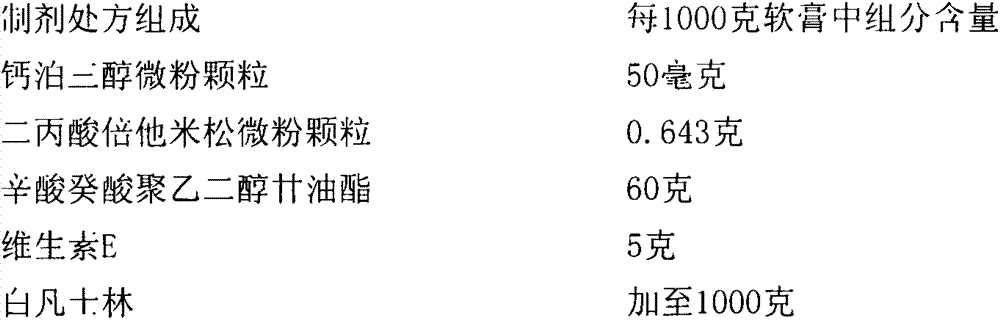
Calcipotriol betamethasone ointment and preparation method thereof
InactiveCN103110648AOrganic active ingredientsAerosol deliveryBetamethasone propionatePropanoic acid
The invention a calcipotriol betamethasone ointment prepared from caprylocaproyl macrogolglycerides. Under the conditions of high speed cutting and a homogeneous state, calcipotriol micronized particles with particle size range being 5-110 microns and the micronized particles of betamethasone dipropionate are uniformly dispersed to caprylocaproyl macrogolglycerides, affinities of caprylocaproyl macrogolglycerides and a commonly used ointment matrix are utilized to obtain the uniform ointment with uniformly distributed particle size. Furthermore, the stability of resisting high temperature (50 DEG C) is better, and is beneficial to improving the quality and the stability of a medicine.
Owner:JIANGSU SEMPOLL PHARMA
Preparation containing calcipotriol and betamethasone dipropionate
ActiveCN104666312AEnsure safetyGuaranteed curative effectOrganic active ingredientsAerosol deliveryForeign - SimilarPharmaceutical formulation
The invention belongs to the field of medicine preparations, and particularly relates to a preparation technology of calcipotriol. According to the preparation technology, the problem of the stability of calcipotriol and betamethasone dipropionate is solved. Benzyl alcohol and triethanolamine are added to auxiliary materials. The paste prepared by the technology is stable in property. Compared with foreign similar products, the cost is lower; and the method is suitable for industrialized production.
Owner:CHONGQING HUAPONT PHARMA
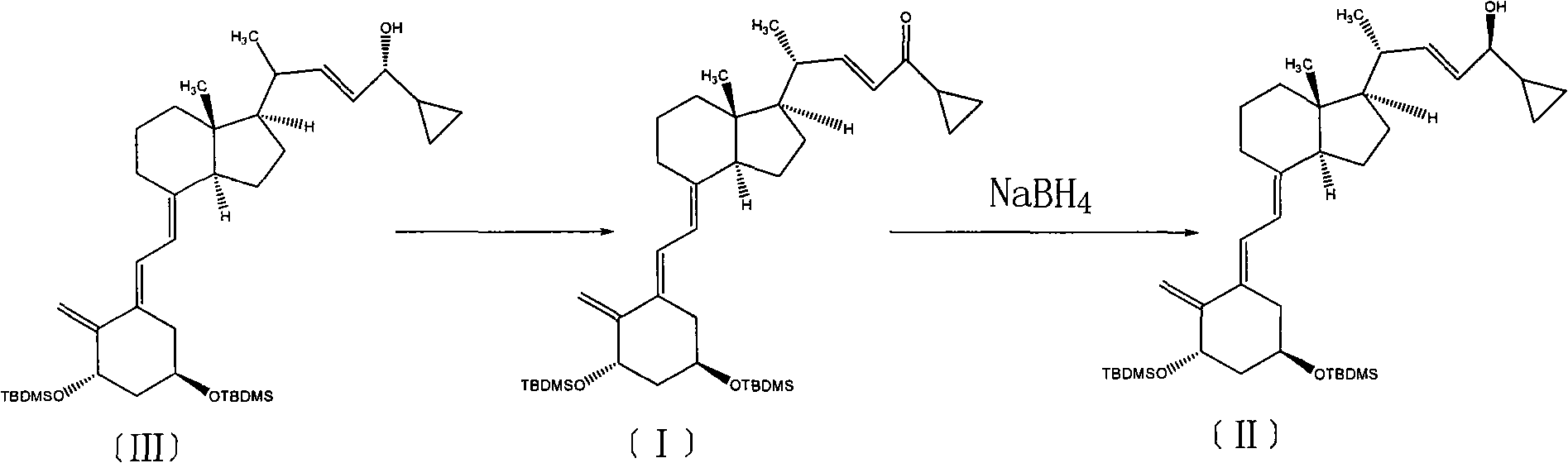
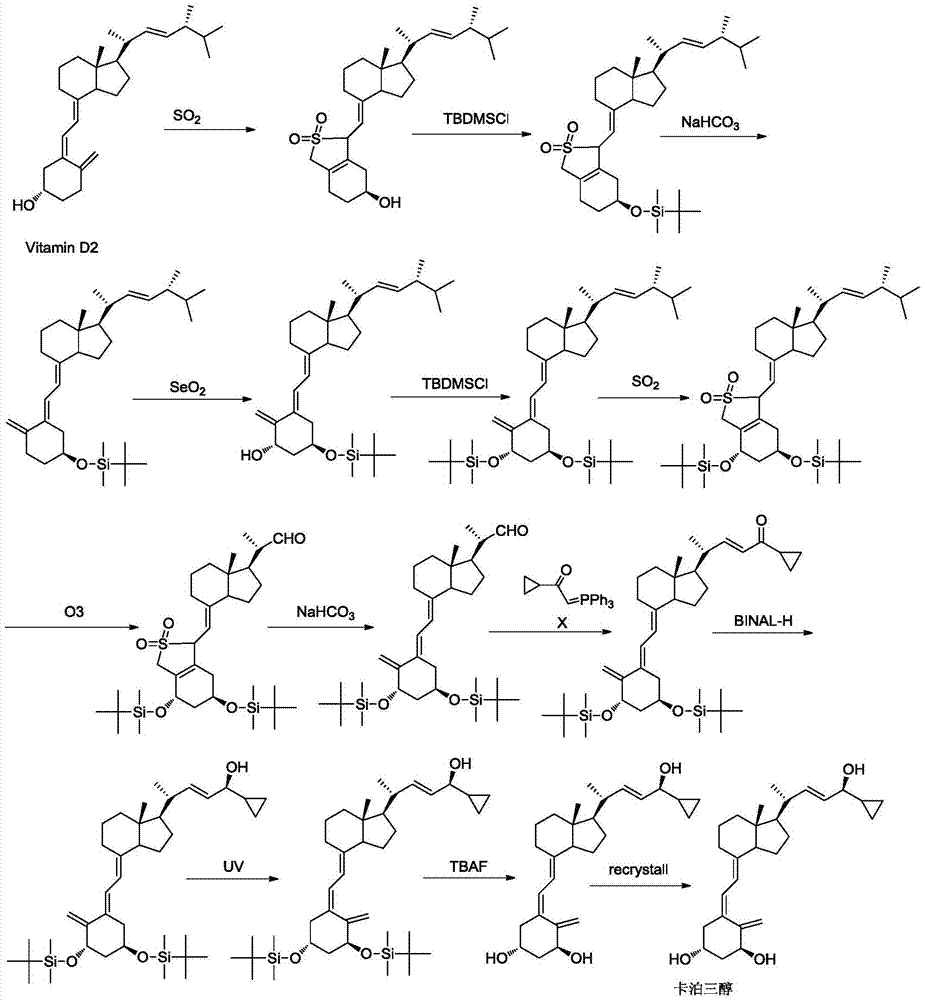
Method for preparing vitamin D2 derivative
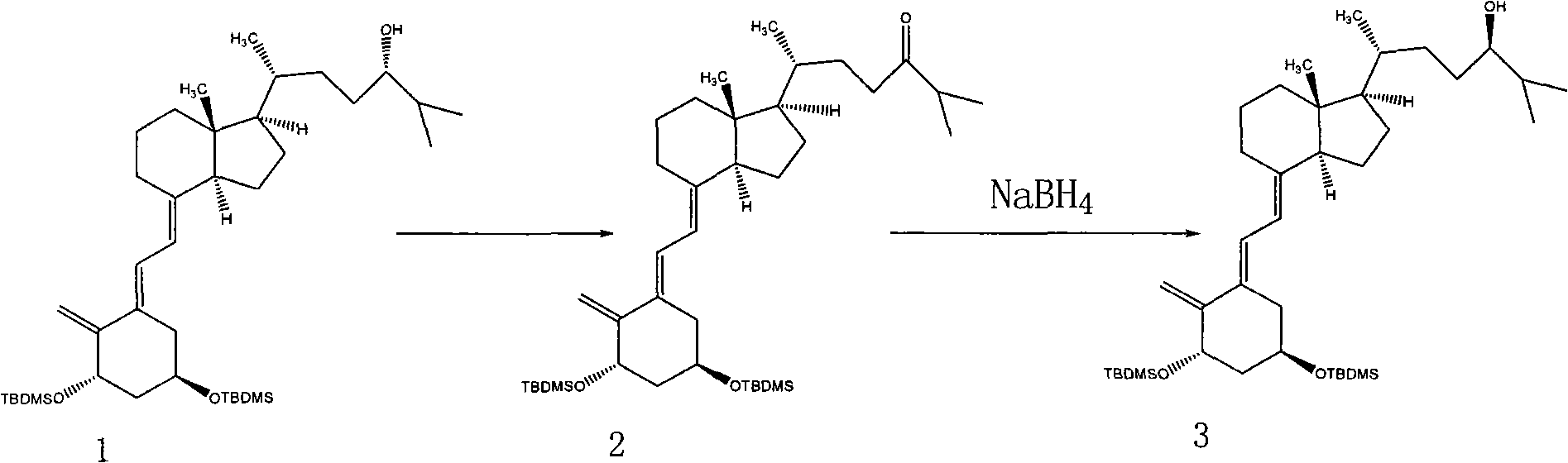
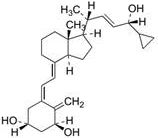
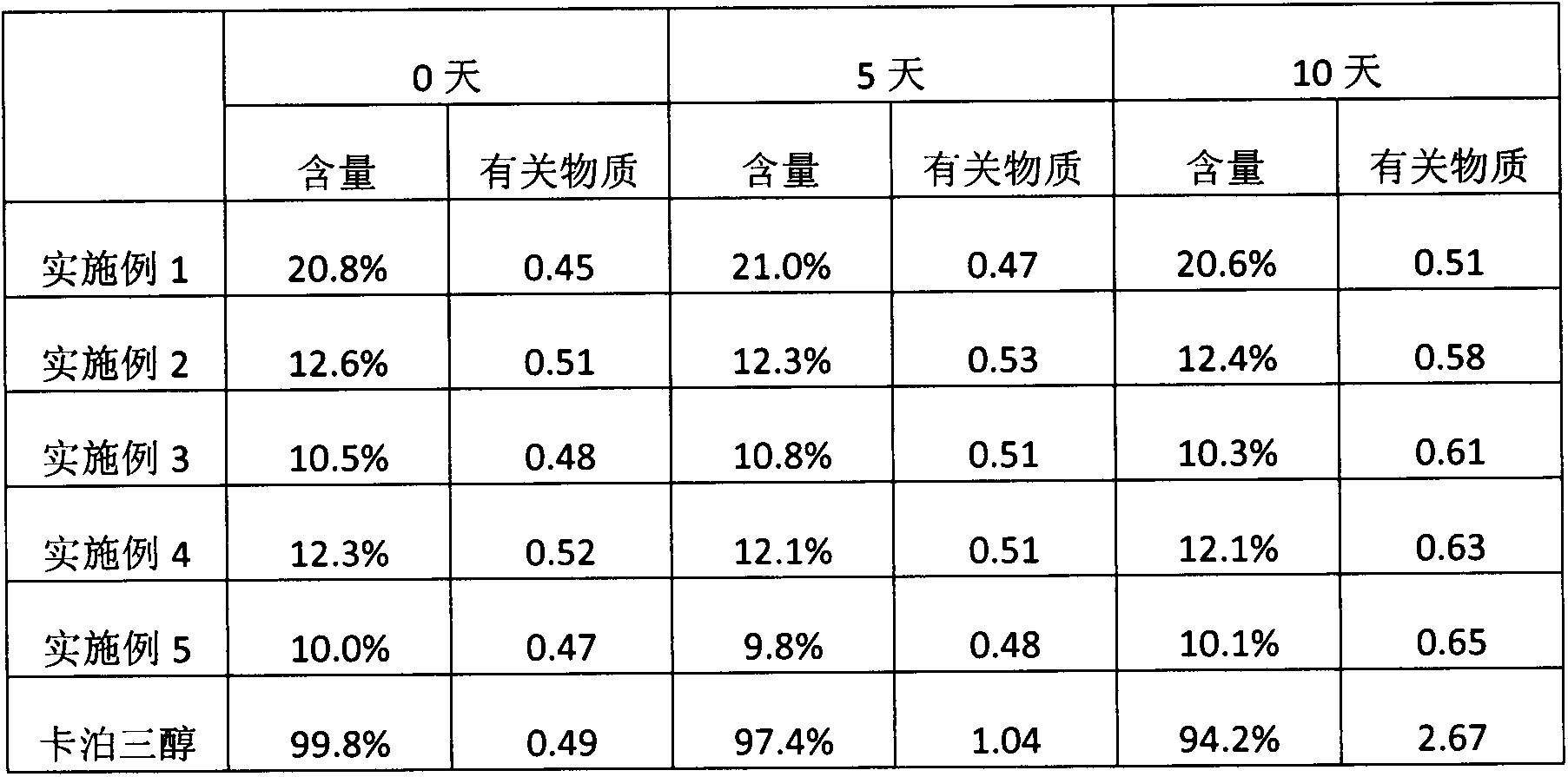
The invention discloses a method for preparing a vitamin D2 derivative. In the conventional method for preparing Calcipotriol, C-24 hydroxy epimeric alcohol (a compound III) is mainly obtained, the yield of the Calcipotriol is greatly reduced, and the C-24 hydroxy epimeric alcohol is a waste intermediate and does not have any utilization value so far. In the method of the invention, the waste intermediate compound III generated in the conventional process of preparing the Calcipotriol is taken as a starting material, and the method comprises the following steps of: in an organic solvent and in the presence of an oxidizer capable of oxidizing hydroxyl into carbonyl, performing heat preservation reaction at the temperature of between 20 and 120 DEG C, after complete reaction, cooling to room temperature, and treating to obtain a compound I; and performing reduction reaction on the compound I to obtain a compound II, namely the Calcipotriol. In the method, the waste intermediate compound III is recycled, the cost of raw materials is low, the utilization rate of the materials is improved, and the pollution is reduced; and by combining the conventional preparation process, the method can obviously improve the yield of the Calcipotriol in the conventional preparation process.
Owner:ZHEJIANG JINGXIN PHARMA
Epimerisation of Allylic Alcohols
InactiveUS20070255066A1Easy to operateImprove processing productivityOrganic compound preparationSteroidsAlcoholAllylic alcohol
The present invention relates to processes for epimerising alcohols of compounds having a hydroxyl substituent on an asymmetric allylic carbon, such as compounds useful for the synthesis of vitamin D analogues where the epimeric hydroxyl substituent is at the 24 position. The Invention further relates to methods of producing intermediates useful for the synthesis of calcipotriol by said epimerisation processes.
Owner:LEO PHARMA AS
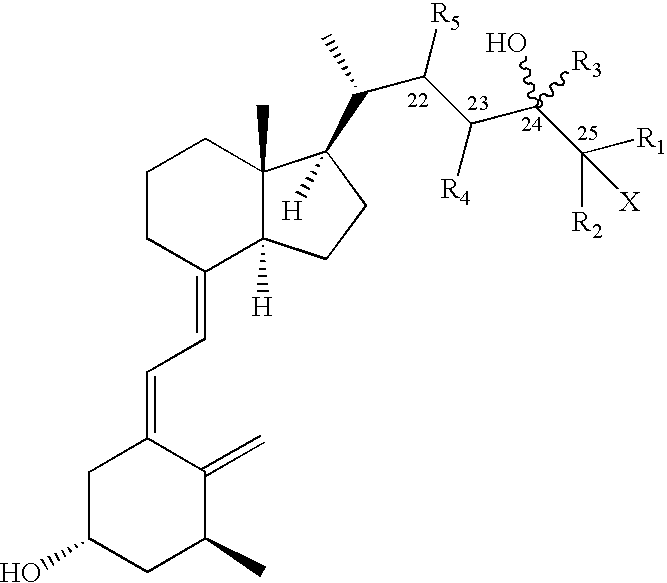
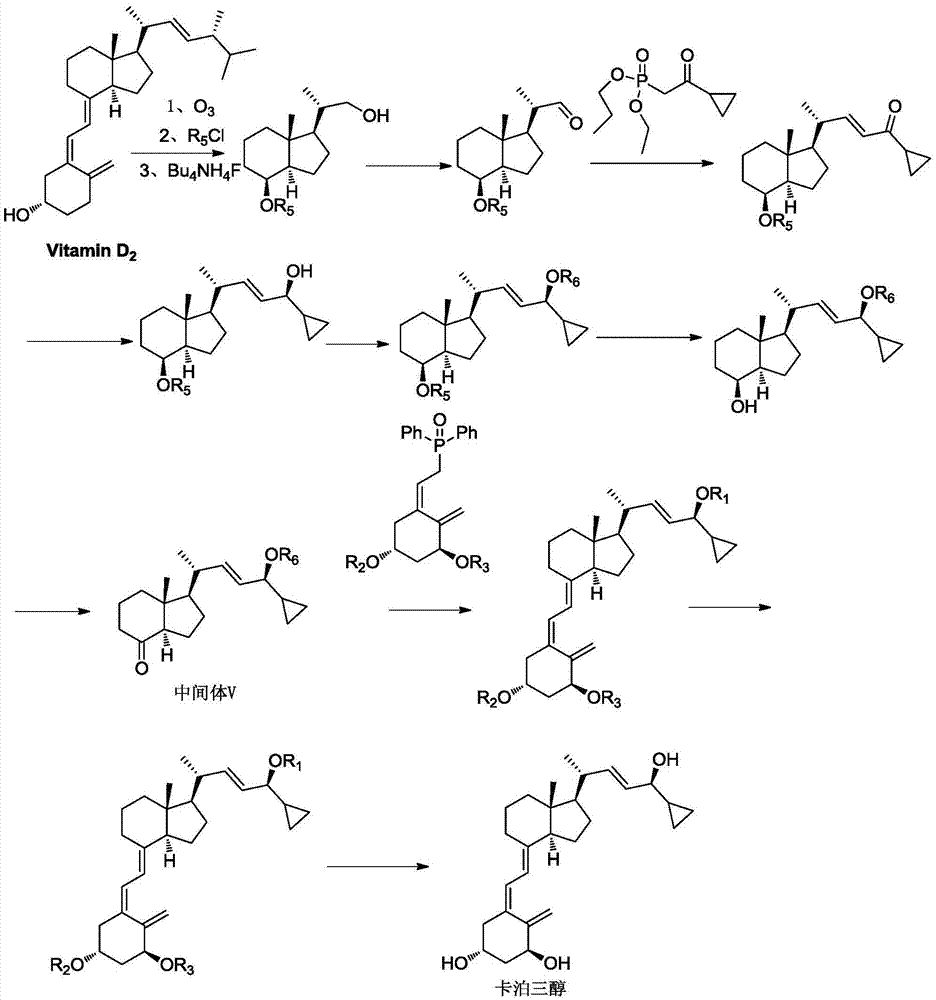
Preparation of 24 alkyl analogs of cholecalciferol and non-racemic compounds
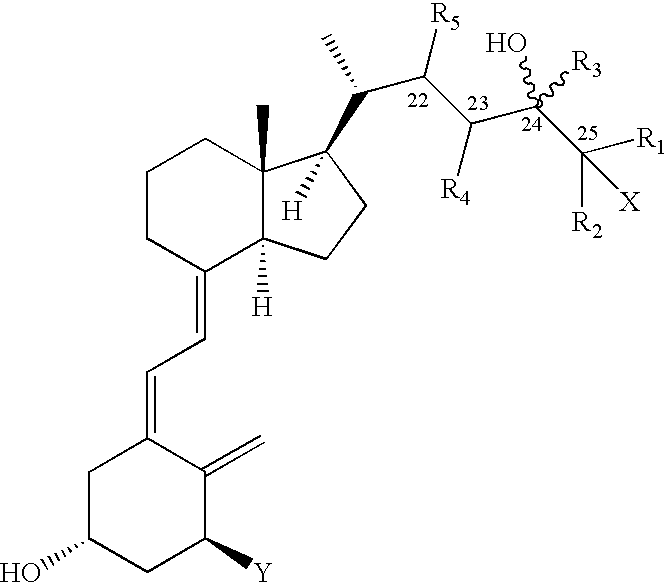
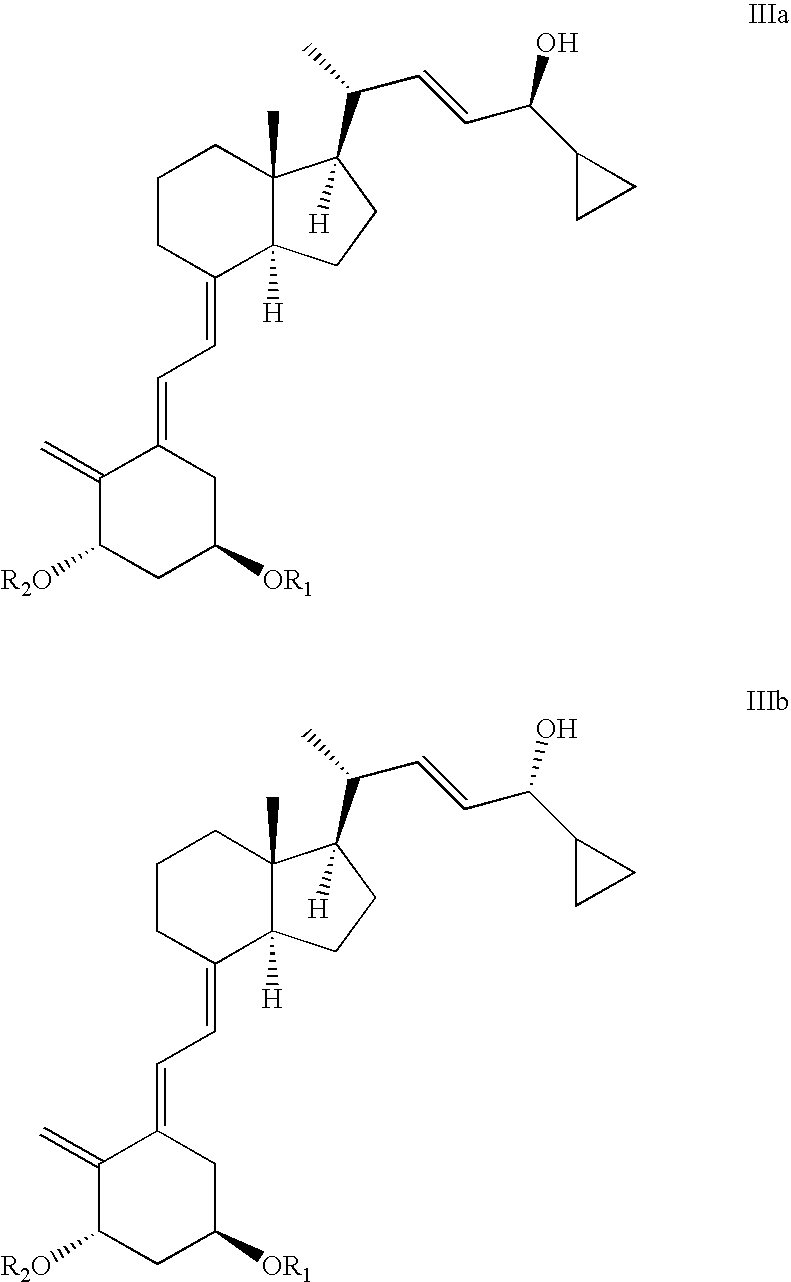
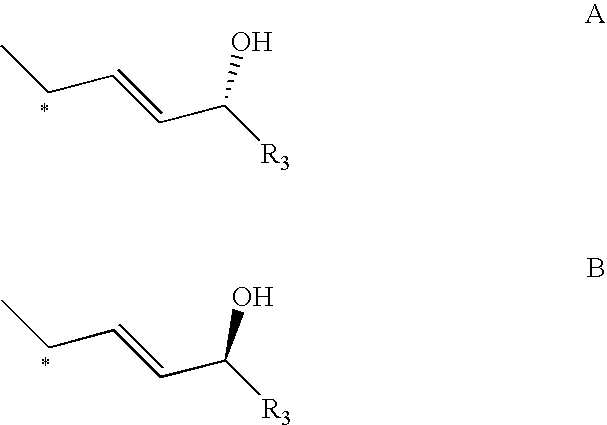
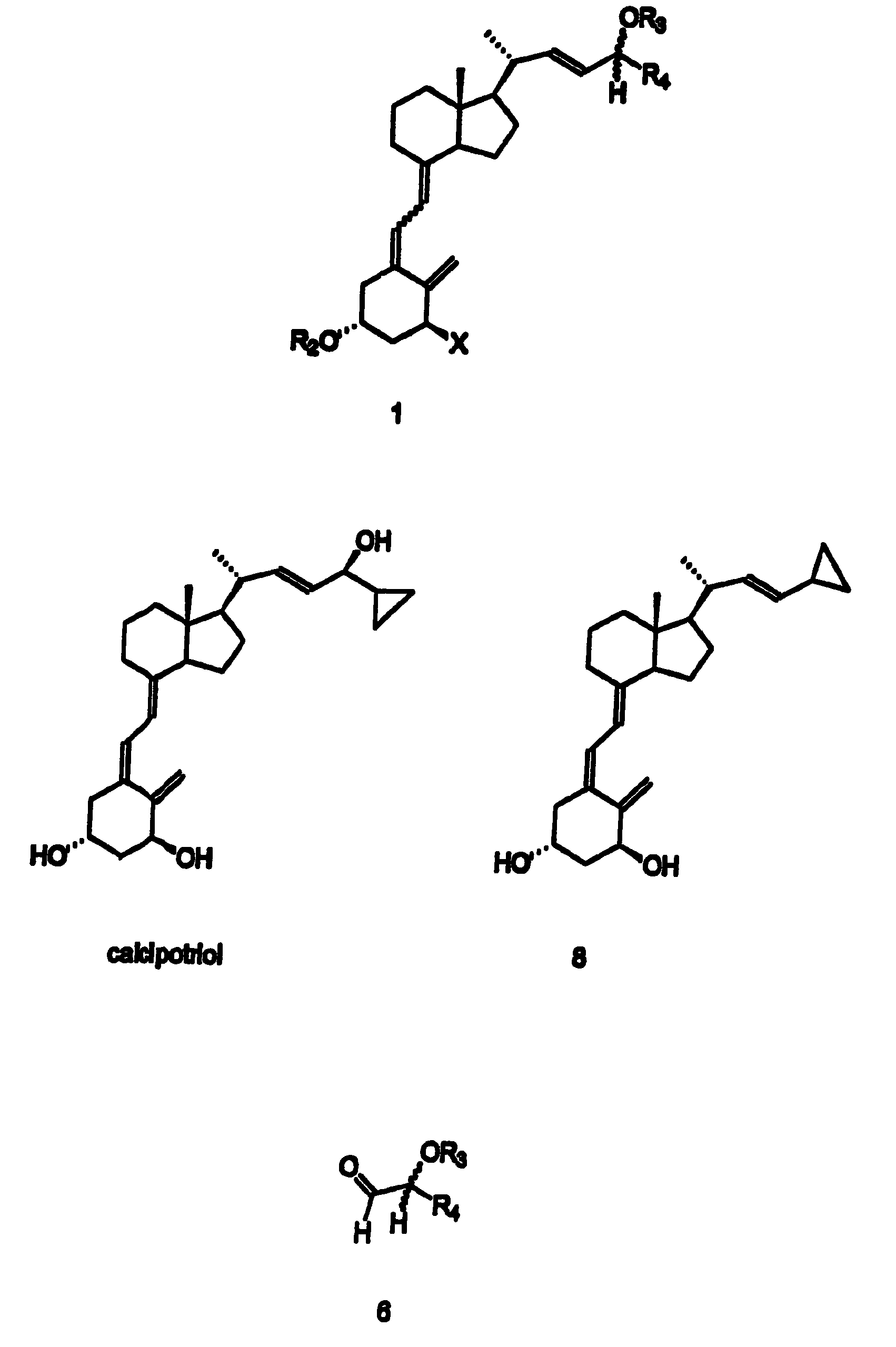
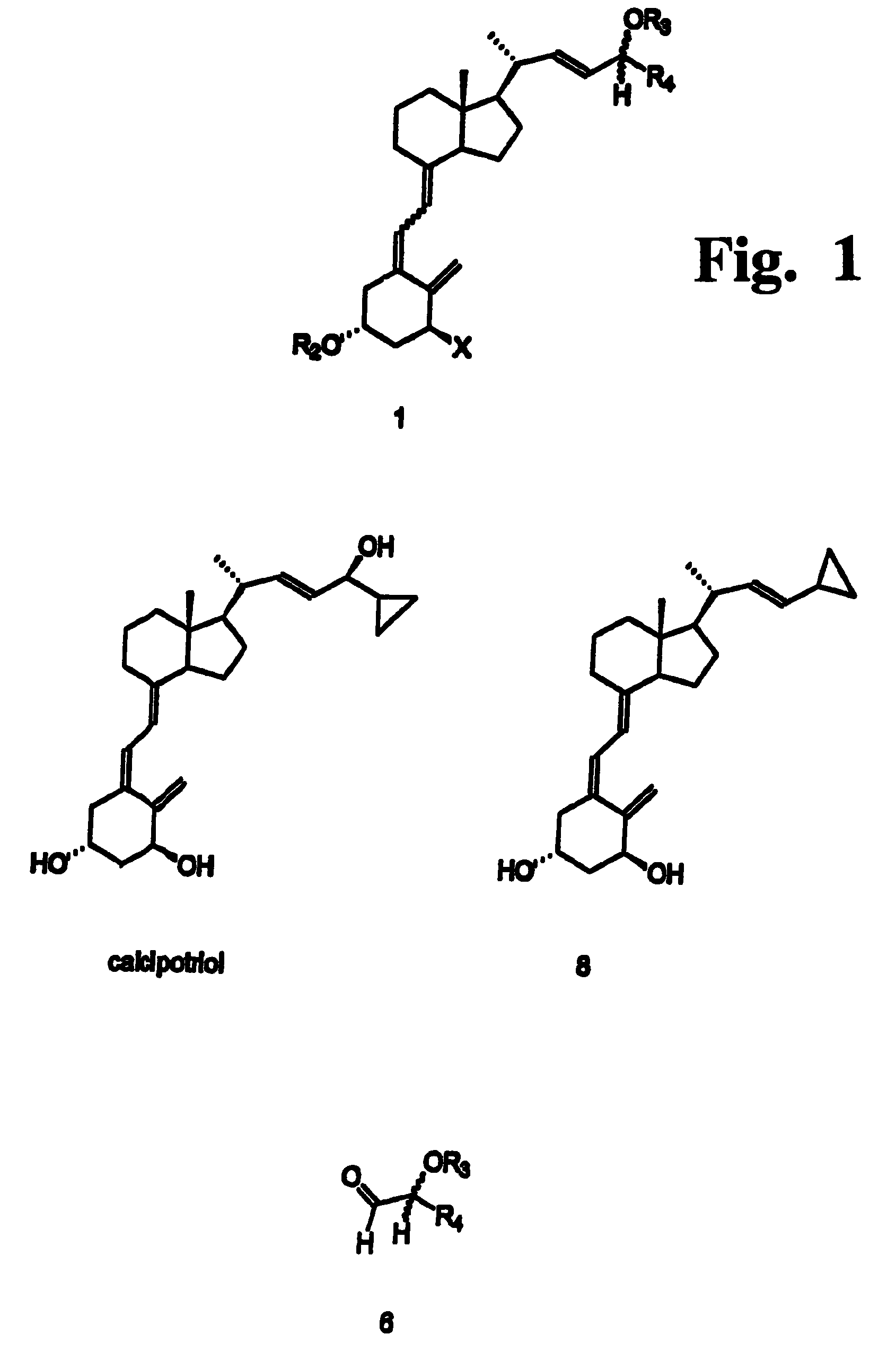
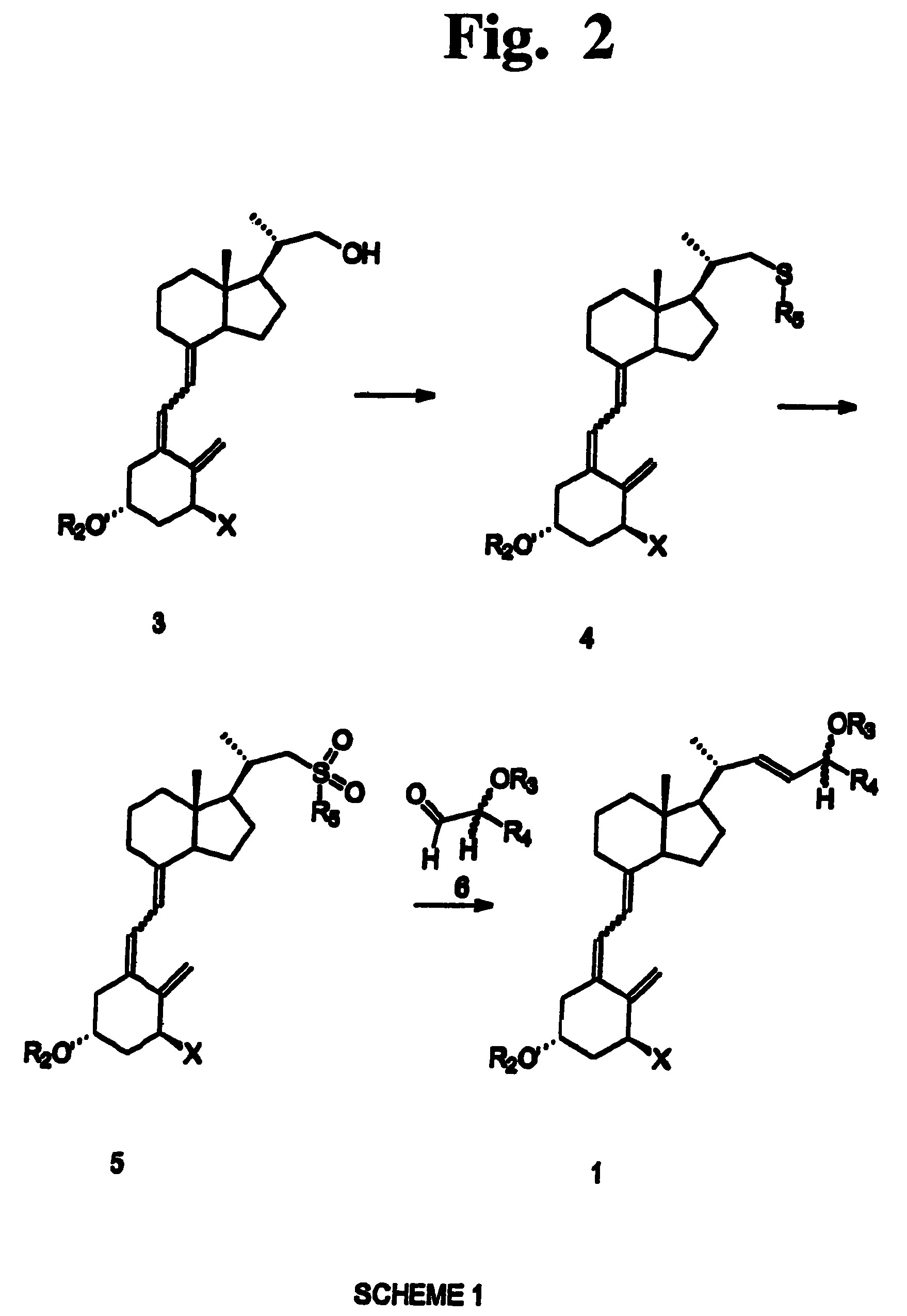
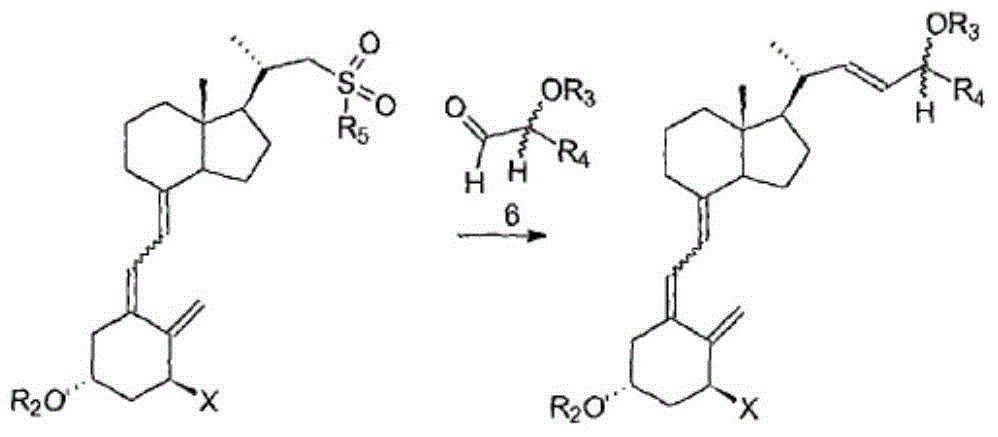
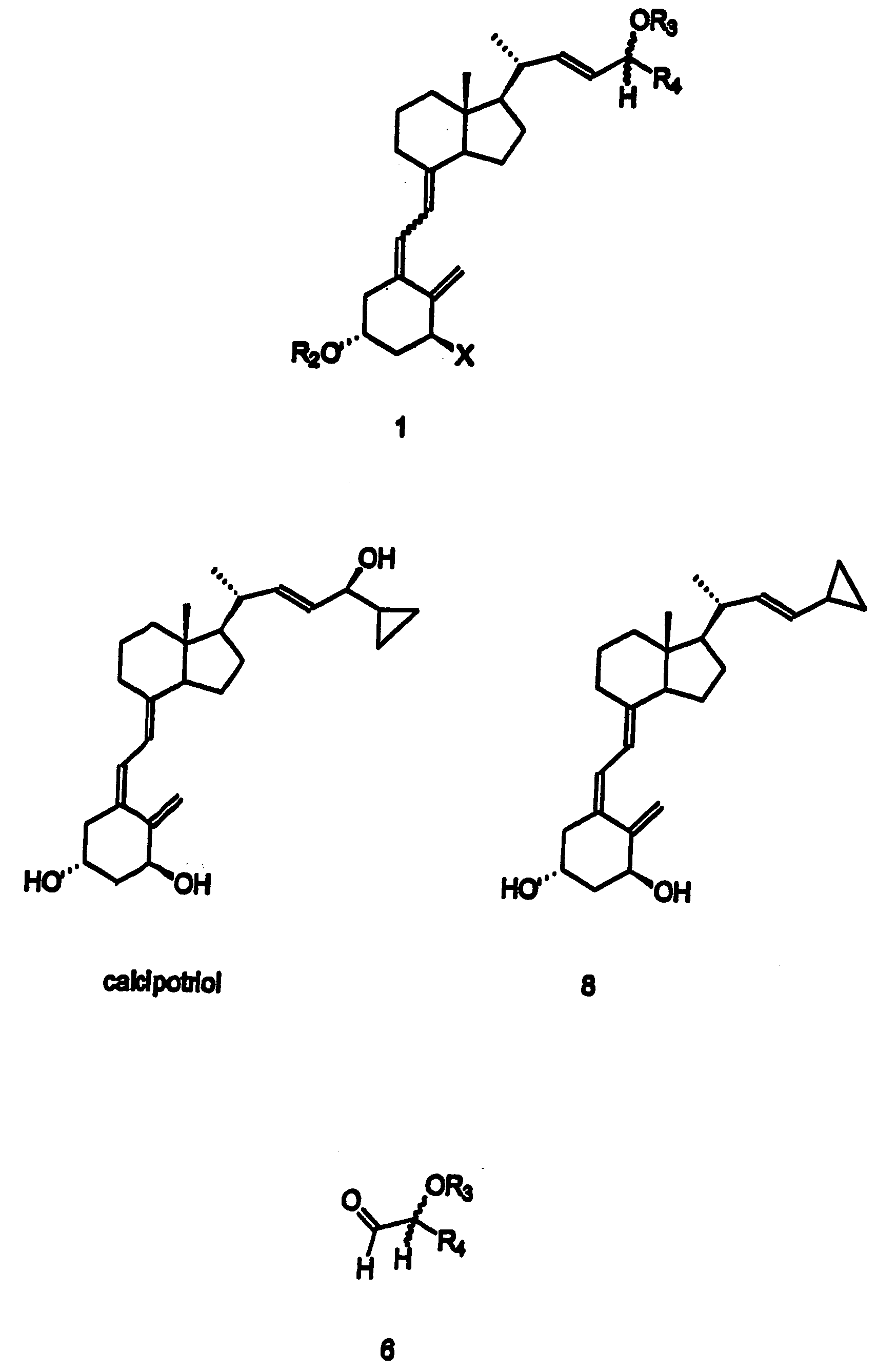
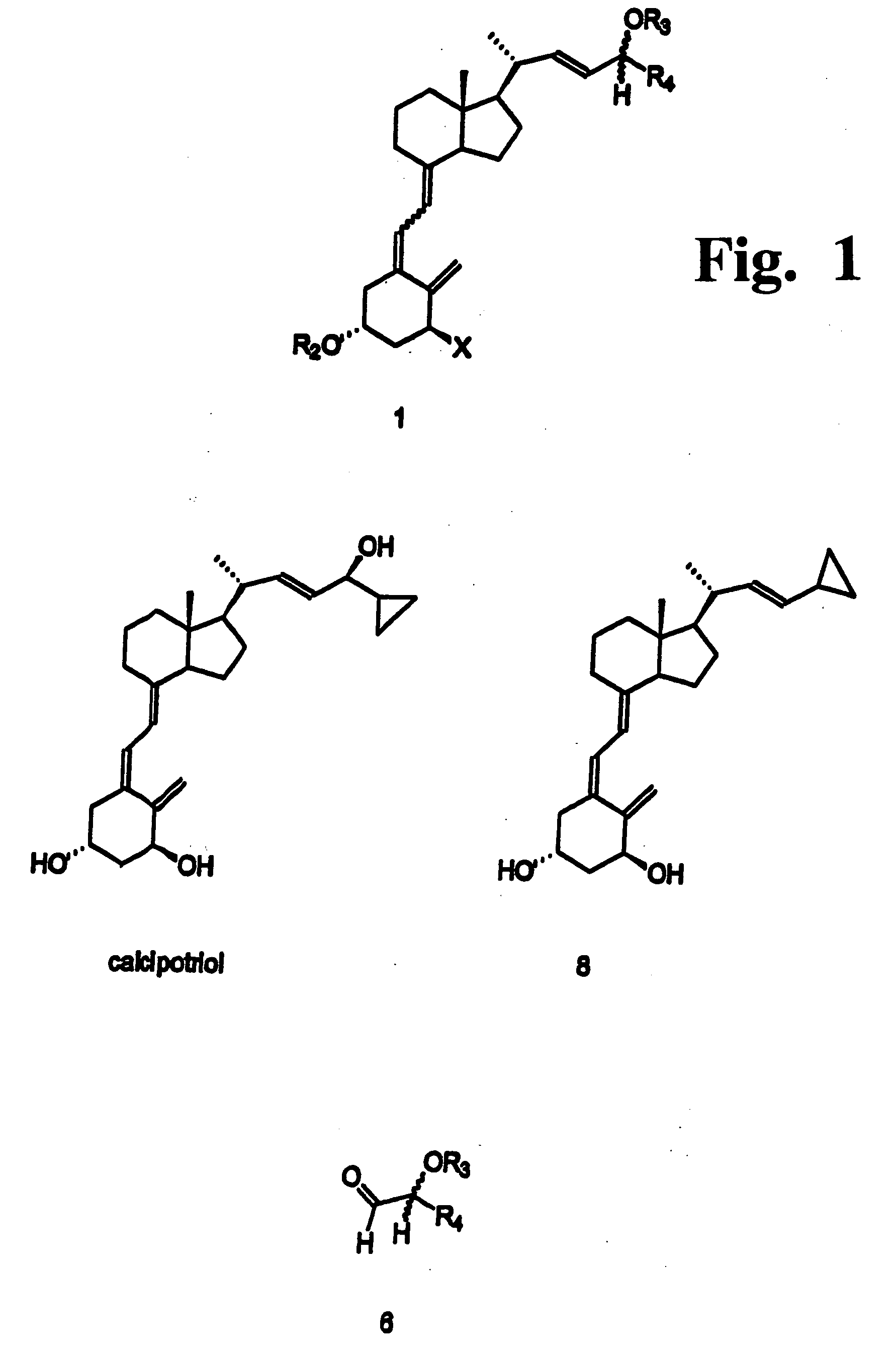
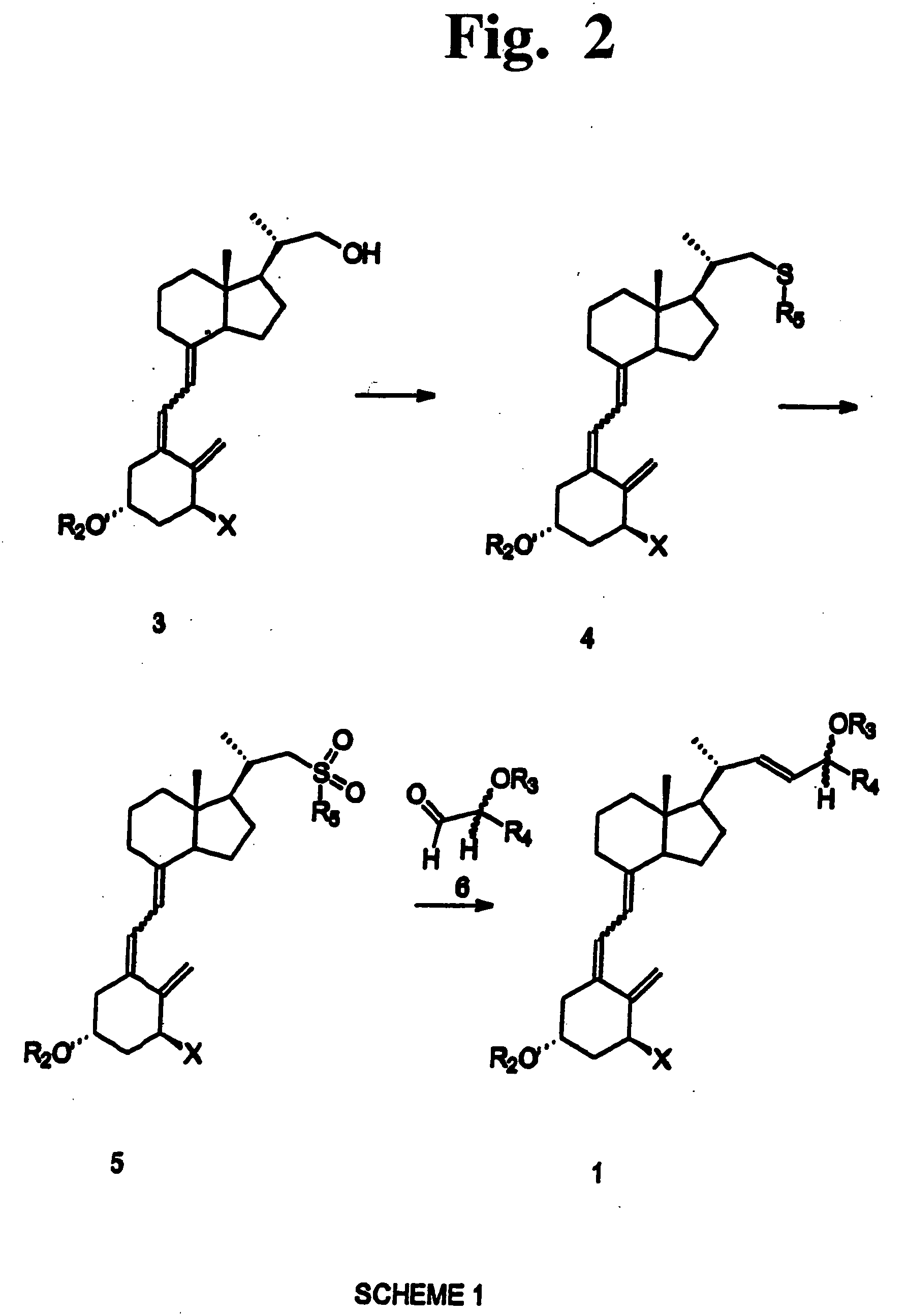
Disclosed is a process for the preparation of 24-alkyl analogs of cholecalcyferol of Formula 1 having a (5E) or (5Z) configuration, wherein X represents a hydrogen atom, a hydroxy group or an OR1 group, where R1, R2 and R3 may be the same or different and represent groups suitable for hydroxyl protection, and R4 is a C1-6 alkyl chain or a C1-6 cykloalkyl group, optionally substituted with C1-3 alkyl groups, especially for calcipotriol.The invention also provides new intermediates and non-racemic compounds being valuable synthones for the synthesis of pharmacologically active substances.
Owner:INSTITUT FARMACEUTYCZNY
Calcipotriol intermediate compound and preparation method thereof
ActiveCN105777665AAvoid it happening againIncrease profitGroup 4/14 element organic compoundsTrimethylsilylCalcipotriol
The invention discloses a calcipotriol intermediate compound and a preparation method thereof. The structure of the compound is represented by general formula I shown in the description. In the general formula I, R1 is selected from a carbobenzoxy group (Cbz), a trimethylsilyl group (TMS), a triethylsilyl group (TES), a t-butyldimethylsilyl group (TBDMS), a triisopropylsilyl group (TIPS), a t-butyl-diphenylsilyl group (TBDPS) or a methoxymethyl group (MOM); and R2 is one of groups shown in the description.
Owner:SHANGYU JINGXIN PHARMA +2
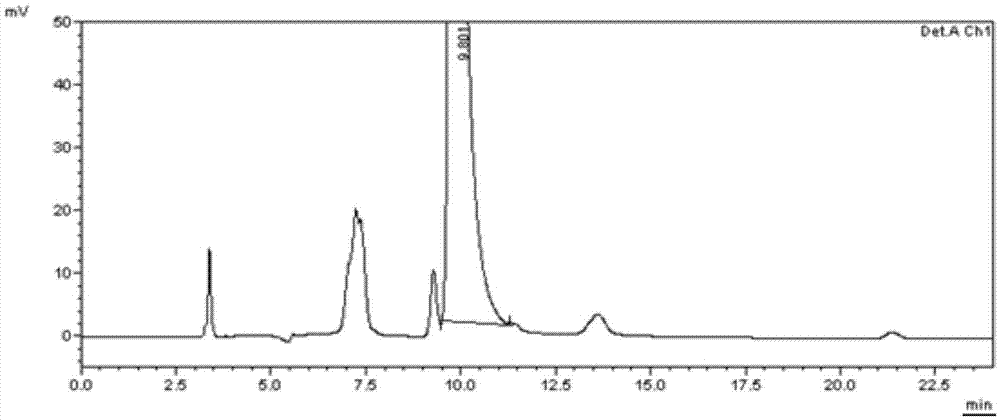
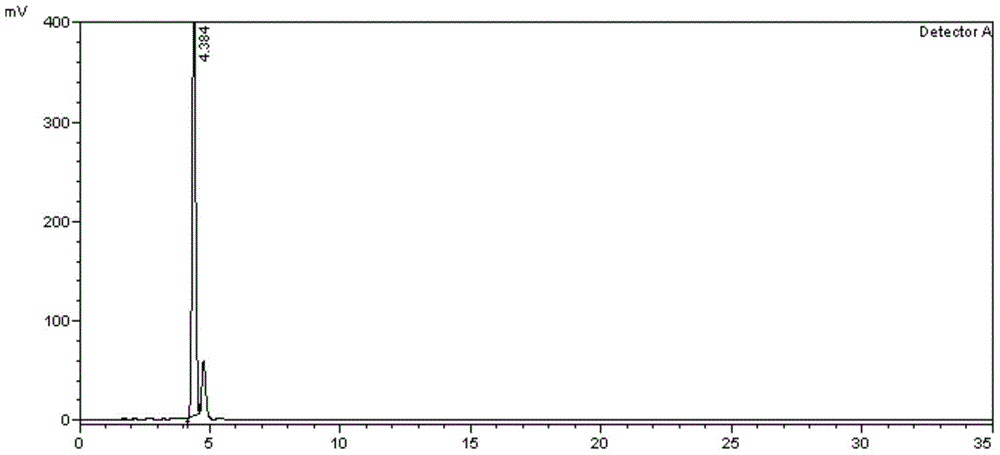
Method for separating and determining calcipotriol midbody L and related impurities
The invention belongs to the field of analytical chemistry and specifically relates to a method for separating and determining calcipotriol midbody L and related impurities. According to the method for separating and determining calcipotriol midbody L and related impurities, silica gel is taken as a solid phase and ethyl acetate-n-hexane is taken as a flowing phase for separating. According to the method, the calcipotriol midbody L and the related impurities can be effectively separated. The determining method comprises the following steps: by adopting a high performance liquid chromatography method, taking calcipotriol midbody L and related impurities reference substances and respectively preparing into a reference substance solution and a sample solution, performing high performance liquid chromatography analysis, recording chromatograms and comparing the impurity peak areas at corresponding peak time in the reference substance solution and the sample solution, thereby realizing the effective separation and detection of the calcipotriol midbody L and related impurities. The operation is simple and the accuracy is high.
Owner:CHONGQING HUABANGSHENGKAI PHARM
Epimerisation of allylic alcohols
The present invention relates to processes for epimerising alcohols of compounds having a hydroxyl substituent on an asymmetric allylic carbon, such as compounds useful for the synthesis of vitamin D analogues where the epimeric hydroxyl substituent is at the 24 position. The invention further relates to methods of producing intermediates useful for the synthesis of calcipotriol by said epimerisation processes.
Owner:LEO PHARMA AS
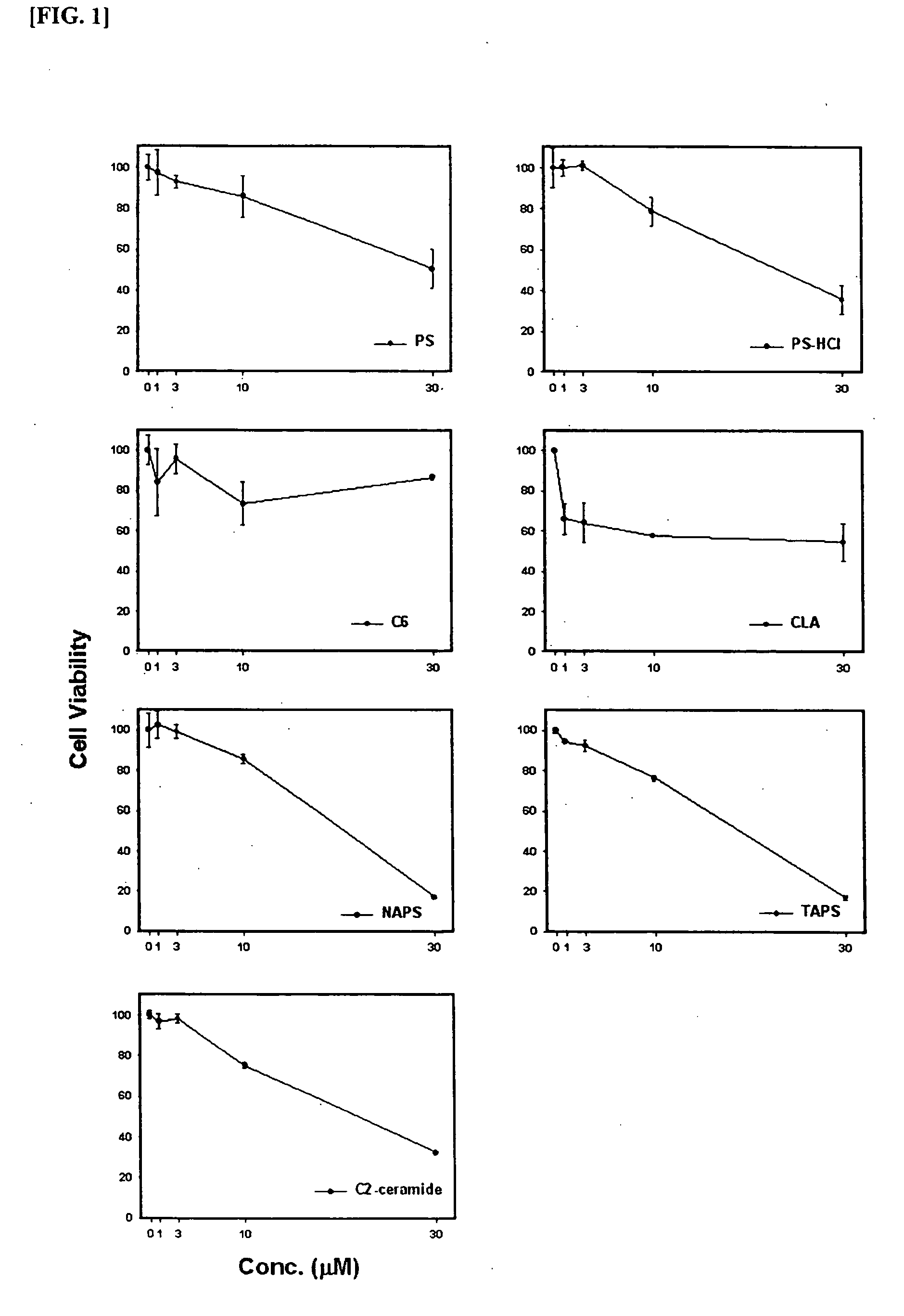
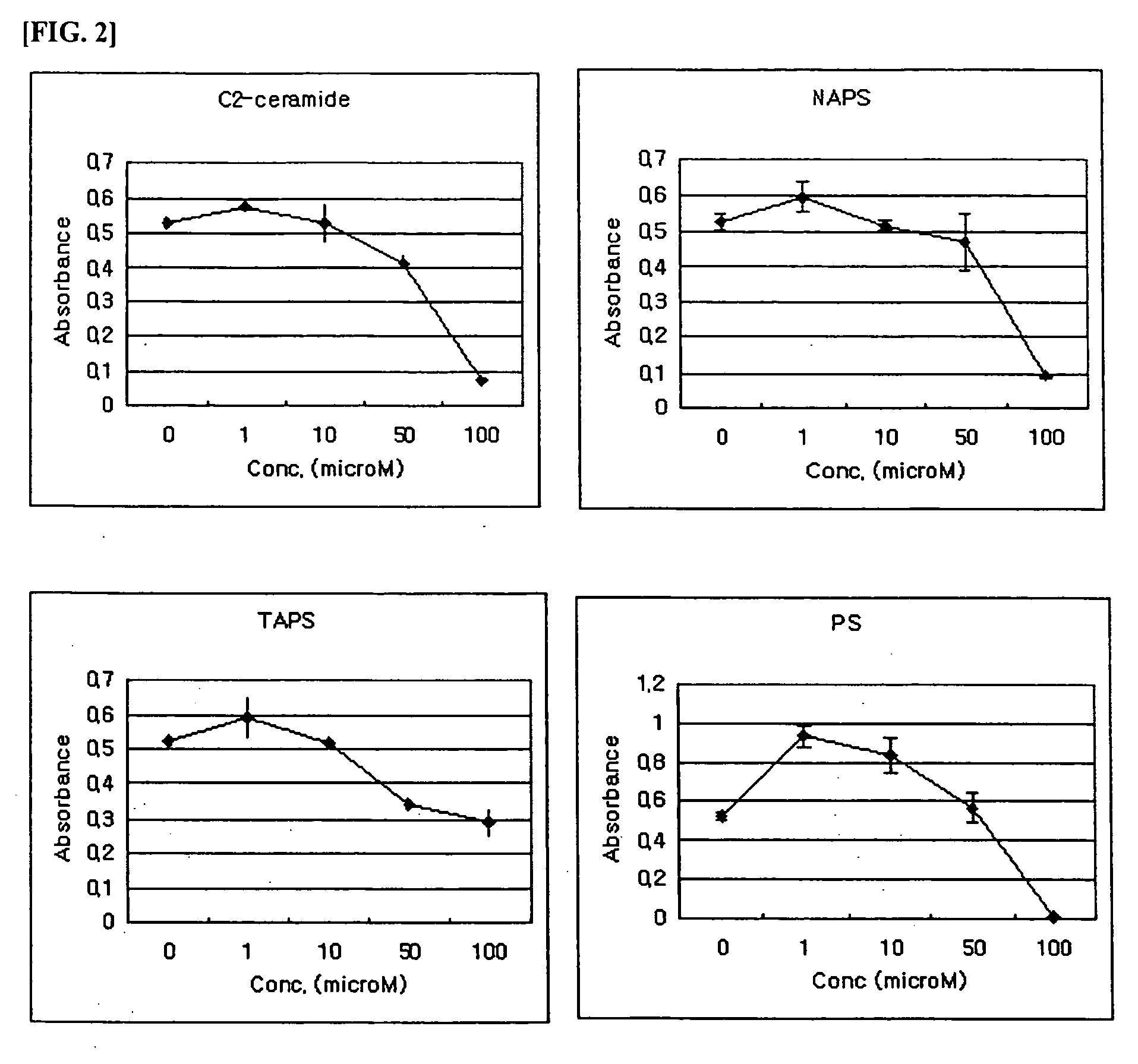
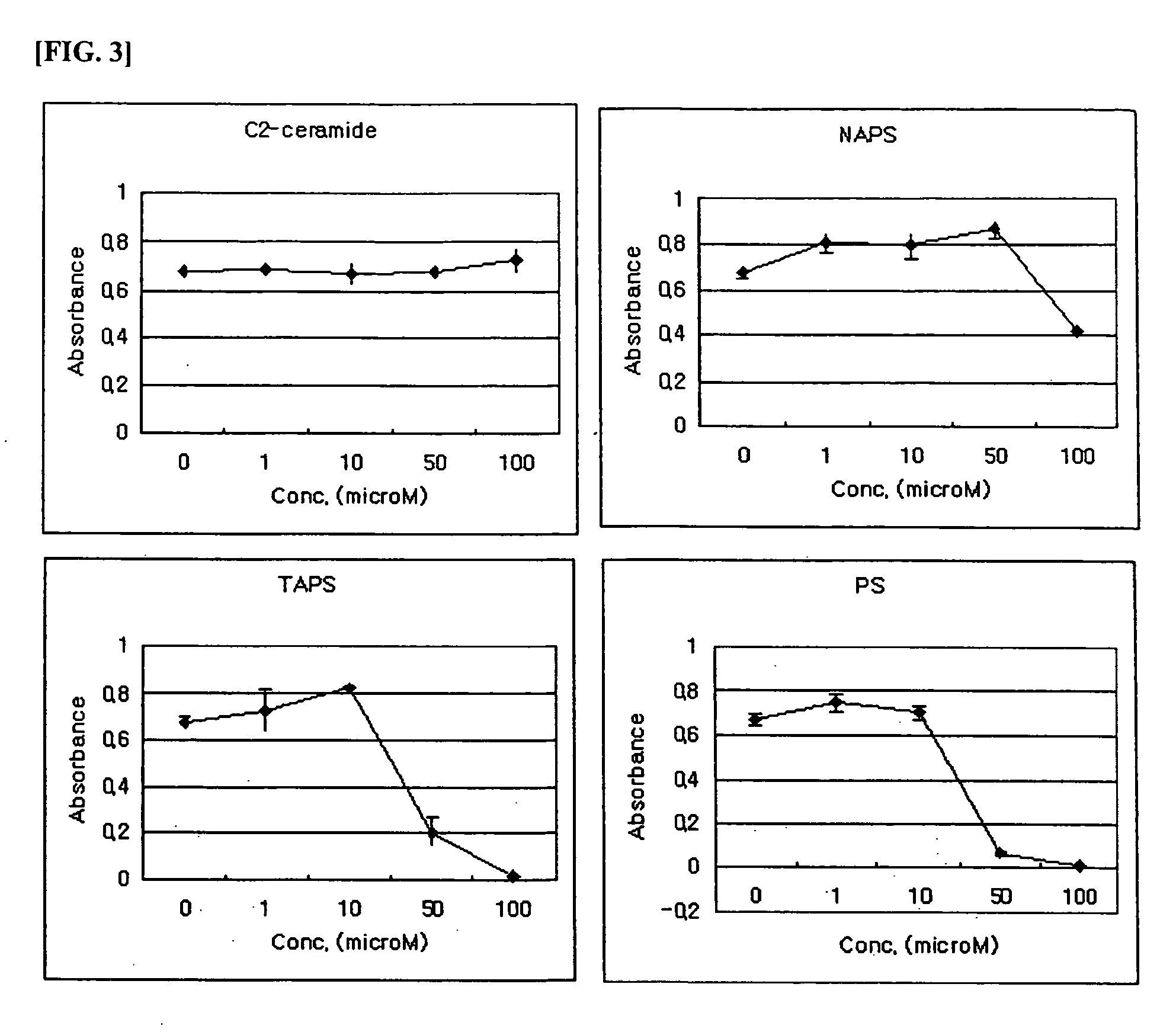
Composition comprising phytosphingosine derivatives for apoptosis induction
InactiveUS20060166934A1Remarkable effectInhibitory activityBiocideCosmetic preparationsAbnormal tissue growthMedicine
The present invention is related to compositions for the induction of apoptosis containing phytosphingosine derivatives as effective components. The present invention is related to compositions for the induction of apoptosis containing Vit D3 or calcipotriol as an effective component in addition to phytosphingosine derivatives. The compositions of the present invention include pharmaceutical compositions or cosmetic compositions having an activity to induce apoptosis. The present invention offers a method of prevention or treatment of various skin diseases, various tumors, various cancers, etc. that may be prevented or cured by the induction of the activity of apoptosis in living bodies, which is comprised of the steps of administration of compositions for the induction of apoptosis containing phytosphingosine derivatives as effective components and irradiation of UVB to psoriatic lesions. Therefore, the compositions of the present invention are useful for the prevention or treatment of various skin diseases, various tumors, various cancers, etc. that may be prevented or cured by the induction of the activity of apoptosis in living bodies.
Owner:KIM EE
Cyclopropyl substituted allyl alcohol and asymmetric synthesis method thereof
ActiveCN107473941AEasy to synthesizeLow priceEther separation/purificationOrganic compound preparationIridiumSynthesis methods
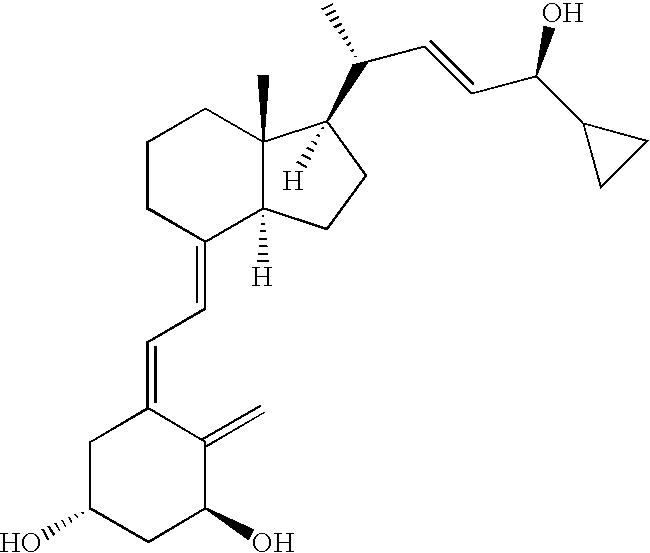
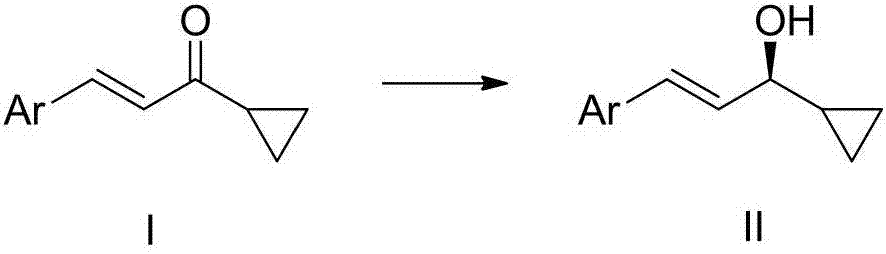
The invention relates to cyclopropyl substituted allyl alcohol and an asymmetric synthesis method thereof. The method comprises the following steps: by taking monosulfonyl chiral diamine and a complex of metals ruthenium, rhodium and iridium as a catalyst, carrying out an asymmetric transfer hydrogenation reaction, thereby obtaining the cyclopropyl substituted allyl alcohol. The method is mild in reaction conditions, simple and convenient to operate, readily available in raw materials, wide in substrate application range and high in enantioselectivity and has important application prospects in the aspect of synthesizing a psoriasis treatment drug calcipotriol.
Owner:CHINA THREE GORGES UNIV
Modified fat-soluble composition and preparation method thereof
InactiveCN108815174AReduced risk of hydrolysisAffect stabilityOrganic active ingredientsOrganic non-active ingredientsUse medicationPretreatment method
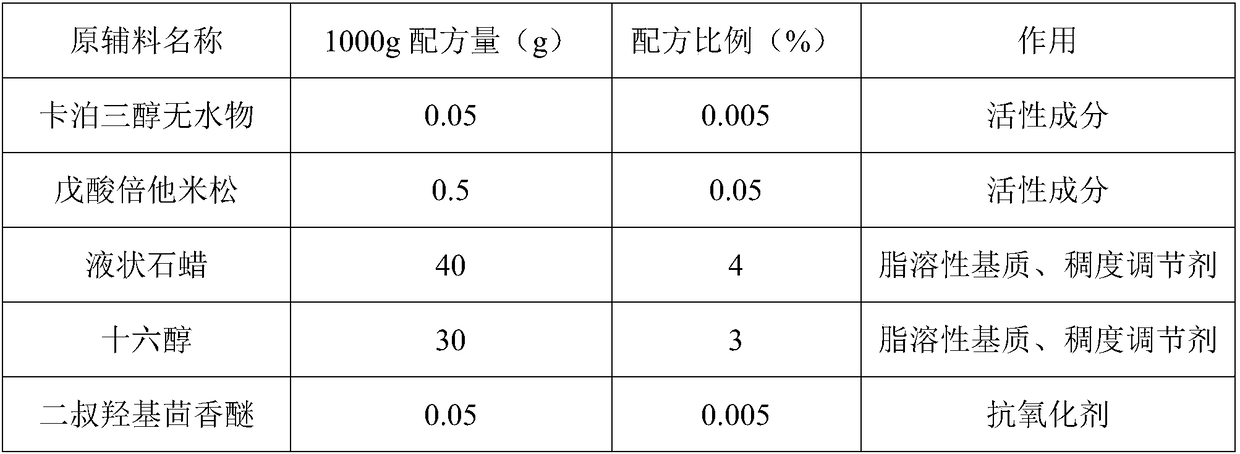
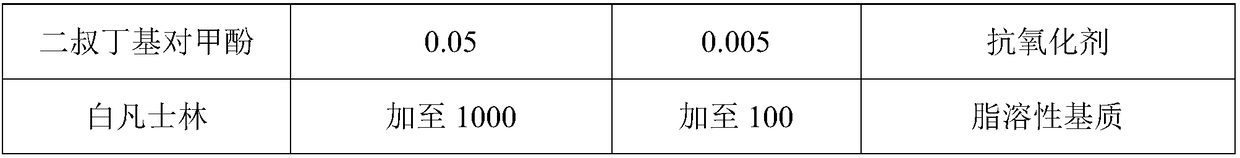
The invention discloses a modified fat-soluble composition and a preparation method thereof. In the composition, an effective component comprises a vitamin D derivative and a betamethasone ester; an adjuvant material comprises a fat-soluble matrix and an antioxidant. In the composition, the fat-soluble matrix is selected, and the water content of the matrix is controlled, so that the hydrolysis risk of the betamethasone ester is reduced, the stability of calcipotriol anhydride is prevented from being affected by hydrolysed acid, and the stability of the modified fat-soluble composition is alsoimproved. In the composition, the more reasonable betamethasone ester is optimized and is used for drug combination treatment for plaque psoriasis, so that more clinical selections are provided for patients. The invention provides an optimized pretreatment method of the calcipotriol anhydride, so that the content uniformity of calcipotriol in a product is ensured, and the safety and the effectiveness of the product are further improved. The invention provides the stable composition through the optimization of the formula and the process.
Owner:昆山普瑞凯纳米技术有限公司
Method for separating and determining calcipotriol intermediate F and impurities thereof
ActiveCN105717206AEffective controlSolving Effective Separation ProblemsGroup 4/14 element organic compoundsComponent separationEnantiomerSilica gel
The invention belongs to the field of analytical chemistry, and particularly relates to a method for separating and determining a calcipotriol intermediate F and impurities thereof. According to the method, solid-liquid separation is performed by adopting octadecylsilane chemically bonded silica as a chromatographic column of a filling agent and taking a mixed solution of methanol and ethyl acetate as a mobile phase, and the impurities comprise one or more of VD2, E, D and an isomer F' of F; effective separation of the intermediate F and the enantiomer impurity thereof can be achieved, effective separation of the intermediate F and the other impurities also can be achieved, the content of the intermediate F and the impurities thereof can be further accurately determined, and the specificity and the sensitivity are high. The operation method is simple, has the advantages of being simple, convenient and rapid and has the extremely important significance on quality control and safety guarantee of the calcipotriol intermediate F.
Owner:CHONGQING HUAPONT PHARMA
Preparation of 24 alkyl analogs of cholecalciferol and non-racemic compounds
Disclosed is a process for the preparation of 24-alkyl analogs of cholecalcyferol of Formula 1 having a (5E) or (5Z) configuration, wherein X represents a hydrogen atom, a hydroxy group or an OR1 group, where R1, R2 and R3 may be the same or different and represent groups suitable for hydroxyl protection, and R4 is a C1-6 alkyl chain or a C1-6 cykloalkyl group, optionally substituted with C1-3 alkyl groups, especially for calcipotriol. The invention also provides new intermediates and non-racemic compounds being valuable synthones for the synthesis of pharmacologically active substances.
Owner:INSTITUT FARMACEUTYCZNY
Method for preparing vitamin D3 analogue intermediate
ActiveCN106905358AEasy to removeShort method stepsGroup 4/14 element organic compoundsOrganic compound preparationChemical synthesisUnit operation
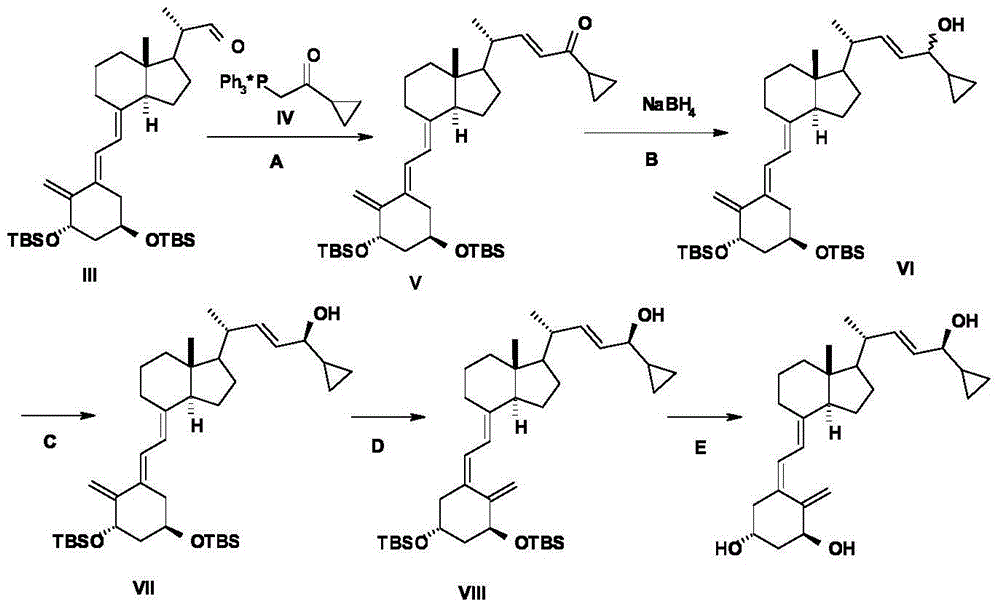
The invention belongs to the technical field of drug chemical synthesis, and particularly relates to a method for preparing a vitamin D3 analogue intermediate, specifically relating to a method for preparing calcipotriol intermediate. The method comprises the following steps: performing WITTIG-HORER reaction to a compound shown in the formula II with raw materials shown in the formula III, to obtain an intermediate shown in the formula IV; performing hydroxyl protection reaction to the intermediate shown in the formula IV, to obtain the vitamin D3 analogue intermediate shown in the formula V. The method has fewer steps and fewer unit operations, the yield is high, and the total yield is up to 40% above, and the cost is low; isomers are fewer, so that impurities are easily removed, purification is easy, the purity of the intermediate V is up to 95%, the long reaction time, low yield, more impurities and high cost problems in the prior art can be overcome, and the industrial large-scale production is facilitated.
Owner:CHONGQING HUABANGSHENGKAI PHARM
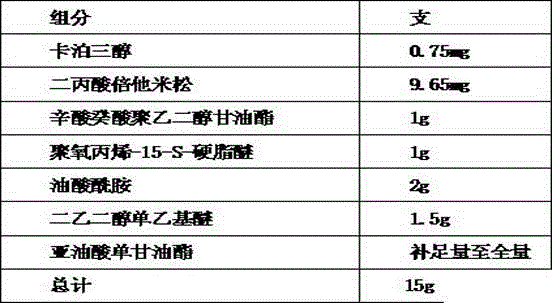
Calcipotriol betamethasone self-microemulsion preparation with excellent performance
InactiveCN106265511AImprove solubilityIncrease dissolution rateOrganic active ingredientsPharmaceutical non-active ingredientsOctanoic AcidsPolyethylene glycol
The invention discloses a calcipotriol betamethasone self-microemulsion preparation with excellent performance, and the self-micromulsion preparation contains main drugs (namely the derivative or analogue of vitamin D (such as calcipotriol) and betamethasone), glycerides, C8-10 mono-and di-, propylene oxygen-15-S-stearyl ether, oleamide, oil phase and co-emulsifier. In the molecular structure of the glycerides, C8-10 mono-and di-, the degree of polymerization of the ethylene glycol is 2-4, and the composition ratio of the aliphatic acid in the molecular structure of the glycerides, C8-10 mono-and di- is that the percentage of octanoic acid is no less than 88%, the percentage of decanoic acid is no more than 10%, and the total proportions of lauric acid, aliphatic acid which are more advanced than lauric acid and aliphatic acid which are more lower than octanoic acid are no less than 2%. The stability, particularly the clinical effect of the composition, is improved.
Owner:JIANGSU SEMPOLL PHARMA
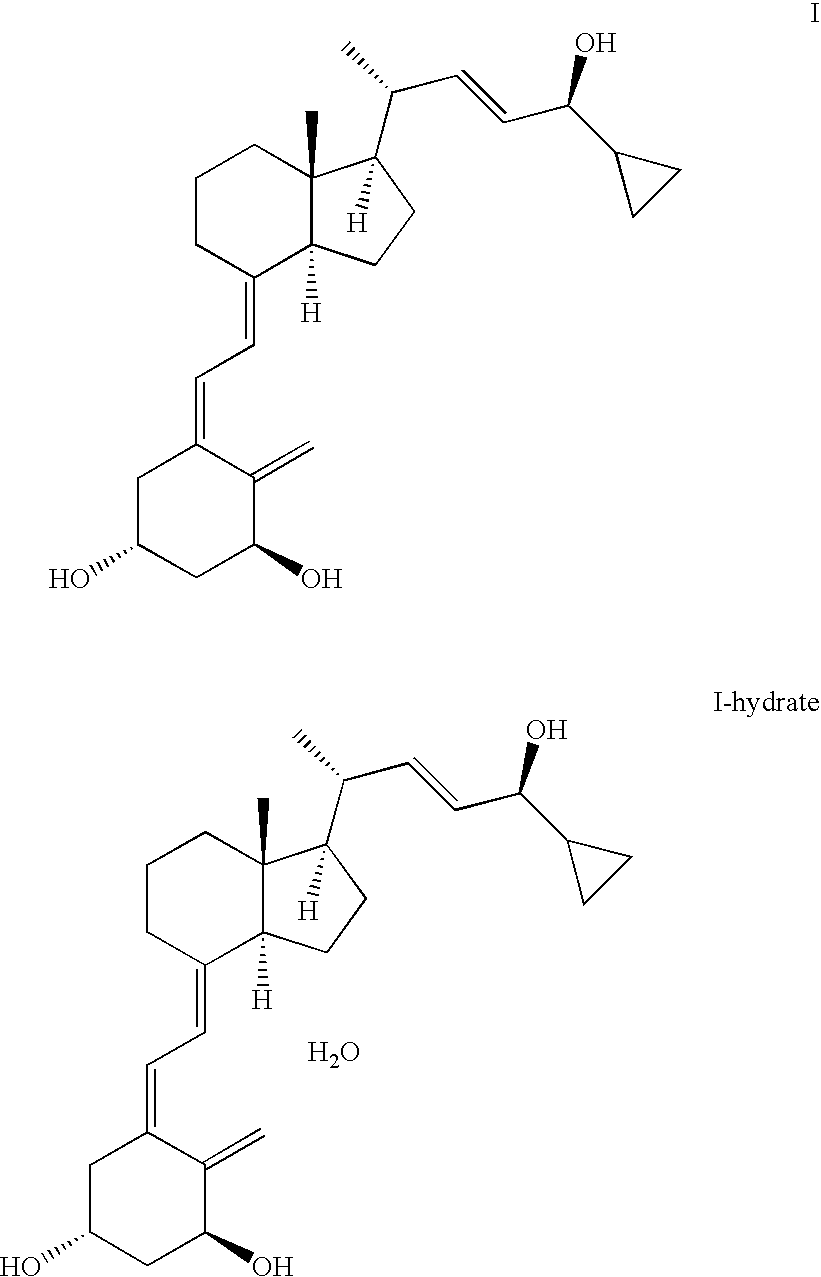
Method for obtaining calcipotriol hydrate
InactiveUS7507865B2Loss of product yieldIncrease heightOrganic active ingredientsOrganic chemistryOrganic solventCalcipotriol
Owner:LAB VINAS SA
Calcipotriol composition with improved stability
The invention discloses a calcipotriol composition with the improved stability. The composition is prepared from a vitamin D derivative or an analog (like calcipotriol), caprylic / capric glycerides arginine esterase and a pharmaceutically receivable additive. The stability of the composition is improved.
Owner:JIANGSU SEMPOLL PHARMA
A kind of pharmaceutical composition for treating psoriasis
ActiveCN105534996BOintment deliveryPharmaceutical non-active ingredientsCurative effectMedical prescription
Owner:CHANGSHA BAISHUN BIOTECH
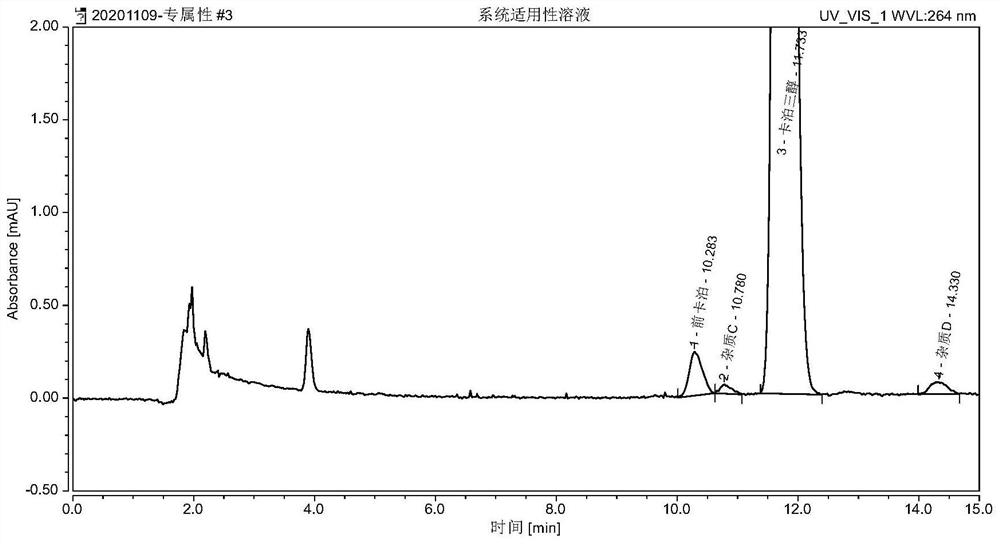
Method for detecting procalcipotriol, impurity C and impurity D in calcipotriol ointment
ActiveCN113533568AGuaranteed testingGuaranteed separation effectComponent separationPhysical chemistryCalcipotriol
The invention discloses a method for detecting procalcipotriol, an impurity C and an impurity D. According to the detection method, a high performance liquid chromatograph is adopted for detection, and the method comprises the following steps: (1) preparation of a solution: preparing a test solution; diluting the test solution by using a mixed solution of a mobile phase A and a mobile phase B according to the test solution to prepare a reference solution; (2) measuring the test solution and the reference solution, respectively injecting the test solution and the reference solution into a high performance liquid chromatograph, and recording chromatograms; (3) taking a proper amount of an impurity C, an impurity D and a calcipotriol reference substance, dissolving and diluting by using a mobile phase, and uniformly shaking to obtain a system applicable solution; and (4) measuring the system applicable solution, injecting the system applicable solution into a high performance liquid chromatograph, and recording a chromatogram. The method is good in precision, accurate, sensitive and reliable in result and wide in application range; meanwhile, the method is simple and easy to implement, low in cost and suitable for application and popularization.
Owner:JIANGSU SEMPOLL PHARMA
Novel synthesis method of calcipotriol
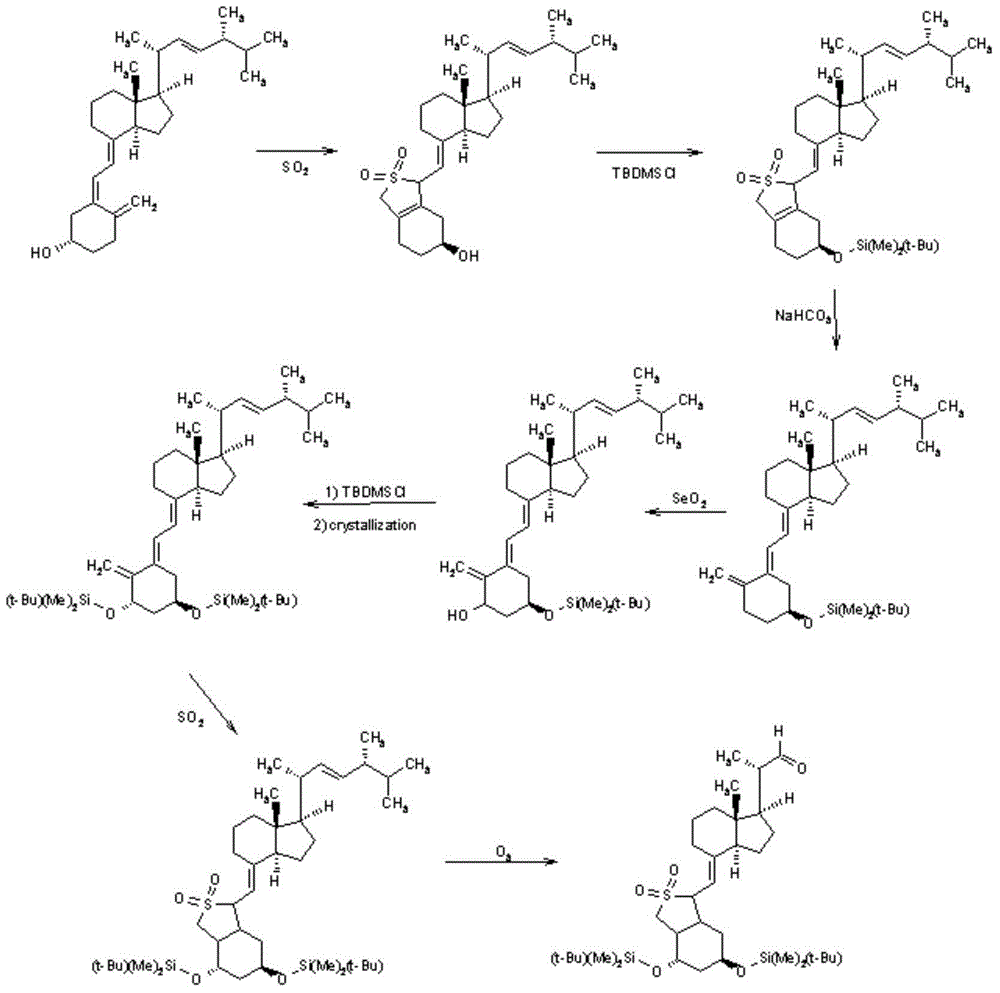
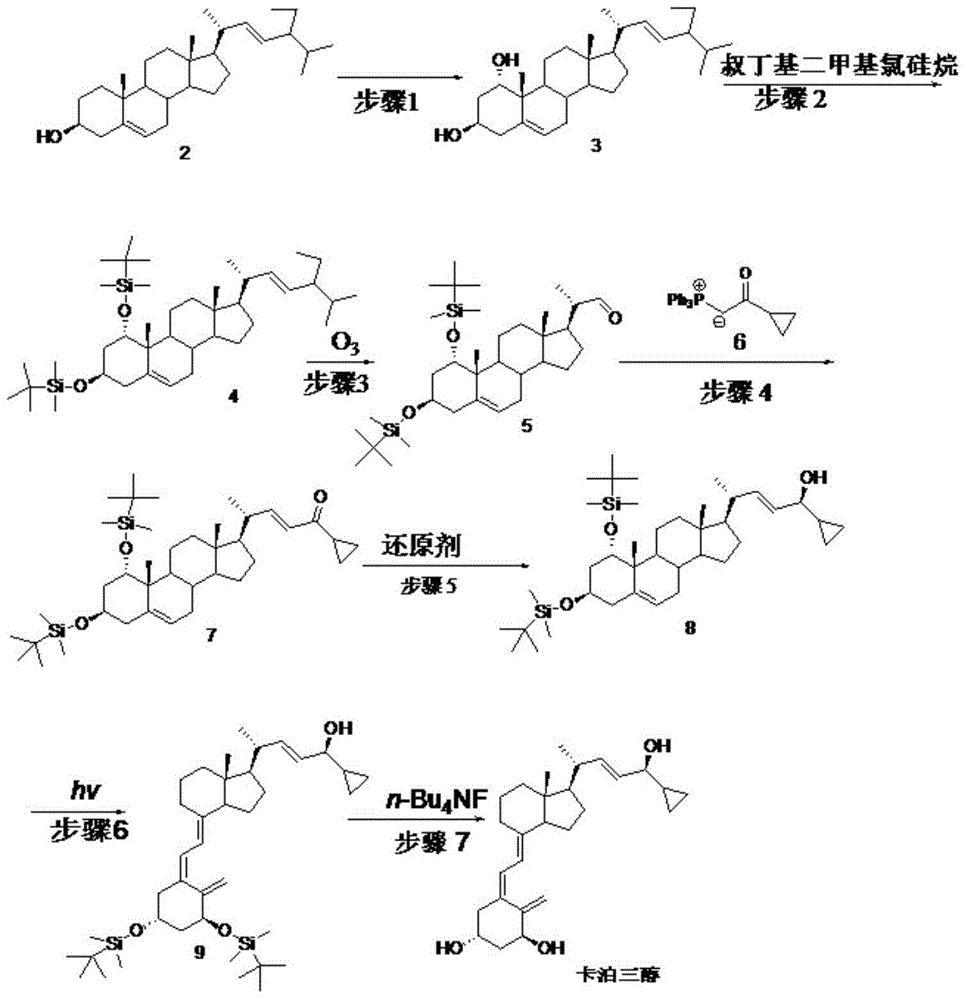
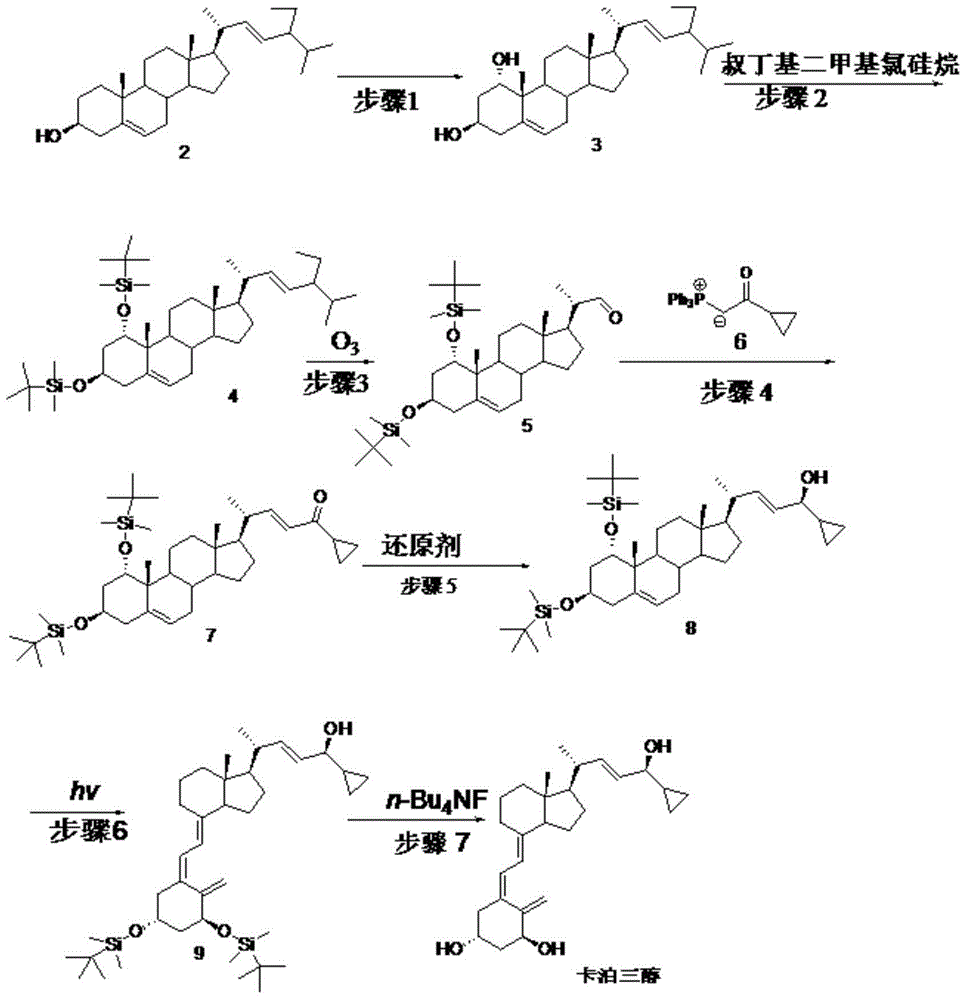
ActiveCN105624215AMethod route shortEasy to operateMicroorganism based processesFermentationIsomerizationTert-butyldimethylsilyl chloride
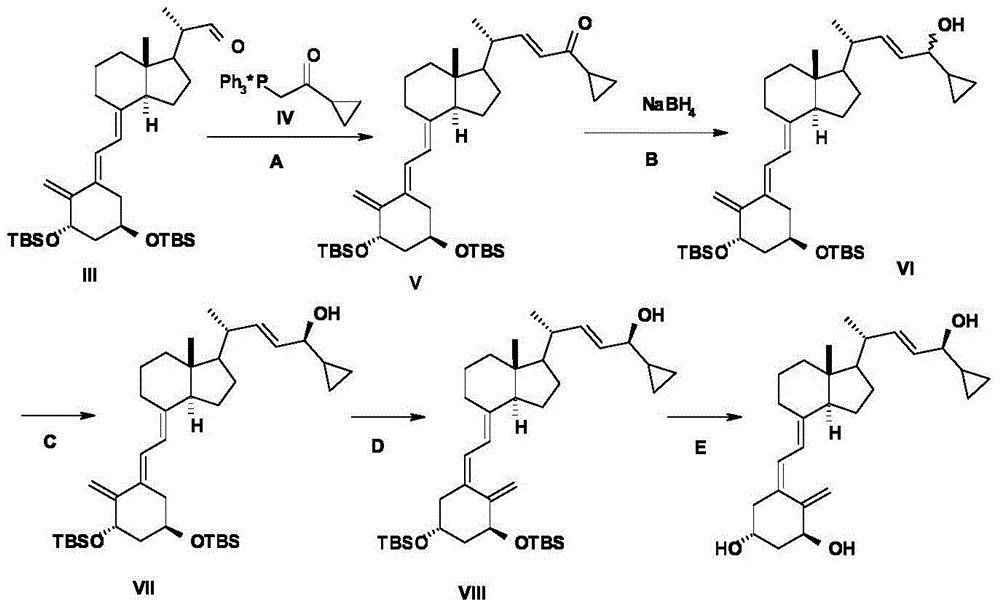
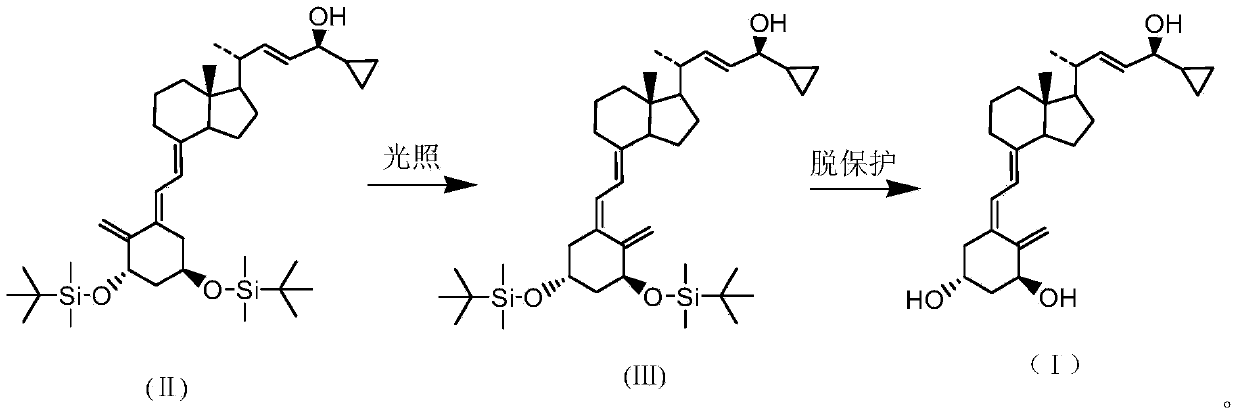
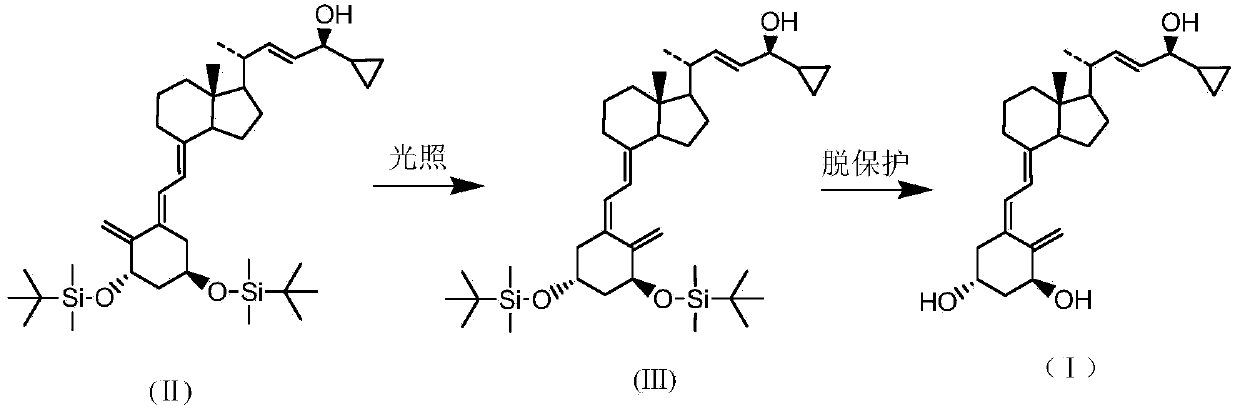
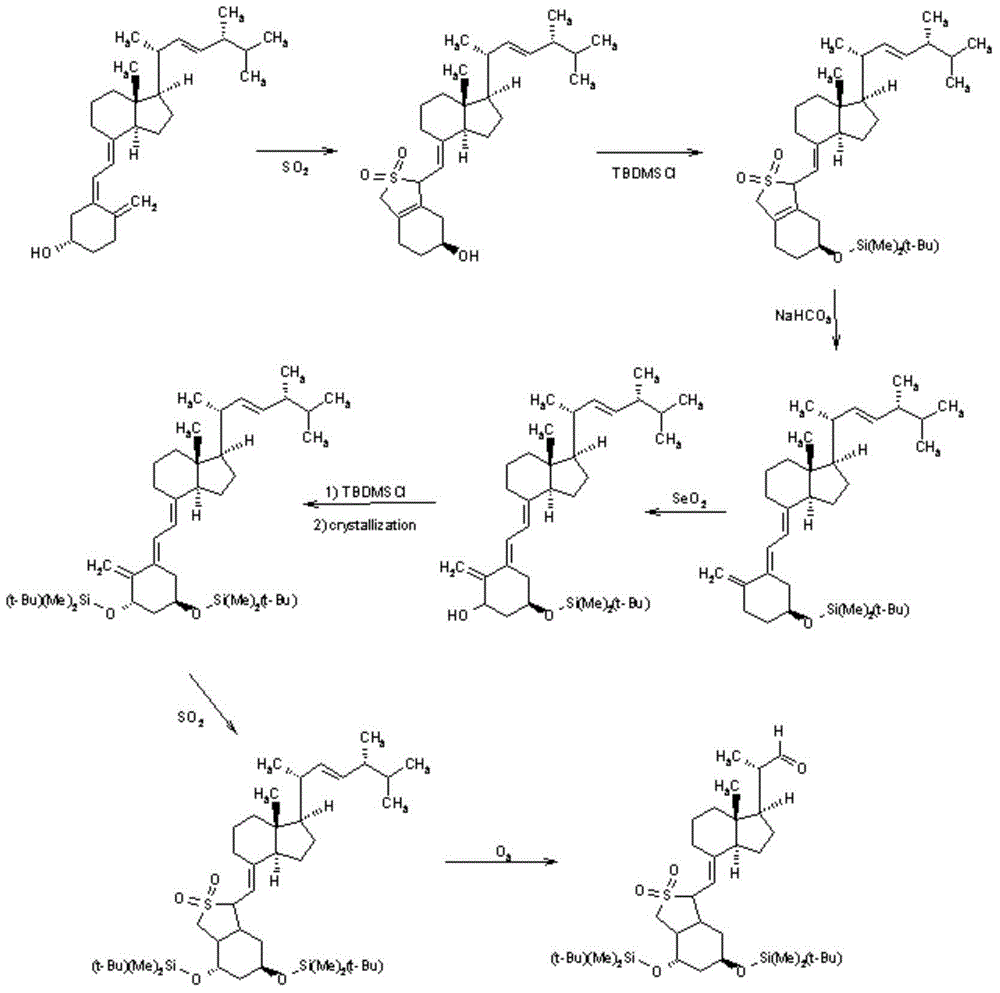
The invention discloses a novel preparation method of calcipotriol. According to the preparation method, stigmasterol-2 is adopted as a starting material, alpha hydroxyl and tert-butyldimethylsilyl chloride are introduced in through microbial fermentation for carrying out seven steps of reactions including hydroxy protection, O3 oxidation, witting reaction, reduction, illumination isomerization, deprotection of hydroxys, and the like, so as to obtain calcipotriol.
Owner:TIANJIN JINYAO GRP
Epimerisation of allylic alcohols
Owner:LEO PHARMA AS
Preparation method of psoriasis therapeutic medicine calcipotriol
The invention discloses a preparation method of a psoriasis therapeutic medicine calcipotriol. The preparation method comprises the following steps: (1) carrying out configuration conversion on (5E, 7E, 22E, 24S)-24-cyclopropyl-1, 3-di[[(1, 1-dimethyl) tertiary butyl silicyl]oxo]-9, 10-open-loop cholest-5, 7, 10 (19), 22-tetraene-ol and anthracene in a proper solvent under ultraviolet-irradiation to obtain an intermediate compound; and (2) de-protecting the intermediate compound in the presence of a de-protecting agent to obtain calcipotriol. The method is simple in process, high in yield and suitable for industrial production.
Owner:CHENGDU YILUKANG MEDICAL TECH & SERVICE
Calcipotriol beta-cyclodextrin inclusion compound and preparation method thereof
InactiveCN103705941AImprove stabilityImproved formulation content uniformityOrganic active ingredientsPharmaceutical non-active ingredientsAqueous solutionPharmaceutical formulation
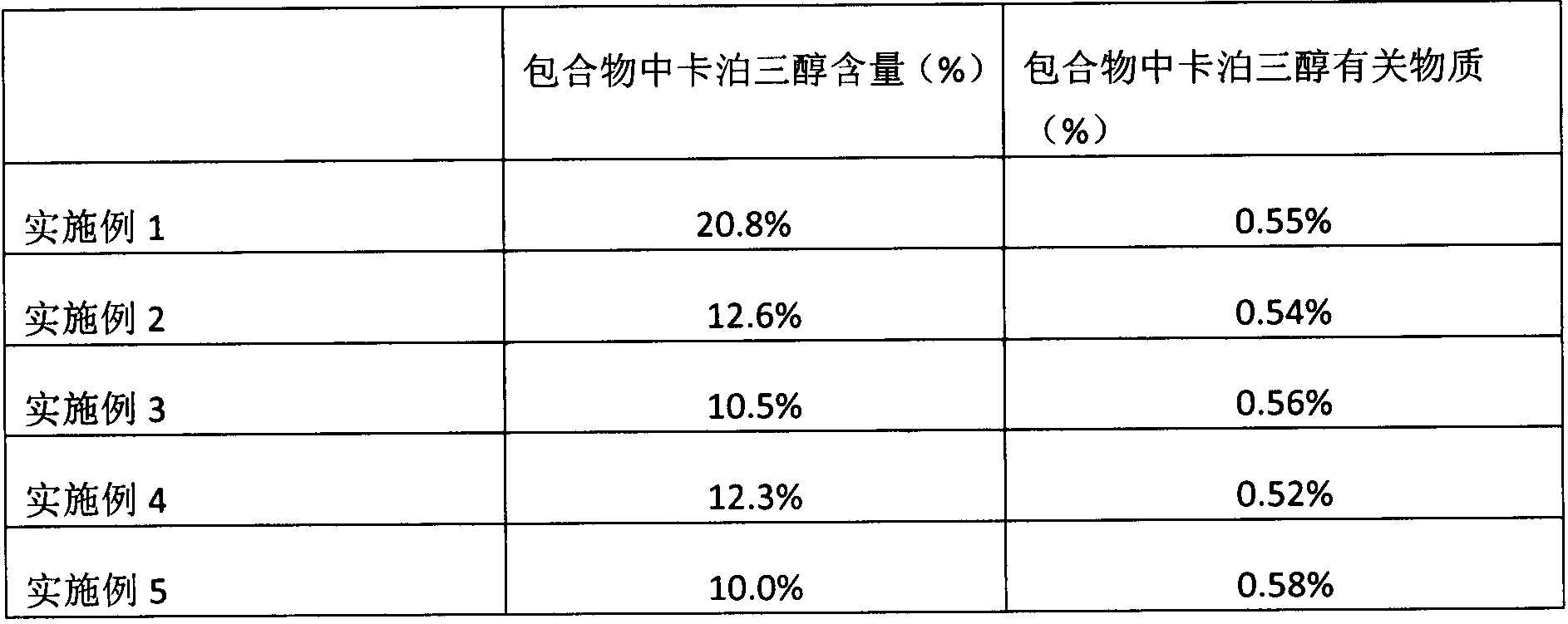
The invention relates to a calcipotriol beta-cyclodextrin inclusion compound and a preparation method thereof. The inclusion compound comprises 4.5%-21% of calcipotriol. The preparation method comprises step of adding one volume of 0.3mol / L calcipotriol ethanol solution into 15-20 volumes of 0.012-0.016mol / L beta-cyclodextrin aqueous solution for clathration. The preparation method finally prepares the calcipotriol inclusion compound meeting the requirement of medicines in grain size and uniformity. The inclusion compound can be further prepared into cream, ointment and suspension solution. Compared with non-covered calcipotriol, the calcipotriol beta-cyclodextrin inclusion compound and the preparations prepared from the inclusion compound are remarkably improved in stability, and the problem of uneven content caused by trace feeding capacity is solved.
Owner:JIANGSU SEMPOLL PHARMA
Preparation method of calcipotriol
ActiveCN105753758AAvoid it happening againReduce production efficiencyOrganic chemistryBulk chemical productionPhotosensitizerOrganic solvent
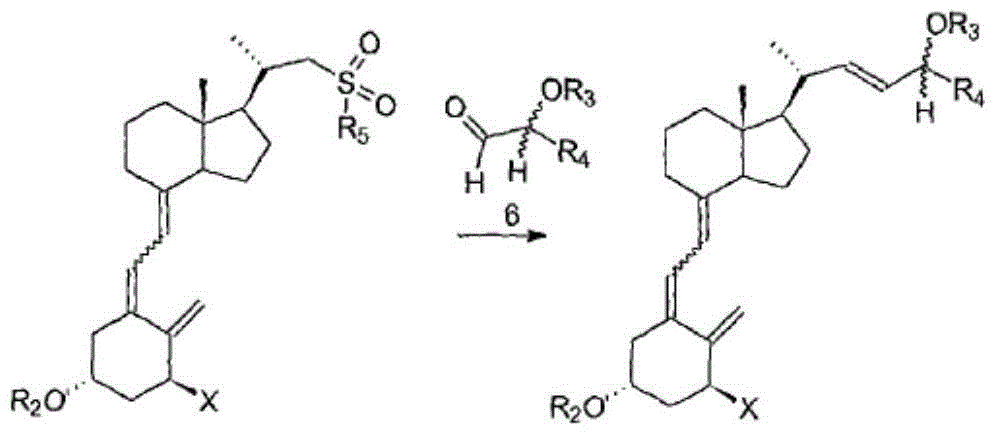
The invention discloses a preparation method of calcipotriol. The method comprises the following steps: step (1), a compound represented by the formula III reacts with a compound represented by the formula I in an inert organic solvent in the presence of alkali to generate a compound represented by the formula XV; step (2) the compound represented by the formula XV is subjected to a photoreaction in toluene in the presence of a photosensitizer to generate a compound represented by the formula XVI; and step (3) the compound represented by the formula XVI is used to prepare calcipotriol compound represented by the formula II in an organic reagent in the presence of a hydroxyl protective group removal reagent. The formula I, formula II, formula III, formula XV, and formula XVI are shown in the description.
Owner:SHANGYU JINGXIN PHARMA +2
Novel pharmaceutical composition for topical treatment of skin psoriasis and the treatment method thereof
The present invention provides the combination of topical 3,3,′5,5′-tetraiodothyroacetic acid and betamethasone for the treatment of skin psoriasis results in a better outcome than the combination of topical calcipotriol and betamethasone. The present invention also provide a method for treatment of skin psoriasis by using the composition of 3,3,′5,5′-tetraiodothyroacetic acid and betamethasone.
Owner:HEINO PEKKA
Compositions and methods for treating hyperproliferative epidermal diseases
InactiveUS9173835B2Relieve symptomsHighly efficaciousBiocideHydroxy compound active ingredientsDiseaseVitamin D metabolism
The present invention provides compositions and methods for use in the treatment of hyperproliferative dermal diseases. Specifically, the present invention teaches pharmaceutical compositions for topical administration where the compositions contain nicotinamide and a vitamin D metabolite, calcipotriol, which are particularly effective in treating and in the maintenance treatment of psoriasis and other related dermal disorders and diseases.
Owner:DERMIPSOR LTD
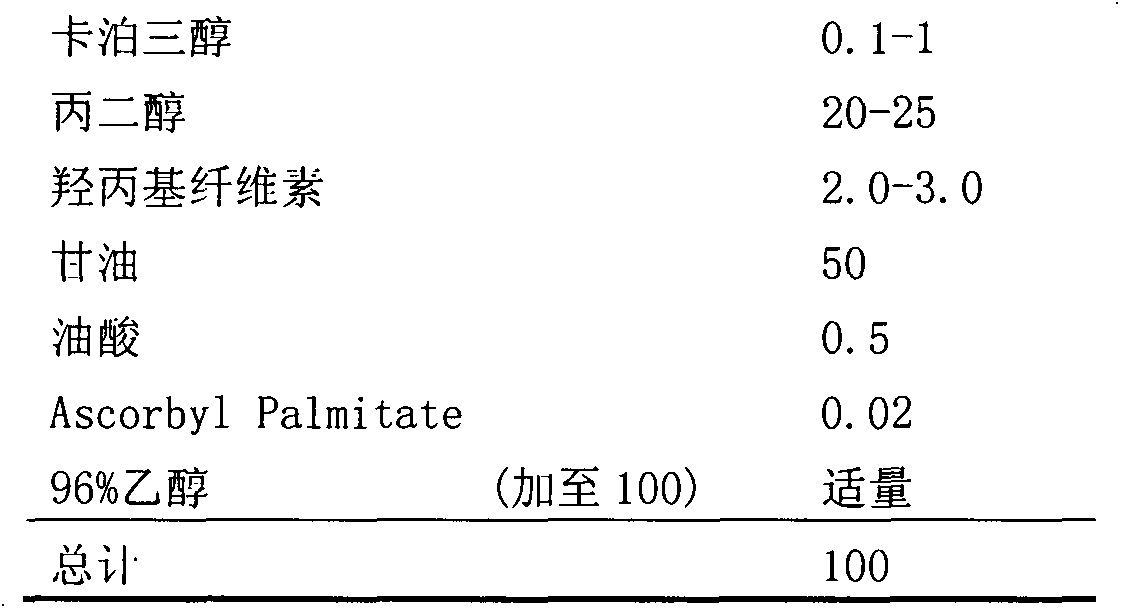
Calcipotriol non-water gel
ActiveCN104138352AGood drug releaseImprove bioavailabilityOrganic active ingredientsAerosol deliveryMedicineCurative effect
A composition for local delivery of calcipotriol is disclosed. The composition comprises (a) not more than 1 wt% of calcipotriol, (b) ethanol, (c) propylene glycol, (d) oleic acid, (e) hydroxy propyl cellulose, (f) glycerol, and (g) 0-5 wt% of water, with the contents being based on the total weight of the composition. The composition has characteristics of high curative effects, low toxicity, stability, capability of guiding or reinforcing delivery of active medicines to skin, and high therapeutic index. The invention also relates to methods of manufacturing and using the composition.
Owner:JIANGSU SEMPOLL PHARMA
Medicine composition for treating psoriasis
ActiveCN105534996AOintment deliveryPharmaceutical non-active ingredientsCurative effectMedical prescription
The invention discloses a medicine composition for treating psoriasis. The medicine composition is characterized by being prepared from, by weight, 1-10 parts of calcipotriol, 1-10 parts of hydroxyurea, 1-20 parts of glycerol monostearate, 1-20 parts of fat-soluble matrix, 1-20 parts of an emulsifier, 5-20 parts of a moisturizing agent, 1-8 parts of a transdermal absorbent and 20-70 parts of purified water. The medicine composition is scientific in formula and simple in preparation technology and has the remarkable treatment curative effect on psoriasis vulgaris and psoriasis inveterate.
Owner:CHANGSHA BAISHUN BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com